Abstract
Eucommia ulmoides is a small tree that has evolved strong pest resistance. This study investigated the mechanism of this resistance by evaluating pest damage and antifeedant activity of the secondary metabolite gutta-percha. In the field, E. ulmoides displayed an average plant resistance of 93.3%, with most damage confined to leaves. We assessed the effects of gutta-percha as a feeding deterrent against Hyphantria cunea and Anoplophora glabripennis larvae by mixing it into or daubing it onto the surface of artificial diets. Diets containing more than 6% gutta-percha significantly reduced the amount of feeding by A. glabripennis larvae and the body length of H. cunea larvae. In addition, approximately 95% of the artificial diet was not consumed compared to the control without gutta-percha. The content, distribution, properties, and dynamics of gutta-percha in E. ulmoides indicate that gutta-percha may function as a physical-chemical barrier against insect pests.
Introduction
Secondary metabolites have a key role in plant resistance to pests and herbivores. Plant secondary metabolites can act as feeding deterrents or inhibit pest growth and development (Pavela & Herda Citation2007; Pavela et al. Citation2009; Rani & Murty Citation2009). Plants may adopt various chemical defenses, among them, the amount of defense chemicals intrinsic to the plant and not induced by the feeding of insects (Shi Citation1994; Maleck & Dietrich Citation1999).
Eucommia ulmoides is the only member of the family Eucommiaceae. It is a typical relict plant native to China, where now 95% of the E. ulmoides resources are located. It grows in most areas of China, and has a high economic value (Zhang & Xue Citation2011). Gutta-percha is a unique secondary metabolite of E. ulmoides; it is an isomer of natural rubber that has great industrial value. Gutta-percha is found in all E. ulmoides dry tissues, and accumulates to 3% in the leaves and 6% in the bark (Song et al. Citation2006). Following long-term investigations, it was discovered that E. ulmoides has a strong resistance to pests, and has rarely suffered disastrous pest infestations (Li Citation2002; Zeng Citation2004; Jiao & Fu Citation2009; Jiang Citation2012).
We investigated the mechanism of E. ulmoides resistance to Hyphantria cunea (Lepidoptera, Arctiidae), which is an invasive defoliator, and Anoplophora glabripennis (Coleoptera, Cerambycidae), which is a typical native borer that has co-evolved with domestic plants like E. ulmoides. Pest infestation patterns on E. ulmoides were investigated in Beijing, and the feeding-deterrent activity of Gutta-percha against the two species was studied. This will provide a base-line reference for future studies on the mechanism of pest resistance of E. ulmoides, and additional strategies for general pest control and prevention.
Materials and methods
Analysis of pest feeding
The investigation study was conducted in June 2014 in urban areas of Beijing. Five sample plots containing 30 E. ulmoides trees per plot were selected. Three distinct classes rating tree health were established to analyze pest infestation () (Wu et al. Citation2002; Liu et al. Citation2003; Qie et al. Citation2013). Tree height, diameter, health status, and injuries were measured and recorded. The pest resistance percentage was calculated as follows: the number of pest-free trees/the total number of trees in the plot (Chen et al. Citation2008; Zhang et al. Citation2014).
Methods for determining the antifeedant activity of gutta-percha
Insect pests
H. cunea egg masses were provided by the China Academy of Forestry Sciences, Beijing. The second- and third-instars that hatched from the same egg mass were used. A. glabripennis larvae were collected from the Ningxia Province, China, from June to September, 2013, and 210 third-stage larvae that fed well on artificial diet were used. Both pests were fed artificial diets in an incubator with 25°C constant temperature, 75% humidity, and were starved for 3 days before experiments to ensure uniform feeding status.
Chemical reagent
Gutta-percha (purity > 99%) was purchased from Sigma-Aldrich (182168-50G, USA), dissolved in petroleum ether (boiling in the range 30−60°C), and stored at 4°C.
Artificial diets
The artificial diet for H. cunea was provided by the Chinese Academy of Forestry, Beijing (Zhang et al. Citation2007). The diet for A. glabripennis was prepared in our lab according to previously described protocols (Zhao et al. Citation1999). At the end of diet preparation, it was poured into a 50 mL transparent plastic box and allowed to solidify. To feed H. cunea, the diet covered the bottom of the culture box. To feed A. glabripennis, the diet that had solidified in the 50 mL box was cut into four pieces (diameter = 6 cm, height = 2.5 cm). One piece was placed into each of four culture boxes as described in the next section.
Antifeedant activity of gutta-percha when mixed into diets
A. glabripennis larvae were separated into four groups with 10 larvae per group, and were fed in individual chambers with artificial diet containing 0%, 3%, 6%, or 9% gutta-percha for 5 days. Second-instars H. cunea were separated into four groups of approximately 50 larvae per group, and were fed in individual chambers with artificial diet containing 0%, 1%, 3%, or 6% gutta-percha for 10 days. The amount of feeding by A. glabripennis larvae was recorded, and the amount of feeding was graded to facilitate statistical analysis. No feeding was defined as class 1, while, 1−50% of the diet consuming was defined as class 2, more than 50% was defined as class 3. Ten H. cunea larvae were randomly selected to measure body length, and the average was calculated. All experiments were independently repeated three times.
In this initial study, we mixed petroleum ether in the artificial diets, and detected no effect on the feeding behavior of H. cunea or A. glabripennis larvae, which is probably due to the low-boiling point and fast evaporation of petroleum ether. Therefore, it was selected as the organic solvent for gutta-percha.
Antifeedant activity of a surface coating of gutta-percha
A. glabripennis larvae were separated into three groups of 10 larvae each, and every larvae was fed in individual chambers with artificial diet daubed with gutta-percha on the surface, or daubed on half of the diet and then covered with the remaining untreated diet (designated as interlayer), or not daubed (control), for 5 days. The amount of unconsumed diet was calculated as ‘the number of larvae that failed to break through the gutta-percha layer/the total number of larvae'. Third-instar H. cunea larvae were separated into three groups of 30 larvae each, and were fed with artificial diet daubed with gutta-percha on the surface for 3 days. Then, the feeding area was measured using a 1 mm × 1 mm paper grid. The amount of unconsumed diet was calculated as ‘the uneaten surface area of the diet / the total area of the diet' (Hu et al. Citation2011). All experiments were independently repeated three times.
Statistical analysis
Data were analyzed using SPSS v16.0 and Prism v6.0.
Results and discussion
Characteristics of pest damage to E. ulmoides
The height of E. ulmoides trees in the five sample plots was 6.3−11.5 m, and the diameter was 15.4−24.1 cm. Symptoms of pest feeding on the trees are summarized as described in . The percentage of trees that exhibited pest resistance averaged 93.3%, and only 1 E. ulmoides tree had poor health status (Class 3) out of the total 150 trees in all five plots. Most of the pest damage occurred on leaves. Only one E. ulmoides tree in Plot 4 had trunk damage by borers, and the area around the emergence hole was not covered by bark.
Antifeedant activity of gutta-percha when mixed into diets
The amount of feeding by A. glabripennis larvae significantly decreased in a dose-dependent manner when gutta-percha was mixed into the artificial diet (). Some of the larvae in the 3% gutta-percha group apparently bored into the diet on the fifth day, and the feeding on that day increased. This behavior was not observed in the groups fed with diet containing 6% or 9% gutta-percha.
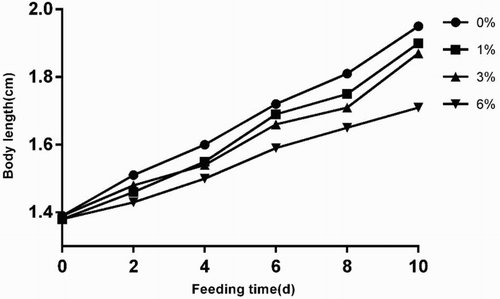
The length of H. cunea larvae fed with artificial diet containing gutta-percha dramatically decreased in a dose-dependent manner after day 4 of the experiment (). The reduction of body length was most obvious in the group that was fed diet containing 6% gutta-percha. Compared with larval length in the control group (0% gutta-percha), there was no significant difference on day 10 in the groups fed 1% and 3% gutta-percha. The body length curves had fluctuations on days 4 and 8 due to molting. Analysis of variance indicated that the body length difference in the group fed with diet containing 6% gutta-percha compared with that of control was statistically significant (P < .05).
Antifeedant activity of a surface coating of gutta-percha
Both H. cunea and A. glabripennis larvae normally feed on the artificial diet. Daubing gutta-percha on the surface of the diet significantly deterred feeding for both insect species (). The antifeedant effect of gutta-percha was more effective if it was daubed on the surface than if it was daubed on half of the diet and then covered with the remaining untreated diet (designated as interlayer). The amount of unconsumed diet for both H. cunea and A. glabripennis larvae was more than 95%.
Conclusion
Gutta-percha is synthesized by a group of E. ulmoides cells that are linked into a web-like network. Gutta-percha has significant antifeedant activity against H. cunea and A. glabripennis larvae. The gutta-percha content in E. ulmoides consistently increases in parallel with the seasonal increase in pest activity (Cui et al. Citation1999). The gutta-percha content in different parts of E. ulmoides also varies. The content was higher in bark than in leaves (Tangpakdee Citation1997; Du et al. Citation2003), which may explain why more pest damage occurs in leaves than in bark. Higher gutta-percha contents in artificial diet had a stronger antifeedant activity against both larval species. Lower gutta-percha contents in artificial diet had limited antifeedant activity during the later stages of the experiment, which may be attributed to increased hunger and improved boring ability as the insects aged. Gutta-percha significantly deterred larval feeding when mixed into artificial diet at ≥6% concentration, or when daubed on the feeding surface of the diet. This indicates that gutta-percha deters insect feeding by acting as a physical or chemical barrier. Higher gutta-percha concentrations would generate stronger barriers.
Figure A1. Analysis of artificial diet consumption by A. glabripennis larvae when diet contained 0−9% gutta-percha.
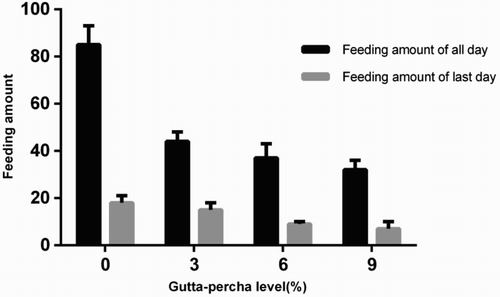
Figure A2. Analysis of E. ulmoides larval length after eating artificial diet containing 0−6% gutta-percha.
E. ulmoides is an ancient plant, which shows stronger resistance to pests than trees that evolved after the Ice Age mass destruction. As a secondary metabolite, gutta-percha has no effect on growth and development of the plant, but plays an important role in pest resistance. The long-term co-evolution of E. ulmoides and pests provided E. ulmoides with this physical barrier (Turlings & Benrey Citation1998; Qin & Wang Citation2001; Zhang & Liu Citation2003; Zhao et al. Citation2006; Felton & Tumlinson Citation2008; Koul Citation2008), but how it evolved still requires further study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chen PC, Li YS, Li YZ, Lu C. 2008. The damage of two kinds of Cerambycidae on the maple and its control measures. Plant Protect (Chinese). 34:158–161.
- Cui YH, Wang M, Sun KL. 1999. Morphological study of gutta-containing cells in Eucommia ulmoides Oliv. Chinese Bull Bot. 16:439–443.
- Du HY, Xie BX, Shao SY. 2003. Prospects and research progress of gutta-percha. J Cent S For Univ(Chinese). 4:95–98.
- Felton GW, Tumlinson JH. 2008. Plant-insect dialogs: complex interactions at the plant-insect interface. Curr Opin Plant Biol. 11:457–463.
- Hu X, Yan SC, Lu YF, Liu D. 2011. Antifeedant activity of the secondary metabolic compounds of yew against Lymantria dispar L. larvae. J Beijing For Univ. 33:151–154.
- Jiang YD. 2012. Investigation of diseases and insect pests for Eucommia ulmoides Oliver in Jishou City and its control measures. Hunan Agr Sci. 11:82–86.
- Jiao YC, Fu PY. 2009. Investigation of diseases and insect pests for Eucommia ulmoides in Zunyi and its control measures. Chinese Countryside Well-off Tech. 7:63–65.
- Koul O. 2008. Phytochemicals and insect control: an antifeedant approach. Crit Rev Plant Sci. 27:1–24.
- Li J. 2002. Application of Eucommia ulmoides prevention and control of plant pests. China: 99121649.0.
- Liu CF, He XY, Chen W, Xu WD, Zhao GL. 2003. Selection of tree species composition in Shenyang's urban forest communities. Chinese J Appl Ecol. 14:2103–2107.
- Maleck K, Dietrich RA. 1999. Defense on multiple fronts: how do plants cope with diverse enemies. Trends Plant Sci. 4:215–219.
- Pavela R, Herda G. 2007. Effect of pongam oil on adults of the greenhouse whitefly Trialeurodes vaporariorum (Homoptera: Trialeurodidae). Entomol Gen. 30:193–201.
- Pavela R, Kazda J, Herda G. 2009. Effectiveness of neem (Azadirachta indica) insecticides against brassica pod midge (Dasineura brassicae Winn). J Pest Sci. 82:235–240.
- Qie GF, Peng ZH, Wang C. 2013. Growth and health status of Ginkgo biloba in Beijing urban street area. Forest Res(Chinese). 26:511–516.
- Qin JD, Wang CZ. 2001. The relation of interaction between insects and plants to evolution. Acta Entomol Sin(Chinese). 44:258–264.
- Rani AS, Murty US. 2009. Antifeedant activity of Spilanthes acmella flower head extract against Spodoptera litura (Fabricius). J Entomol Res. 33:55–57.
- Shi GR. 1994. Significance of secondary substances in collaborative evolutionary between plants and insects. J Biol(Chinese). 44:11–16.
- Song L, Zhang XJ, Dong DP, Wang QH. 2006. Gutta-percha properties and research progress of extraction. Guizhou Chem Ind. 31:6–8.
- Tangpakdee J, Tanaka Y, Shiba K, Kawahara S, Sakurai K, Suzuki Y. 1997. Structure and biosynthesis of trans-polyisoprene from Eucommia ulmoides. Phytochemistry. 45:75–80.
- Turlings TCJ, Benrey B. 1998. Effects of plant metabolites on the behaviour and development of parasitic wasps. Ecoscience. 5:321–333.
- Wu ZM, Huang CL, Bai LB, Wu WY. 2002. Urban forest structure of Hefei city. Sci Silvae Sinicae(Chinese). 38:7–13.
- Zeng LX. 2004. Control of major diseases and pest in Eucommia ulmoides. Guizhou Agr Sci. 32:75–77.
- Zhang GX, Yuan ZY, Hu NN, Zhao GJ, Li XY. 2014. The resistance of different poplar varieties to Cryptorrhynchus lapathi research in Shenyang area. J Liaoning For Sci T. 3:55–56.
- Zhang JC, Xue ZH. 2011. The research progress of natural polymer materials – gutta-percha. Acta Polym Sin(Chinese). 2011:1105–1121.
- Zhang WH, Liu GJ. 2003. A review on plant secondary substances in plant resistance to insect pests. Chinese Bull Bot. 20:522–530.
- Zhang YA, Wang YZ, Xu BM, Qu LJ. 2007. Artificial feeding and rational method of Hyphantria cunea and the method of making the larva artificial feed (P). CN1994241. 2007–07–11.
- Zhao GQ, Liu XG, Luo MH. 2006. Chemical sensory mechanisms of insects selecting host plants. J Henan Univ Sci T: Nat Sci. 27:80–82.
- Zhao J, Nobuo O, Masahiro I. 1999. Artificial rearing of Anoplophora glabripennis. J Beijing For Univ. 21:58–62.
