Abstract
Pre-dispersal predation of seeds of exotic Asteraceae by foraging birds is understudied. Using phenological records and photo-assisted analysis of damages made to seed heads, this paper provides evidence that the black-faced canary Serinus capistratus Finsch and Hartlaub feeds on immature achenes of Tithonia diversifolia (Hemsley) A. Gray and may control its propagule pressure up to 18% of the total number of achenes aged between 20–30 days counted from petal fall.
Introduction
Tithonia diversifolia (Hemsley) A. Gray (also hereafter referred to as TD) was first introduced into Rwanda during the colonial period as an ornamental plant. It soon escaped from cultivation and became naturalized in agro-systems, parklands, and fallows under 2000 m of altitude, where it forms evergreen hedges and invasive bushes (Troupin Citation1985). Although its modern distribution grain and extent has not been investigated, this plant seems to have already crossed the thresholds for an invasive status since early 1970s. Elsewhere in the region, it appears on the checklist of noxious plants in neighboring Democratic Republic of Congo (Ministère de l'Environnement, Conservation de la Nature et Tourisme [Ministry of Environment, Nature Conservation and Tourism] Citation1997, our translation) and composes the invasive flora in Burundi (Bigirimana et al. Citation2012) and in eastern Uganda (Eilu et al. Citation2007) as well as in southern Nigeria (Ayeni et al. Citation1997), and in Côte d’Ivoire (Ipou et al. Citation2011).
Well adapted to wetlands environments, where it readily colonizes agro-systems and young fallows (Ipou et al. Citation2011), TD has the ability to outcompete other plants and reduce the importance of native taxa that once characterized the areas it conquers. Incredibly high per stem seed production is one of the characteristics cited as best predictors of its high capacity of range expansion. In addition, within agro-systems, its ability to regenerate from roots or cuttings makes it a requirement to uproot the plants if the weeding activity is to be effective (Ipou et al. Citation2011).
Regulation of TD biomass accumulation through utilization and predation has been documented. In fact, the use of TD as a source of fodder (Ramírez-Rivera et al. Citation2010) and manure (Nabahungu et al. Citation2011) may contribute to reducing its biomass and energy potential. Foraging cattle in Kenya (Roothaert & Franzel Citation2001) and goats in Rwanda’s fodder scarce regions (Pers. Obs.) were also observed browsing on TD, worsening its struggle to maintain self-sustained populations and spread across the landscape (Catorci et al. Citation2014). On the other hand, it was suggested that insect species such as the leaf-feeding butterfly Chlosyne sp. (Lepidoptera: Nymphalidae) could be used to biologically control its ability to spread (Simelane et al. Citation2011). However, these uses are extremely limited, or non-existent, in the study area and could not be used to explain why this plant took relatively long time to invade this valley, in spite of the seemingly high suitability of the area to TD establishment. This question had remained unanswered until when Serinus capistratus Finsch and Hartlaub (the black-faced canary or, for the purpose of this study, BFC) was observed feeding on immature seeds of this plant (), which gave a clue to the ‘reduced propagule pressure’ hypothesis. Since then it has been suggested that the predation of TD seeds by this species of bird at their immature stage may adversely affect its seed production, which is well in accord with Muoghalu (Citation2008) who indicated that TD invasiveness would be better dealt with by controlling its seedling recruitment and Pearson et al. (Citation2011) who suggested that the resistance of a plant community to an invasion may result from the reduction of the invader’s seed stock through granivory. The role of seed predation as a predictor of biotic resistance to plant invasion was also discussed in Nuñez et al. (Citation2008) for invasive pines in South America and by Preukschas et al. (Citation2014) for grassland species in Switzerland.
This paper provides evidence that BFC feeds on immature achenes of TD. It also assesses the extent of removal of TD seeds by this species of bird as a measure of its contribution to controlling the propagule pressure of this plant.
Materials and methods
Study area
The study was carried out in Rwanda, Central Africa, between 1°03−2°50 South latitude and 28°52−30°54 East longitude. Field data collection was conducted within the fourth sampling grid cell () along the riparian communities of Nyabarongo River, within the official boundaries of Kigali, the capital city of the Republic of Rwanda. The study area falls within both the Albertine Rift, a globally-recognized biodiversity hotspot with many endemic and endangered species (Plumptre et al. Citation2007), and the Nile River and Lake Victoria Basin, one of world’s great rivers and inland lakes. Since 2010, Nyabarongo and associated wetlands have been classified by the Ministerial Order No 008/16.01 of 13/10/2010 in the category of those to be offered fully protection. The process of assigning part of these wetlands the RAMSAR site statute has been under consideration.
Data collection
Two patches of TD of approximately 25 m2 each were selected in the fourth sampling grid cell () and daily monitored for 12 weeks to cover the 4-month long seed production cycle (Ayeni et al. Citation1997; Muoghalu Citation2008). Flower and fruit heads were diligently counted on three sample stems per patch, each time separating canary-damaged from non-damaged seed heads. Photo snapshots were taken as supporting evidence for BFC forage on TD.Footnote1 Plant nomenclature followed Troupin (Citation1985). Bird identification was based on Stevenson and Fanshawe (Citation2002).
Data analysis
Collected data were stratified into weekly averages and normalized by converting raw numbers into proportions. The rate of achene removal (RA ) by BFC at any stage was computed using the formula below: RA (%) = 100 A × C, where A stands for the proportion of removed achenes per capitulum and C represents the proportion of canary-damaged seed heads. The rate of seed removal (RS ) was derived by RS = RA × S, with S representing the seed set.Footnote2 Pearson’s Correlation test was used to analyze the possible relationship between canary-damaged and non-damaged seed heads. The normality of data was assessed using the Shapiro-Wilk test.
Assumptions and limitations
Building upon direct observations of BFC caught feeding on TD immature achenes (weeks 7−10; ) and photo-assisted analysis of patterns of seed head damage (), this study assumes that the bulk – and possibly totality − of pre-dispersal removal of immature seeds of TD that occurred between end of December and mid-February can be attributed to predation by BFC. This assumption is supported by the fact that during the entire period of data collection, no other seedeater of seed destroyer was observed foraging on the monitored TD patches during the 12-week long period of data collection. Only the presence of immature empty seed heads was related to predation by BFC because otherwise they could obviously be the result of normal dispersal of mature seeds had the heads been mature.
Due to financial constraints, the study of differences between early and late mature seed morphology, which could have helped analyze BFC feeding behavior in relation TD seed traits at different stages of the fructification event, was not conducted.
Results
The presence of immature seed-bearing heads was recorded from week 2. However, canary-caused damage on any flower heads was observed after week 7 and concerned achenes aged between 20–30 days counted from petal fall. Since then, the cumulative number of damaged seed heads continued to increase until week 11, when the rate of increase was significantly reduced (). The overall rate of seed removal was estimated at 18.3% of the total number of achenes formed between weeks 1 and 12 (RA = 26.11%). All seed heads resulting from flower heads that developed after 15 January (after week 8) did not show any sign of damage by BFC.
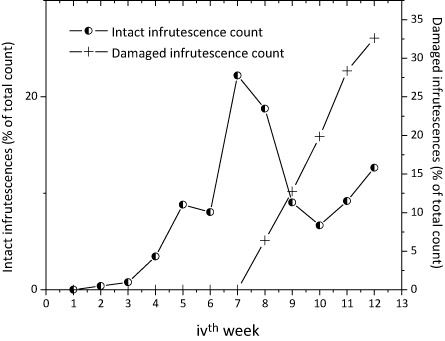
A Pearson’s Correlation test performed over the full range of data showed a very weak (statically not significant) relationship between intact and canary-damaged seed heads counts (r 2 N = 8 = – 0.221, p = 0.5). However, partitioned correlation tests revealed a strongly negative and statistically significant relationship between the two distributions from week 7 to week 10 (r 2 N = 4 = −0.967, p = 0.03); that is, for the period during which the predation of TD seeds was most apparent.
Discussion
Pre-dispersal predation of seeds of Asteraceae has been studied (Cummings et al. Citation1999; Fenner & Lee Citation2001) and its impact on the host-plant species’ reproductive ecology documented (Briese Citation2000; Weppler & Stöcklin Citation2006). On the other hand, pre-dispersal seed predation by bird species has also been reported, in some instances with enormous seed loss (Villaseñor-Sánchez et al. Citation2010). However, pre-dispersal seed predation of exotic Asteraceae by foraging birds is understudied.
This study provided evidence for pre-dispersal predation of seeds of exotic TD (Hemsley) A. Gray (Asteraceae) by the native BFC S. capistratus Finsch and Hartlaub (Fringillidae). BFC was observed feeding on TD (). TD heads were emptied before seeds could reach maturity, 20–30 days from petal fall (). Because seed loss was localized in time (a statistically significant negative correlation was found between intact and damaged heads between weeks 7 and 10), the event of immature seeds disappearance was not attributable to normal dispersal ().


The ecological interaction between BFC and TD is quite revealing in the light of the ‘enemy release’ hypothesis, supporting the idea that despite their escape from natural enemies (Keane & Crawley Citation2002; Colautti et al. Citation2004), alien species may still be vulnerable to enemies found in their introduced range (Nuñez et al. Citation2008).
It is arguable that BFC interferes with the regeneration capacity of TD by feeding on its immature achenes. The rate of seed removal was high enough (18%) for the event to be considered as leading to a significant bias of TD propagule pressure, which, in accord with Steeves et al. (Citation2008) may pose a threat to TD reproductive investment, disrupt its normal course of seed dispersal (Yoshikawa et al. Citation2012), and affect its seedling recruitment (Pitcairn et al. Citation2011). Indeed, the above-described pattern of seed predation is critical to TD capacity of range expansion given the fact that the idea of TD being rewarded by BFC in the form of endozoochorous dispersal of seeds is not envisageable in this case because not only the seeds are eaten before maturity but also have little chance of not being destroyed in the bird’s gizzard.
Because BFCs do not destroy the entire amount of achenes produced ( and ), all else being equal, it could be hypothesized that established populations of TD will have to expand modestly until a threshold beyond which the bird’s influence (on TD potential for seed rain) is overwhelmed. Once that step is passed, TD may become a very serious invader in the study area and possibly one of the valley’s most prominent plant species.
The study sheds some light on a relationship between the date of appearance of a new flower head and the likelihood of the resulting seed head to be attacked by BFC. However, it was not conclusive about the predictors of the likelihood of any TD seed head to be attacked by BFC (Haught & Myster Citation2008). The impact of seed removal by BFC on TD invasiveness as well as the role of its ability to clonally propagate in balancing the effects of seed loss should be empirically researched (Wang et al. Citation2004; Weppler & Stöcklin Citation2006). Finally, understanding that intensity of seed predation may significantly vary over time and among microhabitats within BFC home range (Myster Citation2013), further study should be conducted to investigate TD seed removal in relation to spatial and temporal patterns of TD seed set (Wang et al. Citation2004) and BFC density (Haught & Myster Citation2008).
Conclusion
Through this paper, it was shown that, despite their escape from natural enemies, introduced species may still be vulnerable to enemies native to the introduced range. The study provided evidence that native BFC feeds on immature seeds of exotic TD and contributes to weakening its ability to spread by reducing its seed rain by 18%.
New research questions were opened. These include the study of the impact of seed removal by BFC on TD invasiveness, the role of TD ability to clonally propagate in balancing its seed loss as well as the study of TD seed removal in relation to spatial and temporal patterns of TD seed set and BFC density.
Acknowledgments
The study was conducted with academic support from Dr Beth A. Kaplin (Antioch University New England), Dr Elias Bizuru (University of Rwanda), and Dr Edward N. Mwavu (University of Makerere). Mrs Theodette Gatesire, Research Assistant at the Dian Fossey Gorilla Fund International, offered field assistance throughout the study period and was resourceful in bird identification.
Notes
1. By seed head we refer to a type infrutescence that results from a fertilized flower head (typical inflorescence in the Compositae family in which flowers are grouped together to form a single flower-like structure; Imbert & Ronce Citation2001), which in reality should be referred to as a ‘fruit head’ because the achenes it carries are not seeds but fruits. However, for the purpose of this study, the terms ‘seed head’ and ‘fruit head’ are used interchangeably because predation took place when the achenes were still immature and, consequently, the seeds they contained would not be viable should they manage to survive food grinding in the bird’s digestive tractus.
2. TD seed set in tropical and subtropical environment was estimated at 70% or more by Wang et al. (Citation2004).
References
- Ayeni AO, Lordbanjou DT, Majek BA. 1997. Tithonia diversifolia (Mexican sunflower) in south-western Nigeria: occurrence and growth habit. Weed Res. 37:443–449. 10.1046/j.1365-3180.1997.d01-72.x
- Bigirimana J, Bogaert J, De Cannière C, Bigendako M-J, Parmentier I. 2012. Domestic garden plant diversity in Bujumbura, Burundi: role of the socio-economical status of the neighborhood and alien species invasion risk. Landscape Urban Plann. 107:118–126. 10.1016/j.landurbplan.2012.05.008
- Briese DT. 2000. Impact of the Onopordum capitulum weevil Larinus latus on seed production by its host-plant. J Appl Ecol. 37:238–246. 10.1046/j.1365-2664.2000.00489.x
- Catorci A, Antolini E, Tardella FM, Scocco P. 2014. Assessment of interaction between sheep and poorly palatable grass: a key tool for grassland management and restoration. J Plant Interact. 9:112–121. 10.1080/17429145.2013.776706
- Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ. 2004. Is invasion success explained by the enemy release hypothesis? Ecol Lett. 7:721–733. 10.1111/j.1461-0248.2004.00616.x
- Cummings CL, Alexander HM, Snow AA. 1999. Increased pre-dispersal seed predation in sunflower crop-wild hybrids. Oecologia. 121:330–338. 10.1007/s004420050936
- Eilu G, Oriekot J, Tushabe H. 2007. Conservation of indigenous plants outside protected areas in Tororo District, eastern Uganda. Afr J Ecol. 45:73–78.
- Fenner M, Lee WG. 2001. Lack of pre-dispersal seed predators in introduced Asteraceae in New Zealand. N Z J Ecol. 25:95–99.
- Haught JE, Myster RW. 2008. Effects of species, density, season and prairie-type on post-dispersal seed removal in Oklahoma. Am Midland Nat. 159:482–488. 10.1674/0003-0031(2008)159[482:EOSDSA]2.0.CO;2
- Imbert E, Ronce O. 2001. Phenotypic plasticity for dispersal ability in the seed heteromorphic Crepis sancta (Asteraceae). Oikos. 93:126–134. 10.1034/j.1600-0706.2001.930114.x
- Ipou J, Toure A, Adou ML, Kouame KF, Gue A. 2011. A new invasive species of the agrosystems in the south of Côte d’Ivoire: Tithonia diversifolia (Hemsl.) A. Gray (Asteraceae). Afr J Food Sci Technol. 1:146–150.
- Keane RM, Crawley MJ. 2002. Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol. 17:164–170. 10.1016/S0169-5347(02)02499-0
- Ministère de l'Environnement, Conservation de la Nature et Tourisme [Ministry of Environment, Nature Conservation and Tourism]. 1997. Rapport intermédiaire sur la mise en oeuvre de la convention relative à la biodiversité en Republique Democratique du Congo [Mid-term report on the implementation of the convention on biodiversity in the Democratic Republic of Congo]. Technical Report, Democratic Republic of Congo (Kinshasa). Montreal, Canada: CBD (Convention on Biological Diversity) Secretariat.
- Muoghalu JI. 2008. Growth, reproduction and resource allocation of Tithonia diversifolia and Tithonia rotundifolia. Weed Res. 48:157–162. 10.1111/j.1365-3180.2007.00613.x
- Myster RW. 2013. The role of seed predation in the maintenance of the cross timbers ecotone of Oklahoma, USA. J Plant Interact. 8:134–139. 10.1080/17429145.2012.707234
- Nabahungu NL, Mowo JG, Uwiragiye A, Nsengumuremyi E. 2011. Use of Tithonia biomass, maize residues and inorganic phosphate in climbing bean yield and soil properties in Rwanda. In: Bationo A, Waswa B, Okeyo JM, Maina F, Kihara JM, editors. Innovations as key to the green revolution in Africa. Dordrecht: Springer; p. 335–341.
- Nuñez MA, Simberloff D, Relva MA. 2008. Seed predation as a barrier to alien conifer invasions. Biol Invasions. 10:1389–1398. 10.1007/s10530-007-9214-x
- Pearson DE, Callaway RM, Maron JL. 2011. Biotic resistance via granivory: establishment by invasive, naturalized, and native asters reflects generalist preference. Ecology. 92:1748–1757. 10.1890/11-0164.1
- Pitcairn MJ, Woods DM, Popescu V. 2011. Impact of pre-dispersal seed predation on seedling recruitment by yellow starthistle in California. In: Wu Y, Johnson T, Sing S, Raghu S, Wheeler G, Pratt P, Warner K, Center T, Goolsby J, Reardon R, editors. XIII International symposium on biological control of weeds. Waikoloa (HI): USDA Forest Service; p. 472.
- Plumptre AJ, Davenport TRB, Behangana M, Kityo R, Eilu G, Ssegawa P, Ewango C, Meirte D, Kahindo C, Herremans M, et al. 2007. The biodiversity of the Albertine Rift. Biol Conserv. 134:178–194. 10.1016/j.biocon.2006.08.021
- Preukschas J, Zeiter M, Fischer M, Stampfli A. 2014. Biotic resistance to plant invasion in grassland: does seed predation increase with resident plant diversity? Basic Appl Ecol. 15:133–141. 10.1016/j.baae.2014.01.004
- Ramírez-Rivera U, Sanginés-García JR, Escobedo-Mex JG, Cen-Chuc F, Rivera-Lorca JA, Lara-Lara PE. 2010. Effect of diet inclusion of Tithonia diversifolia on feed intake, digestibility and nitrogen balance in tropical sheep. Agroforestry Syst. 80:295–302. 10.1007/s10457-010-9320-0
- Roothaert RL, Franzel S. 2001. Farmers’ preferences and use of local fodder trees and shrubs in Kenya. Agroforestry Syst. 52:239–252. 10.1023/A:1011896921398
- Seburanga JL, Kaplin BA, Bizuru E, Mwavu EN. 2013. The folk-biology of South American-native shrub, Mimosa pigra L. [Leguminosae] and its invasive success in Rwanda. Int J Biodivers Conserv. 5:486−497.
- Simelane DO, Mawela KV, Fourie A. (2011). Prospective Agents for the Biological Control of Tithonia rotundifolia (Mill.) S.F. Blake and Tithonia diversifolia (Hemsl.) A. Gray (Asteraceae) in South Africa. Afr Entomol. 19:443–450.
- Steeves R, Nazari V, Landry J-F, Lacroix CR. 2008. Predispersal seed predation by a coleophorid on the threatened Gulf of St. Lawrence aster. Can Entomol. 140:297–305. 10.4039/n07-040
- Stevenson T, Fanshawe J. 2002. Field guide: birds of East Africa (Kenya, Tanzania, Uganda, Rwanda, Burundi). London: T. & A. D. Poyser Ltd.
- Troupin G. 1985. Flore du Rwanda: spermatophytes III. Tervuren: Musée Royal de l’Afrique Centrale.
- Villaseñor-Sánchez EI, Dirzoa R, Rentona K. 2010. Importance of the lilac-crowned parrot in pre-dispersal seed predation of Astronium graveolens in a Mexican tropical dry forest. J Trop Ecol. 26:227−236. 10.1017/S0266467409990447
- Wang SH, Sun WB, Cheng X. 2004. Attributes of plant proliferation, geographic spread and the natural communities invaded by the naturalized alien plant species Tithonia diversifolia in Yunnan, China. Acta Ecol Sin. 24:444−449.
- Weppler T, Stöcklin J. 2006. Does pre-dispersal seed predation limit reproduction and population growth in the alpine clonal plant Geum reptans? Plant Ecol. 187:277–287. 10.1007/s11258-006-9141-4
- Yoshikawa T, Masaki T, Isagi Y, Kikuzawa K. (2012). Interspecific and annual variation in pre-dispersal seed predation by a granivorous bird in two East Asian hackberries, Celtis biondii and Celtis sinensis. Plant Biol. 14:506–514. 10.1111/j.1438-8677.2011.00528.x
