Abstract
Chlorophorus caragana (Coleoptera: Cerambycidae) is a trunk borer that feeds on Caragana shrubs in the desert. There are five species of Caragana plant in the distribution area of Ch. caragana. We investigated damaged Caragana plants in the field. Olfactory responses of female Ch. caragana to plants and identified volatile compounds from Caragana plants were further evaluated. Caragana davazamcii was severely damaged in the field, followed by Caragana microphylla. No damage was found to the other three species. Behavioral experiments showed that C. davazamcii, C. microphylla, and Caragana korshinskii were attractive to female insects. Caragana ordosica could repel and avoid female insects. Caragana brachypoda had no effect on the orientation behavior of female insects. Seventy volatile components were identified from the Caragana plants, and (Z)-β-ocimene, 1,3-pentadiene, (Z)-3-hexenyl acetate, perillene, chrysanthenone, and limonene were the most abundant volatiles identified from the Caragana plants. The volatiles were categorized into three groups. Those most attractive to Ch. caragana consisted of chrysanthenone, 1,3-pentadiene, and (Z)-β-ocimene. Those repelling Ch. caragana consisted of perillene, dibutyl phthalate, nonanal, and pentadecane, and those irrelevant to each other consisted of (Z)-3-hexenyl acetate, 1-octene, nonene, decanal, (Z)-3-hexenol, and α-pinene.
Introduction
Volatile organic compounds (VOCs) are released by plants and consist of a mixture of multiple compounds. The chemical composition, proportion, and rate of release vary due to factors such as the plant type and its physiological status. Even if VOCs were from the same family or genera of plants, significant qualitative and quantitative differences were still present (Gao et al. Citation2005). Plant volatiles played a significant role in the orientation of phytophagous insects to host plant (Pettersson et al. Citation1998; Bruce et al. Citation2005). Phytophagous insects recognized their hosts by the specific VOC mixture which was used to decide whether the plants were suitable for feeding and laying eggs (Mustaparta Citation2002; Tasin et al. Citation2006; Anton et al. Citation2007; Cardé & Willis Citation2008; Piñero & Dorn Citation2009; Najar-Rodriguez et al. Citation2013).
Pests damaged several host plants based on certain common volatiles released by these plants (Bruce et al. Citation2005; Rajapakse et al. Citation2006; Leppik & Frérot Citation2012). On the other hand, VOCs released by nonhost plants were always perceived as irrelevant or repellant cues and led to nondirectional movement of phytophagous insects in feeding or laying eggs. Therefore, the similarity of volatiles released by different host plants could provide more information for volatiles essential for insects' attractiveness; however, the volatiles released by nonhost plants could be used for the development of insect repellents (Unsicker et al. Citation2009; Jactel et al. Citation2011).
Caragana plants are shrubs, which are crucial for water and soil conservation in deserts, and are widely distributed in the desert area of Ningxia, China. The larvae of Chlorophorus caragana feed on the wooden part of the plant, which leads to the death of the shrubs due to wind-breakage (Zong et al. Citation2012). Recently, the damage caused by Ch. caragana has been serious in Caragana plants. There are five species of Caragana plants, Caragana davazamcii, Caragana korshinskii, Caragana microphylla, Caragana brachypoda, and Caragana ordosica, distributed in the desert area of Ningxia, China. However, up to now, it is unclear which plant species are most attractive or repellant to Ch. caragana.
Here we (1) investigated natural insect damage on the five species of Caragana in the field, (2) collected and analyzed the VOCs from branches and leaves of the five species of Caragana, (3) evaluated the attractiveness of these VOCs to Ch. caragana females by olfaction selection experiments under laboratory conditions, and (4) applied principal component analysis (PCA) and clustering analysis of these VOCs to determine the common and noncommon components between VOCs derived from the five species of Caragana.
Materials and methods
Plant materials and insects
Five plant species were evenly distributed in the desert woods in Lingwu City. There C. davazamcii, C. microphylla, and C. brachypoda were distributed close to the urbanized city. C. ordosica and C. korshinskii were distributed in the suburbs. The distribution details are shown in .
Table 1. Distribution details of five Caragana species from Lingwu City.
Ch. caragana larvae were collected from the desert woods in Lingwu City and were maintained in laboratory artificial climate chests (Blue Pard MGC-2500, Shanghai, China) at L:D 14:10 and 60% relative humidity. The temperatures of day and night were 30°C and 25°C, respectively. The artificial diet consisted of yeast extract, agar, sugar, Wesson's salt mixture, casein, Vanderzant's vitamin mixture, sorbic acid, methylparaben, and distilled water. The artificial diet was replaced weekly and feces were removed at that time until larvae became pupae. Upon emergence, adults to be tested were sexed. Each adult was kept singly in a transparent plastic disposable cup (upper diameter × height × bottom diameter = 75 mm × 30 mm × 60 mm) and was provided with honey water. Robust unmated female adults were chosen for subsequent behavioral experiments.
Investigation of insect population density in the field
Five Caragana species were chosen in the corresponding desert woods in Lingwu City which is indicated in . For each plant species, six sample plots were investigated. Each plot (20 × 20 m2) included at least 30 plant individuals. Plants were individually investigated on each of four directions (N, S, E, and W) to elucidate the wood anatomy. The pest population density, various forms of insects, and the number of eclosion holes were recorded. The proportion of damaged plants and the pest population density per plant were calculated as an index of measurable damages by Ch. caragana.
Attraction of five species of Caragana to Ch. caragana
The attraction of Caragana plants to unmated female Ch. caragana was measured with a Y-tube olfactometer in June and July 2013. Healthy twigs from five Caragana species were obtained from the desert woodland in Lingwu (). Twigs and leaves from the five species of Caragana were cut off in the field at 6:00 am, and then placed in a clean zip lock bag with a wet cotton ball to prevent dehydration. We did not further cut the leaves from the excised twigs to avoid further adding new artificial wound, so the number of leaves from the twigs from the five species was not absolutely equal. The weight and length were also different (). These twigs were then taken back to the Management Quarantine Station of Lingwu City to perform behavioral tests.
The olfactometer was a Y-shaped glass tube (diameter: 7.5 cm; selective arm length: 40 cm; common arm length: 50 cm). The selective arms were connected to two glass chambers (diameter: 7.5 cm; length: 10 cm), one with plant material and the other being a methylene chloride control. Tested insects were placed into the common arm. Air velocity was 1 L/min, controlled by an air sampler (type QC-1, the Beijing Municipal Institute of Labor Protection Science). Airflow was filtered through activated carbon and a gas-washing bottle, then pushed into the two glass chambers, finally reaching the test insects. The airflow was started 10 min before the test insects were placed into the chamber to ensure that the plant odor was sufficiently spread within the selective arm.
Experiments were performed under even light (incandescent simulation of sunlight) and temperatures of 24–25°C with 60–70% relative humidity. All experiments were carried out between 8:00 am and 6:00 pm. During the time, Ch. caragana was active in the field. An unmated female insect was randomly selected and placed at the entrance of the Y-tube. Each female was observed for a maximum of 10 min. When the insect had passed the 20 cm mark on the selective arm, it was considered to have made a choice; if the insect did not reach the mark, it was considered not to make a choice (Bertschy et al. Citation1997). For each plant sample, 30 female insects were tested, and the tests were repeated three times. After five insects were tested, the position of both glass arms and selective chambers were changed simultaneously to avoid deviation of odor. After a plant sample was tested, the entire device was cleaned in detergent solution, rinsed with ethanol, and then dried at 120°C.
Collection of VOCs from five species of Caragana plants
From 8:00 am to 1:00 pm on July 20–26, 2013, healthy plants of C. davazamcii, C. microphylla, C. korshinskii, C. ordosica, and C. brachypoda were selected for headspace collection of VOCs (Kappers et al. Citation2011). The plant twigs were placed into a sealed Teflon oven bag (Reynolds, 406 mm × 444 mm, Richmond, VA, USA). After being quickly evacuated, the bag was filled with air filtered by activated carbon. The closed loop system collected plant volatiles repeatedly, and finally, the VOCs were adsorbed into an adsorption tube (CAMSCO, Houston, TX, USA; length: 8.89 cm; diameter: 0.635 cm) with 200 mg Tenax TA (60/80 mesh; Supelco, Bellefonte, DE, USA). Before the collection, the absorption tube was activated for 120 min at 270°C using nitrogen blowing at 100 mL/min. The connecting tubes of the devices were made from Teflon (inner diameter: 0.6 cm; outer diameter: 0.8 cm). During collection, plants were checked to confirm that they were not damaged. Six samples from each plant were collected at the same time. The six samples were as adjacent as possible to ensure the similar habitat (Vallat et al. Citation2005). Meanwhile, collection using an empty bag was performed as a control. The flow rate was 500 mL/min, and each sample was collected consecutively over 4 h. To avoid the direct sunlight and excessive vapor from plants, the entire collection device was covered by a sunshade. After sampling, the adsorption tubes were maintained at –20°C.
Automatic thermal desorption/gas chromatography/mass spectrometry (ATD/GC/MS)
Automatic thermal desorption equipment (ATD 650 Turbo Matrix; PerkinElmer, Waltham, MA) was directly connected to the GC (Clarus 600; Perkin Elmer). The injection rate of VOCs dissociated from the adsorption tube to GC was 5.0%.
Automatic thermal desorption (ATD)
The first dissociation temperature of the sample was 260°C, which was maintained for 10 min. Before entering a cold trap (–25°C), the sample was heated to 300°C at a heating rate of 40°C/s, then maintained for 5 min.
Gas chromatography (GC)
A DB-5 chromatographic column was used (30 m long; internal diameter: 0.25 mm; film thickness: 0.15 μm: Agilent Technologies, Santa Clara, CA). The carrier gas was He (1.5 mL/min). The initial temperature of 40°C was maintained for 2 min. Next, the temperature was increased to 160°C at a rate of at 4°C/min, before heating to 270°C in 20°C/min steps where it was maintained for 3 min. The split ratio was 2:1.
Mass spectrometry (MS)
The electrons were from an EI source; the electron was 70 ev; scanned mass-to-charge ratio range was 29–600 amu; interface temperature was 25°C; ion source temperature was 220°C; and quadrupole temperature was 150°C. Emission current was 150 uA. Using the full scan mode, each scan was performed for 0.2 s.
Chromatographic retention time and MS data in NIST of identified compounds and 34 standards () were compared. Then, C5–C27 straight-chain alkanes were injected into the same equipment and analyzed by the same temperature programming. Retention time was used to calculate the retention index (KI value). Through comparisons with published KI values, a qualitative diagnosis was performed (Ruther Citation2000; Adams Citation2007). No quantitative analysis was performed, and any statistical assessment was related to these ‘peak areas.’ Using total ion current peak area normalization, the relative amount of each identified component was calculated. The software Turbo Mass 5.4.2 (PerkinElmer, Waltham, MA) was used for data analysis.
Chemicals
Nonene (99.5%), heptaldehyde (95.0%), 1-heptanol (98.0%), 1-decene (99.5%), limonene (95.0%), nonanal (95.0%), (E,E)-alloocimene (90.0%), 1-dodecene (99.5%), decanal (95.0%), 1-tridecene (99.5%), and pentadecane (99.0%) were obtained from CNW technologies GmbH (Düsseldorf, Germany); methyl butyrate (99.0%), 1-octene (99.0%), octane (99.0%), (Z)-3-hexenol (99.5%), nonane (99.0%), benzaldehyde (95.0%), β-pinene (95.0%), decane (99.0%), octanal (97.0%), (Z)-3-hexenyl acetate (95.0%), 1-hexanol,2-ethyl- (99.0%), isophorone (99.0%), dodecane (99.0%), tridecane (98.0%), tetradecane (97.0%), hexadecane (98.0%), and dibutyl phthalate (99.0%) were obtained from Dr Ehrenstorfer (Augsburg, Germany); chrysanthenone (85.0%) was obtained from CHEMOS GmbH (Czech Republic, Germany); α-pinene (98.0%) was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA); β-elemene (82.0%) was obtained from Skyrun Industrial Co. Limited (CSR Ind, China); 1,3-pentadiene (99.0%) was obtained from Tokyo Chemical Industry Co. (Tokyo, Japan); (Z)-β-ocimene (95.0%) was obtained from BOC Sciences (New York, USA); and perillene (98.0%) was obtained from Shanghai BeiZhuo Biotechnology Co., Ltd (Shanghai, China). Compounds for which no standards were available were tentatively identified using the NIST database.
Statistical analysis
Chi-square test was applied to analyze results from the behavioral tests, including preference (percentage of adult insects choosing the odor source or the clean air), nonpreference (percentage of adult insects with no choice), and responsiveness (the ratio of adult insects making a choice). By means of one-way variance, the different response percentages of Ch. caragana to different odor sources from five Caragana species were compared (SPSS 16.0, SPSS Inc., Chicago, IL, USA). Multivariate analysis of variance (ANOVA) was performed to identify significant differences between volatiles (dependent variables) emitted by different Caragana species. If significant, one-way ANOVA and Tukey's HSD post hoc tests were further conducted to test for quantitative differences in concentrations of individual and total headspace volatiles emitted by different Caragana species (SPSS 16.0, SPSS Inc., Chicago, IL, USA).
Different plant VOCs were summarized with PCA and cluster analysis. PCA was applied to yield a 2D display of the multivariable set of data and to graphically determine whether clustering of five different Caragana species occurred based on their overall volatile profiles. Hierarchical clustering analysis of each sample were carried out using the between-group linkage method and Euclidean distance (SPSS 16.0, SPSS Inc., Chicago, IL, USA).
Result and discussion
Natural damage to five Caragana species by Ch. caragana
The result showed that C. davazamcii had the largest average larvae per plant rate (74.00% ± 13.00%) and population density of insects per plant (1.16 ± 0.84). It was followed by C. microphylla, with average larvae per plant rate (11.00% ± 2.00%) and population density of insects per plant (0.13 ± 0.08; ). No damage was found on C. ordosica, C. brachypoda, and C. korshinskii.
Table 2. Investigation of damage on five species of Caragana in the field.
Olfactory response of Ch. caragana to five Caragana species
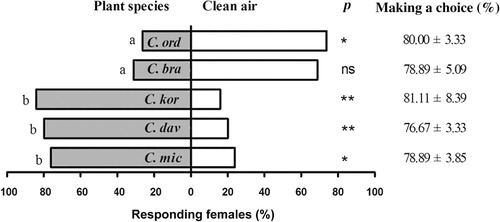
Female Ch. caragana was attracted by the odor of C. korshinskii (χ2 = 10.67, N = 24, P < 0.01), C. davazamcii (χ2 = 7.35, N = 23, P < 0.01), and C. microphylla (χ2 = 6.00, N = 24, P < 0.05). The odor of C. ordosica (χ2 = 6.00, N = 24, P < 0.05) repelled Ch. caragana. The effect of C. brachypoda odor was not significant (χ2 = 3.52, N = 24, P > 0.05). Results from the one-way ANOVA showed that olfactory response rates of Ch. caragana to the five species VOCs were significant (F = 37.68, P < 0.01). The difference between the attraction of C. korshinskii, C. davazamcii, and C. microphylla was not significant. The difference between C. ordosica and C. brachypoda was also not significant. However, the difference between these two groups was found to be significant ().
VOC composition of five Caragana species
Through headspace collection and GC-MS analysis, 70 different compounds were identified from the twigs of five Caragana species. The identified components were divided into eight chemical classes, which comprised 5 alcohols, 7 ketones, 10 alkanes, 8 olefins, 11 esters, and 1 aromatic compound. No quantitative analysis were performed. The relative amount of each identified component was based on peak area normalization of total ion current ().
Table 3. Relative TIC-peak areas of VOCs collected in the headspace of the twigs and leaves from species of Caragana plants.
Thirty-five types of compounds were identified from C. microphylla. The most abundant compounds included eight terpenes, two ketones, and seven esters, with relative amounts of 55.43%, 17.49%, and 15.97%, respectively. In addition, C. microphylla also included four olefins, eight alkanes, five aldehydes, and one alcohol. All of these four classes accounted for only 10.8% of the total amount. (Z)-β-ocimene (34.42% ± 2.78%), chrysanthenone (17.27% ± 2.39%), (Z)-3-hexenyl acetate (15.32% ± 0.96%), 1,3-pentadiene (10.81% ± 3.24%), and β-elemene (7.27% ± 2.39%) were the principal components of C. microphylla, accounting for 85.09% of the total amount of VOCs identified ().
Thirty-six types of compounds were identified from C. davazamcii, including eight abundant terpenoid compounds, accounting for 75.2% of total amount. The relative amounts of terpenoids compounds in C. davazamcii were significantly higher than that in the other four plants (F = 677.48, P < 0.05). In addition, nine kinds of alkanes and eight kinds of olefins were identified, accounting for 10.7% and 6.1% of total amount, respectively. Then, six aldehydes, two ketones, one alcohol, and two esters were identified, with 8.0% of the total relative amounts. (Z)-β-ocimene (24.88% ± 4.44%), 1,3-pentadiene (22.29% ± 3.46%), limonene (20.66% ± 1.24%), and β-pinene (5.61% ± 1.33%) were the principal components of C. davazamcii, accounting for 73.44% of the total amount ().
Thirty types of compounds were identified from C. korshinskii, including four abundant terpenoid compounds, accounting for 56.8% of total amount, followed by nine alkanes with19.1% of total amount. In additions, five olefin (7.0%), two esters (6.0%), three ketones (5.1%), six aldehydes (4.5%), and one aromatic compound (0.7%) were also identified. (Z)-β-ocimene (35.13% ± 3.32%), 1,3-pentadiene (20.6% ± 3.81%), (Z)-3-hexenyl acetate (5.05% ± 0.55%), pentadecane (4.89% ± 0.41%), and chrysanthenone (4.49% ± 0.82%) were the principal components of C. korshinskii, accounting for 70.16% of the total amount ().
Twenty-one types of compounds were identified from C. brachypoda, including three esters and four olefins, accounting for 31.1% and 25% of total amount, respectively. The two classes were significantly higher than those in the other four species (esters: F = 730.31, P < 0.05; olefins: F = 579.81, P < 0.05). Four aldehydes were identified, which were significantly higher than in the other four plants (F = 180.4, P < 0.05), accounting for 14.8% of total amount. In addition, three terpenes and four alkanes were identified, with 13.1% and 9.6% of total amount, respectively. Two ketones and one alcohol were identified, with 6.3% of total relative amount. (Z)-3-hexenyl acetate (27.45% ± 1.39%), nonene (10.35% ± 0.89%),1-octene (9.56% ± 0.77%), perillene (6.05% ± 0.35%), decanal (5.75% ± 0.29%), and α-pinene (5.51% ± 1.28%) were the principal components of C. brachypoda, accounting for 64.67% of the total amount ().
Forty-eight compounds were identified in C. ordosica, including 14 identified abundant terpenes, accounting for 60.8% of total amount. The other compounds present at high relative amounts were seven aldehydes (11.9%), seven alkanes (9.6%), and six esters (8.8%). In addition, five alcohols, four olefins, and five ketones were identified, accounting for 8.5% of total amount. Perillene (40.46% ± 2.58%), dibutyl phthalate (7.24% ± 1.2%), nonanal (7.24% ± 0.9%), (Z)-β-ocimene (6.6% ± 1.02%), pentadecane (5.9% ± 1.54%), and α-pinene (4.76% ± 0.59%) were the principal components of C. ordosica, accounting for 72.2% of the total amount ().
PCA and hierarchical cluster analysis
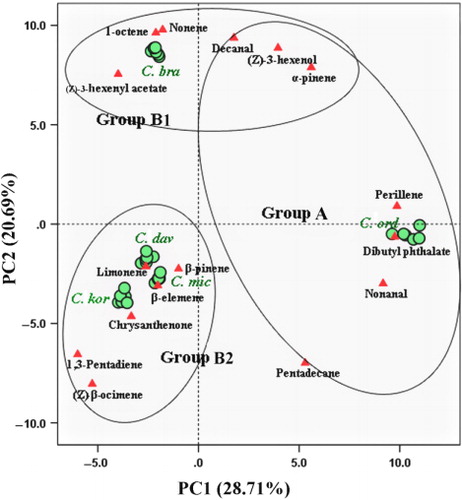
PCA clearly divided the VOCs from the five plant species into three groups. The PCA horizontal axis explained 28.71% of the total variance and the vertical axis a further 20.69% (). From five Caragana VOCs, 16 compounds were selected based on three principles. First of all, these compounds should account for a relatively high percentage in one or several plant species, and their mean percentages in total VOCs were not less than 4.3%. Second, the amounts of selected VOCs were significantly different between the five plant species (ANOVA: P < 0.05). Third, these compounds should cover most of the chemical classes. According to these principles, 16 compounds were marked in the biplot (1,3-pentadiene, 1-octene, (Z)-3-hexenol, nonene, α-pinene, β-pinene, (Z)-3-hexenyl acetate, limonene, (Z)-β-ocimene, nonanal, perillene, chrysanthenone, decanal, β-elemene, pentadecane, and dibutyl phthalate).
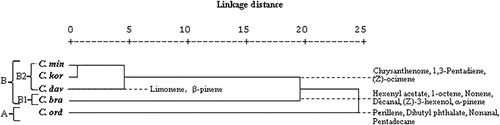
Hierarchical cluster analysis between-groups linkage (squared Euclidean distance) was used to analyze these volatiles derived from five Caragana plant species, at a distance >5 and <20. They were divided into three clusters (B1, B2, and A; ).
PCA and system clustering results were consistent. The results showed VOC composition between the groups was significantly different. According to PCA, C. davazamcii, C. korshinskii, and C. microphylla, which attracted Ch. caragana in the behavioral test, belonged to Group B2. The common characteristics of the three species were the high percentage of (Z)-β-ocimene (35.13% ± 3.32% to 24.88% ± 4.44%), chrysanthenone (17.27% ± 2.39% to 2.47% ± 0.60%), and 1,3-pentadiene (22.29% ± 3.46% to 10.81% ± 3.24%). Furthermore, (Z)-3-hexenyl acetate (15.32% ± 0.96%) and β-elemene (7.27% ± 2.39%) of C. microphylla were also abundant. The VOCs of C. davazamcii differed slightly from C. microphylla and C. korshinskii, in terms of the proportions of limonene (20.66% ± 1.24%) and β-pinene (5.61% ± 1.33%; ). Overall, the main chemical characteristics of this Group B2 were the high proportions of terpenoids, ketone, and ester compounds.
Damage to C. korshinskii by Ch. caragana was not observable during the field investigation; however, this plant species was attractive to female insects during behavior experiments. The phylogenetic distance between C. davazamcii, C. microphylla, and C. korshinskii was close, as reported by Hou et al. (Citation2006). In the present study, PCA and cluster analysis also indicated that plant VOCs of C. korshinskii are similar to C. davazamcii and C. microphylla. The behavior results from our study supported the powerful attraction ability of C. davazamcii, C. microphylla, and C. korshinskii to the beetle during the behavior experiment. However, why no damage was observed on C. korshinskii plants? In our investigation, no Ch. caragana population was found in the C. korshinskii suburbs (). Lower population densities in C. korshinskii suburbs of Lingwu City may explain the low damage rate by Ch. caragana on C. korshinskii.
Phytophagous insects select host plants on the basis of one or several substances released by the plant (Chin Citation1980; Zhang et al. Citation2001). Previous studies have shown that ocimene is an important defensive substance for plants. External stimuli could induce plants to release ocimene (Arimura et al. Citation2004). Using a Y-shaped tube, it was found that (E/Z)-β-ocimene and (Z)-3-hexenyl acetate attracted Myllocerinus aurolineatus (Coleoptera: Curculionidae; Sun et al. Citation2010). Fewer studies have been performed on chemical communication by chrysanthenone and 1,3-pentadienes (Kostyk et al. Citation1993). (Z)-3-hexenyl acetate attracted Pantomorus cervinus (Coleoptera: Curculionidae; Wee et al. Citation2008); and β-elemene and limonene as kairomones affected the behavior of Anoplophora glabripennis (Coleoptera: Cerambycidae; Yasui et al. Citation2007; Yasui et al. Citation2011; Wei et al. Citation2013). Several studies have showed that some monoterpenes, such as α-pinene and β-pinene, can attract Cerambycidae, Curculionidae, and Scolytidae, which feed on coniferous trees. Furthermore, mixing ethanol with the chemical attractant strengthened its action (Mizell et al. Citation1984; Byers et al. Citation1985; Siegfried Citation1987; Sweeney et al. Citation2004; Wei et al. Citation2013). In total, all above-mentioned studies indicated that these compounds (e.g. ocimene, chrysanthenone, 1,3-pentadienes, β-elemene, limonene, and β-pinene) have biological activities on Coleoptera. So, we hypothesize they were also attractive to Ch. caragana. Furthermore, several other studies had shown that single compounds had no effect on phytophagous insects, but a mixture of multiple components formed a specific chemical signature to herbivores (Dicke Citation2000; Hammack Citation2001).
Group B1 (C. brachypoda) was the nonhost plant of Ch. caragana, and VOCs were characterized by high levels of (Z)-3-hexenyl acetate (27.45% ± 1.39%). Other species, except for C. microphylla, contained less of this substance. The contents of nonene (10.35% ± 0.89%) and 1-octene (9.56% ± 0.77%) were also more abundant than the other plant species. The contents of decanal (5.75% ± 0.29%), α-pinene (5.51% ± 1.28%), and (Z)-3-hexenols (3.05% ± 0.16%) were higher than in the other four plant species. It was clear that the chemical components of Group B1 mainly consisted of esters and olefin, and small amounts of aldehydes, alcohols, and terpenes were also present. In the bioassay, Ch. caragana did not perform significant orientation behavior to VOCs of C. brachypoda.
The nonhost plant C. ordosica that repelled Ch. caragana belonged to Group A. One of the characteristics of the VOCs in this group was the high level of perillene (40.46% ± 2.58%), which was less in other species. Next, the contents of dibutyl phthalate (7.24% ± 1.20%), nonanal (7.24% ± 0.90%), and pentadecane (5.90% ± 1.54%) were all found to be higher in this species than in the others. In addition, the percentages of α-pinene (4.76% ± 0.59%), (Z)-3-hexenol (2.02% ± 0.12%), and decanal (2.83% ± 0.78%) were slightly higher than that in C. davazamcii, C. microphylla, and C. korshinskii. The chemical components of Group A were mainly terpenes, with a slightly higher percentage of esters, aldehydes, and alkanes, as well as a small amount of alcohol.
The components of nonhost plant VOCs did not induce orientation behavior and even led to anti-directional movements. Poland and Haack (Citation2000) indicated nonhost plant VOCs, including 1-hexanol, (Z)-3-hexen-1-ol, (E)-2-hexen-1-ol,3-octanol, and verbenone, interfered with host plant orientation by Tomicus piniperda (Coleoptera: Scolytidae). Erbilgin et al. (Citation2007) showed that acetophenone had a strong repellent activity on western pine beetles. In our studies, perillene, dibutyl phthalate, nonanal, pentadecane, α-pinene, (Z)-3-hexenol, and decanal, derived from C. ordosica, were repellant to Ch. caragana. However, we found Group B1 and Group A shared three common components, including α-pinene, (Z)-3-hexenols, and decanal. Group B1 did not exhibit significantly repellant behavior by Ch. caragana, so these three compounds might not be the main repellent substances.
Conclusion
Among the three Caragana species, C. korshinskii, C. davazamcii, and C. microphylla attracted Ch. caragana in laboratory. The common substance of the three species was a high proportion of terpene compounds ((Z)-β-ocimene, 1,3-pentadiene, β-elemene, limonene, and β-pinene), as well as ketone compounds (chrysanthenone), and ester compounds ((Z)-3-hexenyl acetate). In the field, C. davazamcii and C. microphylla were damaged by Ch. caragana, but no damage to C. korshinskii was found in the field. The inconsistence for C. korshinskii might contribute to the inappropriate physical characteristics and nutrient status of C. korshinskii. C. ordosica repelled Ch. caragana and did not suffer from damage in the field. The chemical characteristics of this species mainly consisted of terpenes (perillene and α-pinene), a slightly higher percentage of esters (dibutyl phthalate), aldehydes (nonanal and decanal), alkanes (pentadecane), and a small amount of alcohol ((Z)-3-hexenol). C. brachypoda did not suffer from damage in the field, and its chemical characteristics were mainly esters ((Z)-3-hexenyl acetate), olefinic (nonene and 1-octene), a small concentrations of aldehyde (decanal), alcohols ((Z)-3-hexenols), and terpenes (α-pinene).
Additional information
Funding
References
- Adams RP. 2007. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream (IL): Allured Publishing Corporation.
- Anton S, Dufour MC, Gadenne C. 2007. Plasticity of olfactory-guided behaviour and its neurobiological basis: lessons from moths and locusts. Entomol Exp Appl. 123:1–11. 10.1111/j.1570-7458.2007.00516.x
- Arimura G-I, Ozawa R, Kugimiya S, Takabayashi J, Bohlmann J. 2004. Herbivore-induced defense response in a model legume. Two-spotted spider mites induce emission of (E)-β-ocimene and transcript accumulation of (E)-β-ocimene synthase in Lotus japonicus. Plant Physiol. 135:1976–1983. 10.1104/pp.104.042929
- Bertschy C, Turlings TC, Bellotti AC, Dorn S. 1997. Chemically-mediated attraction of three parasitoid species to mealybug-infested cassava leaves. Fla Entomol. 80:383–395. 10.1104/pp.104.042929
- Bruce TJ, Wadhams LJ, Woodcock CM. 2005. Insect host location: a volatile situation. Trends Plant Sci. 10:269–274. 10.1016/j.tplants.2005.04.003
- Byers JA, Lanne BS, Löfqvist J, Schlyter F, Bergström G. 1985. Olfactory recognition of host-tree susceptibility by pine shoot beetles. Naturwissenschaften. 72:324–326. 10.1007/BF00454776
- Cardé R, Willis M. 2008. Navigational strategies used by insects to find distant, wind-borne sources of odor. J Chem Ecol. 34:854–866.
- Chin C-T. 1980. The physiological bases of host-plant specificity of phytophagous insects. Acta Entomol Sinica. 23:106–122.
- Dicke M. 2000. Chemical ecology of host-plant selection by herbivorous arthropods: a multitrophic perspective. Biochem Syst Ecol. 28:601–617. 10.1016/S0305-1978(99)00106-4
- Erbilgin N, Krokene P, Kvamme T, Christiansen E. 2007. A host monoterpene influences Ips typographus (Coleoptera: Curculionidae, Scolytinae) responses to its aggregation pheromone. Agr Forest Entomol. 9:135–140. 10.1111/j.1461-9563.2007.00329.x
- Gao Y, Jin YJ, Li HD, Chen HJ. 2005. Volatile organic compounds and their roles in bacteriostasis in five conifer species. J Integr Plant Biol. 47:499–507. 10.1111/j.1744-7909.2005.00081.x
- Hammack L. 2001. Single and blended maize volatiles as attractants for diabroticite corn rootworm beetles. J Chem Ecol. 27:1373–1390. 10.1023/A:1010365225957
- Hou X, Liu J, Zhao Y, Zhao L. 2006. Interspecific relationships of Caragana microphylla, C. davazamcii and C. korshinskii (Leguminosae) based on ITS and trn L-F datasets. Acta Phytotaxon Sin. 44:126–134.
- Jactel H, Birgersson G, Andersson S, Schlyter F. 2011. Non-host volatiles mediate associational resistance to the pine processionary moth. Oecologia. 166:703–711. 10.1007/s00442-011-1918-z
- Kappers I, Hoogerbrugge H, Bouwmeester H, Dicke M. 2011. Variation in herbivory-induced volatiles among cucumber (Cucumis sativus L.) varieties has consequences for the attraction of carnivorous natural enemies. J Chem Ecol. 37:150–160. 10.1007/s10886-011-9906-7
- Kostyk BC, Borden JH, Gries G. 1993. Photoisomerization of antiaggregation pheromone verbenone: biological and practical implications with respect to the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). J Chem Ecol. 19:1749–1759. 10.1007/BF00982305
- Leppik E, Frérot B. 2012. Volatile organic compounds and host-plant specialization in European corn borer E and Z pheromone races. Chemoecology. 22:119–129. 10.1007/s00049-012-0104-z
- Mizell R, III, Frazier J, Nebeker TE. 1984. Response of the clerid predator Thanasimus dubius (F.) to bark beetle pheromones and tree volatiles in a wind tunnel. J Chem Ecol. 10:177–187. 10.1007/BF00987655
- Mustaparta H. 2002. Encoding of plant odour information in insects: peripheral and central mechanisms. Entomol Exp Appl. 104:1–13. 10.1046/j.1570-7458.2002.00985.x
- Najar-Rodriguez A, Bellutti N, Dorn S. 2013. Larval performance of the oriental fruit moth across fruits from primary and secondary hosts. Physiol Entomol. 38:63–70. 10.1111/phen.12003
- Pettersson J, Karunaratne S, Ahmed E, Kumar V. 1998. The cowpea aphid, Aphis craccivora, host plant odours and pheromones. Entomol Exp Appl. 88:177–184. 10.1046/j.1570-7458.1998.00360.x
- Piñero JC, Dorn S. 2009. Response of female oriental fruit moth to volatiles from apple and peach trees at three phenological stages. Entomol Exp Appl. 131:67–74.
- Poland TM, Haack RA. 2000. Pine shoot beetle, Tomicus piniperda (Col., Scolytidae), responses to common green leaf volatiles. J Appl Entomol. 124:63–69. 10.1046/j.1439-0418.2000.00448.x
- Rajapakse CNK, Walter GH, Moore CJ, Hull CD, Cribb BW. 2006. Host recognition by a polyphagous lepidopteran (Helicoverpa armigera): primary host plants, host produced volatiles and neurosensory stimulation. Physiol Entomol. 31:270–277. 10.1111/j.1365-3032.2006.00517.x
- Ruther J. 2000. Retention index database for identification of general green leaf volatiles in plants by coupled capillary gas chromatography−mass spectrometry. J Chromatogr A. 890:313–319. 10.1016/S0021-9673(00)00618-X
- Siegfried BD. 1987. In-flight responses of the pales weevil, Hylobius pales (Coleoptera: Curculionidae) to monoterpene constituents of southern pine gum turpentine. Fla Entomol. 70:97–102. 10.2307/3495095
- Sun XL, Wang GC, Cai XM, Jin S, Gao Y, Chen ZM. 2010. The tea weevil, Myllocerinus aurolineatus, is attracted to volatiles induced by conspecifics. J Chem Ecol. 36:388–395. 10.1007/s10886-010-9771-9
- Sweeney J, De Groot P, MacDonald L, Smith S, Cocquempot C, Kenis M, Gutowski JM. 2004. Host volatile attractants and traps for detection of Tetropium fuscum (F.), Tetropium castaneum L., and other longhorned beetles (Coleoptera: Cerambycidae). Environ Entomol. 33:844–854. 10.1603/0046-225X-33.4.844
- Tasin M, Bäckman AC, Bengtsson M, Varela N, Ioriatti C, Witzgall P. 2006. Wind tunnel attraction of grapevine moth females, Lobesia botrana, to natural and artificial grape odour. Chemoecology. 16:87–92. 10.1007/s00049-005-0332-6
- Unsicker SB, Kunert G, Gershenzon J. 2009. Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Curr Opin Plant Biol. 12:479–485. 10.1016/j.pbi.2009.04.001
- Vallat A, Gu H, Dorn S. 2005. How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochemistry. 66:1540–1550. 10.1016/j.phytochem.2005.04.038
- Wee SL, El-Sayed AM, Gibb AR, Mitchell V, Suckling DM. 2008. Behavioural and electrophysiological responses of Pantomorus cervinus (Boheman) (Coleoptera: Curculionidae) to host plant volatiles. Aust J Entomol. 47:24–31. 10.1111/j.1440-6055.2007.00624.x
- Wei JR, Lu XP, Jiang L. 2013. Monoterpenes from larval frass of two cerambycids as chemical cues for a parasitoid, Dastarcus helophoroides. J Insect Sci. 13:1–12. 10.1673/031.013.5901
- Yasui H, Fujiwara-Tsujii N, Wakamura S. 2011. Volatile attractant phytochemicals for a population of white-spotted longicorn beetles Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae) fed on willow differ from attractants for a population fed on citrus. Chemoecology. 21:51–58. 10.1007/s00049-010-0065-z
- Yasui H, Yasuda T, Fukaya M, Akino T, Wakamura S, Hirai Y, Kawasaki K, Ono H, Narahara M, Kousa K. 2007. Host plant chemicals serve intraspecific communication in the white-spotted longicorn beetle, Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae). Appl Entomol Zool. 42:255–268. 10.1303/aez.2007.255
- Zhang F, Kan W, Zhang ZN. 2001. Progress in chemical ecology of tritrophic interactions among host-plants, aphids and natural enemies. Acta Ecologica Sinica. 21:1025–1033.
- Zong SX, Xie GL, Wang W, Luo Y, Cao CJ. 2012. A new species of Chlorophorus Chevrolat (Coleoptera: Cerambycidae: Cerambycinae) from China with description of biology. Zootaxa. 3157:54–60.
