ABSTRACT
The application of atoxigenic Aspergillus flavus strains in maize fields has been shown to be an effective strategy for controlling contamination of aflatoxins, potent carcinogens produced by the fungus. This study monitored the expression levels of 18 defense genes against toxigenic and atoxigenic A. flavus strains in developing maize kernels over a time course of 96 h after inoculation. A stronger upregulation of genes encoding pathogenesis-related proteins, oxidative stress-related proteins, transcriptional factors and lipoxygenases were observed in response to the atoxigenic strain. On the other side, this strain showed a significant enhanced growth in the later stages of infection, measured as copy number of the constitutive calmodulin gene. These results suggest that overexpression of maize-defense-associated genes observed in response to the atoxigenic strain could contribute to an aflatoxin reduction. The identification of genes significantly affecting the resistance to A. flavus or aflatoxin accumulation would accelerate the development of resistant cultivars.
1. Introduction
Aflatoxins are polyketide-derived secondary metabolites primarily produced by Aspergillus flavus in several host crops, highly toxigenic and carcinogenic to humans and animals consuming contaminated food and feed (Bhatnagar et al. Citation2006). Aflatoxin contamination of maize is a serious safety issue worldwide but, until few years ago, it has not been signaled as a matter of concern in Europe (Logrieco & Moretti Citation2008). However, the alarming contaminations reported in 2003, 2012 and 2015 for Italy and south Europe, respectively, must be mentioned (Giorni et al. Citation2007; Levic et al. Citation2013).
Aflatoxin mitigation in maize through proper cropping systems, such as tillage, irrigation, early sowing and harvesting, is commonly not sufficient for the production of grain compliant with legal limits. Several attempts to develop varieties resistant to fungal growth and/or aflatoxin contamination have been carried out using breeding strategies (conventional and based on genetic modifications). As a result, many new sources of resistance to Aspergillus infection and the subsequent accumulation of aflatoxins in corn have been identified and released (Chen et al. Citation2015). In the long term, the creation of resistant germplasm with desirable agronomic characteristics could be the most economically efficient control measure for all markets (Williams et al. Citation2015).
The biocontrol strategy, based on the competitive exclusion of toxigenic by applying atoxigenic A. flavus strains in field, has been shown to significantly reduce aflatoxin contamination in the USA and African countries and may reduce the problem in the short term as well (Ehrlich Citation2014). Atoxigenic strains of A. flavus were reported to reduce the contamination of aflatoxin up to 90% in maize in in-vitro studies carried out with Italian strains (Mauro et al. Citation2015). Comparable results are reported in field trials managed in the USA and Africa (Abbas et al. Citation2006; Atehnkeng et al. Citation2016). Use of biocontrol products with atoxigenic A. flavus active ingredients is a proven method for reducing the aflatoxin content and will be crucial in the future; in fact, A. flavus and other aflatoxin-producing fungi thrive under the hot and dry environmental conditions recently experienced and predicted due to the ongoing climate change (Battilani et al. Citation2016). However, for this biocompetition strategy to achieve its full potential in mitigating the issue of aflatoxin contamination, management programs that optimize both biocontrol’s long-term and area-wide benefits are still needed.
Plants respond to fungal pathogens through various defense mechanisms that are host–pathogen dependent (Kelley et al. Citation2012). The fungal pathogens are basically classified into groups of biotrophs, hemibiotrophs and necrotrophs (Oliver & Ipcho Citation2004). The plant resistance to necrotrophic fungi is known to be quantitative and controlled by multiple genes (St Clair Citation2010). Toxigenic strains of A. flavus are characterized with the features of necrotrophic fungal pathogens (Kelley et al. Citation2012) and jasmonate- and ethylene-dependent signaling pathways are likely involved in such resistance systems, triggered by fungal toxins or other fungal effectors (Oliver & Ipcho Citation2004). The identification of specific genes playing a precise role in host resistance to A. flavus or aflatoxin accumulation would enable more rapid selection of resistant maize inbred lines and hybrids. Previous gene mapping studies led to the identification of several A. flavus resistance-related quantitative trait loci (QTLs) in maize (Brown et al. Citation2013). Interestingly, QTLs for resistance to aflatoxin accumulation appeared to be derived by both resistant and susceptible parental lines (Willcox et al. Citation2013). Nevertheless, the molecular mechanisms underlying the significant aflatoxin reduction in resistant maize lines or the significant aflatoxin accumulation in susceptible maize lines are yet to be determined.
The aim of this study was to understand the molecular response of maize after infection with toxigenic and atoxigenic A. flavus strains. Real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed by assaying a set of selected defense-related candidate genes on developing kernel samples of a susceptible inbred line over a time course of 96 h. The results report interesting differences between toxigenic and atoxigenic strains in the expression level of the assayed genes and will help in understanding the complex genetics involved in plant–pathogen interaction.
2. Materials and methods
2.1. Plant and fungal materials
The maize inbred line CO354 was used as a susceptible genotype to A. flavus infection (Lanubile et al. Citation2015). The line was developed by the Eastern Cereal and Oilseed Research Centre, Agriculture and Agri-Food Canada, Ottawa, Canada (Reid et al. Citation2009), and was maintained by pollinating sister plants at the Department of Sustainable Crop Production, Università Cattolica del Sacro Cuore, Piacenza, Italy. The study was carried out in open air and a randomized block design with three treatments (inoculation with water, toxigenic and atoxigenic strains of A. flavus) and three replicates were adopted. Six plants distributed in three plastic pots were used for each replication. Each pot (40 cm diameter and 35 cm height) contained 25 kg of dry loam soil with the following properties: pH 7.5, organic matter 1.80%, available P 15.5 mg kg−1 and exchangeable K 130 mg kg−1. Maize seeds were sown in late April 2013 and plants were grown with an unlimited water supply and were fertilized with NH4NO3 corresponding to 150 kg N ha−1. The fertilizer was dressed twice. Flowering was on in early July. Ears were hand self-pollinated. The maximum and minimum temperatures (°C), respectively, were 30.6 and 18.3 in the period occurring from flowering to harvest.
The aflatoxin-producing MPVP A2092, belonging to vegetative compatibility group (VCG) IT03 and the atoxigenic MUCL54911 (MPVP A2085; VCG IT06) strains of A. flavus originating from Italy were used in this study. The isolates were previously examined for aflatoxin production and categorized into VCGs (Giorni et al. Citation2007; Mauro et al. Citation2013) and included in the culture collection of the Department of Sustainable Crop Production, Università Cattolica del Sacro Cuore, Piacenza, Italy. Cultures were inoculated on Petri plates (9 cm diameter) in Czapek Agar (CZ; sucrose 30 g; NaNO3 2 g; KCl 0.5 g; MgSO4·7H2O 0.5 g; FeSO4·7H2O 0.01 g; K2HPO4 1 g; ZnSO4·7H2O 0.001 g; CuSO4·7H2O 0.005 g; agar 15 g; H2O to 1 L) and incubated at 25°C with a 12 h photoperiod for 14 days. Conidia were collected by rinsing plates with sterile water, scraping the agar surface with a scalpel and filtering the suspension through sterile cloth. The suspension was adjusted to a final concentration of 3.5 × 106 conidia/ml using a Bürker chamber.
2.2. Fungal inoculation and sample collection
Maize ears were inoculated 15 days after hand-pollination using a side-needle inoculator, as reported by Lanubile et al. (Citation2015). The inoculated and immediately adjacent kernels were collected at 24, 48, 72 and 96 h post-inoculation (hpi). Control ears were mock-inoculated (inoculation with water) at the same inoculation time listed above. Three biological replicates were prepared for each time point, collecting kernels from six different maize ears. They were ground in liquid nitrogen with a pestle and mortar and used both for gene expression analyses and aflatoxin quantification.
2.3. RNA extraction and real-time RT-PCR expression analysis
Total RNA extraction and purification were performed according to Lanubile et al. (Citation2015). Real-time RT-PCR experiments were performed on kernels collected at 24, 48, 72 and 96 hpi using the 2x iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) and the CFX-96 device (Bio-Rad). One microgram of total RNA was used for cDNA synthesis following the iScript cDNA synthesis kit protocol (Bio-Rad). Twenty nanogram of single strand cDNA, determined by fluorometric assay (Qubit, Invitrogen), were used for real-time RT-PCR. Relative quantitative analysis was performed under the following conditions: 95°C for 3 min and 40 cycles at 95°C 15 s, 57–63°C for 30 s. A melting curve analysis, from 60°C to 95°C with a 0.5°C increment each 5 s, was used to identify different amplicons, including nonspecific products. Three technical replicates (within each biological replicate) were employed for each tested sample and template-free negative controls. Gene-specific primers were downloaded from the literature or designed possibly within consecutive exons, separated by an intron, using Primer3 software (supplemental Table 1). Relative quantification of maize genes was normalized to the housekeeping gene β-actin (supplemental Table 1) and fold change (FC) values in gene expression were analyzed by using the 2–ΔΔCt method (Schmittgen & Livak Citation2008).
To quantify fungal growth, the copy number of calmodulin transcript was detected using real-time RT-PCR; thermal cycling conditions were same as those reported above. The calmodulin number of copies is related to the ng of cDNA obtained from kernel tissues according to the equation of the linear regression reported in the technical manual (Bio-Rad). Fungal cDNA (20 ng) was serially diluted [1:1, 1:5, 1:52, 1:53, 1:54, 1:55] in sterile water and 20 ng of each kernel cDNA sample was compared to the dilution standard curve to determine fungal cDNA copy number.
2.4. Aflatoxin analysis
Aflatoxin content in kernels was measured according to Bertuzzi et al. (Citation2012). Briefly, 5 g of kernel sample, homogenized at 96 hpi, was extracted for 60 min with 20 mL of acetone–water 7 + 3 v/v using a rotary-shaking stirrer. After purification through an immunoaffinity column, the extract was filtered (Millex HV 0.45 mm) before high-performance liquid chromatography- fluorescence detection analysis (Jasco Corporation, Tokyo, Japan). Detected aflatoxins B1 (AFB1) and B2 (AFB2) were expressed in µg kg−1. The limit of detection was 0.1 µg kg−1.
2.5. Statistics
Two-factor analysis of variance (ANOVA) was performed on all genes, considering the sampling time points (24, 48, 72 and 96 hpi) and treatments (aflatoxin-producing and atoxigenic-inoculated samples) as fixed factors, in order to test the significance (P ≤ .05) of sampling, treatments and their interactions. Differences between aflatoxin-producing and atoxigenic strains within the same time of sampling were performed using two-way ANOVA and considered to be significant at *P ≤ .05; **P ≤ .01; ***P ≤ .001.
3. Results and discussion
3.1. Fungal growth and aflatoxin accumulation in kernels challenged with toxigenic and atoxigenic strains of A. flavus
In order to determine differences in fungal colonization between the toxigenic (MPVP A2092) and atoxigenic (MUCL54911)-inoculated maize kernels from ears of the susceptible line CO354, quantification of the fungal calmodulin transcript by real-time RT-PCR was carried out in this study (). Fungal growth of both A. flavus strains increased over the time course of 96 h, with the highest levels of calmodulin copy number at 72 hpi (818 vs. 900 copies for the toxigenic and atoxigenic strains, respectively) (). The decrease in calmodulin transcripts from 72 to 96 hpi probably coincided with the beginning of the necrosis of infected maize kernel. No significant differences were observed in the ability to colonize maize ears at 24 and 48 hpi between the two strains. On the other hand, in the later stages of infection the atoxigenic strain showed a significant higher copy number compared to the toxigenic one (900 and 545 copies at 72 and 96 hpi, respectively, for the atoxigenic strain compared to 818 and 400 copies for the toxigenic one). Aflatoxin levels in kernels inoculated at 96 h with the toxigenic strain were 3900.2 and 3.1 µg kg−1 for AFB1 and AFB2, respectively, while, as expected, no aflatoxins were produced by the atoxigenic strain. The toxigenic strain A2092 was reported as one of the best producers of AFB1 on coconut-based medium (Giorni et al. Citation2007). Aflatoxin production in the Aspergilli is a complex process under a high degree of regulation through multiple mechanisms (Amaike & Keller Citation2011). Currently, the molecular mechanisms responsible for the loss of aflatoxin production in A. flavus are not well understood (Schmidt-Heydt et al. Citation2008). Atoxigenic A. flavus isolates were commonly deleted of a part or the entire aflatoxin gene cluster (Chang et al. Citation2005). The atoxigenic isolate MUCL54911 belongs to the VCG IT6 that was observed most frequently in infected maize produced in Italy and showed the deletion of all 16 genes of the aflatoxin biosynthesis cluster (Mauro et al. Citation2013). The inability to produce aflatoxins in kernels of the susceptible maize line at 96 hpi with the atoxigenic isolate MUCL54911 was confirmed in previous studies, where no aflatoxin production was detected in kernels at 7 days after inoculation with the same A. flavus strain (Mauro et al. Citation2013, Citation2015). Nevertheless, the absence of aflatoxin production for the atoxigenic strain did not change its growth ability on maize kernels, rather it increased in the later time-points compared to the toxigenic strain. According to these findings, previous genetic analyses of fumonisin-non-producing mutants of Fusarium verticillioides demonstrated that mutants were more virulent on maize ears than their wild-type progenitor strains (Proctor et al. Citation2002; Lanubile et al. Citation2013). On the other hand, trichothecene-non-producing mutants of Fusarium graminearum showed a reduced virulence on wheat and maize probably caused by the loss of mycotoxin production (Proctor et al. Citation2002). In light of these evidences further investigations should address more critically the relationships of aflatoxin and virulence of A. flavus.
Table 1. Analysis of A. flavus infection steps.
3.2. Time course expression analysis on selected candidate genes
Determination of mechanisms underlying maize host resistance to aflatoxin accumulation has been proved difficult due to the complex nature of this quantitative trait. Many genes were found to be involved in the maize host resistance (Kelley et al. Citation2012). The identification of genes having larger effects on the resistance or susceptibility is important to facilitate marker-assisted selection breeding of resistant maize lines. Without aflatoxin-resistant commercial varieties, farmers rely on cultural practices and lately biocontrols to manage A. flavus infection and to reduce aflatoxin contamination. Our study provides the first investigation of gene expression changes that occur when maize is inoculated with A. flavus by comparing toxigenic and atoxigenic interactions in the same genetic background, using the susceptible CO354 genotype. Eighteen potentially relevant genes related to maize defense to A. flavus infection were selected, and then real-time RT-PCR expression analysis was conducted to take a closer look at the highly expressed host plant genes and to strengthen our knowledge concerning novel details of infection by using two different host–pathogen combinations.
3.3. Expression of pathogenesis-related genes
Upon pathogen attack, plants react with complex defense responses including the production of pathogenesis-related (PR) proteins. Among the 17 PR protein families the expression profiles of five PR genes (PR1, PRm3, PR5, PRm6 and PR10) were investigated after inoculation of maize kernels with toxigenic and atoxigenic strains of A. flavus ((a)–(e)). Compared to mock-inoculated control kernels, PR gene expression was induced by the inoculation of maize ears with both A. flavus strains as early as 24 up to 96 hpi. Interestingly, significantly higher FCs were observed in response to the atoxigenic strain for all tested genes starting from 48 until 96 hpi. The atoxigenic strain caused drastic induction of PR1 and PR10 genes (201.4 and 34.4 FCs, respectively) at 48 hpi that declined in the further time-points ((a) and 1(e)). Similarly, PR5 and PRm6 reached the highest level of induction at 48 hpi that remained high at 72 hpi, whereas declined at 96 hpi ((c) and 1(d)). Conversely, the expression of PRm3 was different and peaked at 96 hpi with an FC value of 187.2 ((b)). The most striking differences in the host response following inoculation with the atoxigenic and toxigenic strains of A. flavus were notably evident at 48 hpi, where the magnitude of induction was significantly higher in response to the atoxigenic one of 6.4 fold for PR10 gene, followed by a 5.1, 3.7 and 2.6 fold induction for PRm6, PR1 and PR5 genes, respectively. In the later time-points (72–96 hpi) less pronounced differences were observed, with the exception of PRm3 gene expression that was 5.5-fold significantly upregulated at 96 hpi with the atoxigenic strain compared to the toxigenic one ((b)). Even though PR10 gene displayed the lower FC values compared to the other PR genes, however, the most marked significant differences between the atoxigenic and toxigenic strains were observed for this gene all over the considered time course. The expression of PR10 gene has been reported to be induced in fungal-infected maize seed (Cordero et al. Citation1994). Furthermore, studies by Chen et al. (Citation2010) showed that PR10 has antifungal activity against A. flavus in vitro. They also showed that repression of maize PR10 by RNAi gene silencing resulted in increased susceptibility to A. flavus and aflatoxin production (Chen et al. Citation2010).
Figure 1. FC of differentially expressed genes PR1 (a), PRm3 (b), PR5 (C), PRm6 (d) and PR10 (e), in kernels of the susceptible CO354 maize line at 24, 48, 72 and 96 hpi with aflatoxin-producing and atoxigenic strains of A. flavus (white and gray bar charts, respectively). Vertical bars indicate ±standard deviation. *Significant differences between aflatoxin-producing and atoxigenic-inoculated means within the same time of sampling, according to two-way ANOVA (*P ≤ .05; **P ≤ .01; ***P ≤ .001).
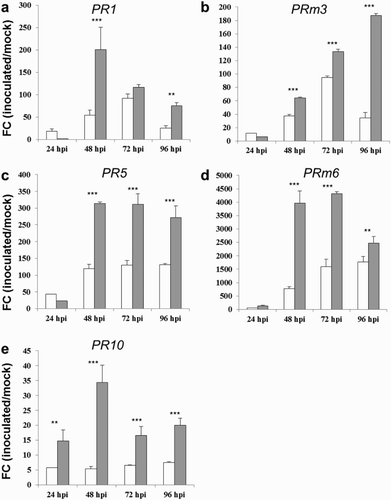
PRm6 was the gene with the highest induction values ranging from 128.7 to 4320.9 with the atoxigenic A. flavus and from 54.2 to 1771.7 with the toxigenic one over the time course of four days ((d)). It is well known that plant β-1,3-glucanases, such as PRm6, play a role in plant defense against various pathogen invasions. Several studies have revealed the presence of a relationship between maize β-1,3-glucanase and A. flavus infection. It was reported that the growth of A. flavus was inhibited more by callus of a resistant maize genotype (Tex 6 x Mo17) than by a susceptible genotype (Pa91) (Lozovaya et al. Citation1998). This inhibition was correlated with the activity levels of β-1,3-glucanase in the callus and in the culture medium. Bedre and co-workers (Citation2015) reported that two β-1,3-glucanases (BG1 and BG3) were highly induced by an atoxigenic strain of A. flavus on cotton seed, whereas another BG3 transcript was upregulated specifically by the toxigenic strain (Bedre et al. Citation2015). The hydrolytic enzymes β-1,3-glucanase and chitinase, such as PRm3, possess antifungal activity by degrading the fungal cell wall containing chitins (Cleveland et al. Citation2004). Plant chitinases possess lysozyme activity and are highly active in inhibiting fungal growth (Prasad et al. Citation2013). Different isoforms of chitinases were induced by infection with both atoxigenic and toxigenic A. flavus in cotton pericarp and seed (Bedre et al. Citation2015). Furthermore, a chitinase A positioned in bin 2.04 was associated with a large aflatoxin reducing QTL, as reported by Hawkins et al. (Citation2015).
Overall, the induction and greater accumulation of PR genes confirmed their essential role in the response against A. flavus pathogen. Furthermore, these data provided the first evidence that the transcriptional response of maize to an atoxigenic strain of A. flavus was more pronounced in FC values compared to the toxigenic one.
3.4. Modulation of reactive oxygen species scavenging-related genes
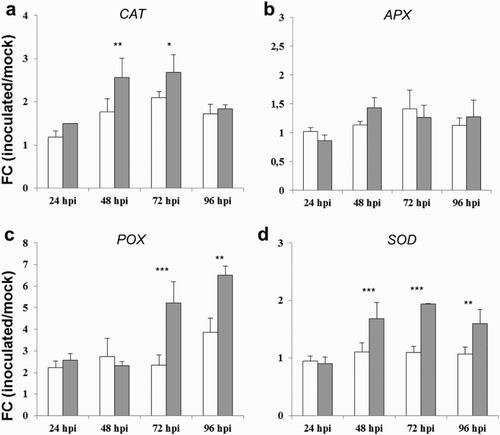
Oxidative burst is one of the earliest defense responses of plants against pathogen infection and wounding, and it is associated with production of reactive oxygen species (ROS; Dickman & Fluhr Citation2013). ROS are involved not only in direct defense reaction by killing the pathogens, but also in the activation of defense-related genes through a signaling mechanism (Torres Citation2010). ROS can regulate the transcription factors (TFs) and produce antimicrobial phytoalexins and other secondary metabolites, which have inhibitory activity on pathogen growth (Torres Citation2010). The transcripts encoding catalase (CAT), ascorbate peroxidase (APX), peroxidase (POX) and superoxide dismutase (SOD), which are involved in ROS processing and scavenging, showed increased expression levels under A. flavus infection in maize ((a)–(d)). In general, the susceptible line showed a stronger induction for almost all antioxidant genes analyzed in this study in response to the atoxigenic strain, even though at less extend levels, as previously reported for PR genes. POX was the most induced gene with FC values ranging from 2.6 to 6.5 and from 2.2 to 3.9 over the time course following atoxigenic and toxigenic strain inoculation, respectively ((c)), and the atoxigenic A. flavus inoculation elicited a reaction of about two times higher at the later time-points (72–96 hpi) compared to the toxigenic one ((c)). Less marked significant differences were observed in favor of atoxigenic strain for CAT gene between 48 and 72 hpi and SOD gene from 48 to 96 hpi ((a) and 2(d)), while the expression patterns for APX gene were similar under the atoxigenic and toxigenic strain inoculation ((b)). The observed enhanced expression level of antioxidant mechanisms against the atoxigenic strain in maize tissues leads to the hypothesis that the increase in defense responses to ROS-induced oxidative stress may correlate to resistance to A. flavus and aflatoxin reduction. Prior research exploring the roles of oxidative stress in regulating aflatoxin biosynthesis as well as recent discoveries into the upstream regulation of major pathway regulatory factors has begun to elucidate the biological function of aflatoxins (Roze et al. Citation2013). Numerous studies have shown that aflatoxin production can be exacerbated by ROS and their reactive products, including oxylipins (Gao & Kolomiets Citation2009). Several works have demonstrated that A. flavus toxigenic isolates exhibited elevated oxygen consumption, greater mycelial ROS accumulation and greater peroxisome number than atoxigenic isolates, indicating a reasonable link between ROS and aflatoxin accumulation (Reverberi et al. Citation2012). Given the possible role of oxidative stress in the promotion of aflatoxin biosynthesis, the hypothesis has been proposed that aflatoxin may function as a form of antioxidant protection to toxigenic Aspergilli (Reverberi et al. Citation2010), and as a consequence the toxigenic strain could use aflatoxin to inhibit and reduce the expression level of maize oxidative stress-related responses. This would provide an explanation to the biological significance of aflatoxin, although the potential antioxidant mechanism of action of this mycotoxin has yet to be fully elicited.
Figure 2. FC of differentially expressed genes CAT (a), APX (b), POD (c) and SOD (d), in kernels of the susceptible CO354 maize line at 24, 48, 72 and 96 hpi with aflatoxin-producing and atoxigenic strains of A. flavus (white and gray bar charts, respectively). Vertical bars indicate ±standard deviation. *Significant differences between aflatoxin-producing and atoxigenic-inoculated means within the same time of sampling, according to two-way ANOVA (*P ≤ .05; **P ≤ .01; ***P ≤ .001).
3.5. Changes in genes encoding TFs
The expression of downstream genes in response to pathogen infection is normally controlled by TFs, direct or indirect targets of various signal transduction pathways. Many components of these pathways are regulated by TFs of a type known as WRKY TFs (Rushton et al. Citation2010). WRKY TFs are abundant in many model and crop species, such as Arabidopsis (74), rice (109), soybean (176) and maize (136) (Wu et al. Citation2005; Pandey & Somssich Citation2009; Wei et al. Citation2012; Song et al. Citation2016). In the present study the expression levels of three WRKY and one Myb-like DNA-binding protein TF genes were also examined over the time course following toxigenic and atoxigenic A. flavus inoculation ((a)–(d)). The expression of WRKY1 increased in inoculated kernels from 24 to 48 hpi and thereafter for both strain treatments ((a)), but the atoxigenic strain caused a stronger significant induction compared to the toxigenic one from 48 to 96 hpi. The highest expression levels were observed for WRKY29 gene where significant differences were displayed between the two strains at 24 and 72 hpi (FC of 1 vs. 4.2 and 9 vs. 24.7 in response to the toxigenic and atoxigenic strains, respectively; (b)). No significant variation in the expression levels between the two strain treatments was detected for WRKY110 gene, except at 72 hpi, which showed a marginally significant induction in response to atoxigenic A. flavus inoculation (FC of 1.4 vs. 2.1 against the toxigenic and atoxigenic strains, respectively; (c)). This is in agreement with previous results reported by Wei et al. (Citation2012), that also found a gradual increasing trend in the expression levels of maize WRKY29 gene (designated as WRKY39) during the infection with Ustilago maydis. On the other hand no expression changes were observed for WRKY110 gene (designated as WRKY30), suggesting that it may respond primarily to environmental stress and not to pathogen infections (Wei et al. Citation2012). Recent transcriptional profiling studies have shown that 28 WRKY-related transcripts were differentially expressed under toxigenic and atoxigenic strains of A. flavus in cotton seed and pericarp tissues (Bedre et al. Citation2015). Most of the WRKY TFs were upregulated under the atoxigenic strain infection in pericarp and the toxigenic strain infection in seed, with the exception of WRKY75 and WRKY40 genes that were specifically upregulated under the atoxigenic strain in both tissues. Furthermore, Fountain et al. (Citation2015) examined the expression of several WRKY TFs in both resistant and susceptible maize inbred lines in response to A. flavus inoculation under field conditions over a time course of 18 days. Interestingly, elevated expression levels of WRKY53.1 were observed in the susceptible line B73. The significant induction of this gene could be due to the presence of at least two paralogous copies of the gene in the genome of B73 or to a defect in feedback inhibition of expression mediated by the WRKY53.1 protein (Fountain et al. Citation2015). Identifying downstream target genes of WRKY factors will be crucial in understanding their biological functions. Transient overexpression of Petroselinum crispum WRKY1 in parsley protoplast led to the activation of a reporter gene driven by the promoters of three potential target genes, namely Pc Pathogenesis-related 1-1 (PcPR1-1), PcWRKY1 and PcWRKY3 (Eulgem et al. Citation1999). Consistent with this, a strong induction of PR genes was also observed in our study, more marked in response to the atoxigenic A. flavus strain, as discussed in the previous paragraph.
Figure 3. FC of differentially expressed genes WRKY1 (a), WRKY29 (b), WRKY110 (c) and Myb-like DNA binding protein (d), in kernels of the susceptible CO354 maize line at 24, 48, 72 and 96 hpi with aflatoxin-producing and atoxigenic strains of A. flavus (white and gray bar charts, respectively). Vertical bars indicate ±standard deviation. *Significant differences between aflatoxin-producing and atoxigenic-inoculated means within the same time of sampling, according to two-way ANOVA (*P ≤ .05; **P ≤ .01; ***P ≤ .001).
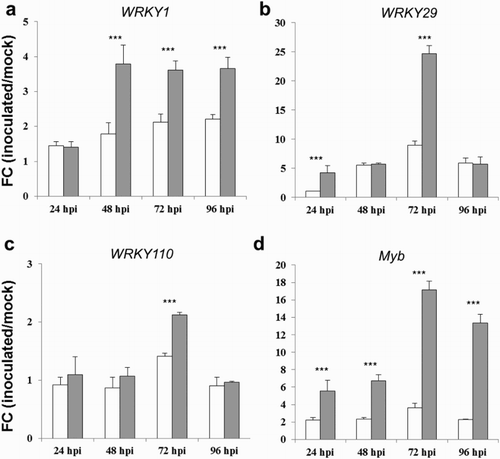
Among TFs a pivotal role in biotic responses is also played by Myb genes. In the current study, A. flavus inoculation induced the Myb expression from 24 to 96 hpi, and significant different expression patterns were found between the two strains all over the time course considered ((d)). The strongest induction was detected against the atoxigenic strain and transcripts were from 2.5 to 5.8-fold higher than that in response to the toxigenic one ((d)). The Myb TFs regulate the downstream expression of flavonoid genes, PR genes and genes involved in secondary metabolism (Ambawat et al. Citation2013). Taken together these results suggest that atoxigenic A. flavus may have an enhancing effect on maize kernel TFs, which in turn could determine the increase in downstream PR and ROS-related gene expression.
3.6. Expression of lipoxygenase genes
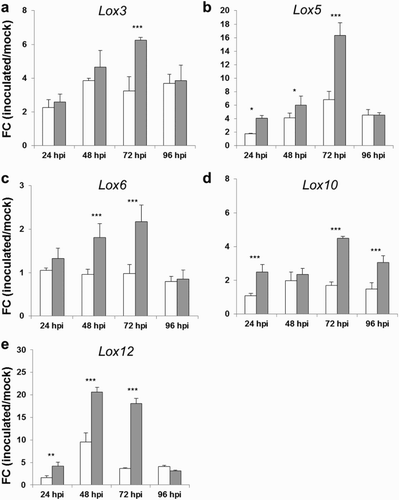
Functional and physiological analyses of lipoxygenases (LOXs) were first addressed to dicots and recent studies aimed to unravel the role of LOX in plant–pathogen crosstalk with the use of maize mutants (Gao et al. Citation2009; Christensen et al. Citation2014; Maschietto et al. Citation2015) and fungal mutants (Lanubile et al. Citation2013; Scala et al. Citation2014). In this work the expression levels of three selected 9-LOX genes (Lox3, Lox5 and Lox12), one 13-LOX gene (Lox10), and the plastidial Lox6 gene were analyzed by real-time RT-PCR after treatment with toxigenic and atoxigenic A. flavus pathogens ((a)–(e)). The most striking significant differences between the atoxigenic and the toxigenic strain were given at 72 hpi for all assayed genes. At this time-point atoxigenic strain inoculation caused a higher induction in the expression level of 1.9-fold for Lox3 ((a)), 2.4-fold for Lox5 ((b)), 2.2-fold for Lox6 ((c)), 2.6-fold for Lox10 ((d)) and about 5-fold for Lox12 ((e)), compared to the toxigenic one. The expression level of Lox3, Lox5, Lox6 and Lox10 genes peaked at 72 hpi in response to the atoxigenic strain and declined to the toxigenic strain levels at 96 hpi, with the exception of Lox10; significant variations of Lox10 were still observed between the two strains at 96 hpi ((d)). Overall, Lox12 gene showed the strongest induction at 48 hpi against both pathogens, with FC values of 9.5 and 20.6 for the toxigenic and atoxigenic strains, respectively ((e)). Previous studies reported the identification of three differentially expressed Lox genes (Lox1, Lox2 and Lox3) in pericarp cotton tissue under toxigenic and atoxigenic A. flavus interaction, suggesting their role in the mechanism of resistance against this pathogen (Bedre et al. Citation2015). Prior research illustrated the role of lipids, particularly oxylipins, in the specific interaction between maize and A. flavus and in regulating aflatoxin biosynthesis (Gao & Kolomiets Citation2009). It was found that disruption of the ZmLOX3 caused enhanced resistance to F. verticillioides, Colletotrichum graminicola, Cochliobolus heterostrophus, and Exserohilum pedicellatum (Isakeit et al. Citation2007; Gao et al. Citation2009). However, the maize lox3 mutant showed increased susceptibility to A. flavus and Aspergillus nidulans, indicating that this gene regulated disease resistance in a pathogen-specific manner (Gao et al. Citation2009). Fabbri et al. (Citation1983) found that seeds of high-oil crops such as peanut supported higher levels of aflatoxin production by Aspergillus parasiticus than seeds of graminaceous plants, such as maize or wheat, which contain higher levels of starch. In addition, they found that culturing A. parasiticus amended with peroxidized lipids resulted in significantly elevated aflatoxin production with no significant effect on fungal biomass (Fabbri et al. Citation1983). These findings indicate that certain 9-oxylipins can play important roles in suppressing aflatoxin biosynthesis while other oxylipins may promote aflatoxin biosynthesis (Gao et al. Citation2009). Results of this study suggest that low expression levels of maize defense-associated genes, such as Lox genes, displayed in response to the toxigenic A. flavus strain, may be related to the negative impact of aflatoxin. As a consequence the mycotoxin could provide a competitive advantage for the aflatoxigenic strain and predispose the susceptible plant to a greater infection.
Figure 4. FC of differentially expressed genes Lipoxygenase 3 (Lox3) (a), Lox5 (b), Lox6 (c), Lox10 (d) and Lox12 (e), in kernels of the susceptible CO354 maize line at 24, 48, 72 and 96 hpi with aflatoxin-producing and atoxigenic strains of A. flavus (white and gray bar charts, respectively). Vertical bars indicate ±standard deviation. *Significant differences between aflatoxin-producing and atoxigenic-inoculated means within the same time of sampling, according to two-way ANOVA (*P ≤ .05; **P ≤ .01; ***P ≤ .001).
4. Conclusions
The contamination of agricultural crops with aflatoxins is a major concern for global food safety and security, particularly in developing countries and in climate change scenarios. Numerous studies have shown that the resistant maize inbred lines exhibited significantly low levels of aflatoxin accumulation. Determination of the mechanisms underlying such maize host resistance to aflatoxin contamination has been proved difficult due to the complex nature of this quantitative trait. Use of non-aflatoxigenic strains has emerged as the best management practice for reducing aflatoxin contamination, and has led to commercially registered products, first in cotton (2003), then in peanut (2004), and more recently in maize (2008). In this study, we conducted real-time RT-PCR on 18 genes that comprise the potential components of maize host resistance. Four different maize functional groups of genes were considered, including PR, oxidative stress-related, TFs, and Lox genes, in response to atoxigenic and toxigenic A. flavus strains over a time course of 96 h. The transcriptional analysis provided a clue to the molecular mechanisms of the interactions of a susceptible maize genotype with atoxigenic/toxigenic strains of A. flavus and more drastic and active reactions were observed in response to the atoxigenic A. flavus for almost all assayed genes. These results suggest that aflatoxin could contribute to A. flavus virulence in maize. How the toxin enhances virulence of this fungus is not clear, however, it seems likely that the inhibitory effects of aflatoxins could impair plant defense responses. On the other hand, the more elevated significant levels of disease caused by the aflatoxin-nonproducing strain in the later stages of inoculation may demonstrate that other factors contribute to the ability of the A. flavus to cause disease and enhanced differential plant responses. Indeed, A. flavus produces other toxins (e.g. cyclopiazonic acid and G-aflatoxins) and factors that may contribute to the virulence of this pathogen. The differences observed in plant gene expression levels between the two strains cannot be exclusively attributed to the absence and/or presence of aflatoxin. It could be speculated that other deletions in regions outside the aflatoxin gene pathway cluster can be involved in the atoxigenic strain. Furthermore, in the cluster for aflatoxin biosynthesis the functions of 19 genes have been assigned, but for 6 of them the functions are still unassigned (Yu et al. Citation2004). The lack of these genes with unknown function may compromise or alter the functionality of additional downstream genes. For this reason the atoxigenic strain could reinforce other metabolic pathways in order to recover the lack of these genes. Further investigations on the behavior of these atoxigenic and toxigenic strains co-inoculated during crop infection will provide new information on the ability of the atoxigenic strain to inhibit aflatoxin production by toxigenic strain. Different biocontrol mechanisms may contribute to the definitively decreased aflatoxin contamination observed in previous co-inoculation experiments. It could be speculated that the physical exclusion and/or the competition for nutrients are insufficient elements to explain the reduction of aflatoxin in biological control, and other unknown factors perhaps involving interactions with the host, such as the elevated expression levels of plant defense genes, suppress aflatoxin synthesis in maize. For this reason, knowing which genes are typically expressed in response to A. flavus attack will lead to a better management of aflatoxin contamination and enhancement of host resistance to aflatoxin contamination through conventional breeding and biotechnology applications.
Supplemental_Data.docx
Download MS Word (23.2 KB)Acknowledgements
The authors greatly acknowledge Irene Falasconi for her contribution to gene expression analyses and Terenzio Bertuzzi for mycotoxin analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abbas HK, Zablotowicz RM, Bruns HA, Abel CA. 2006. Biocontrol of aflatoxin in corn by inoculation with non-aflatoxigenic Aspergillus flavus isolates. Biocontrol Sci Technol. 16:437–449. doi: 10.1080/09583150500532477
- Amaike S, Keller NP. 2011. Aspergillus flavus. Annu Rev Phytopathol. 49:107–133. doi: 10.1146/annurev-phyto-072910-095221
- Ambawat S, Sharma P, Yadav NR, Yadav RC. 2013. MYB transcription factor genes as regulators for plant responses: an overview. Physiol Mol Biol Plants. 19:307–321. doi: 10.1007/s12298-013-0179-1
- Atehnkeng J, Donner M, Ojiambo PS, Ikotun B, Augusto J, Cotty PJ, Bandyopadhyay R. 2016. Environmental distribution and genetic diversity of vegetative compatibility groups determine biocontrol strategies to mitigate aflatoxin contamination of maize by Aspergillus flavus. Microb Biotechnol. 9:75–88. doi: 10.1111/1751-7915.12324
- Battilani P, Toscano P, Van der Fels-Klerx HJ, Moretti A, Camardo Leggieri M, Brera C, Rortais A, Goumperis T, Robinson T. 2016. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci Rep. doi:10.1038/srep24328
- Bedre R, Rajasekaran K, Mangu VR, Sanchez-Timm LE, Bhatnagar D, Baisakh N. 2015. Genome-wide transcriptome analysis of cotton (Gossypium hirsutum L.) identifies candidate gene signatures in response to aflatoxin producing fungus Aspergillus flavus. PLoS ONE. 10:e0138025. doi: 10.1371/journal.pone.0138025
- Bertuzzi T, Rastelli S, Mulazzi A, Pietri A. 2012. Evaluation and improvement of extraction methods for the analysis of aflatoxins B1, B2, G1 and G2 from naturally contaminated maize. Food Anal Method. 5:512–519. doi: 10.1007/s12161-011-9274-5
- Bhatnagar D, Cary JW, Ehrlich K, Yu J, Cleveland TE. 2006. Understanding the genetics of regulation of aflatoxin production and Aspergillus flavus development. Mycopathologia. 162:155–166. doi: 10.1007/s11046-006-0050-9
- Brown RL, Menkir A, Chen Z-Y, Bhatnagar D, Yu J, Yao H, Cleveland TE. 2013. Breeding aflatoxin resistant maize lines using recent advances in technologies – a review. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 30:1382–1391. doi: 10.1080/19440049.2013.812808
- Chang P-K, Horn BW, Dorner JW. 2005. Sequence breakpoints in the aflatoxin biosynthesis gene cluster and flanking regions in non aflatoxigenic Aspergillus flavus isolates. Fungal Genet Biol. 42:914–923. doi: 10.1016/j.fgb.2005.07.004
- Chen Z-Y, Brown RL, Damann KE, Cleveland TE. 2010. PR10 expression in maize and its effect on host resistance against Aspergillus flavus infection and aflatoxin production. Mol Plant Pathol. 11:69–81. doi: 10.1111/j.1364-3703.2009.00574.x
- Chen Z-Y, Rajasekaran K, Brown RL, Sayler RJ, Bhatnagar D. 2015. Discovery and confirmation of genes/proteins associated with maize aflatoxin resistance. World Mycotoxin J. 8:211–224. doi: 10.3920/WMJ2014.1732
- Christensen SA, Nemchenko A, Park Y, Borrego E, Huang P, Schmelz EA, Kunze S, Feussner I, Yalpani N, Meeley R, Kolomiets MV. 2014. The novel monocot-specific 9-lipoxygenase ZmLOX12 is required to mount an effective jasmonate-mediated defence against Fusarium verticillioides in maize. Mol Plant Microbe Interact. 27:1263–1276. doi: 10.1094/MPMI-06-13-0184-R
- Cleveland TE, Yu JJ, Bhatnagar D, Chen Z-Y, Brown RL, Chang P-K, Cary JW. 2004. Progress in elucidating the molecular basis of the host plant-Aspergillus flavus interaction, a basis for devising strategies to reduce aflatoxin contamination in crops. J Toxicol-Toxin Rev. 23:345–380. doi: 10.1081/TXR-200027892
- Cordero M, Raventos D, Segundo B. 1994. Differential expression and induction of chitinases and β-1,3-glucanases in response to fungal infection during germination of maize seeds. Mol Plant Microbe Interact. 7:23–31. doi: 10.1094/MPMI-7-0023
- Dickman MB, Fluhr R. 2013. Centrality of host cell death in plant–microbe interactions. Annu Rev Phytopathol. 51:543–570. doi: 10.1146/annurev-phyto-081211-173027
- Ehrlich KC. 2014. Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: advantages and limitations. Front Microbiol. 5:50.
- Eulgem T, Rushton PJ, Schmelzer E, Hahlbrock K, Somssich IE. 1999. Early nuclear events in plant defence signalling: rapid gene activation by WRKY transcription factors. EMBO J. 18:4689–4699. doi: 10.1093/emboj/18.17.4689
- Fabbri A, Fanelli C, Panfili G, Passi S, Fasella P. 1983. Lipoperoxidation and aflatoxin biosynthesis by Aspergillus parasiticus and A. flavus. J Gen Microbiol. 129:3447–3452.
- Fountain JC, Raruang Y, Luo M, Brown RL, Guo BZ, Chen Z-Y. 2015. Potential roles of WRKY transcription factors in regulating host defense responses during Aspergillus flavus infection of immature maize kernels. Physiol Mol Plant Pathol. 89:31–40. doi: 10.1016/j.pmpp.2014.11.005
- Gao X, Brodhagen M, Isakeit T, Brown SH, Göbel C, Betran J, Feussner I, Keller NP, Kolomiets MV. 2009. Inactivation of the lipoxygenase ZmLOX3 increases susceptibility of maize to Aspergillus spp. Mol Plant Microbe Interact. 22:222–231. doi: 10.1094/MPMI-22-2-0222
- Gao X, Kolomiets MV. 2009. Host-derived lipids and oxylipins are crucial signals in modulating mycotoxin production by fungi. Toxin Rev. 28:79–88. doi: 10.1080/15569540802420584
- Giorni P, Magan N, Pietri A, Bertuzzi T, Battilani P. 2007. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int J Food Microbiol. 113:330–338. doi: 10.1016/j.ijfoodmicro.2006.09.007
- Hawkins LK, Mylroie JE, Oliveira DA, Smith JS, Ozkan S, Windham GL, Williams WP, Warburton ML. 2015. Characterization of the maize chitinase genes and their effect on Aspergillus flavus and aflatoxin accumulation resistance. PLoS ONE. 10:e0126185. doi: 10.1371/journal.pone.0126185
- Isakeit T, Gao X, Kolomiets M. 2007. Increased resistance of a maize mutant lacking the 9-lipoxygenase gene, ZmLOX3, to root rot caused by Exserohilum pedicellatum. Phytopathology. 155:758–760. doi: 10.1111/j.1439-0434.2007.01301.x
- Kelley RY, Williams WP, Mylroie JE, Boykin DL, Harper JW, Windham GL, Ankala A, Shan X. 2012. Identification of maize genes associated with host plant resistance or susceptibility to Aspergillus flavus infection and aflatoxin accumulation. PLoS ONE. 7:e36892. doi: 10.1371/journal.pone.0036892
- Lanubile A, Logrieco A, Battilani P, Proctor RH, Marocco A. 2013. Transcriptional changes in developing maize kernels in response to fumonisin-producing and nonproducing strains of Fusarium verticillioides. Plant Sci. 210:183–192. doi: 10.1016/j.plantsci.2013.05.020
- Lanubile A, Maschietto V, De Leonardis S, Battilani P, Paciolla C, Marocco A. 2015. Defence responses to mycotoxin-producing fungi Fusarium proliferatum, F. subglutinans, and Aspergillus flavus in kernels of susceptible and resistant maize genotypes. Mol Plant Microbe Interact. 28:546–557. doi: 10.1094/MPMI-09-14-0269-R
- Levic J, Gošić-Dondo S, Ivanović D, Stanković S, Krnjaja V, Bočarov-Stančić A, Stepanić A. 2013. An outbreak of Aspergillus species in response to environmental conditions in Serbia. Pesticidi i Fitomedicina. 28:167–179. doi: 10.2298/PIF1303167L
- Logrieco AF, Moretti A. 2008. Between emerging and historical problems: an overview of the main toxigenic fungi and mycotoxin concerns in Europe. In: Bandyopadhyay R, Leslie JF, Visconti A, editors. Mycotoxins: detection methods, management, public health and agricultural trade. Trowbridge: Cromwell Press; p. 139–153.
- Lozovaya VV, Waranyuwat A, Widholm JM. 1998. β-1,3-Glucanase and resistance to Aspergillus flavus infection in maize. Crop Sci. 38:1255–1260. doi: 10.2135/cropsci1998.0011183X003800050024x
- Maschietto V, Marocco A, Malachova A, Lanubile A. 2015. Resistance to Fusarium verticillioides and fumonisin accumulation in maize inbred lines involves an earlier and enhanced expression of lipoxygenase (LOX) genes. J Plant Physiol. 188:9–18. doi: 10.1016/j.jplph.2015.09.003
- Mauro A, Battilani P, Callicott KA, Giorni P, Pietri A, Cotty PJ. 2013. Structure of an Aspergillus flavus population from maize kernels in northern Italy. Int J Food Microbiol. 162:1–7. doi: 10.1016/j.ijfoodmicro.2012.12.021
- Mauro A, Battilani P, Cotty PJ. 2015. Atoxigenic Aspergillus flavus endemic to Italy for biocontrol of aflatoxins in maize. BioControl. 60:125–134. doi: 10.1007/s10526-014-9624-5
- Oliver RP, Ipcho SVS. 2004. Arabidopsis pathology breathes new life into the necrotrophs-vs.-biotrophs classification of fungal pathogens. Mol Plant Pathol. 5:347–352. doi: 10.1111/j.1364-3703.2004.00228.x
- Pandey SP, Somssich IE. 2009. The role of WRKY transcription factors in plant immunity. Plant Physiol. 150:1648–1655. doi: 10.1104/pp.109.138990
- Prasad K, Bhatnagar-Mathur P, Waliyar F, Sharma KK. 2013. Overexpression of a chitinase gene in transgenic peanut confers enhanced resistance to major soil borne and foliar fungal pathogens. J Plant Biochem Biotechnol. 22:222–233. doi: 10.1007/s13562-012-0155-9
- Proctor RH, Desjardins AE, McCormick SP, Plattner RD, Alexander NJ, Brown DW. 2002. Genetic analysis of the role of trichothecene and fumonisin mycotoxins in the virulence of Fusarium. Eur J Plant Pathol. 108:691–698. doi: 10.1023/A:1020637832371
- Reid LM, Zhu X, Parker A, Yan W. 2009. Increased resistance to Ustilago zeae and Fusarium verticilliodes in maize inbred lines bred for Fusarium graminearum resistance. Euphytica. 165:567–578. doi: 10.1007/s10681-008-9782-6
- Reverberi M, Punelli M, Smith CA, Zjalic S, Scarpari M, Scala V, Cardinali G, Aspite N, Pinzari F, Payne GA. 2012. How peroxisomes affect aflatoxin biosynthesis in Aspergillus flavus. PLoS ONE. 7:e48097. doi: 10.1371/journal.pone.0048097
- Reverberi M, Ricelli A, Zjalic S, Fabbri AA, Fanelli C. 2010. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl Microbiol Biotechnol. 87:899–911. doi: 10.1007/s00253-010-2657-5
- Roze LV, Hong S-Y, Linz JE. 2013. Aflatoxin biosynthesis: current frontiers. Annu Rev Food Sci Technol. 4:293–311. doi: 10.1146/annurev-food-083012-123702
- Rushton PJ, Somssich IE, Ringler P, Shen QJ. 2010. WRKY transcription factors. Trends Plant Sci. 15:247–258. doi: 10.1016/j.tplants.2010.02.006
- Scala V, Giorni P, Cirlini M, Ludovici M, Visentin I, Cardinale F, Fabbri AA, Fanelli C, Reverberi M, Battilani P, et al. 2014. LDS1-produced oxylipins are negative regulators of growth, conidiation and fumonisin synthesis in the fungal maize pathogen Fusarium verticillioides. Front Microbiol. 5:669. doi: 10.3389/fmicb.2014.00669
- Schmidt-Heydt M, Magan N, Geisen R. 2008. Stress induction of mycotoxin biosynthesis genes by abiotic factors. FEMS Microbiol Lett. 284:142–149. doi: 10.1111/j.1574-6968.2008.01182.x
- Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 3:1101–1118. doi: 10.1038/nprot.2008.73
- Song H, Wang P, Hou L, Zhao S, Zhao C, Xia H, Li P, Zhang Y, Bian X, Wang X. 2016. Global analysis of WRKY genes and their response to dehydration and salt stress in soybean. Front Plant Sci. 7:9.
- St. Clair DA. 2010. Quantitative disease resistance and quantitative resistance loci in breeding. Annu Rev Phytopathol. 48:247–268. doi: 10.1146/annurev-phyto-080508-081904
- Torres MA. 2010. ROS in biotic interactions. Physiol Plant. 138:414–429. doi: 10.1111/j.1399-3054.2009.01326.x
- Wei K-F, Chen J, Chen Y-F, Wu L-J, Xie D-X. 2012. Molecular phylogenetic and expression analysis of the complete WRKY transcription factor family in maize. DNA Res. 19:153–164. doi: 10.1093/dnares/dsr048
- Willcox M, Davis GL, Windham GL, Abbas HK, Betrán J, Holland JB, Williams WP. 2013. Confirming QTL for aflatoxin resistance from Mp313E in different genetic backgrounds. Mol Breed. 32:15–26. doi: 10.1007/s11032-012-9821-9
- Williams WP, Krakowsky MD, Scully BT, Brown RL, Menkir A, Warburton ML, Windham GL. 2015. Identifying and developing maize germplasm with resistance to accumulation of aflatoxins. World Mycotoxin J. 8:193–209. doi: 10.3920/WMJ2014.1751
- Wu K-L, Guo Z, Wang H, Li J. 2005. The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res. 12:9–26. doi: 10.1093/dnares/12.1.9
- Yu J, Chang P-K, Ehrlich KC, Cary JW, Bhatnagar D, Cleveland TE, Payne GA, Linz JE, Woloshuk CP, Bennett JW. 2004. Clustered pathway genes in aflatoxin biosynthesis. Appl Environ Microbiol. 70:1253–1262. doi: 10.1128/AEM.70.3.1253-1262.2004
