ABSTRACT
Durum wheat (Triticum turgidum L. ssp. durum (Desf.) Husnot) was grown under conditions to promote mineral biofortification at the grain level. Along plant development, biomass accumulation and the kinetics of nutrients accumulation were assessed, identifying the nutrient fluxes of roots and shoots, and the timescale constraints of crop biofortification. Plants were grown under environmentally controlled conditions, submitted to four increasing concentrations of nutrient solutions (1-, 2-, 4- and 6-fold) of micro- (Fe, Zn, Cu and Mn) and macronutrients (Ca, K, P and Mg). The threshold of mineral toxicity was not reached as evaluated through plant biomass accumulation, but considering grain yield, the twofold nutrient concentration was the best treatment for biofortification. In the different treatments, the contents and the mineral unrests of roots uptake and shoots translocation varied, at different magnitudes and trends, before the onset of booting and from the physiological maturity onwards. Except for Cu, all mineral nutrients were mainly detected in the bran and embryo of the grains; therefore, the production of biofortified pasta for human consumption requires the use of integral semolina.
1. Introduction
Nutritional deficiencies are serious problems of public health, which affect more than half of the world’s population, particularly in developing countries. Among strategies to control such deficiencies is biofortification, a process for increasing the mineral nutrient content in crops, either by means of fertilization (agronomic biofortification), or by conventional breeding and/or genetic engineering to obtain cultivars with higher mineral absorption and accumulation capabilities (genetic biofortification). Accordingly, biofortification aims to complement existing interventions, providing nutrients in a sustainable way for the most vulnerable population groups (Lidon et al. Citation2015).
Accumulation of nutrients in plants is closely linked to homeostasis, comprising a network of coordinated uptake, transport, trafficking and sequestration activities. Accordingly, an adequate amount of nutrients is provided to all cell types, at all stages of development and under different environmental conditions (Lidon & Henriques Citation1994; Lidon Citation2001; Tsonev & Lidon Citation2012; Martins et al. Citation2014). This whole process is influenced by plant genetic background and environmental interactions. Indeed, environmental factors, such as soil properties, water availability and fertilizers application, have a huge impact on genotype factors, and thereby on mineral concentrations (Cakmak et al. Citation2004; White & Broadley Citation2009). Additionally, as root–soil interface further limits nutrients uptake to increase their absorption by roots, the available levels of nutrients in the rhizosphere must increase. The uptake mechanisms must also be sufficiently functional, and with enough specificity, to allow the accumulation of nutrients, once they enter the apoplast or symplast of root cells. Once absorbed by roots, nutrients must further be efficiently translocated and accumulated in plant organs (e.g. in the grains). Nevertheless, if a threshold is reached, critical impairments might occur, including a wide range of plant responses to stress, with negative implications at the uptake and translocation kinetics (Lidon & Henriques Citation1992; Lidon Citation2000). Additionally, under stress photoassimilates mobilization becomes affected. In the particular case of wheat grains, the yield and nutritional properties will be further affected at different levels if the threshold of toxicity is reached.
Durum wheat (Triticum turgidum L. ssp. durum (Desf.) Husnot) production comprises approximately 30 million tons on 16 million cultivated hectares (Pataco et al. Citation2015). Although the production of this cereal represents only 8% of the world production of wheat (Pataco et al. Citation2015), as a staple food durum wheat is one of the major targets of biofortification. Accordingly, the aim of this study was the biofortification of durum wheat grains considering the contents and kinetics of uptake, translocation and accumulation of micro- and macronutrients, as well as their localization in grain tissues.
2. Materials and methods
2.1. Experimental design
Seeds of the durum wheat, Triticum turgidum L. ssp. durum (Desf.) Husnot cv. Marialva, were washed, sterilized and sown in pots (volume 3 L; 4 plants per pot). Peat was used as substrate (Siro – Substrato Universal, Portugal), having the following characteristics: pH 5.5–6.5; conductivity 0.6–1.2 mS cm−1; 70% organic matter; 46.18 Ca, 9.75 K, 4.25 P, 5.72 Mg and 1.81 Fe (all in g kg−1); 13.28 Cu and 40.33 Zn (both in mg kg−1). Germination and growth were carried out in a walk-in growth chamber (EHHF 10000, ARALAB, Portugal), under environmentally controlled conditions (80% RH; 500 ppm CO2; 22/18°C, day/night temperature; photosynthetic photon flux density of ca. 800 µmol Q m−2 s−1, 12 h photoperiod). The plants were then supplemented twice a week with a complete nutrient solution (pH 5.5) prepared from stock solutions A and B. They were filled up to a final volume of 1000 mL with distillated water. Stock A contained 0.09 g MnCl2.4H2O (Merck 1173874), 0.12 g (NH4)6.MO7O24.4H2O (Merck 1182), 0.01 g H3BO3 (Merck 10043353), 0.016 g ZnSO4.7H2O (Merck 7446200), 0.08 g CuSO4.5H2O (Merck 7758987), 0.16 g FeCl3.6H2O (Sigma-Aldrich 44943) and 12.00 g C6H8O7.H2O (Merck 902), dissolved with 50 mL de H2SO4 95–97% (Merck 7664939). Stock B contained 111 g NH4NO3 (Merck 6484522), 30 g Na2HPO4.2H2O (Panreac 122507), 65 g K2SO4 (Scharlau PO02871000), 17 g CaCl2.2H2O (Scharlau CA01941000) and 4.8 g MgSO4 (Merck 7487889), dissolved in 700 mL of water.
The experimental design considered four nutritional treatments (1 – which is the control, 2-, 4-, and 6-fold contents of micro- and macronutrients, therefore application of 15, 30, 60 and 90 mL of the nutrient solution dissolved in 5 L of distilled water – treatments [15], [30], [60] and [90], respectively) and four analytical dates (16, 70, 92 and 126 days after germination, corresponding to t1, t2, t3 and t4, respectively).
2.2. Nutrients content
In each treatment and experimental period, shoot and root dry weight was determined after drying at 105°C, for 10 days, until weight constant. Plant parts of one organ were chosen at random after all physically damaged or deformed plants were discarded.
The concentrations of Fe, Cu, Mn, Zn, Ca, K, Mg and P in roots, shoots and grains were determined in dried samples ground and passed through a 0.7-mm sieve (Jones Citation2001) after wet digestion in a digester VELP DKL. In each digester tube, 0.5 g of plant material and 8 mL of nitric acid were mixed. The acid was in contact with the sample overnight. Thereafter, the tubes were placed in the digester at 100°C, for 1 h, and the temperature was increased gradually until 150°C. After cooling, 2 mL of perchloric acid was added and the samples were further heated up to 200°C until the solution became colorless. After cooling, 10 mL of water was added and samples were heated again at 100°C for about 5 min (until white smoke appeared). After digestion, the solution was quantitatively transferred to 25 mL volumetric flasks. The concentration of each element was determined by Inductively Coupled Plasma - Atomic Emission Spectrometry system (model IRIS Interprid II XSP Radial, Thermo Scientific). Reference samples, SRM 1567th, certified by the National Institute of Standards and Technology (NIST, U.S.A.), were used.
2.3. Nutrients kinetics
In each experimental period, being r, s and g, the root, shoot and grain, respectively, total uptake (TU) of each nutrient was determined by multiplying the biomass of each organ (Br,s,g) of the plant with the concentration of the nutrient (Cr,s,g), followed by the sum of these values – TU = (Br × Cr) + (Bs × Cs) + (Bg × Cg). Translocation to the shoot (TS) of each element was calculated on a percentage basis, considering the division of the product of shoot and grain biomass and the concentration of the nutrient in these tissues [(Bs × Cs) + (Bg × Cg)] by total absorption – TS = {[(Bs × Cs) + (Bg × Cg)]/TU} × 100. The mobilization rate (MR) of each element to the grains was also calculated on a percentage basis, dividing the product of grains biomass and the content of the element (Bg × Cg) by total absorption – MR = [(Bg × Cg)/TU] × 100.
2.4. Nutrients deposition
Micro X-ray fluorescence evaluation of mineral localization of nutrients in wheat grain was determined using a µ-Energy Dispersive X-ray Fluorescence system (M4 Tornado™, Bruker, Germany), as previously optimized (Ramos et al. Citation2016). Briefly, the X-ray generator was operated at 50 kV and 100 µA without the use of filters, to enhance the ionization of low-Z elements. For a better quantification of the mineral heavy elements, a set of filters between the X-ray tube and the sample was used, composed of three foils of Al/Ti/Cu with a thickness of 100/50/25 µm, respectively. All the measurements with filters were performed at 600 µA. Detection of fluorescence radiation was performed by an energy-dispersive silicon drift detector (XFlash™), with 30 mm2 sensitive area and 142 eV energy resolution for Mn Kα. In order to obtain a better distribution mapping of the elements, the wheat grain was cut in two halves with a stainless steel surgical blade. Measurements were taken under 20 mbar vacuum conditions and performed directly on the two sides of each grain, first in the mapping mode, and then with point analysis on interest sites. These point spectra were acquired during 200 s. Quantification of the point spectra was performed using the WinAXIL™ software package (Canberra, Belgium), with three reference samples in the compare mode.
2.5. Statistical analysis
Data were statistically analyzed through an analysis of variance (ANOVA), using a two-way ANOVA (p ≤ .05), in order to evaluate differences between the four nutritional treatments, between the four analytical dates, and their interaction. Based on the ANOVA results, a Tukey’s test for mean comparisons was performed at a 95% confidence level. Different statistical indexes on the tables indicate significant differences for the 95% confidence level.
3. Results
Throughout the life cycle of Triticum turgidum L. ssp. durum (Desf.) Husnot cv. Marialva, which ended 126 days following germination, the onset of booting, flowering, anthesis and beginning of the physiological maturity occurred at the 35th–39th, 43rd–47th, 52nd–56th and 69th–73rd days, respectively. In this context, at the 70th and 126th days following germination, the average dry root weight decreased significantly between treatments [30] and [60], but thereafter an increase was found in treatment [30] (). Between the 16th and 70th days after germination, the control (i.e. treatment [15]) also displayed a significant accumulation of dry biomass, but thereafter no significant variations occurred. Root biomass of treatment [30] further showed a significant increase at the 126th day after germination, whereas in treatment [60] the highest dry weight was found at the 92nd day. Moreover, root dry weight of treatment [90] did not show significant variations (). Shoot dry matter showed no significant differences in all treatments throughout the life cycle, except for treatment [15] at the end of the physiological maturity ().
Table 1. Dry weight of roots and shoots. Each value represents the mean ± S.E. (n = 3, being each sample from a different pot). For a 95% confidence level, letters a, b, c and r, s indicate significant differences among treatments within each experimental period and among different experimental periods times for each treatment, respectively.
Root iron concentrations of treatments [15] and [30] did not vary significantly among the tested experimental periods, but treatments [60] and [90] showed significantly higher values (). It was also found that among all treatments, there were no significant variations at the beginning of the physiological maturity (t2). In t3 significantly higher values occurred in treatment [90]. In the end of the physiological maturity (t4), significantly higher concentrations prevailed in treatments [60] and [90]. In the shoot, Fe concentrations varied significantly among treatments and experimental periods (). In the control, higher Fe levels were detected at the end of the physiological maturity (t4), whereas in treatments [30] and [90] this nutrient concentration prevailed at t3 and t2/t3, respectively ().
Table 2. Micronutrients of roots and shoots. Each value represents the mean ± S.E. (n = 3, being each sample from a different pot). For a 95% confidence level, letters a, b, c, d and r, s, t, u indicate significant differences between treatments within each experimental period and between different experimental periods times for each treatment, respectively.
Within each treatment, Cu concentration in the roots did not vary significantly over time (). Moreover, considering the variations among experimental periods, at t2 and t3 significant changes occurred, with treatment [60] showing the highest values. At the end of the physiological maturity (t4), the levels of this element did not vary significantly among the different treatments (). The concentrations of Cu in the shoots remained constant within treatments [15] and [30], yet in treatment [60] higher values were found at t2 (). Among all the experimental periods, t2 of treatment [60] had the highest levels of this element.
In the roots, among the experimental periods, the concentration of Mn did not vary significantly in treatment [30]. In treatment [90], significantly higher values were found at the end of the physiological maturity (t4). In the control and treatment [60], higher concentrations were found at t2 and beginning of the physiological maturity (t2), respectively (). During t2, the highest concentrations were also found in treatment [60], but at the end of the physiological maturity (t4) the highest levels were measured in treatment [90] (). In the shoot, Mn concentration of treatments [15] and [30] varied significantly over the life cycle of the durum wheat, but significantly higher values prevailed in treatments [60] and [90] (). Considering the different experimental periods, it was found that t4 had significantly higher values in treatment [60], but at t3 significantly higher concentrations occurred in treatment [90].
In root Zn levels, in each experimental period, a decrease was found between the control and treatment [30]. Considering each treatment, significant differences were found at the end of the physiological maturity (excepting for treatments [15] and [90]). In all cases, the highest Zn levels were found at t3 (). In the shoot, the highest values of Zn concentrations in the control were found at t3 and t4, but in treatments [60] and [90] these were found at the end of the physiological maturity (t4 – ).
During all the life cycle of durum wheat, Ca levels in the roots did not vary significantly in treatments [15] and [30]; in contrast, treatments [60] and [90] displayed significantly higher values at the end of the physiological maturity (t4 – ). Considering the experimental periods, it was found that in all treatments there were no significant variations in t2, but at t3 the highest concentrations were found in treatments [30] and [90]. At the end of the physiological maturity (t4), significantly higher concentrations were further detected in treatments [60] and [90]. In the shoot, the concentrations of Ca progressively increased within treatments [15], [60] and [90], whereas treatment [30] displayed a significant increase from t2 to t3 (). Between treatments [15] and [90], there were no significant changes at t2, but in the following experimental periods Ca levels increased significantly.
Table 3. Macronutrients of roots and shoots. Each value represents the mean ± S.E. (n = 3, being each sample from a different pot). For a 95% confidence level, letters a, b, c and r, s, t indicate significant differences between treatments with in each experimental period and between different experimental periods times for each treatment, respectively.
The K-levels in the roots and shoots, within each experimental period, did not vary significantly, except in the roots for treatment [90], at the end of the physiological maturity (t4) and in the shoot for the control treatment, at t3 and t4. In the roots, considering each treatment, significant differences were found throughout the different experimental periods, but in all cases the highest K levels were detected at t3 (). In the shoot of all treatments, at t3, also prevailed a higher K concentration ().
In the root, within each treatment, Mg levels varied significantly during all the life cycle of durum wheat (). Comparing the levels of Mg among the experimental periods, it was found that all treatments (except treatment [90]) displayed the highest values at t3. In the shoot, Mg concentration of the control and treatment [30] varied significantly during all the life cycle of durum wheat, with treatments [60] and [90] displaying the highest values ().
In the roots, considering each experimental period, P concentrations did not show significant changes (). Considering each treatment in all cases, the highest concentration was found at t3. In the shoots, considering each experimental period, it was found that t2 had the significantly lowest values in treatments [60] and [90], whereas in t4 the highest values were found in all treatments, except in [30] ().
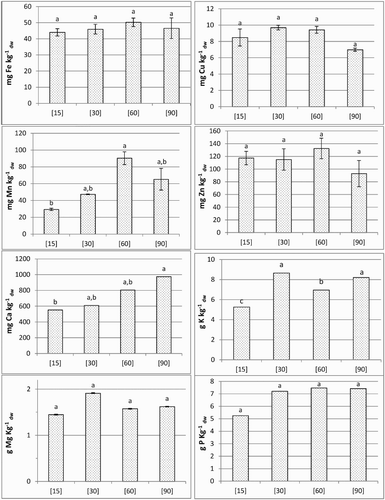
In the grain (), between treatments [15] and [60], Fe concentration increased 1.14-fold (from 44.03 to 50.23 mg kg−1 dw), followed by a decrease to 93% in treatment [90]. The levels of Cu did not vary significantly among the different treatments, ranging between 6.99 and 9.70 mg kg−1 dw. The concentration of Mn, relatively to the control, displayed the significantly highest value in treatment [60]. Zinc concentrations did not differ significantly among treatments, varying between 92.96 and 132.54 mg kg−1 dw. Calcium concentration, between treatments [15] and [90], increased by about 76.5%. The concentrations of K were found to be minimum and maximum in the control and treatment [30] (5.26–8.65 g kg−1 dw), respectively. Nonsignificant variations were found between treatments [30] and [90]. Magnesium concentrations did not vary significantly among the treatments, with minimum and maximum concentrations being found in the control and treatment [30] (1.45–1,91 g kg−1 dw), respectively. Phosphorus showed no significant differences among treatments, varying between 5.26 and 7.47 g kg−1 dw.
Figure 1. Nutrients contents in the grains. Each value represent the mean ± S.E. (n = 2). For a 95% confidence level, letters a, b indicate significant differences between treatments.
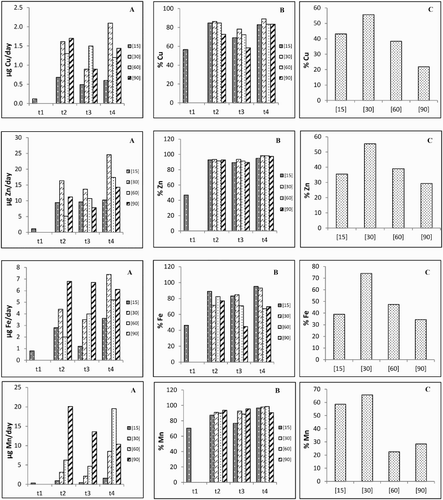
Within the experimental periods t2, t3 and t4, the interaction between biomass yields and the levels of nutrients revealed () that, from treatments [15] to [90], total accumulation of Fe, Cu and Mn increased progressively in the roots (except for Fe and Cu in t2, treatment [60], and for Cu in t3 and t4, treatments [90] and [60], respectively). Moreover, a clear trend could not be found for Zn (). In this context, during the life cycle, in these tissues the average for total accumulation of Fe, Cu, Mn and Zn varied among treatments (in µg) in the range 8.9–378.8, 1.2–42.0, 2.0–147.0 and 12.4–105.7, respectively ().
Table 4. Total accumulation of micronutrients in roots, shoots and grains. In each experimental period, values were determined multiplying the average of each micronutrient and total biomass of each organ.
Considering the pattern of macronutrients (), it was found that total accumulation of Ca in the roots, within the experimental periods t2, t3 and t4, increased from treatments [15] to [90] (except in treatment [60] in t2). Moreover, K, although showing a similar trend in t2 (except in treatment [60]) and t3, displayed an opposite trend in t4, from treatment [30] onwards. Total accumulation of Mg did not show a clear trend, but P increased among treatments of t3, and in t4, a decrease was found from treatment [30] onwards (). Until the end of the cultivation period, total accumulation of Ca, K, Mg and P in root tissues ranged (in mg) 0.3–22.5, 2.9–56.7, 0.1–0.5 and 0.5–12.4 mg, respectively ().
Table 5. Total accumulation of macronutrients in roots, shoots and grains. In each experimental period, values were determined multiplying the average of each micronutrient and total biomass of each organ.
In the shoot tissues, total accumulation of Fe also increased within the experimental periods t2, t3 and t4 (), from treatments [15] to [90] (except in treatment [60] of t2 and t3 and treatments [15]–[60] of t4). Mn showed a similar pattern, increasing in each experimental period between treatments [15] and [90] (except in t4, treatment [90]), but Cu and Zn did not reveal a clear pattern (), although substantial increases were detected in the highest treatment, relatively to the control (excepting for Zn, in treatment [90] of t3). During all the life cycle, in shoot tissues, the average of total accumulation of Fe, Cu, Mn and Zn varied in the range of 7.7–390.5, 1.5–136.7, 4.8–2285.4 and 11.0–1631.2 µg, respectively (). Total accumulation of Ca in the shoots increased in t2, t3 and t4, between treatments [15] and [90] (except in treatments [30]–[60], in t2 and t3), but K did not show a clear trend in t2 and increased in t3 and t4 (except in treatment [60]). Additionally, total accumulation of Mg increased progressively among treatments of the experimental periods t2, t3 and t4 (except in treatment [60]), whereas P did not reveal a clear trend (). Accordingly, until the end of the life cycle, total accumulation of Ca, K, Mg and P in the shoots ranged 0.2–238.3, 4.5–754.8, 0.2–14.6 and 1.0–156.8 mg, respectively ().
In the grains, total accumulation of all nutrients displayed the highest value in [30], decreasing progressively thereafter ( and ). Among micronutrients, the amounts of Fe, Cu, Mn and Zn varied in the range 214.3–838.1, 41.3–176.8, 142.9–863.6 and 557.6–2098.3 µg, respectively (). The average for total accumulation of Ca, K, Mg and P ranged 2.7–11.1, 25.6–157.6, 7.0–34.9 and 25.6–131.4 mg, respectively ().
Considering all the treatments, in each experimental period, it was found that ((A) and (B)) the uptake and translocation rates of Cu maintained similar patterns (t2 > t4 > t3 > t1) for treatment [15]. The other treatments showed different patterns among them. Zinc uptake rates revealed different patterns among the experimental periods (t4 > t3 > t2 > t1 for treatments [15] and [60], whereas treatments [30] and [90] attained for t4 > t2 > t3; additionally, t3 and t4 maintained similar values and increased, respectively), but the translocation rates to the shoot after t1 did not vary substantially ((A) and (B)). The patterns of the Fe uptake rates remained similar to Cu for treatment [30], but antagonistic trends were found for treatments [60] and [90] (t4 > t3 > t2 and t2 > t3 > t4, respectively). The translocation rates of Fe to the shoot followed different orders: in treatments [15] (t4 > t2 > t3) and [90] (t2 > t4 > t3). Nevertheless, divergent patterns were found for treatments [30] and [60] (t4 > t3 > t2 and t2 > t3 > t4, respectively). Relatively to Mn, the uptake rates of treatments [15], [30] and [60] remained similar (t4 > t2 > t3 > t1), but treatment [90] decreased from t2 onwards ((A)). The translocation rates of Mn to the shoot showed ((B)) minor variations (except in treatment [15] in t1 and t4).
Figure 2. Roots uptake (A) and shoot translocation (B) rates as well as mobilization (C) rate to the grains of micronutrients in each experimental period and for the different treatments.
Ca uptake rates between t2 and t4 increased progressively in treatments [15], [60] and [90], yet in treatment [30] it slightly decreased between t3 and t4 ((A)). Potassium uptake rates also decreased progressively between t2 and t4 in treatments [15] and [30], but the pattern of the remaining treatments was found to be t3 > t2 > t4. In treatments [15], [30] and [60], the trends of P uptake rate were also found to be t2 > t4 > t3 > t1, t4 > t2 > t3 and t3 > t4 > t2, respectively, whereas treatment [90] increased from t1 onwards ((A)). Magnesium uptake rates progressively increased from t1 onwards in treatments [30] and [60], but the trend of the remaining treatments was found to be t4 > t2 > t3 ((A)). The translocation rates to the shoot of all the macronutrients ((B)) showed minimum values in t1, but in the other experimental periods the patterns were quite similar (excepting in t2 for Ca and treatment [15] in t3 for P).
Figure 3. Roots uptake (A) and shoot translocation (B) rates as well as mobilization (C) rate to the grains of macronutrients in each experimental period and for the different treatments.
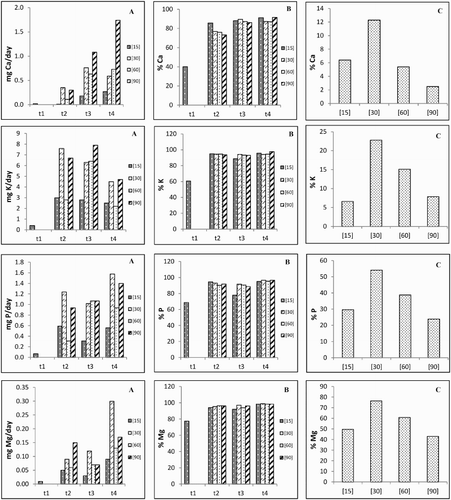
The MRs of all nutrients to the grains showed maximum values in treatment [30], decreasing (except for Mn) progressively until treatment [90] ((C) and (C)).
Comparing the control to the highest treatment, it was found that in both cases the accumulation of Fe, Zn and Mn as well as K, P and Ca prevailed in the bran and embryo, while Cu disseminated all over the grain ().
4. Discussion
Mineral concentration in plant organs is influenced by environmental interactions, namely, complex connections between the composition and physicochemical properties of the soil, as well as fertilization and transpiration flow, which govern most of translocation of minerals from the root to the upper parts of the plants (Reboredo & Ribeiro Citation1984; Lidon & Henriques Citation1992; Cakmak et al. Citation2004; Carelli et al. Citation2006). Plant growth and development largely depend on the combination and concentration of available mineral nutrients, with the plants often facing significant challenges to obtain an adequate supply of nutrients to meet the demands of basic cellular processes. Indeed, nutrient deficiency or toxicity may result in decreased plant productivity and/or fertility. In this context, the higher biomass production in the roots of treatment [30] from the 70th day onwards () consistently pointed the most efficient association between cell growth and endogenously generated mineral fluxes derived from the nutrient solution. Moreover, higher levels of all nutrients in the growth solution, far from promoting a wide-ranging plant response to stress and beyond prompting higher interferences at the uptake and translocation kinetics (Kabata-Pendias & Pendias Citation2001), indicated that the threshold of toxicity was not reached. Indeed, the higher shoot biomass yield was observed in treatment [90] from the 70th day after germination onwards (). Nitrogen in the nutrient growth solution can promote root uptake and translocation of other nutrients (Kutman et al. Citation2010; Aciksoz et al. Citation2011; Erenoglu et al. Citation2011), determining the homeostasis of minerals and, therefore, a network of a coordinated functioning of nutrients transport, trafficking and sequestration. This feature provided an unstressful amount of nutrients to all cell types, at all stages of development ( and ; ), which is a requirement for grain biofortification.
In vascular plants, ions concentration at the root surface level is determined by the properties of the rooting medium and by the plant ion uptake kinetics. Roots can take up Fe, Cu, Mn and Zn in their cationic forms or as metal chelates (White Citation2003; Marschner Citation2011), but their concentrations in the rhizosphere, although determined by soil-specific precipitation, complexation and adsorption reactions, followed a common pathway. Indeed, transition metals are mostly absorbed as divalent ions via ion channels with considerable specificity or homeostasis. Thus, the pattern of these micronutrient contents (–) suggests that, within the experimental periods, its contents are largely achieved by specific active-excretion mechanisms controlled by cytoplasmic concentrations.
Regardless of the variations in the different treatments and experimental periods (), the limited solubility of Fe and the mobilization of its ferric form in the rhizosphere by phytosiderophores to the root cells (Ishimaru et al. Citation2006) did not reach critical levels (above 500 mg kg−1 dw) (Foy et al. Citation1978; Reboredo et al. Citation2005; Marschner Citation2011). The low mobility of Fe as Fe3+-citrate within the xylem (Reboredo Citation1997; Mukherjee et al. Citation2006; Marschner Citation2011) seemed to have determined nonsignificant variations in the grains (; ), but its contents, relatively to worldwide field trials (Ebens & Shacklette Citation1982; Kabata-Pendias & Pendias Citation2001; Marschner Citation2011), doubled or tripled under the imposed optimal growth conditions (). Thus, this tendency clearly evidenced the elastic metabolism of Fe accumulation required for biofortification.
Cu is taken up by high-affinity transporters (White & Broadley Citation2009), and eventually bound by metallothioneins inside plant cells (Lidon & Henriques Citation1994; Guo et al. Citation2008). In our experimental conditions, Cu did not trigger critical levels () of deficiency or toxicity (1–5 mg K−1 dw and 20–40 mg kg−1 dw, respectively) (Robson & Reuter Citation1981; Kabata-Pendias & Pendias Citation2001). Possibly, this pattern was achieved because Cu translocation from roots is highly restricted (Reboredo & Henriques Citation1991; Lidon & Henriques Citation1992), and stress effects on photosynthates mobilization when occurring in the shoot are high (Lidon & Henriques Citation1993a, Citation1993b). Still, the amount of Cu that accumulated in grains (between 6.99 and 9.70 mg kg−1 dw, ) remained within the range usually found in field trials (0.6–10.3 mg kg−1 dw) (Kabata-Pendias & Pendias Citation2001; Marschner Citation2011).
Likewise, regardless of plant species, variety or growth conditions, Mn contents depend on the concentration of this diffusible element in the root’s nutrient strata (Lidon Citation2000, Citation2001, Citation2002). In this study, the critical levels of deficiency and toxicity (10–20 mg kg−1 dw and 380–1600 mg kg−1 dw, respectively), of this particularly important mineral for C-assimilation pathway and several enzymes’ activation (Lidon et al. Citation2004), were not as well surpassed () (Kabata-Pendias & Pendias Citation2001). In fact, the significant variations found in this study for Mn () remained within typical values, since, depending on the geographical area, Mn contents in cereal grains typically vary between 7.5 and 103 mg kg−1 dw (Lidon Citation2000; Kabata-Pendias & Pendias Citation2001).
Among treatments and during all the experimental periods, the symplastic (White & Broadley Citation2009) and apoplastic (Broadley et al. Citation2007; Reboredo Citation2012) transport of Zn from the root to the xylem, through Zn2+ or as a Zn–phytosiderophore complex, showed typical Zn contents (). It has been reported that the critical levels of deficiency and toxicity for roots and shoot tissues are in the range of 10–20 mg kg−1 dw (lower level) and 100–300 mg kg−1 dw (upper level), respectively (Cakmak et al. Citation1994). Besides, it was also pointed that the average for durum wheat is close to 140 mg kg−1 dw (Ruano et al. Citation1988; Kabata-Pendias & Pendias Citation2001), and that the worldwide average of Zn content in the grain oscillates between 22 and 33 mg kg−1 dw (Kabata-Pendias & Pendias Citation2001). Accordingly, our study supports that growth conditions and genotype characteristics may have caused a substantial augmentation of this nutrient (), which, as found for Fe, strongly supports a high potential for Zn biofortification.
From the beginning of the physiological maturity onwards, the increasing Ca contents in the roots () suggested that Ca2+-permeable cation channels (Wheeler & Brownlee Citation2008; White & Broadley Citation2009) passively allow Ca influx to root cells. Besides, through the apoplast (White Citation2001; Moore et al. Citation2002), this nutrient may also reach the stele. Additionally, this study also points that Ca might enter in the xylem, being translocated passively to the shoot and grains, reaching significantly higher values (; ). Indeed, considering that Ca content in most vascular plant tissues is about 5 g kg−1 dw (Raven et al. Citation2002), in the highest treatment and after t2, about a 2.6-fold increase was found in the shoots, whereas this nutrient content in the grain almost doubled. Accordingly, following the growth conditions imposed in our experimental design, Ca accumulation at treatments [60] and [90] strongly reflects the metabolic aptitude of this nutrient for biofortification.
Through high- and low-affinity transport systems to directly acquire K from the soil, the average levels of this nutrient in most vascular plant tissues are within the range from 10 to 53 g kg−1 dw (Bergmann Citation1992; Raven et al. Citation2002; Marschner Citation2011). However, higher values were found in all treatments along the vegetative cycle (), showing a high affinity for K+ uptake, eventually associated with, and driven by, transmembrane proton transport in ion channels of the plasma membrane in the root cells (Maathuis & Sanders Citation1997). Moreover, K contents in the shoots revealed a different trend among treatments, reinforcing the hypothesis that xylem loading of K is regulated separately from K uptake (Al-Karaki Citation2000), and further determining the significantly higher contents of this nutrient in the grain (), which further strengthens the biofortification capability of durum wheat with K.
Mg contents in most tissues of vascular plants oscillate around 1–2 g kg−1 dw (Kabata-Pendias & Pendias Citation2001; Raven et al. Citation2002), but in the present study its content in the roots displayed lower values () without significant variations (except in treatment [90] at anthesis). The antagonistic trend found between Mg and K contents supports previous findings (Gransee & Führs Citation2013) about their competition in the affinity transport system and eventually that Mg2+-permeable cation channels of the root cells have an energy cost (White Citation2000; White & Broadley Citation2009). Still, the consistently low amounts of Mg in the shoot tissues irrespective of mineral doses and analytical date () suggested that Mg translocation from the root to the shoot could be also impaired by high K levels (Gransee & Führs Citation2013). Finally, significant variation in Mg contents could not be found in the grain (), probably because Mg is a phloem-mobile element readily translocated to the grain (Wilkinson et al. Citation1990).
As the concentration of P in most soils is low, but plant requirements are high, its uptake requires an energized transport of Pi across the plasma membrane (Schachtman et al. Citation1998). In our study, this homeostatic control probably caused the absence of significant variations of P contents in the roots among treatments, but not during the life cycle of the plants (). Considering the average levels (2–3.1 g kg−1 dw) in shoot tissues of most vascular plants (Bergmann Citation1992; Raven et al. Citation2002; Marschner Citation2011), the higher and significantly different values found among treatments and experimental periods () seemed to underlie the existence of complex interactions at the level of influx and efflux from/to the roots, but without significant impact to the P content in the grains ().
Variations in biomass yields of roots and shoots () and nutrients accumulation showed different trends ( and ). This suggested different uptake kinetics (i.e. the net result between simultaneous influx from the nutrient solution to the roots and efflux from the roots to the solution) and translocation mechanisms developed from the beginning of physiological maturity onwards ( and ). In the roots, total accumulation of each micronutrient (), as an outcome of specific uptake kinetics () during the life cycle of the plant, faced progressive deviations (except in Fe(t2)). Moreover, during all the experimental periods, the uptake mechanisms coupled to total accumulation of each macronutrient () were not synergistically directed, suggesting that isomorphous substitution prevailed mostly between K(t1), Ca(t1) and P(t1). Nevertheless, Zn and Mn translocation kinetics to the shoot did not vary substantially ( and ), whereas Cu and Fe showed major variations in t3 and from t3 onwards, respectively (). Accordingly, data suggest that the absence of nutritional stress prompted metabolic elasticity. In this context, a cross-linkage prevailed between grain production and treatment [30], since the highest accumulation of all nutrients occurred ( and ) as a result of an utmost translocation rate ( and ).
5. Conclusion
Within a wheat genotype, screening treatments for high nutrient concentrations, without considering the grain yield capacity of genotypes, will not necessarily result in selecting the targeted nutritional treatments with best capacity for grain accumulation of nutrients. Accordingly, for meeting the daily requirements to feed humans, all treated grains of durum wheat (Triticum turgidum L. ssp. durum (Desf.) Husnot) cv. Marialva, treatments [30] and [90], consistently showed the high content for micro- and macronutrients, without being significantly different. However, considering the grain yield, the highest accumulation was found in treatment [30], which, therefore, must be considered the best and more efficient biofortification treatment. Considering nutrients distribution in the wholegrain (therefore including the bran) of durum wheat, the production of biofortified pasta for human consumption requires the use of integral semolina.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aciksoz SB, Yazici A, Ozturk L, Cakmak I. 2011. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil. 349: 215–225. doi:10.1007/s11104-011-0863-2
- Al-Karaki GN. 2000. Growth, sodium, and potassium uptake and translocation in salt stressed tomato. J Plant Nutr. 23:69–379. doi:10.1080/01904160009382023
- Bergmann W. 1992. Nutritional disorders of plants: development, visual and analytical diagnosis. Stuttgart: Gustav Fischer Verlag Jena.
- Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. 2007. Zinc in plants. New Phytol. 173:677–702. doi:10.1111/j.1469-8137.2007.01996.x
- Cakmak I, Gulut KY, Marschner H, Graham RD. 1994. Effect of zinc and iron deficiency on phytosiderophore release in wheat genotypes differing in zinc efficiency. J Plant Nutr. 17:1–17. doi:10.1080/01904169409364706
- Cakmak I, Torun A, Millet E, Feldman M, Fahima T, Korol A, Nevo E, Braun HJ, Özkan H. 2004. Triticum dicoccoides: an important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. J Soil Sci Plant Nutr. 50:1047–1054. doi:10.1080/00380768.2004.10408573
- Carelli ML, Fahl JI, Ramalho JC. 2006. Aspects of nitrogen metabolism in coffee plants. Theor Exp Plant Physiol.(ex-Braz. J Plant Physiol.) 18:9–21. doi:10.1590/S1677-04202006000100002
- Ebens R, Shacklette HT. 1982. Geochemistry of some rocks, mine spoils, stream sediments, soils, plants, and waters in the western energy region of the conterminous United States, US Geological Survey of Professional Paper, p. 1237.
- Erenoglu EB, Kutman UB, Ceylan Y, Yildiz B, Cakmak I. 2011. Improved nitrogen nutrition enhances root uptake, root-to-shoot translocation and remobilization of zinc in wheat. New Phytol. 189:438–448. doi:10.1111/j.1469-8137.2010.03488.x
- Foy CD, Chaney RL, White MC. 1978. The physiology of metal toxicity in plants. Ann Rev Plant Physiol. 29:511–566. doi:10.1146/annurev.pp.29.060178.002455
- Gransee A, Führs H. 2013. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil. 368: 5–21. doi:10.1007/s11104-012-1567-y
- Guo WJ, Meetam M, Goldsbrough PB. 2008. Examining the specific contributions of individual Arabidopsis metallothioneins to copper distribution and metal tolerance. Plant Physiol. 146:1697–1706. doi:10.1104/pp.108.115782
- Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M. 2006. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J. 45:335–346. doi:10.1111/j.1365-313X.2005.02624.x
- Jones Jr JB. 2001. Laboratory guide for conducting soil tests and plant analysis. Plant tissue analysis for micronutrients. Boca Raton (FL): CRC Press; p. 362.
- Kabata-Pendias A, Pendias H. 2001. Trace elements in soils and plants. Boca Raton (FL): CRC Press.
- Kutman UB, Yildiz B, Ozturk L, Cakmak I. 2010. Biofortification of durum wheat with zinc through soil and foliar applications of nitrogen. Cereal Chem. 87(1):1–9. doi:10.1094/CCHEM-87-1-0001
- Lidon FC, Almeida AS, Costa AR, Bagulho AS, Scotti-Campos P, Semedo J, Maçãs B, Pinheiro N, Gomes C, Leitão AE, et al. 2015. Sequential Zn and Fe biofortification of bread wheat grains – from controlled to uncontrolled environments. Crop and Pasture Sci. 66:1097–1104. doi:10.1080/10.1071/CP14270
- Lidon FC, Henriques FS. 1992. Copper toxicity in rice: a diagnostic criterium and its effect on Mn and Fe contents. Soil Sci. 154:130–135. doi: 10.1097/00010694-199208000-00006
- Lidon FC, Henriques FS. 1993a. Changes in the contents of photosynthetic electron carriers, RNAse activity and membrane permeability triggered by excess Cu in rice. Photosynthetica. 28:99–108.
- Lidon FC, Henriques FS. 1993b. Changes in the thylakoid membrane polypeptide patterns triggered by excess copper in rice. Photosynthetica. 28:109–117.
- Lidon FC, Henriques FS. 1994. Subcellular localization of copper and partial isolation of copper proteins in roots from rice plants exposed to excess copper. Aust J Plant Physiol. 21:427–436. doi:10.1071/PP9940427
- Lidon FC, Ramalho JC, Barreiro MG. 2004. Manganese accumulation in rice: implications on the photosynthetic functioning. J Plant Physiol. 161:1235–1244. doi:10.1016/j.jplph.2004.02.003
- Lidon FC. 2000. Rice adaptation to excess manganese: nutrients accumulation and implications of the quality of crops. J Plant Physiol. 156:652–658. doi:10.1016/S0176-1617(00)80227-8
- Lidon FC. 2001. Tolerance of rice to excess manganese in the early stages of vegetative growth. Characterisation of manganese accumulation. J Plant Physiol. 158:1341–1348. doi:10.1078/0176-1617-00507
- Lidon FC. 2002. Micronutrient uptake and translocation in Mn-treated rice. J Plant Nutr. 25:757–768. doi:10.1081/PLN-120002957
- Maathuis FJM, Sanders D. 1997. Regulation of K + absorption in plant root cells by external K+: Interplay of different plasma membrane K+ transporters. J Exp Bot. 48(Special Issue):451–458. doi:10.1093/jxb/48.Special_Issue.451
- Marschner H. 2011. Mineral nutrition of higher plants. 3rd ed. New York: Academic Press; p. 672.
- Martins LD, Tomaz MA, Lidon FC, DaMatta FM, Ramalho JC. 2014. Combined effects of elevated [CO2] and high temperature on leaf mineral balance in Coffea spp. plants. Climatic Change. 126:365–379. doi:10.1007/s10584-014-1236-7
- Moore CA, Bowen HC, Scrase-Field S, Knight MR, White PJ. 2002. The deposition of suberin lamellae determines the magnitude of cytosolic Ca2+ elevations in root endodermal cells subjected to cooling. Plant J. 30:457–466. doi:10.1046/j.1365-313X.2002.01306.x
- Mukherjee I, Campbell NH, Ash JS, Connolly EL. 2006. Expression profiling of the Arabidopsis ferric chelate reductase (FRO) gene family reveals differential regulation by iron and copper. Planta. 223:1178–1190. doi:10.1007/s00425-005-0165-0
- Pataco IM, Mourinho MP, Oliveira K, Santos C, Pelica J, Ramalho JC, Leitão AE, Scotti-Campos P, Lidon FC, Reboredo FH, Pessoa MF. 2015. Durum wheat (Triticum durum) biofortification in iron and definition of quality parameters for the industrial production of pasta – a review. Emirates J Food Agric. 27:242–249. doi:10.9755/ejfa.v27i3.19284
- Ramos I, Pataco IM, Mourinho MP, Lidon F, Reboredo F, Pessoa MF, Carvalho ML, Santos JP, Guerra M. 2016. Elemental mapping of biofortified wheat grains using micro X-ray fluorescence. Spectrochimica Acta Part B. 120:30–36. doi:10.1016/j.sab.2016.03.014
- Raven PH, Evert RF, Eichhorn SE. 2002. Biology of plants. 7th ed. New York: Freeman WH and Company; p. 686.
- Reboredo F, Fernando AL, Oliveira JFS. 2005. Bioaccurnulation of copper, iron, and zinc by Pinus halepensis (Miller). Bull Env Cont Toxicol. 74: 698–705. doi:10.1007/s00128-005-0639-6
- Reboredo F, Henriques F. 1991. Some observations on the leaf ultrastructure of Halimione portulacoides (L.) Aellen grown in a medium containing copper. J Plant Physiol. 137:717–722. doi:10.1016/S0176-1617(11)81228-9
- Reboredo F. 1997. Some observations on the effects of iron on the leaf ultrastructure of Halimione portulacoides. J Plant Physiol. 151:581–589. doi:10.1016/S0176-1617(97)80234-9
- Reboredo F. 2012. Zinc compartmentation in Halimione portulacoides (L.) Aellen and some effects on leaf ultrastructure. Env Sci Poll Res. 19:2644–2657. doi:10.1007/s11356-012-0757-8
- Reboredo FHS, Ribeiro C. 1984. Vertical distribution of Al, Cu, Fe and Zn in soil salt marshes of the Sado estuary, Portugal. Int J Env Studies. 23:249–253. doi: 10.1080/00207238408710160
- Robson AD, Reuter DJ. 1981. Diagnosis of copper deficiency and toxicity. In: Loneragan JF, Robson AD, Graham RD, editors. Copper in soils and plants. London: Academic Press; p. 287–312.
- Ruano A, Poschenrieder CH, Barcelo J. 1988. Growth and biomass partitioning in zinc-toxic bush beans. J Plant Nutr. 11:577–588. doi:10.1080/01904168809363824
- Schachtman DP, Reid RJ, Ayling SM. 1998. Phosphorus uptake by plants: from soil to cell. Plant Physiol. 116:447–453. doi:10.1104/pp.116.2.447
- Tsonev T, Lidon FC. 2012. Zinc in plants – an overview. Emir J Food and Agric. 24:322–333.
- Wheeler GL, Brownlee C. 2008. Ca2+ signaling in plants and green algae – changing channels. Trends in Plant Sci. 13:506–514. doi:10.1016/j.tplants.2008.06.004
- White PJ, Broadley MR. 2009. Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 182:49–84. doi:10.1111/j.1469-8137.2008.02738.x
- White PJ. 2000. Calcium channels in higher plants. Biochim Biophys Acta (Biomembranes). 1465:171–189. doi:10.1016/S0005-2736(00)00137-1
- White PJ. 2001. The pathways of calcium movement to the xylem. J Exp Bot. 52:891–899. doi:10.1093/jexbot/52.358.891
- White PJ. 2003. Ion transport. In: Thomas B, Murphy DJ, Murray BG, editors. Encyclopedia of applied plant sciences. London: Academic Press; p. 625–634.
- Wilkinson SR, Welch RM, Mayland HF, Grunes DL. 1990. Magnesium in plants: uptake, distribution, function, and utilization by man and animals. Metal Ions Biol Syst. 26:33–56.

![Figure 4. Location of nutrients in wheat grains halved by fluorescence radiation of X rays. For treatments [15] and [90], respectively: scanning electron micrographs (1 and 1a); Fe (2 and 2a); Zn (3 and 3a); Mn (4 and 4a); Cu (5 and 5a); K (6 and 6a); P (7 and 7a); Ca (8 and 8a).](/cms/asset/8b07d141-e790-49b7-9064-a01619a93ae0/tjpi_a_1278049_f0004_c.jpg)