Abstract
We tested the hypothesis that reducing the carbon (C):Phosphorus (P) ratio in rhizosphere soil would reduce bacterial competition with the plant for P from phytin, which would then increase phytin use efficiency for the plant. A three-factor pot experiment was carried out to study the effect of inoculation with a phytin-mineralizing bacterium, Pseudomonas alcaligenes (PA), on maize P uptake from phytin. Two levels of organic P, two levels of inorganic P, and three different PA inoculation treatments were used. When maize plants were grown in low available P soil with phytin, PA transformed soil P into microbial biomass P, which caused competition for available P with plant and inhibited plant uptake. When 5 mg P kg−1 as KH2PO4 was added, inoculation with PA increased soil acid phosphatase activity which enhanced the mineralization rate of phytin. PA mobilized more P than it immobilized in microbial pool and enhanced plant P uptake. We conclude that the decreased C:P ratio by adding small amount of inorganic P in the rhizosphere could drive phytin mineralization by the bacteria and improve plant P nutrition.
Introduction
Plants harbor specific bacterial communities in their rhizospheres (Lundberg et al. Citation2012) that play crucial roles in mediating various soil chemical and biochemical processes, e.g. phosphorus (P) and nitrogen (N) transformations (Richardson Citation2001; Hayatsu et al. Citation2008). In recent years there has been increasing concern over P as it is becoming the limiting resource in agriculture (Vance et al. Citation2003; Cordell et al. Citation2009; Veneklaas et al. Citation2012). In the field P mainly exists in sparingly available forms e.g. organic P, which contributes a large proportion of the total soil P (Harrison Citation1987) and is also usually adsorbed by soil clays, in the form of humic-associated P and precipitates (Richardson & Simpson Citation2011). It is therefore of vital importance to harness the functions of bacteria to mobilize organic P for plant utilization.
Rhizosphere soils are usually P deficient (Gahoonia & Nielsen Citation1992; Zoysa et al. Citation1997) and bacteria are involved in two reverse processes in organic P turnover at the plant–soil interface. On the one hand, more than 40% of culturable bacteria have the ability to mobilize organic P (Jorquera et al. Citation2008) to release orthophosphates, usually H2PO4 − and HPO4 2−, which can be absorbed by roots (Rodriguez & Fraga Citation1999). On the other hand, bacteria may transform available P into microbial biomass P (MBP) using soil organic carbon (C; Wu et al. Citation2007). Plant root exudates provide readily available C substrates for bacteria (Hodge et al. Citation1997) that stimulate bacterial growth and therefore increase MBP in the rhizosphere. Therefore, regulating these two processes in the rhizosphere to make bacteria mobilize more insoluble phosphates from the soil but immobilize less themselves is the central issue in enhancing soil P use efficiency.
Introducing inoculants of phosphate solubilizing bacteria (PSB) into the soil is considered to be an efficient way to increase the availability of organic or insoluble inorganic phosphates. Studies of inoculation with PSB have been widely conducted under laboratory or controlled glasshouse conditions (Shobirin et al. Citation2009; Krey et al. Citation2011). However, limited success has been achieved to date in the field because of our poor understanding of the biological processes of the inoculated bacteria in the soil, and future opportunities with specific microbial inoculants require ecological consideration of their interaction with plant roots and rhizosphere microbes (Richardson & Simpson Citation2011).
The interactions between roots and PSB have been recognized for some time. PSB interact with plant roots intimately and their relationship to plant P uptake can be both positive and negative to cause a change in allocation of soil available P between plants and bacteria. Such processes can be regulated by the ratio of soil organic C and available P content (Marschner Citation2008). In high C:P conditions bacteria have a net immobilization effect on soil P while in low C:P conditions they have a net mobilization effect (Stevenson Citation1986). Therefore, adding organic C to the soil can increase the ratio of C:P and has been demonstrated to increase MBP and subsequently decrease plant-available P. Marschner (Citation2007) reported that adding glucose to the soil stimulated the bacterial transformation of available P into MBP, indicating that MBP competed for available P with plants. As C was depleted and C:P declined, the bacteria-fixed P would be released from the MBP pool and became available for the plant again. On the other hand, adding P-rich compounds to the soil can decrease the C:P ratio and increase soil available P by promoting organic P mineralization. Spohn and Kuzyakov (Citation2013) found that when glucose-6-phosphate, which has a low C:P of 2.3, was added to the soil it enhanced P mineralization that was driven by the microbial need for C. However, to our knowledge, there is no direct evidence to show how and to what extent the rhizodeposition and available P ratio affect the allocation of soil organic phosphates between plants and bacteria in the rhizosphere.
Starter P fertilizer technology, applying a small amount of inorganic P fertilizer near the seed, is widely used in crop production to ensure good seedling establishment and, ultimately, crop yield (Costigan Citation1984; Cahill et al. Citation2008). The applied P fertilizers increase soil available P for plants (Attar et al. Citation2012; Djodjic & Mattsson Citation2013) and subsequently decrease the C:P ratio in the rhizosphere. In addition, increasing soil available P can also directly (DeForest et al. Citation2012) or indirectly affect the C:P ratio by reducing the amount of carbohydrates released per unit of plant root (Schilling et al. Citation1998), which influences the activity of MBP and soil P turnover in the rhizosphere. However, previous studies have mainly focused on the direct function of inorganic P in improving plant P nutrition (Bittman et al. Citation2006; Cahill et al. Citation2008), neglecting its effects on inducing organic P mineralization and utilization by plants roots mediated through soil bacteria. The aim of this study was to investigate the effect of reducing the C:P ratio in the rhizosphere by adding a small amount of inorganic P on plant P uptake from phytin, which is the main form in the soil organic P pool. We hypothesized that in low available P soil bacteria compete for P with plants and thus inhibit root uptake though they can mobilize phytin to release inorganic P. However, when a small amount of inorganic P is applied to the soil, it relieves the competition because the bacteria mobilize more phytin than is immobilized and as a result increase plant-available P.
Materials and methods
Biological materials and soil
The P-efficient maize (Zea mays L.) genotype 181, which was developed for cultivation on calcareous soils by Professor Fanjun Chen in China Agricultural University, was used in this experiment. Pseudomonas alcaligenes (PA) M20 (Zhang et al. Citation2014) which can mobilize and utilize phytin was selected as the bacterial inoculum (see Supplementary Figure S1).
The growth medium used for growing plants was a mixture of clay soil (Alfisols, collected from Tai'an, Shandong, China, pHwater 6.4, air-dried and 2 mm sieved) and fine river sand (9:1, w/w). This clay soil was collected from a woodland which had not been used to grow crops before and the labile organic P (extracted by 0.5 M NaHCO3, pH 8.5) content was 3 mg kg−1. It also contains 7.27 g kg−1 organic matter, 7.2 mg kg−1 mineral N, 3.3 mg kg−1 Olsen-P (NaHCO3-extractable P), 142 mg kg−1 organic P, and 97.6 mg kg−1 NH4Cl-exchangeable K. The mixed culture medium (referred to as soil throughout) was supplemented with 200 mg kg−1 N (as KNO3), 50 mg kg−1 Mg (as MgSO4), 5 mg kg−1 Cu (as CuSO4), 5 mg kg−1 Mn (as MnSO4), and 5 mg kg−1 Zn (as ZnSO4) and then treated with 10 kGy 60Co γ-ray radiation (Beijing Radiation Application Research Center, Beijing, China) in the dry status to kill the indigenous microbes. The soil was stored two weeks after radiation to stabilize the nutrients.
Experimental design and plant growth
The experiment was conducted in a greenhouse for six weeks during April to May in 2011 with temperatures ranging from 24°C to 30°C and natural light and was setup in a randomized block design with three factors: (1) two inorganic P levels, P0 (no added P), P5 (5 mg kg−1 P added as KH2PO4); (2) two organic P levels, −phytin (0 mg kg−1 P added as phytin) and +phytin (100 mg kg−1 P added as phytin); and (3) three PA inoculation treatments, −PA (no PA inoculation), I+PA (PA inoculation at seedling initiation) and 2w+PA (PA inoculation 2 weeks after sowing). Thus, there were 12 treatments, each of which was replicated four times resulting in 48 pots in total.
The microcosm unit used in the experiment was a plastic pot with a diameter of 15 cm and a height of 20 cm and each pot received 2 kg of soil. Two kg of soil was weighed in a plastic bag for each pot and then KH2PO4 (5 mg P kg−1 soil) and/or phytin (100 mg P kg−1 soil) was added or not to each bag. After thorough mixing in the bags, the soil was transferred into the pots. Half of the pots received 5 mg kg−1 inorganic P (in the form of KH2PO4) and the other half did not receive the inorganic P. For each inorganic P treatment the pots were divided into two groups. Half of the pots received 100 mg kg−1 organic P (in the form of phytin with 19.6% P content, P0410, Tokyo Chemical Industry, Tokyo, Japan), while the other half did not receive organic P.
Maize seeds were disinfected with 10% (v/v) H2O2 for 10 min and 70% (v/v) ethanol for 3 min and then rinsed eight times with sterile deionized water. After imbibing water at 27°C in the dark for 2 d, three pre-germinated seeds were sown in each pot and thinned to one seedling 7 d after sowing. During the experiment soil moisture was kept at 18–20% (w/w, c. 70% of field moisture capacity) as determined gravimetrically by weighing the pots every two days and adding water as necessary.
The inoculum of PA was pre-cultured in a 250 ml flask with 100 ml liquid Luria-Bertani (LB) medium (with 1% bacterial inoculum) at 37°C at 180 rpm for 24 h and then diluted to 109 CFUs ml−1 with sterilized 0.85% NaCl solution. For the I+PA treatment, PA suspension was inoculated at sowing. Five ml of suspension which contained 5 × 109 CFUs was added to the pre-germinated seeds. For the 2w+PA treatment, the same amount of inoculum was inoculated around the roots of maize seedling two weeks after sowing by removing the top 2 cm soil. The roots were covered with soil afterward. In the –PA treatments, 5 ml of autoclaved PA suspension was inoculated in the same way as in the I+PA treatment.
The data for total organic C and available P contents were collected by analyzing the soil and the culture medium C:P ratio was calculated as follows: for the zero KH2PO4 and zero phytin added treatment, the medium C:P = (C in medium)/(Olsen-P in medium) = 1278; for the no KH2PO4 but phytin added treatment, the medium C:P = (C in medium + C in phytin)/(Olsen-P in medium) = 1287; for the KH2PO4 but no phytin added treatment, the medium C:P = (C in medium)/(Olsen-P in medium + P in KH2PO4) = 476; for the KH2PO4 and phytin added treatment, the medium C:P = (C in medium + C in phytin)/(Olsen-P in medium + P in KH2PO4) = 482.
Harvest and sample analysis
Plants were separately harvested into shoot and root tissues. Rhizosphere soil was collected as follows. Roots were removed from the pots and any soil loosely adhering to the roots was gently shaken off back into the pot. Soil adhering to the roots was swept up with a brush and then mixed with a blender to obtain a uniform mixture for subsequent analysis (see below). The roots were then washed with tap water to remove the possibly attached soil. Shoot and root tissues were dried at 65°C for 72 h and ground. Shoot and root P concentrations were determined by the vanadomolybdate method (Jackson Citation1958) after digestion with H2SO4 and 30% H2O2 (Thomas et al. Citation1967) from which the P contents were calculated. The rhizosphere soil was analyzed as follows.
Determination of available inorganic P was performed using Olsen's method (Olsen et al. Citation1954). MBP was determined with the chloroform fumigation–extraction method (Brookes et al. Citation1982). Briefly, MBP was calculated based on the difference in soil inorganic P before and after 24 h fumigation. A kp-value of 0.40 was used.
Phosphatases are classified into different groups basing on different methods. For example, phosphatases can be classified into acid and alkaline phosphatase basing on pH, be classified into phosphomonoesterase and phosphodiesterase basing on combined bond of the enzyme, or be classified into phytase etc. by substrate. Therefore, there are overlaps among different groups. Considering all kinds of phosphatase are pH dependence, acid phosphatase and alkaline phosphatase activity are usually used to represent the general phosphatase activity, and to assess the mineralization of organic phosphates in soil in many previous studies. Here, phosphatase activity was determined according to Tabatabai and Bremner (Citation1969) and Neumann (Citation2006). The pH of the rhizosphere soil was measured in a water solution (soil:water = 1:5, w/v) and was used to adjust the buffer pH in the phosphatase activity measurement. Soil (0.5 g) was transferred into 2 mL Eppendorf reaction-vials (using a micropipette with cut tips) and then 0.4 mL Na-acetate buffer (200 mM) and 0.1 mL p-nitrophenyl phosphate (150 mM, Sigma St. Louis, MO, USA) solution were added. After incubating for 30 min at 30°C (mixing occasionally) the reaction was terminated by addition of 0.5 mL 0.5 M NaOH. The phosphatase activity was expressed as μg p-nitrophenyl phosphate hydrolyzed per min per g dry soil.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). All data were checked for normality using the Kolmogorov–Smirnov test and Levene's test was used to check equality of variance. Three-way analyses of variance (ANOVAs) were performed to compare the effects of +/− KH2PO4, +/− phytin and different PA inoculations on plant P content and MBP and phosphatase activity in soil. To compare the effects of PA under different KH2PO4 and phytin application conditions the significance of differences between treatments was evaluated by Tukey's honestly significant difference (HSD) test t at P ≤ 0.05 in each KH2PO4 × phytin treatment.
Results
The P values of three-way ANOVAs are shown in . The results show the effects of KH2PO4, phytin, and PA inoculation treatments and their interactions on plant P content and MBP and phosphatase activity in the rhizosphere soil.
Table 1. Three-way ANOVA of measured variables.
Plant P content and phytin use efficiency
Plant P content was increased significantly by the addition of PA, KH2PO4, and phytin (PA: P = 0.046; KH2PO4 and phytin: P < 0.001; ). The significant interaction between PA and KH2PO4 shows that the effect of PA on plant P content was most pronounced when KH2PO4 was added (PA × KH2PO4: P = 0.001). PA decreased plant P content in the absence of KH2PO4 but in the presence of phytin, while it increased plant P content in the presence of both KH2PO4 and phytin (PA × KH2PO4 × Phytin: P = 0.001).
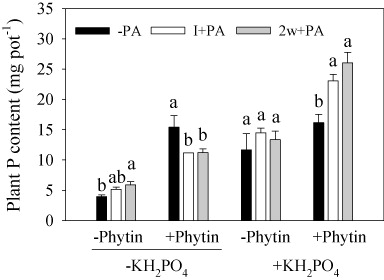
The phytin use efficiencies for maize mediated through PA were different under conditions of with or without the addition of KH2PO4 (). In the absence of KH2PO4, the −PA treatment had the highest phytin use efficiency of 5.7%, while I+PA and 2w+PA decreased it to 3.0% and 2.6%. However, in the presence of KH2PO4, the −PA treatment had the lowest phytin use efficiency of 2.2%, while I+PA and 2w+PA increased it to 4.3% and 6.3%.
Table 2. Increase in plant P content and phytin P use efficiency in plants grown in phytin amended soil with additional KH2PO4 or not.
Microbial biomass P
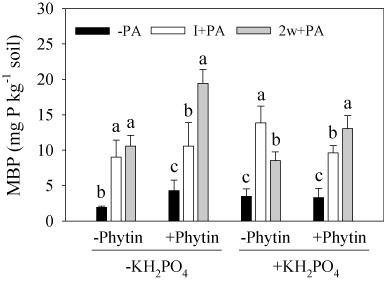
There was no significant difference in the soil MBP regardless of the presence of KH2PO4 or phytin (KH2PO4: P = 0.517; phytin: P = 0.120), while MBP was more than doubled by inoculation with PA compared with noninoculation (PA: P < 0.001; ). The addition of phytin produced a positive effect on MBP when KH2PO4 was absent (KH2PO4 × phytin: P = 0.002). Apart from these, with the addition of phytin, 2w+PA showed higher MBP than I+PA regardless of the presence of KH2PO4. Without the addition of phytin, I+PA and 2w+PA had the same MBP in the absence of KH2PO4, while I+PA showed higher MBP than 2w+PA in the presence of KH2PO4.
Phosphatase activity
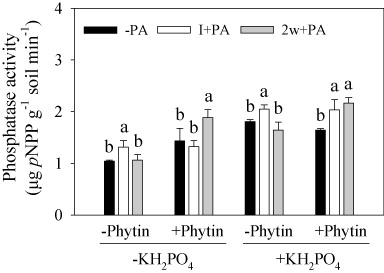
To measure phosphatase activities as local soil pH condition, we used the rhizosphere pH of each pot to adjust pH buffer which ranged from 6.3 to 6.5. Inoculating with PA had no significant influence on rhizosphere pH. All three factors, PA, KH2PO4, and phytin, had significant influence on phosphatase activities (PA, P = 0.011; KH2PO4 and phytin, P < 0.001; ). The effect of PA depended on phytin and was considerably more pronounced when phytin was absent (PA × Phytin: P < 0.001). A significant interaction between KH2PO4 and phytin (KH2PO4 × Phytin: P = 0.017) shows that phosphatase activity was increased significantly by the addition of phytin when KH2PO4 was absent.
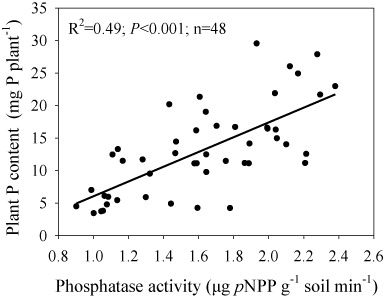
Relationship between phosphatase activity and plant P content
Plant P content was significantly correlated with soil phosphatase activity (R2 = 0.49 and P < 0.001, ).
Discussion
Despite the importance of organic P mineralization to maintain soil fertility, less is understood about the mechanisms that regulate microbial P mineralization in rhizosphere. Recent studies suggest that microbial C demand is able to drive organic P mineralization with potential benefits for plants (Spohn & Kuzyakov Citation2013) and changing soil C:P ratio by adding sugars to the rhizosphere altered MBP and the available P content in the soil (Marschner Citation2007). Here, in the maize rhizosphere, our results demonstrate, in terms of plant P uptake, that adding a small amount of inorganic P to decrease the C:P ratio in a low P soil was able to significantly change the relationship between maize roots and rhizosphere PSB from negative to positive. In the field soil, there are numerous bacteria which can mobilize organic P, and decreasing the ratio of C:P may also influence interactions among themselves, which have potential effects on plant P uptake and should be considered in the future.
Utilization of phytin by maize
Phytate-P may constitute up to 80% of organic P in soil (Turner et al. Citation2002) which represents a large potential P source for plants but it must be hydrolyzed by the phosphatase exuded from plants or soil microbes to release orthophosphate before it can be used by plants and/or microorganisms (Richardson et al. Citation2000). In our experiment adding phytin increased the plant P content () and dry weight (Supplementary Table S1) of maize plants significantly. Phosphatase activity in the rhizosphere soil showed a significantly positive linear correlation with plant P content (). These results indicate that the added phytin was hydrolyzed by phosphatase and then the inorganic phosphate released was absorbed by maize plants and thereafter increased their growth.
Maize P uptake mediated through PA at different KH2PO4 addition rates
To investigate the mechanisms of nonsoluble phosphate mobilization by PSB in the soil and their contribution to plant P uptake the culture substrates are usually pretreated with steam or 60Co gamma radiation to reduce the influence of indigenous soil microorganisms (Wolf & Skipper Citation1994; Richardson et al. Citation2000). Otherwise, they will compete with the inoculated PSB and inhibit their colonization in the soil (Richardson & Simpson Citation2011). In our present study, though phosphatase activities were detected in the −PA treatments which may be derived from root exudates of maize and bacteria in dry-precipitation or irrigation water, inoculation with PA had significant higher phosphatase activities than –PA. Such result suggested that inoculation with PA gave substantial contribution to the increase of phosphatase activities (; ). In addition, soil available inorganic P (Olsen-P) also increased by more than 10 mg kg−1 due to addition of phytin (Supplementary Figure S2). Addition of 5mg kg−1 KH2PO4 might give some contribution to the increasing of available P, but the contribution should be small in such a clay soil. Firstly, clay soil normally has relative high P fixing capacity. Secondly, the addition of KH2PO4 was far less than the increase of available P. Therefore, these above results proved that the inoculated PA played main role in enhancing the mineralization rate of phytin by increasing phosphatase activity, which might improve plant P nutrition (). Many plants, including maize, have limited capacity to obtain P directly from phytate in soil because they do not produce and exude phytase to the rhizosphere (Hayes et al. Citation1999; Richardson et al. Citation2000). Therefore, the successful colonization of PSB in the rhizosphere is important for phytate utilization by plants.
MBP was higher in both PA inoculation treatments compared with −PA treatment, especially in 2w+PA treatment, regardless of KH2PO4 addition (). This result suggests that though PA promoted phytin-P mineralization by increasing phosphatase activities () it also converted a substantial amount of the soil P into MBP, which might compete with the plant for P. MBP represents a potential bioavailable P source (Brookes et al. Citation1982) that can prevent P fixing by soil clays and can be released again for root acquisition when the soil conditions change, e.g. drying, freezing, or decomposition of organic matter (Ross et al. Citation1999; Chen et al. Citation2002; Butterly et al. Citation2009). Converting biological P sources to chemical P sources for plant uptake is of interest in enhancing plant P nutrition (Richardson & Simpson Citation2011).
Maize P uptake from phytin was affected to different degrees by inoculated PA at different KH2PO4 addition rates (). Compared with –PA treatment, both I+PA and 2w+PA treatments decreased plant P content and shoot dry weight when no KH2PO4 was added while they increased plant P content and shoot P concentration when 5 mg P kg−1 KH2PO4 was added (, Supplementary Table S1). This indicates that the relationship between PA and maize P uptake was regulated from negative to positive by adding a small amount of inorganic P. This regulation was related to the changes in phosphatase activity and MBP caused by the addition of KH2PO4 ( and ). It is generally accepted that the phosphatase activity increases when soil solution inorganic concentration is low and is inhibited by high solution inorganic P concentration (Speir & Ross Citation1978). But Radersma and Grierson found phosphatase activity in the rhizosphere of the plants in their study was not suppressed in the soils amended with P (Radersma & Grierson Citation2004). Our results also showed that applying inorganic P in the low available P soil could increase the bacteria derived phosphatase activity by stimulating the bacterial growth. This suggests that addition of inorganic P to the rhizosphere not only directly supplies available P to plant roots but also indirectly enhances the bioavailability of soil organic P via priming soil PSB activity and thereafter improves plant P nutrition.
Regulating the plant–bacterium relationship by changing the C:P ratio
Maize roots can exude sugars, carboxylates, amino acids, and other trace substances into the rhizosphere when they are suffering P deficiency (Schilling et al. Citation1998). These compounds can be used as active C for bacteria and promote their growth which is defined as a ‘priming effect’ (Kuzyakov et al. Citation2000). Root exudates increase the ratio of C:P in the rhizosphere, which regulates the mobilization and immobilization of P by PSB. At a low C:P ratio, bacteria mobilize more P than they immobilize and enhance plant P uptake, while at a high C:P ratio bacteria immobilize more P than they mobilize and inhibit plant P uptake (Song et al. Citation2003). Our results show that in the low P soil without addition of KH2PO4, inoculation with PA caused competition for P with maize and thereafter decreased P content of maize () at a relative higher C:P ratio. However, addition of 5 mg kg−1 KH2PO4 decreased the C:P ratio in rhizosphere and thereafter promoted P acquisition of maize from soil organic P pool ().
Conclusions
Starter P fertilizer, e.g. applying a small amount of uniformly mixed fertilizer and placed fertilizer which also contains inorganic P, is widely used in crop production to ensure good seedling establishment and, ultimately, crop yield (Costigan Citation1984; Cahill et al. Citation2008). These P fertilizers are able to not only increase soil available P but also subsequently decrease the ratio of C:P in the rhizosphere. In agricultural ecosystems more than 40% of soil culturable bacteria have the ability to mobilize insoluble and organic P (Jorquera et al. Citation2008) which can be regulated by adding a small amount of inorganic P fertilizer as our results show. This indicates that in addition the direct P nutritional supply effect, the inorganic P fertilizer may indirectly enhance soil organic P utilization of plants mediated by soil bacteria. This may represent another possible mechanism for the promotion of crop growth and production by starter P fertilizer and other similar P application methods. But comparing with the field soil which contains a complete food web (Buchan et al. Citation2013), the bacterial grazers in our experiment has been removed. These microbes (e.g. nematodes and protozoa) can release P from bacterial biomass and affect turnover of P in the field. This aspect should be considered in the future study.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/17429145.2014.977831.
Supplementary material.doc
Download MS Word (301 KB)Acknowledgments
We thank Dr Peter Christie, China Agricultural University, for his kind help in editorial revision of this paper.
Funding
This work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education [grant number 20120008130001]; the National Natural Science Foundation of China [grant number 31372139]; Beijing Natural Science Foundation [grant number 6132019]; and the Innovative Group Grant of the National Science Foundation of China [grant number 31121062].
Additional information
Funding
References
- Attar HA, Blavet D, Selim EM, Abdelhamid MT, Drevon JJ. 2012. Relationship between phosphorus status and nitrogen fixation by common beans (Phaseolus vulgaris L.) under drip irrigation. Int J Environ Sci Technol. 9:1–13. 10.1007/s13762-011-0001-y
- Bittman S, Kowalenko CG, Hunt DE, Forge TA, Wu X. 2006. Starter phosphorus and broadcast nutrients on corn with contrasting colonization by mycorrhizae. Agron J. 98:394–401. 10.2134/agronj2005.0093
- Brookes PC, Powlson DS, Jenkinson DS. 1982. Measurement of microbial biomass phosphorus in soil. Soil Biol Biochem. 14:319–329. 10.1016/0038-0717(82)90001-3
- Buchan D, Gebremikael MT, Ameloot N, Sleutel S, De Neve S. 2013. The effect of free-living nematodes on nitrogen mineralisation in undisturbed and disturbed soil cores. Soil Biol Biochem. 60:142–155. 10.1016/j.soilbio.2013.01.022
- Butterly CR, Bunemann EK, McNeill AM, Baldock JA, Marschner P. 2009. Carbon pulses but not phosphorus pulses are related to decreases in microbial biomass during repeated drying and rewetting of soils. Soil Biol Biochem. 41:1406–1416. 10.1016/j.soilbio.2009.03.018
- Cahill S, Johnson A, Osmond D, Hardy D. 2008. Response of corn and cotton to starter phosphorus on soils testing very high in phosphorus. Agron J. 100:537–542. 10.2134/agronj2007.0202
- Chen CR, Condron LM, Davis MR, Sherlock RR. 2002. Phosphorus dynamics in the rhizosphere of perennial ryegrass (Lolium perenne L.) and radiata pine (Pinus radiata D. Don.). Soil Biol Biochem. 34:487–499. 10.1016/S0038-0717(01)00207-3
- Cordell D, Drangert J-O, White S. 2009. The story of phosphorus: global food security and food for thought. Global Environ Change. 19:292–305. 10.1016/j.gloenvcha.2008.10.009
- Costigan PA. 1984. The effects of placing small amounts of phosphate fertilizer close to the seed on growth and nutrient concentrations of lettuce. Plant Soil. 79:191–201. 10.1007/BF02182341
- DeForest JL, Smemo KA, Burke DJ, Elliott HL, Becker JC. 2012. Soil microbial responses to elevated phosphorus and pH in acidic temperate deciduous forests. Biogeochemistry. 109:189–202. 10.1007/s10533-011-9619-6
- Djodjic F, Mattsson L. 2013. Changes in plant-available and easily soluble phosphorus within 1 year after P amendment. Soil Use Manage. 29:45–54.
- Gahoonia T, Nielsen N. 1992. The effects of root-induced pH changes on the depletion of inorganic and organic phosphorus in the rhizosphere. Plant Soil. 143:185–191. 10.1007/BF00007872
- Harrison AF. 1987. Soil organic phosphorus: a review of world literature. Wallingford: CAB International.
- Hayatsu M, Tago K, Saito M. 2008. Various players in the nitrogen cycle: diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci Plant Nutr. 54:33–45. 10.1111/j.1747-0765.2007.00195.x
- Hayes JE, Richardson AE, Simpson RJ. 1999. Phytase and acid phosphatase activities in extracts from roots of temperate pasture grass and legume seedlings. Aust J Plant Physiol. 26:801–809.
- Hodge A, Paterson E, Thornton B, Millard P, Killham K. 1997. Effects of photon flux density on carbon partitioning and rhizosphere carbon flow of Lolium perenne. J Exp Bot. 48:1797–1805.
- Jackson ML. 1958. Soil chemical analysis. Englewood Cliffs: Prentice-Hall.
- Jorquera MA, Hernández MT, Rengel Z, Marschner P, de la Luz Mora M. 2008. Isolation of culturable phosphobacteria with both phytate-mineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol Fertil Soils. 44:1025–1034. 10.1007/s00374-008-0288-0
- Krey T, Caus M, Baum C, Ruppel S, Eichler-Lobermann B. 2011. Interactive effects of plant growth-promoting rhizobacteria and organic fertilization on P nutrition of Zea mays L. and Brassica napus L. J Plant Nutr Soil Sci. 174:602–613. 10.1002/jpln.200900349
- Kuzyakov Y, Friedel JK, Stahr K. 2000. Review of mechanisms and quantification of priming effects. Soil Biol Biochem. 32:1485–1498. 10.1016/S0038-0717(00)00084-5
- Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, Tremblay J, Engelbrektson A, Kunin V, del Rio TG, et al. 2012. Defining the core Arabidopsis thaliana root microbiome. Nature 488:86–90. 10.1038/nature11237
- Marschner P. 2007. Plant microbe interactions in the rhizosphere and nutrient cycling. In: Marschner P, Rengel Z, editors. Nutrient cycling in terrestrial ecosystems, soil biology series. Heidelberg: Springer; p. 159–182.
- Marschner P. 2008. The role of rhizosphere microorganisms in relation to P uptake by plants. In: White PJ, Hammond JP, editors. The ecophysiology of plant-phosphorus interactions. Heidelberg: Springer; p. 165–176.
- Neumann G. 2006. Quantitative determination of acid phosphatase activity in the rhizosphere and on the root surface. In: Jones DL. 4.2 Biochemistry. In: Luster J, Finlay R, editors. Handbook of methods used in rhizosphere research, Online Edition. Available from: http://www.rhizo.at/handbook.
- Olsen SR, Cole CV, Watanabe FS, Dean LA. 1954. Estimation of available phosphorus in soils by extraction with sodium carbonate. US Dep Agric Circ; p. 939.
- Radersma S, Grierson PF. 2004. Phosphorus mobilization in agroforestry: organic anions, phosphatase activity and phosphorus fractions in the rhizosphere. Plant Soil. 259:209–219. 10.1023/B:PLSO.0000020970.40167.40
- Richardson AE. 2001. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J Plant Physiol. 28:897–906. 10.1104/pp.111.175448
- Richardson AE, Hadobas PA, Hayes JE. 2000. Acid phosphomonoesterase and phytase activities of wheat (Triticum aestivum L.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile culture. Plant Cell Environ. 23:397–405. 10.1046/j.1365-3040.2000.00557.x
- Richardson AE, Simpson RJ. 2011. Soil microorganisms mediating phosphorus availability. Plant Physiol. 156:989–996. 10.1104/pp.111.175448
- Rodriguez H, Fraga R. 1999. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 17:319–339. 10.1016/S0734-9750(99)00014-2
- Ross DJ, Tate KR, Scott NA, Feltham CW. 1999. Land-use change: effects on soil carbon, nitrogen and phosphorus pools and fluxes in three adjacent ecosystems. Soil Biol Biochem. 31:803–813. 10.1016/S0038-0717(98)00180-1
- Schilling G, Gransee A, Deubel A, LeZoviE G, Ruppe S. 1998. Phosphorus availability, root exudates, and microbial activity in the rhizosphere. Z Pflanzenernähr Bodenk. 161:465–478.
- Shobirin A, Farouk A, Greiner R. 2009. Potential phytate-degrading enzyme producing bacteria isolated from Malaysian maize plantation. Afr J Biotechnol. 8:3540–3546.
- Song QH, Li FM, Liu HS, Wang J, Li SQ. 2003. Effect of plastic film mulching on soil microbial biomass in spring wheat field in semi-arid loess area. Chin J Appl Ecol. 14:1512–1516.
- Speir TW, Ross DJ. 1978. Soil phosphatase and sulphatase. In: Burns RG, editor. Soil enzymes. New York: Academic Press; p. 198–235.
- Spohn M, Kuzyakov Y. 2013. Phosphorus mineralization can be driven by microbial need for carbon. Soil Biol Biochem. 61:69–75. 10.1016/j.soilbio.2013.02.013
- Stevenson FJ. 1986. Cycles of soil carbon, nitrogen, phosphorus, sulfur, micronutrients. New York: Wiley.
- Tabatabai MA, Bremner JM. 1969. Use of p-nitrophenyl phosphate for assay of phosphatase activity. Soil Biol Biochem. 1:301–307. 10.1016/0038-0717(69)90012-1
- Thomas RL, Sheard RW, Moyer JR. 1967. Comparison of conventional and automated procedures for nitrogen, phosphorus, and potassium analysis of plant material using a single digestion. Agron J. 59:240–243. 10.2134/agronj1967.00021962005900030010x
- Turner BL, Paphazy MJ, Haygarth PM, McKelvie ID. 2002. Inositol phosphates in the environment. Philos Trans R Soc London Ser B. 357:449–469. 10.1098/rstb.2001.0837
- Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol. 157:423–447. 10.1046/j.1469-8137.2003.00695.x
- Veneklaas EJ, Lambers H, Bragg J, Finnegan PM, Lovelock CE, Plaxton WC, Price CA, Scheible WR, Shane MW, White PJ, Raven JA. 2012. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 195:306–320. 10.1111/j.1469-8137.2012.04190.x
- Wolf DC, Skipper HD. 1994. Soil sterilization. In: Weaver RW, Angle JS, Bottomley PS, editors. Methods of soil analysis, part 2: microbiological and biochemical properties. Madison: Soil Science Society of America; p. 41–52.
- Wu JS, Huang M, Xiao HA, Su YR, Tong CL, Huang DY, Syers JK. 2007. Dynamics in microbial immobilization and transformations of phosphorus in highly weathered subtropical soil following organic amendments. Plant Soil. 290:333–342. 10.1007/s11104-006-9165-5
- Zhang L, Fan JQ, Ding XD, He XH, Zhang FS, Feng G. 2014. Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil. Soil Biol Biochem. 74:177–183. 10.1016/j.soilbio.2014.03.004
- Zoysa AKN, Loganathan P, Hedley MJ. 1997. A technique for studying rhizosphere processes in tree crops: soil phosphorus depletion around camellia (Camellia japonica L.) roots. Plant Soil. 190:253–265. 10.1023/A:1004264830936