ABSTRACT
In this study, zinc (Zn) deficiency caused a significant reduction in growth parameters and tissue Zn concentrations in BRRI 33 (sensitive) but not in Pokkali (tolerant). The increase of proton extrusion in both genotypes under high pH suggests that it gets triggered as a common consequence of reducing pH and solubilization of Zn. Real-time PCR showed pronounced upregulation of OsZIP4, OsDMAS1, OsNAS2 and OsPCS1 in Zn-deficient roots of Pokkali, and to a lesser extent in BRRI 33 only for OsZIP4 and OsPCS1. This suggests that OsDMAS1, OsNAS2 and OsPCS1 functions as secondary consequences leading to higher chelation and uptake of Zn under Zn deficiency in Pokkali. Further, a major increase in CAT, POD, SOD, GR and key metabolites suggests that high antioxidant defense plays a critical role in Zn deficiency tolerance in Pokkali. Further, Pokkali self-grafts and plants having Pokkali rootstock combined with BRRI 33 scion showed no significant decline in plant height, root dry matter and Zn concentration along with upregulation of Zn transporters (OsZIP4 and OsIRT1) under Zn deficiency, suggesting that signal driving mechanisms for Zn deficiency tolerance mechanisms are generated in the root and Zn-inefficient BRRI 33 is not capable of producing signals or sensing them.
Introduction
Zn deficiency is a well-known agricultural problem that causes leaf bronzing, growth stunting, delayed maturity and yield loss (Dobermann and Fairhurst Citation2000; Fageria et al. Citation2002). Reasons of Zn deficiency in the soil are the high pH, precipitation or absorption of Zn with other soil particles and redox potential (Kabir et al. Citation2014). Zn deficiency in crops often leads to low-grain Zn content, which in turn leads to malnutrition in children, susceptibility to infectious diseases and birth problems in pregnant women (Prasad Citation2009; Graham et al. Citation2012). Considerable genetic variability has been found in rice in response to Zn deficiency (Wissuwa et al. Citation2006; Höller, Meyer, et al. Citation2014), bean (Hacisalihoglu et al. Citation2004), wheat (Cakmak et al. Citation1994), sorghum (Li et al. Citation2013), barley (Genc et al. Citation2007) and few other crops. Zn efficiency (ZE), which is also termed as Zn deficiency tolerance, refers the ability of a plant to survive under Zn-limited conditions (Graham and Rengel Citation1993; Kalayci et al. Citation1999; Impa et al. Citation2013).
Most of the mechanisms conferring Fe-efficiency and ZE are common in plants, but these need to be specifically investigated for ZE. One mechanism by which plants extrude H+ into the rhizosphere for decreasing soil pH under metal-limiting is called proton extrusion (Santi and Schmidt Citation2009) and thus, it increases Zn solubility (Hajiboland and Beiramzadeh Citation2008; Palmer and Guerinot Citation2009). At molecular level, Zn homeostasis in plants is tightly regulated by Zn sensors and metal chelators involved in Zn acquisition and sequestration (Clemens Citation2001). In rice, OsHMA2 transcript mainly expressed in roots showing no upregulation under Zn deficiency but participated in Zn loading to xylem and root-to-shoot translocation (Takahashi et al. Citation2012). Among the chelators, deoxymugineic acid (DMA) and nicotianamine (NA) play crucial roles in both long and short-distance metal transport under abiotic stress (von Wiren et al. 1999; Takahashi et al. Citation2003). Genes encoding DMA and NA synthase (NAS) showed differential regulation under variable Fe and Zn status in rice, maize, Arabidopsis and barley (Inoue et al. Citation2003; Klatte et al. Citation2009; Johnson et al. Citation2011). However, OsNAS3 may not be involved in DMA secretion as its expression is restricted to the pericycle and companion cells of the roots (Inoue et al. Citation2003). Further, DMA in Zn-deficient rice plants are critically involved with the distribution of Zn and increase of Zn translocation (Suzuki et al. 2008) and overexpression of OsNAS2 gene led a significant increase of Fe and Zn in rice endosperm (Johnson et al. Citation2011). Also, phytochelatins (PCs), glutathione-derived metal-binding peptides, are involved in Zn accumulation and PC-Zn complexes formation in a few plant species but not in rice (Kobayashi and Yoshimura Citation2006; Chekmeneva et al. Citation2008; Tennstedt et al. Citation2008).
Enzymes and amino acids (Rai Citation2009; Apel and Hirt Citation2004; Jelali et al. Citation2010; Kocsy et al. Citation2011; Jin-Hua et al. Citation2012; Mukhopadhyay et al. Citation2013; Kabir et al. Citation2014) having antioxidant properties play key roles for controlling radicals and peroxides at cellular level and protect plants from abiotic stresses (Rai Citation2009; Jelali et al. Citation2010; Kocsy et al. Citation2011; Kabir et al. Citation2014). Höller, Meyer, et al. (Citation2014) further showed that Zn deficiency showed an increase in ROS formation in both roots and leaves to maintain cellular redox homeostasis and avoiding oxidative stress under Zn deficiency in rice. Recently, a five-fold increase of proline accumulation was reported in Zn-efficient RIL47 cultivar under Zn deficiency (Höller, Hajirezaei, et al. Citation2014). Studies revealed that abiotic stress tolerance is associated with ROS scavenging via increased antioxidant defense (Alscher et al. Citation2002; Hacisalihoglu et al. Citation2003; Kabir et al. Citation2016).
In response to the abiotic stress, different signals get activated and transmitted between roots and shoots. Plants undergo complex signaling pathways for sensing nutrient status (Schmidt Citation2003). These deficiency signals could either be originated in the root or shoot leading to both direct and local responses in other tissues through the signal transmission. Evidence also support the utilization of a number of nutrient-regulated mobile signals (Liu et al. Citation2009). Arabidopsis grafts with NgMTP1 overexpressed shoot scions and wild-type roots showed ZE responses in both roots and shoots (Gustin et al. Citation2009). To date, systemic signaling in Zn homeostasis has never been addressed in rice.
Insufficient understanding of ZE hampers our efforts for developing improved Zn-efficient cultivars. Few studies have investigated genetic variations for ZE in rice (Wissuwa et al. Citation2006; Höller, Meyer, et al. Citation2014; Höller, Hajirezaei, et al. Citation2014; Naher et al. Citation2014; Naher Citation2015); however, no report is available on the mechanistic basis for ZE in rice. Therefore, we investigated whether natural variation in ZE can be attributed to Zn concentration, root-shoot development and chlorophyll concentration in contrasting rice genotypes (Pokkali and BRRI 33). Also, the role of proton extrusion activity conferring ZE was studied. We further examined the expression of several genes (OsZIP4, OsHMA2, OsDMAS1, OsNAS2 and OsPCS1) associated with ZE in contrasting rice genotypes. Furthermore, the role of antioxidant systems and metabolites underlying differential ZE were studied. We also sought to determine the origin of Zn-deficiency signal by reciprocal grafting based on physiological and molecular investigations.
Materials and methods
Plant materials
Two cultivars (cv. Pokkali and BRRI 33) of rice (Oryza sativa L. ssp. Indica), with different tolerance to Zn deficiency, were used in this study, the former being efficient and latter inefficient (Naher et al. Citation2014; Naher Citation2015).
Plant cultivation
Seeds of two genotypes (Pokkali-tolerant and BRRI 33-sensitive), with different tolerance to Zn deficiency, were used in this study. Following surface sterilization of seeds with 75% ethanol and deionized water, seeds were germinated in the dark at room temperature. Afterward, uniform seedlings were transplanted to solution culture (Hoagland and Arnon Citation1950) containing the following nutrient concentrations (µM): KNO3 (4000), Ca(NO3)2·4H2O (4000), NH4H2PO4 (1300), MgSO4·7H2O (2000), KCl (50), H3BO3 (25), Fe-EDTA (25), MnSO4·4H2O (2), Na2MoO4·2H2O (0.5), CuSO4·5H2O (0.5) and Zn as ZnSO4 at two levels viz. 0.01 µM (Zn-deficient) and 2.0 µM (Zn-sufficient/control). HCl or KOH was used to maintain the pH (6.0) of the nutrient solution. Plants were grown in 2 L of the aerated solution in a growth chamber maintaining 10 h light and 14 h dark (550–560 μmol s−1 per μA). All control and stressed plants were grown concurrently for 7 days after treatment was imposed and harvested at the same time.
Measurement of growth parameters
Plants grown under both Zn-sufficient and Zn-deficient hydroponic conditions were harvested after 1 week. Root length and shoot height were then measured using a scale. Also, root tissues were washed in deionized water and quickly blotted in tissue paper. Both root and leaf tissues were then dried at 75°C for 2 days before measuring the dry weight in digital balance.
Determination of chlorophyll concentration in leaves
Leaf chlorophyll concentration was determined using spectrophotometer on 1-week old plants as described previously by Lichtenthaler and Wellburn (Citation1983) with some modifications. Briefly, 100 mg leaf tissues were poured in 5 mL falcon tube having 95% acetone. The tissues were then ground with the mortal-pestle and homogenates were centrifuged at 2500 rpm for 10 min. After centrifugation, the supernatant was placed in another falcon tube, and the absorbance was read at 662 (chlorophyll a) and 646 (chlorophyll b) on a spectrophotometer (UV-1650PC, Shimadzu).
Determination of Zn concentration
Harvested tissues (root and shoot) were washed with CaSO4 (1 mM) and deionized water before drying in an oven at 80°C for 3 days (Kabir et al. Citation2015). Once tissues were dried, 3 mL HNO3 and 1 mL of H2O2 were mixed with samples and heated at 75°C for 10 min. The concentration of Zn was then analyzed by Flame Atomic Absorption Spectroscopy outfitted with ASC-6100 autosampler and air-acetylene atomization gas mixture system (Model No. AA-6800, Shimadzu). Standard solutions of Zn were separately prepared from their respective concentration of stock solutions (Shimadzu).
Determination of proton (H+) extrusion
Extrusion of the proton from roots was measured on plants grown in small vials (50 mL) under Zn-sufficient and bicarbonate-induced (NaHCO3 10 mM, pH 8.0) Zn-deficiency as previously described (M’Sehli et al. Citation2008; Kabir et al. Citation2012). Extrusion of H+ efflux was estimated by titration method (Kabir et al. Citation2012).
RNA isolation and real-time PCR analysis
Expression pattern of OsActin (AY212324), OsZIP4 (AB126089.1), OsHMA2 (AB697186.1), OsIRT1 (AB070226.1), OsDMAS1 (AB269906.1), OsNAS2 (AB023818) and OsPCS1 (AF439787.2) was studied by quantitative real-time PCR in roots of Pokkali and BRRI 33. Briefly, harvested roots (50–100 mg) were ground to a fine powder in the presence of liquid nitrogen with a mortar and pestle. Afterward, total RNA was extracted following the instruction is given by SV total RNA Isolation System (cat. no. Z3100), Promega Corporation, United States. The purity of RNA was checked by denaturing agarose gel electrophoresis and quantified by NanoDrop 2000 UV-Vis Spectrophotometer. Subsequently, reverse transcription reactions were performed to convert RNA to the first-strand cDNA using GoScript™ Reverse Transcription System (Cat no. A5001), Promega Corporation, United States. The cDNA was then treated with RNAse for eliminating RNA contamination if any. Real-time PCR analysis was performed using GoTaq® qPCR Master Mix (Promega United States) in Eco™ real-time PCR system (Illumina, United States) platform using gene-specific primers (Supplementary Table S1). The expression data were normalized to the expression level of OsActin followed by ΔΔCT method (Eco Software v4.0.7.0). The real-time PCR program used was as follows: 3 min at 95°C, 40 cycles of 30 s at 94°C, 15 s at 57°C and 30 s at 72°C.
Enzymatic assay
CAT, POD, SOD and GR enzymes were extracted in roots of 1-week old plants as previously described with slight modifications (Goud and Kachole Citation2012). Briefly, roots were ground in 5 mL of 100 mM phosphate buffer (pH 7.0) and the homogenate was centrifuged for 10 min (8000 rpm) before separating the supernatant in Eppendorf tubes. CAT was analyzed in a reaction mixture containing 100 mM potassium phosphate buffer (pH 7.0), 6% H2O2 and 100 µL root extract. Once root extract is added, the changes in absorbance were monitored at 240 nm (extinction coefficient of 0.036 mM−1 cm−1) in a UV spectrophotometer at 30 s intervals up to 1 min. The activity of CAT is expressed as µmol of H2O2 oxidized min−1 (mg protein)−1. For POD analysis, root extract was mixed with 100 mM potassium phosphate buffer (pH 6.5), 1 0.05 M pyrogallol solution, 200 mM H2O2. The change of absorbance was then monitored at 430 nm (extinction coefficient 12 mM–1cm–1) from 30 s up to 1.5 min in a spectrophotometer. The specific activity of the enzyme is expressed as µmol pyrogallol oxidized min−1 (mg protein)−1. In the case of SOD, assay mixture was mixed with 50 mM sodium carbonate/bicarbonate buffer (pH 9.8), 0.1 mM EDTA and 0.6 mM epinephrine (Sun and Zigman Citation1978). The adrenochrome formation for 4 min was then read at 475 nm in a UV-Vis spectrophotometer. For GR activity, 100 μL of root extract was mixed with 0.2 M phosphate buffer (pH 7.0), 1 mM EDTA, 20 mM oxidized glutathione (GSSG) and 0 2 mM NADPH. Oxidation of NADPH by GR was subsequently monitored at 340 nm. The GR activity was calculated using the extinction coefficient of 6.12 mM−1 cm−1 (Halliwell and Foyer Citation1978).
Malondialdehyde (MDA) content, an indicator of ROS was analyzed as previously described (Kosugi and Kikugawa Citation1985). Briefly, harvested tissues were ground in 20% trichloroacetic acid and 0.5% 2-thiobarbituric acid. The homogenate was heated at 95°C for 30 min before transferring to an ice bath. Afterward, the ice-cold mixture was centrifuged at 5000 × g for 10 min at 25°C. The absorbance of the supernatant was subsequently recorded at 532 and 600 nm in a spectrophotometer. The MDA concentration was determined by subtracting the nonspecific turbidity at 600 nm by its molar extinction coefficient (Kosugi and Kikugawa Citation1985).
Determination of antioxidant capacity
The percentage of antioxidant capacity (AA%) of roots harvested from 1-week old plants was assessed by DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical assay (Brand-Williams et al. Citation1995). The reaction mixture contained 0.5 mL of root sample, 3 mL of absolute ethanol and 0.3 mL of DPPH radical solution diluted in 0.5 mM in ethanol. The reaction mixture changed its color from deep violet to light yellow and absorbance was recorded at 517 nm using a spectrophotometer after 100 min. The DPPH scavenging activity was then calculated according to the formula given by Mensor et al. (Citation2001).
Analysis of metabolites by LC-MS (liquid chromatography–mass spectrometry)
Plant metabolites were analyzed in leaves by an LCMS-2020 system equipped with prominence HPLC system (Shimadzu, Kyoto, Japan) and mass spectrometry (4000 Qtrap, AB Sciex) in ESI positive ion mode as previously described with some modifications (Murakami et al. Citation2014; Islam et al. Citation2015). Briefly, a mobile-phase column (EZ:faast AAA-MS column 250 × 2.0 mm) was eluted (0.25 mL/min) with gradient of 68% (0–13 min), 83% (13–13.1 min) and 68% (13–17 min) acetonitrile containing 10 mM ammonium formate in water at 35°C. The MS was operated as follows: drying gas flow of 15 L/min, nebulizer gas flow of 1.5 L/min, ESI voltage of 1.8 kV and temperature of 365°C.
Analysis of PC and DMA by HPLC (high-performance liquid chromatography)
Phytochelatin (PC) and 2-DMA were tested in roots and leaves by HPLC (Binary Gradient HPLC System, Waters Corporation, Milford, Massachusetts, USA) with Empower2™ software as previously described with some modifications (Lindberg et al. Citation2007; Nishiyama et al. Citation2012; Islam et al. Citation2015; Kabir et al. Citation2015). Briefly, samples were ground in chilled mortar pestle in deionized water. The sample mixtures were centrifuged at 1500 g for 10 min before separating the supernatant in Eppendorf tubes. The HPLC systems connected to a Waters 515 HPLC pump and Waters In-line degasser AF and a C18 reverse phase-HPLC column (particle size: 5 µm, pore size: 300 A, pH Range: 1.5–10, Dimension: 250 mm × 10 mm). Buffer A (water and 0.1% TFA) and buffer B (80% acetonitrile and 0.1% TFA) were used at the gradient of: 1–24 min 100% A, 25–34 min 100% B and 35–40 min 100% A in mobile phase. Both standards and samples were diluted (100×) and subsequently filtered through 0.22 µm Minisart Syringe Filters (Sartorius Stedim Biotech, Germany) before injection. Sample peaks were detected with a Waters 2489 dual absorbance detector (Waters Corporation, Milford, Massachusetts, USA) at 280 and 360 nm. Further, PC was detected with Thlaspi arvensis in comparison with the retention times (glycin-PCn). GSH-equivalents of each PC were further used for PC quantification (Lindberg et al. Citation2007).
Reciprocal micrografting of plants
Reciprocal grafting between tolerance and sensitive genotypes was performed on very young plants as previously described (Turnbull et al. Citation2002; Kabir et al. Citation2013) with some modifications (Supplementary Figure S1). The tiny stems of Pokkali and BRRI 33 were diagonally cut (45◦ from the horizontal) 0.4 cm above the germinated seed 2 days after emergence. Scion (the portion removed) separated from each genotype were grafted onto each genotype’s rootstock in four combinations (BRRI 33 rootstock + BRRI 33 scion, Pokkali rootstock + Pokkali scion, BRRI 33 rootstock + Pokkali scion and Pokkali rootstock + BRRI 33 scion). Each graft was held together by thin glass tubing positioned over the graft. Grafted plants were then kept wet on filter paper in Petri dishes for 1 day before transferring to the hydroponic conditions. No adventitious rooting was observed on grafted plants.
Statistical analysis
All experiments had at least three independent biological replications for each sample. Data were subjected to one-way ANOVA by IBM SPSS package v. 20 (IBM Corp., New York, USA) and means were compared by Duncan’s multiple range test at 5% significance level (P < .05). Further, the graphical presentation was prepared using GraphPad Prism 6.
Results
Variations in morpho-physiological traits
Zn deficiency caused a significant decrease in shoot height, shoot dry weight, root dry weight and leaf chlorophyll concentration in BRRI 33 but not in Pokkali compared to the plants grown under Zn-sufficient conditions (). However, only root length was not significantly changed due to Zn deficiency in BRRI 33 and showed no genotypic differences between contrasting genotypes. Furthermore, Zn concentrations in roots and leaves of Pokkali were not affected due to Zn deficiency compared to Zn sufficient plants. In contrast, Zn deficiency caused a severe decline in Zn concentration in both roots and leaves of BRRI 33 compared to the controls (). Under Zn sufficient conditions, proton extrusion was not observed in roots of both genotypes. However, Zn deficiency induced extrusion of the proton in both genotypes; however, the induction of proton extrusion was 3.5-fold higher in Pokkali than BRRI 33 ().
Table 1. Morpho-physiological features in Pokkali and BRRI 33 grown under Zn-sufficient and Zn-deficient solution culture.
Expression of key genes associated with ZE
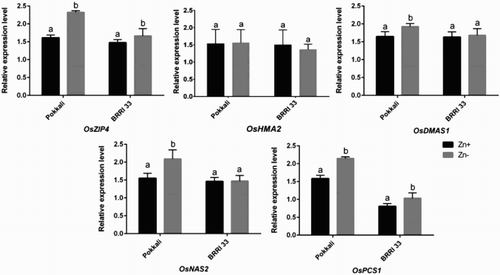
Expression of OsZIP4 significantly increased under Zn deficiency compared with Zn-sufficient controls though the induction was more pronounced in Pokkali than BRRI 33 (). OsHMA2 showed no significant changes in roots of either of the genotypes under Zn deficiency compared with controls. OsDMAS1 and OsNAS2 showed significant upregulation in roots of Pokkali under Zn deficiency compared to the plants grown in Zn sufficient conditions (). However, these transcripts did not differ in roots of BRRI 33 between Zn-sufficient and Zn-deficient conditions. OsPCS1 showed a significant increase in expression under Zn deficiency compared with control plants though the expression was more pronounced in Pokkali than BRRI 33.
Antioxidant enzymatic activities in roots
The activity of CAT (2.6-fold) and POD (2.8-fold) increased significantly by low Zn supply in roots of Pokkali (). In contrast, CAT (1.36-fold) and POD (2.33-fold) activities significantly reduced in BRRI 33 due to Zn deficiency (). The increase of SOD, GR and antioxidant capacity in roots of Pokkali over control values were 4.69, 3.5 and 2.95-fold, respectively. Further, MDA content significantly increased (1.77-fold) under Zn deficiency in roots of BRRI 33 compared with control plants; however, Pokkali showed no significant changes due to Zn deficiency ().
Table 2. Changes in antioxidant enzymes, antioxidant capacity (%) and MDA content in roots of Pokkali and BRRI 33 in response to Zn deficiency.
Changes of metabolites due to Zn deficiency
LC-MS technique was employed to study the changes of metabolites in leaves of Pokkali and BRRI 33 grown in both Zn-sufficient and Zn-deficient conditions. Of these 21 metabolites, numbers of metabolites significantly increased in concentration due to Zn deficiency compared to Zn-sufficient controls were 20 and 9 in Pokkali and BRRI 33, respectively (). Pokkali showed no significant decrease, while nine metabolites significantly decreased in BRRI 33 under Zn-deficiency compared with controls. Isoleucine in Pokkali as well as cysteine, tryptophan and isoleucine in BRRI 33 were not changed between treatments. Under Zn stress, three different metabolites (glutathione, methionine and cysteine) associated with S metabolism significantly increased in leaves of Pokkali in comparison with Zn-sufficient plants (). However, BRRI 33 showed a significant increase and a decrease in glutathione and methionine, respectively, in similar conditions. Among the N-metabolites, tryptophan, glutamic acid, lysine, valine, aspartic acid, hydroxylysine, proline and arginine showed the highest increase (6-18-fold) in Pokkali under Zn deficiency compared with controls. In contrast, Zn deficiency showed a prominent increase (3-10-fold) in hydroxylysine, alanine serine and threonine in BRRI 33. Further, hydroxyproline, hydroxylysine and isoleucine were not significantly varied between the genotypes (). PC and DMA were analyzed in roots and leaves of both Pokkali and BRRI 33 through HPLC system. Both PC and DMA were significantly increased in roots due to Zn deficiency in Pokkali but not in BRRI 33 ( and ). In leaves, PC and DMA were significantly increased in Pokkali but BRRI 33 showed significant increase only for PC under Zn-deficiency compared with controls ( and ).
Figure 2. HPLC analysis of total PC in roots and leaves of Pokkali and BRRI 33 grown under Zn-sufficient and Zn-deficient conditions. Different letters indicate significant differences between means ± SD of treatments (n = 3).
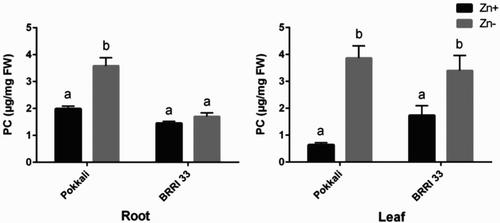
Figure 3. HPLC analysis of DMA in roots and leaves of Pokkali and BRRI 33 grown under Zn-sufficient and Zn-deficient conditions. Different letters indicate significant differences between means ± SD of treatments (n = 3).
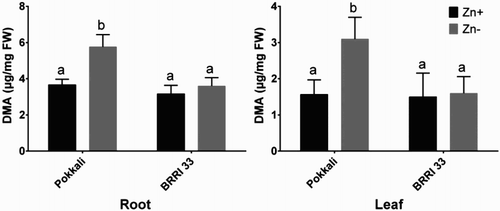
Table 3. LC-MS analysis of plant metabolites (ppm) in fresh leaves of Pokkali and BRRI 33 grown under Zn-sufficient and Zn-deficient conditions.
Grafting experiments
Micrografting between Pokkali and BRRI 33 were performed to locate the origin of the signal(s) that lead response conferring Zn deficiency tolerance. Using self-grafted control plants in the experiment ensured that results were not perplexed by grafting process or any other responses. Morpho-physiological features (plant height, root dry weight, leaf dry weight, Zn concentrations in roots and leaves) in self-grafted BRRI 33 plants showed significant decline under Zn shortage than controls. However, these parameters excluding leaf dry weight were not significantly affected due to Zn deficiency in self-grafted Pokkali (). When Pokkali (Zn-efficient) rootstock was grafted with the BRRI 33 (Zn-inefficient) scion, plants showed no significant changes in morpho-physiological features other than leaf dry weight between Zn-sufficient and Zn-deficient conditions. In contrast, BRRI 33 rootstock grafted to a Pokkali scion showed a significant decline in both root and shoot features under Zn-deficient conditions (). We further investigated the expression of two Zn transporters (OsZIP4 and OsIRT1) if above morpho-physiological data are consistent with the molecular evidence. Grafted plants having BRRI 33 rootstock with either BRRI 33 (Type 1) or Pokkali (Type 3) scion showed no changes of expression in OsZIP4 and OsIRT1 transcript between Zn+ and Zn− conditions. However, plants grafted between Pokkali rootstock and Pokkali/BRRI 33 (Type 2 and Type 4) scion showed a significant increase in OsZIP4 and OsIRT1 expression under Zn deficiency compared to Zn-sufficient control plants ().
Figure 4. Changes in the expression pattern of Zn transporter genes (OsZIP4 and OsIRT1) in roots of different combination of grafted plants (type 1: BRRI 33 rootstock + BRRI 33 scion, type 2: Pokkali rootstock + Pokkali scion, type 3: BRRI 33 rootstock + Pokkali scion, type 4: Pokkali rootstock + BRRI 33 scion) under Zn deficiency. The data are expressed as x-folds, where the control (Zn sufficiency) is set to 1 and the treated (Zn deficiency) are x-fold (ratio) to that. ns, not significantly (P > .05) changed. *Statistically significantly (P < .05) changed.
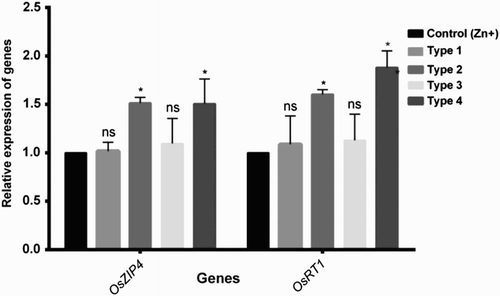
Table 4. Morpho-physiological features in different combination of grafted plants grown under different growth conditions.
Discussion
ZE is an importance trait given the prevalence of Zn-deficient conditions worldwide (Kalayci et al., Citation1999; Kabir et al. Citation2014). To gain insights into the determinants underlying ZE, we combined physiological, molecular and metabolic analyses along with micrografting technique for identifying the origin of Zn-deficiency signal in contrasting rice genotypes.
Physiological variations under Zn deficiency in contrasting genotypes
Morpho-physiological data suggest that genotypic differences exist in Pokkali and BRRI 33 showing no changes in shoot Zn content in Pokkali, while BRRI 33 () showed a significant decrease in shoot Zn in response to Zn deficiency. Impa et al. (Citation2013) also reported similar leaf Zn content in Zn-efficient IR55179 than sensitive KP rice genotypes. Similarly, Zn-efficient RIL46 showed more biomass than Zn-inefficient IR74 under low Zn field conditions (Widodo et al. Citation2010). Several root-related processes, such as efflux of phytosiderophores, proton exudation and formation of Fe plaques may influence higher Zn uptake in Zn-efficient genotypes (Impa et al. Citation2013; Rose et al. Citation2013). Proton extrusion, an important mechanism for mineral acquisition, showed induction under Zn deficiency in both Pokkali and BRRI 33 but relatively more pronounced in Zn-efficient Pokkali (). It may be possible that extrusion of the proton is a common response to Zn deficiency in rice plants.
Molecular mechanisms of ZE
Firstly, Zn-regulated transporter, OsZIP4 strongly expressed in both Pokkali and BRRI 33 under Zn shortage compared to Zn sufficient plants but the transcripts were more abundant in Zn-efficient Pokkali () and suggest the greater ability to absorb Zn. In addition, the slight increase of OsZIP4 in BRRI 33 is not sufficient to withstand Zn-deficiency. Furthermore, Induction of OsZIP1, OsZIP3 and OsZIP4 (Zn transporters) were also reported in rice under Zn shortage (Ramesh et al. Citation2003; Ishimaru et al. Citation2005; Widodo et al. Citation2010) though the expression level was much higher in OsZIP4 than that of OsZIP1 and OsZIP3 (Ishimaru et al. Citation2005) in shoots. In addition, expression of OsZIP4 was observed in root apical meristem and shoot meristem involved with Zn transport within the rice plant (Ishimaru et al. Citation2005). In this study, expression of OsHMA2 was not induced due to Zn deficiency in roots of Pokkali and BRRI 33, suggesting that Zn uptake in roots is not influenced by its activity. As OsHMA2 was reported to be correlated with root to shoot translocation of Zn (Takahashi et al. Citation2012), it might be possible that expression of the gene is genotype-dependent and is only involved in Zn transport and regulation in shoots of rice plants. Further, OsDMAS1 and OsNAS2 genes showed significant upregulation only in Zn-efficient Pokkali, while the expression of these genes was not increased under Zn deficiency in BRRI 33, but they are still expressed. It suggests that higher accumulation of DMA and NA are closely linked with ZE in Pokkali as a secondary consequence and allow Pokkali plants to chelate more Zn when Zn is limited. These data further implicate that the differential expression of OsDMAS1 and OsNAS2 may be one of the most important mechanisms underlying differential ZE in Pokkali and BRRI 33 plants. Previously, the upregulation of three NA-related genes (OsNAS1, OsNAS2, and OsNAS3) was reported due to Fe deficiency in root and shoot tissue in rice (Inoue et al. Citation2003; Bashir et al. Citation2011; Suzuki et al. Citation2012). In a previous study, PC deficiency resulted in significant decrease in root Zn accumulation in Arabidopsis (Tennstedt et al. Citation2008). However, this finding raised the questions whether PC synthesis is also crucial for Zn homeostasis in rice plants. We observed a significant increase in PC accumulation in roots and leaves of Zn-efficient Pokkali as well as leaves of BRRI 33 under Zn deficiency (). At molecular level, upregulation of OsPCS1 was also more pronounced in roots of Pokkali as expected (). These findings are correlated with the evidence observed in grafting experiments showing that a Zn-efficient rootstock is essential for signaling response mechanisms conferring tolerance to Zn deficiency.
Antioxidant defense under Zn deficiency
In our work, increased activity of CAT and POD in roots of Zn-efficient Pokkali under Zn deficiency suggests that these ROS-scavenging antioxidant enzymes play a vital role in removing destructive oxidant species. Increased activities of CAT and POD in response to Zn-deficiency have also been reported in Apple (Jin-Hua et al. Citation2012) and Poncirus trifoliate (Xiao et al. Citation2010). Furthermore, SOD and GR activities notably increased in roots of Pokkali, suggesting better efficiency in converting O2 to H2O2 to prevent plants from oxidative stress. Hacisalihoglu et al. (Citation2003) previously reported that expression of SOD-regulating gene (SOD1.1) increased in Zn-efficient cultivar compared with Zn-inefficient one grown under Zn-limiting conditions in wheat (Hacisalihoglu et al. Citation2003). Increased GR activity due to Zn shortage may activate both antioxidant enzyme activities and ASC–GSH cycle, thus reviving antioxidant metabolites. Frei et al. (Citation2010) reported variations in antioxidant activities (SOD, POX, GR) associated with differential ZE in contrasting rice genotypes. Therefore, it can be inferred that Pokkali is efficient in self-protecting from the oxidative damage generated by Zn deficiency. We also observed no changes in MDA content due to Zn deficiency in Pokkali, which is consistent with the increased antioxidant activities. MDA content has a detrimental effect on the membranes which in turn indicates the severity of stress experienced by any plant (Chakraborty and Pradhan Citation2012). The results obtained in BRRI 33 could be due to an overproduction of ROS and, at the same time, to an inadequate capacity to detoxify it. Scavenging of ROS for restoring redox metabolism, preservation of cellular turgor and structures are actively functioned during abiotic stress in plants (Yancey Citation2005; Mittler Citation2006).
Our LC-MS data showed substantial variations in some key metabolites generally associated with stress tolerance in leaves of Pokkali and BRRI 33 (). Among the S metabolites, elevated glutathione, methionine and cysteine were observed in leaves of Pokkali, while BRRI 33 showed significant increase only in glutathione but less pronouncedly due to Zn shortage (). Therefore, the higher accumulation of S-metabolites may secondarily trigger ZE and in particular, glutathione may function in protecting cells as an antioxidant under Zn-deficiency-induced oxidative injury in Pokkali. Glutathione protects plant cells from oxidative stress through the GSH-ascorbate cycle and as an electron donor to glutathione peroxidase in plants (Zaharieva et al. Citation2004; Zagorchev et al. Citation2013; Kabir et al. Citation2013). Furthermore, cysteine and methionine can mediate ROS-mediated oxidation to methionine sulfoxide (Cabreiro et al. Citation2006). We also found major increases in N metabolites were found for tryptophan, glutamic acid, lysine, valine, aspartic acid, hydroxylysine, proline and arginine (an amino acid with a high N/C ratio) in Pokkali, while these metabolites were not increased in BRRI 33 under Zn shortage, implying that N recycling may involve in C distribution to either organic acids or amino acids. Apart from acting as an osmolyte, proline is involved in scavenging free radicals, stabilizing sub-cellular structures and buffering cellular redox potential under Zn deficiency (Ashraf and Foolad Citation2007; Hayat et al. Citation2012; Höller, Hajirezaei, et al. Citation2014).
Origin of Zn-deficiency signal
Reciprocal grafting of Pokkali and BRRI 33 revealed that Pokkali-type ZE, as indicated by root and leaf morpho-physiological characteristics, was all observed in plants comprising Pokkali rootstock and BRRI 33 scion. In contrast, plants grafted with a Pokkali scion and BRRI 33 rootstock exhibited BRRI 33-type inefficiency to Zn-deficiency. Molecular analysis further revealed the upregulation of Zn transporters genes (OsZIP4 and OsIRT1) in plants grafted with a Pokkali rootstock and Pokkali/BRRI 33 scion under Zn deficiency, suggesting that Pokkali roots might contain mechanisms or signal capable of maintaining normal growth and Zn concentrations in BRRI 33 shoots, and can even exhibit Pokkali-type ZE when grafted to BRRI 33 shoots. On the contrary, Pokkali shoots do not contain any mechanism that may elicit ZE from BRRI 33 roots. It showed an indication of the efficiency for maintaining growth features and Zn uptake in both root and shoot under Zn shortage, as Pokkali does, is an asset of the Pokkali root, and is attributed to any signal or mechanism solely present in Pokkali roots. Similarly, Hussain et al. (Citation2004) investigated the systemic regulation of Zn homeostasis in Arabidopsis hma2hma4 double mutant having Zn-replete root and Zn-deficient shoot. Furthermore, Guimarães et al. (Citation2009) reported that hyperaccumulation of Zn in leaves of Thlaspi caerulescens is largely dictated by its root processes. Our data suggest that ZE in roots of Pokkali and BRRI 33 are driven by signal(s) originating in the roots. However, it may be alternatively possible that BRRI 33 roots are unable to ‘sense’ such signal whatever the nature of this signal is. The grafting experiment, to our knowledge, in rice, has never been reported before in the scientific literature and will be useful for studying stress-induced signaling in rice.
In conclusion, this study suggests that differential ZE in two naturally occurring rice cultivars (Pokkali and BRRI 3) is associated with variations in several biochemical and molecular mechanisms. ZE in Pokkali is mainly attributed to (i) the higher OsZIP4 and OsPCS1 expression as well as the upregulation of OsDMAS1 and OsNAS2 in roots, (ii) strong antioxidant defense through the increase of antioxidant enzymes and metabolites. In contrast, most of these mechanisms and antioxidant enzymes were limiting factors that led to the sensitivity of BRRI 33 to Zn deficiency. Reciprocal grafting demonstrated that BRRI 33 rootstock could not generate a Zn-efficient signal, even when grafted with the Pokkali scion, but it was capable of sensing the signals from the Pokkali rootstock and act as a Zn-efficient line. Therefore, the signals from the root are key in defining ZE of these rice varieties. The results may also motivate further researchers for improvement of Zn biofortification of rice and other stable crops. Moreover, Pokkali may let the breeders develop rice varieties capable of growing in Zn-deficient soils, or containing higher bioavailable Zn for improved human nutrition.
Supplemental_Data.docx
Download MS Word (174.4 KB)Acknowledgements
We also thank Bangladesh Council of Scientific and Industrial Research (BCSIR), Rajshahi Branch for providing LC-MS service. Authors are also grateful to DNA Technology, Denmark, for supplying us primers on a timely basis. We would like to acknowledge the help of Tommy Landberg, Department of Ecology, Environment and Plant Sciences, Stockholm University, Sweden for phytochelatin standard.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alscher RG, Erturk N, Heath LS. 2002. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot. 53:1331–1341. doi: 10.1093/jexbot/53.372.1331
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701
- Ashraf M, Foolad MR. 2007. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 59:206–216. doi: 10.1016/j.envexpbot.2005.12.006
- Bashir K, Ishimaru Y, Shimo H, Nagasaka S, Fujimoto M, Takanashi H, Tsutsumi N, An G, Nakanishi H, Nishizawa NK. 2011. The rice mitochondrial iron transporter is essential for plant growth. Nat Comm. 2:322.
- Brand-Williams W, Cuvelier ME, Berset C. 1995. Use of a free radical method to evaluate antioxidant activity. Food Sci Tech. 28:25–30.
- Cabreiro F, Picot CR, Friguet B, Petropoulos I. 2006. Methionine sulfoxide reductases. Ann NY Acad Sci. 1067:37–44. doi: 10.1196/annals.1354.006
- Cakmak I, Gulut KY, Marschner H, Graham RD. 1994. Effect of zinc and iron deficiency on phytosiderophore release in wheat genotype differing in zinc efficiency. J Plant Nutr. 17: 1–17. doi: 10.1080/01904169409364706
- Chakraborty U, Pradhan B. 2012. Oxidative stress in five wheat varieties (Triticum aestivum L.) exposed to water stress and study of their antioxidant enzyme defense system, water stress responsive metabolites and H2O2 accumulation. Braz J Plant Physiol. 24(2):117–130. doi: 10.1590/S1677-04202012000200005
- Chekmeneva E, Prohens R, Diaz-Cruz JM, Arino C, Esteban M. 2008. Competitive binding of Cd and Zn with the phytochelatin (Gamma-Glu-Cys)4-Gly: comparative study by mass spectrometry, voltammetry-multivariate curve resolution, and isothermal titration calorimetry. Environ Sci Tech. 42:2860–2866. doi: 10.1021/es702725a
- Clemens S. 2001. Molecular mechanisms of plant metal tolerance and homeostasis. Planta. 212(4): 475–486. doi: 10.1007/s004250000458
- Dobermann A, Fairhurst T. 2000. Rice. Nutrient disorders & nutrient management. Handbook series. Potash & Phosphate Institute (PPI), Potash & Phosphate Institute of Canada (PPIC) and International Rice Research Institute, 191 p.
- Fageria NK, Baligar VC, Clark RB. 2002. Micronutrients in crop production. Adv Agron. 77: 185–268. doi: 10.1016/S0065-2113(02)77015-6
- Frei M, Wang Y, Ismail AM, Wissuwa M. 2010. Biochemical factors conferring shoot tolerance to oxidative stress in rice grown in low zinc soil. Func Plant Biol. 37:74–84. doi: 10.1071/FP09079
- Genc Y, Huang CY, Langridge P. 2007. A study of the role of root morphological traits in growth of barley in zinc-deficient soil. J Exp Bot. 58(11):2775–2784. doi: 10.1093/jxb/erm142
- Goud PB, Kachole MS. 2012. Antioxidant enzyme changes in neem, pigeonpea and mulberry leaves in two stages of maturity. Plant Sig Behav. 7:1258–1262. doi: 10.4161/psb.21584
- Graham RD, Knez M, Welch RM. 2012. How much nutritional iron deficiency in humans globally is due to an underlying zinc deficiency? Adv Agron. 115:1–40. doi: 10.1016/B978-0-12-394276-0.00001-9
- Graham RD, Rengel Z. 1993. Genotypic variation in Zn uptake and utilization by plants. In: Robson AD, editor. Zinc in soils and plants. Dordrecht: Kluwer Academic; p. 107–114.
- Guimarães MA, Gustin JL, Salt DE. 2009. Reciprocal grafting separates the roles of the root and shoot in zinc hyperaccumulation in Thlaspi caerulescens. New Phytol. 184(2):323–329. doi: 10.1111/j.1469-8137.2009.02969.x
- Gustin JL, Loureiro ME, Kim D, Na G, Tikhonova M, Salt DE. 2009. MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J. 57(6):1116–1127. doi: 10.1111/j.1365-313X.2008.03754.x
- Hacisalihoglu G, Hart JJ, Wang YH, Cakmak I, Kochian LV. 2003. Zinc efficiency is correlated with enhanced expression and activity of zinc-requiring enzymes in wheat. Plant Physiol 131:595–602. doi: 10.1104/pp.011825
- Hacisalihoglu G, Ozturk L, Cakmak I, Welch RM, Kochian LV. 2004. Genotypic variation in common bean in response to zinc deficiency in calcareous soil. Plant Soil. 259:71–83. doi: 10.1023/B:PLSO.0000020941.90028.2c
- Hajiboland R, Beiramzadeh N. 2008. Rhizosphere properties of rice genotypes as influenced by anoxia and availability of zinc and iron. Pesq Agropec Brasil. 43:613–622. doi: 10.1590/S0100-204X2008000500009
- Halliwell B, Foyer CH. 1978. Properties and physiological function of a glutathion reductase purified from spinach leaves by affinity chromotography. Planta. 139:9–17. doi: 10.1007/BF00390803
- Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. 2012. Role of proline under changing environments. Plant Sig Behav. 7:1456–1466. doi: 10.4161/psb.21949
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. Cali Agric Exp Stat. 347:1950.
- Höller S, Hajirezaei MR, von Wiren N, Frei M. 2014. Ascorbate metabolism in rice genotypes differing in zinc efficiency. Planta. 239:367–379. doi: 10.1007/s00425-013-1978-x
- Höller S, Meyer A, Frei M. 2014. Zinc deficiency differentially affects redox homeostasis of rice genotypes contrasting in ascorbate level. J Plant Physiol. 171:1748–1756. doi: 10.1016/j.jplph.2014.08.012
- Hussain D, Haydon M, Sherson S, Jarvis R, Camakaris J, Cobbett CS. 2004. P-Type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell. 16(5):1327–1339. doi: 10.1105/tpc.020487
- Impa SM, Gramlich A, Tandy S, Schulin R, Frossard E, Johnson-Beebout SE. 2013. Internal Zn allocation influences Zn deficiency tolerance and grain Zn loading in rice (Oryza sativa L.). Front Plant Sci. 4:534. doi: 10.3389/fpls.2013.00534
- Inoue H, Higuchi K, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. 2003. Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J. 36:366–381. doi: 10.1046/j.1365-313X.2003.01878.x
- Ishimaru Y, Suzuki M, Kobayashi T, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. 2005. Oszip4, a novel zinc-regulated zinc transporter in rice. J Exp Bot. 56(422):3207–3214. doi: 10.1093/jxb/eri317
- Islam M, Begum MC, Kabir AH, Alam MF. 2015. Molecular and biochemical mechanisms associated with differential responses to drought tolerance in wheat (Triticum aestivum L.). J Plant Interact. 10(1):195–201. doi: 10.1080/17429145.2015.1064174
- Jelali N, Wissala M, Dell’orto M, Abdellya C, Gharsallia M, Zocchi G. 2010. Changes of metabolic responses to direct and induced Fe deficiency of two Pisum sativum cultivars. Environ Exp Bot. 68:238–246. doi: 10.1016/j.envexpbot.2009.12.003
- Jin-Hua T, Xiu-Shan T, Fei L, Hong-Yi Z, Chun-Xia F, Yan-An T. 2012. Effects of zinc deficiency stress on the antioxidative capability and plant hormone level of the different apple rootstocks. Acta Hort Sin. 39:1429–1436.
- Johnson AA, Kyriacou B, Callahan DL, Carruthers L, Stangoulis J, Lombi E. 2011. Constitutive overexpression of the OsNAS gene family reveals single-gene strategies for effective iron- and zinc-biofortification of rice endosperm. Plos One 6(9):e24476. doi: 10.1371/journal.pone.0024476
- Kabir AH, Begum MC, Haque A, Amin R, Swaraz AM, Haider SA, Paul NK, Hossain MM. 2016. Genetic variation in Fe toxicity tolerance is associated with the regulation of translocation and chelation of iron along with antioxidant defence in shoots of rice. Func Plant Biol. doi:10.1071/FP16068.
- Kabir AH, Paltridge NG, Able AJ, Paull JG, Stangoulis JCR. 2012. Natural variation for Fe-efficiency is associated with upregulation of strategy I mechanisms and enhanced citrate and ethylene synthesis in Pisum sativum L. Planta. 235:1409–1419. doi: 10.1007/s00425-011-1583-9
- Kabir AH, Paltridge NG, Rossener U, Stangoulis JCR. 2013. Mechanisms associated with Fe-deficiency tolerance and signalling in shoots of Pisum sativum L. Physiol Plant. 147:381–395. doi: 10.1111/j.1399-3054.2012.01682.x
- Kabir AH, Rahman MM, Haider SA, Paul NK. 2015. Mechanisms associated with differential tolerance to Fe deficiency in okra (Abelmoschus esculentus Moench). Environ Exp Bot. 112:16–26. doi: 10.1016/j.envexpbot.2014.11.011
- Kabir AH, Swaraz AM, Stangoulis J. 2014. Zinc-deficiency resistance and biofortification in plants. J Plant Nutri Soil Sci. 177:311–319. doi: 10.1002/jpln.201300326
- Kalayci M, Torun B, Eker S, Aydin M, Ozturk L, Cakmak I. 1999. Grain yield, zinc efficiency and zinc concentration of wheat genotypes grown in a zinc-deficient calcareous soil in field and greenhouse. Field Crops Res. 63:87–98. doi: 10.1016/S0378-4290(99)00028-3
- Klatte M, Schuler M, Wirtz M, Fink-Straube C, Hell R, Bauer P. 2009. The analysis of Arabidopsis nicotianamine synthase mutants reveals functions for nicotianamine in seed iron loading and iron deficiency responses. Plant Physiol. 150:257–271. doi: 10.1104/pp.109.136374
- Kobayashi R, Yoshimura E. 2006. Differences in the binding modes of phytochelatin to cadmium(ii) and zinc(ii) ions. Biol Trace Elem Res. 114:313–318. doi: 10.1385/BTER:114:1:313
- Kocsy G, Simon-Sarkadi L, Kovacs Z, Boldizsar A, Sovany C, Kirsch K, Galiba G. 2011. Regulation of free amino acid and polyamine levels during cold acclimation in wheat. Acta Biol Szeged. 55:91–93.
- Kosugi H, Kikugawa K. 1985. Thiobarbituric acid reaction of aldehydes and oxidized lipids in glacial acetic acid. Lipid. 20:915–921. doi: 10.1007/BF02534777
- Li Y, Zhang Y, Shi D, Liu X, Qin J, Ge Q, Xu L, Pan X, Li W, Zhu Y, Xu J. 2013. Spatial-temporal analysis of zinc homeostasis reveals the response mechanisms to acute zinc deficiency in Sorghum bicolor. New Phytol. 200(4):1102–1115. doi: 10.1111/nph.12434
- Lichtenthaler HK, Wellburn AR. 1983. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem Soc Transac. 11:591–592. doi: 10.1042/bst0110591
- Lindberg S, Landberg T, Gregor M. 2007. Cadmium uptake and interaction with phytochelatins in wheat protoplasts. Plant Physiol Biochem. 45(1):47–53. doi: 10.1016/j.plaphy.2007.01.001
- Liu TY, Chang CY, Chiou TJ. 2009. The long-distance signaling of mineral macronutrients. Curr Opin Plant Biol. 12(3):312–319. doi: 10.1016/j.pbi.2009.04.004
- Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS. 2001. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 15:127–130. doi: 10.1002/ptr.687
- Mittler R. 2006. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 11:15–19. doi: 10.1016/j.tplants.2005.11.002
- M’Sehli W, Youssfi S, Donnini S, Dell’Orto M, De Nisi P, Zocchi G, Abdelly C, Gharsalli M. 2008. Root exudation and rhizosphere acidification by two lines of Medicago ciliaris in response to lime-induced iron deficiency. Plant Soil. 312:151–162. doi: 10.1007/s11104-008-9638-9
- Mukhopadhyay M, Das A, Subha P, Bantawa P, Sarkar B, Ghosh P, Mondal TK. 2013. Structural, physiological, and biochemical profiling of tea plantlets under zinc stress. Biol Plant 57:474–480. doi: 10.1007/s10535-012-0300-2
- Murakami S, Nakata R, Aboshi T, Yoshinaga N, Teraishi M, Okumoto Y, Ishihara A, Morisaka H, Huffaker A, Schmelz EA, Mori N. 2014. Insect-induced daidzein, formononetin and their conjugates in soybean leaves. Metabol. 4:532–546. doi: 10.3390/metabo4030532
- Naher T, Sarkar MR, Kabir AH, Haider SA, Paul NK. 2014. Screening of Zn-efficient rice through hydroponic culture. Plant Environ Dev. 3(2):14–18.
- Naher T. 2015. Screening of Zn-efficient genetic lines and mechanisms conferring differential Zn-efficiency in rice (Oryza sativa L.) [MSc thesis]. Department of Botany, University of Rajshahi, Rajshahi.
- Nishiyama R, Kato M, Nagata S, Yanagisawa Y, Yoneyama T. 2012. Identification of Zn–nicotianamine and Fe-2-deoxymugineic acid in the phloem sap from rice plants (Oryza sativa L.). Plant Cell Physiol. 53(2):381–390. doi: 10.1093/pcp/pcr188
- Palmer CM, Guerinot ML. 2009. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat Chem Biol. 5:333–340. doi: 10.1038/nchembio.166
- Prasad AS 2009. Impact of the discovery of human zinc deficiency on health. J Amer Coll Nutri. 28:257–265. doi: 10.1080/07315724.2009.10719780
- Rai VK. 2009. Role of amino acids in plant responses to stresses. Biol Plant. 45:481–487. doi: 10.1023/A:1022308229759
- Ramesh SA, Shin R, Eide DJ, Schachtman DP. 2003. Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiol. 133:126–134. doi: 10.1104/pp.103.026815
- Rose TJ, Impa SM, Rose MT, Pariasca-Tanaka J, Mori A, Heuer S. 2013. Enhancing phosphorous and zinc acquisition efficiency in rice: a critical review of root traits and their potential utility in rice breeding. Ann Bot. 112:331–345. doi: 10.1093/aob/mcs217
- Santi S, Schmidt W. 2009. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol. 183:1072–1084. doi: 10.1111/j.1469-8137.2009.02908.x
- Schmidt W. 2003. Iron solutions: acquisition strategies and signalling pathways in plants. Trends Plant Sci. 8:188–193. doi: 10.1016/S1360-1385(03)00048-7
- Sun M, Zigman S. 1978. An improved spectrophotomeric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem. 90:81–89. doi: 10.1016/0003-2697(78)90010-6
- Suzuki M, Bashir K, Inoue H, Takahashi M, Nakanishi H, Nishizawa NK. 2012. Accumulation of starch in Zn-deficient rice. Rice 5:9.
- Takahashi R, Ishimaru Y, Shimo H, Ogo Y, Senoura T, Nishizawa NK, Nakanishi H. 2012. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 35:1948–1957. doi: 10.1111/j.1365-3040.2012.02527.x
- Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S. 2003. Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell. 15:1263–1280.
- Tennstedt P, Peisker D, Böttcher C, Trampczynska A, Clemens S. 2008. Phytochelatin synthesis is essential for the detoxification of excess zinc and contributes significantly to the accumulation of zinc. Plant Physiol. 149(2):938–948. doi: 10.1104/pp.108.127472
- Turnbull CG, Booker JP, Leyser HM. 2002. Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J. 32:255–262. doi: 10.1046/j.1365-313X.2002.01419.x
- von Wiren N, Marschner H, Römheld V. 1996. Roots of iron-efficient maize also absorb phytosiderophore-chelated zinc. Plant Physiol. 111:1119–1125. doi: 10.1104/pp.111.4.1119
- Widodo B, Broadley MR, Rose T, Frei M, Pariasca-Tanaka J, Yoshihashi T, Thomson M, Hammond JP, Aprile A, Close TJ, et al. 2010. Response to zinc deficiency of two rice lines with contrasting tolerance is determined by root growth maintenance and organic acid exudation rates, and not by zinc-transporter activity. New Phytol. 186(2):400–414. doi: 10.1111/j.1469-8137.2009.03177.x
- Wissuwa M, Ismail AM, Yanagihara S. 2006. Effects of zinc deficiency on rice growth and genetic factors contributing to tolerance. Plant Physiol. 142:731–741. doi: 10.1104/pp.106.085225
- Xiao JX, Qi XX, Zhang SL. 2010. Effects of zinc- and iron deficiency on physiological indices, mineral contents, and leaf ultrastructure of Poncirus trifoliata. J Appl Ecol. 21:1974–1980.
- Yancey PH. 2005. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol. 208:2819–2830. doi: 10.1242/jeb.01730
- Zagorchev L, Seal CE, Kranner I, Odjakova M. 2013. A central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci. 14:7405–7432. doi: 10.3390/ijms14047405
- Zaharieva T, Gogorcena Y, Abadia J. 2004. Dynamics of metabolic responses to iron deficiency in sugar beet roots. Plant Sci. 166:1045–1050. doi: 10.1016/j.plantsci.2003.12.017