 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Drought is an important abiotic stress that limits the plant growth and productivity. Present investigation was aimed that plant growth-promoting rhizobacteria (PGPR) isolated from moisture-stressed area impart drought tolerance in plants and tryptophan may improve their efficiency. Pseudomonas sp. (1), Bacillus cereus and Bacillus pumilus (B. pumilus) were isolated from maize rhizosphere grown in irrigated fields, semi-arid region and arid region, respectively. Proteus sp. and Pseudomonas sp. (2) were isolated from rice rhizosphere grown in irrigated fields and raised bed. B. pumilus produced 5× more abscisic acid (ABA) in culture media than Pseudomonas sp. (1) by the addition of l-tryptophan. These inoculants also modulated the phytohormone content of maize leaves in a pot experiment. Higher ABA was produced by the application of B. pumilus and Pseudomonas sp. (2), while indole 3-acetic acid and gibberellic acid were found higher in Pseudomonas sp. (1) and Proteus sp. treated plants. Addition of l-tryptophan increased the concentration of all phytohormones in soil and leaves of maize. Maximum increase in relative water content, osmotic potential, protein content and photosynthetic pigments was recorded in B. pumilus treated maize plants. Under irrigated condition, response of Pseudomonas sp. co-inoculated with B. pumilus from arid field superseded while under drought stress the effect of later predominated. Bacillus pumilus can be used in the formulation of biofertilizer to alleviate drought stress in arid and semi-arid regions.
Introduction
World comprises about 60% of the arid and semi-arid region and consequently water crisis which badly influence the crop productivity particularly in most of the Asian countries with an economy mainly dependent upon irrigated agriculture (Swain et al. Citation2017). The decrease in rainfall, increase in atmospheric CO2 and more extreme weather events accompanied by a 1.5–4.5°C increase in temperature are expected in the next 100 years (Torres and Henry, Citation2016). Agriculture sector is severely affected by drought stress widespread in the arid and semi-arid area resulting in prominent loss of yield and production of agricultural crops. Like many other countries, Pakistan is also facing the severe problem of drought. In rain-fed areas, the mean annual rainfall is much below than the crop water requirements. Likewise, under irrigated conditions, the availability of water is not ensured for the whole year (Riaz et al. Citation2013). Among various other approaches, the application of plant growth-promoting rhizobacteria (PGPR) is considered as the most sustainable approach to cope with drought situation.
Inoculation of plants with PGPR of the arid or semi-arid area has the potential to combat stress (Sandhya et al. Citation2010). A wide range of rhizobacterial species have been identified as auxin producers (Ahmed et al. Citation2008). Inoculation of plants with PGPR can enhance the drought tolerance (Figueiredo et al. Citation2008). Arshad et al. (Citation2008) reported that Pseudomonas spp. inoculation enhanced the growth, yield and ripening of pea (Pisum sativum L.) under drought stress conditions.
The most effective physiological precursor of auxins in microbes is l-tryptophan (Khalid et al. Citation2006). Addition of l-tryptophan to soils enhances the plant growth and development by increasing the indole 3-acetic acid (IAA) content of leaves (Lee et al. Citation2012).
l-tryptophan as a component of root exudates is released in the rhizosphere soil (Moe, Citation2013). It may also be released as an end-product of protein hydrolysis of dead cells in the soil. The mechanism of growth promotion by the application of l-tryptophan was demonstrated by Quiroz et al. (Citation2012). De Souza et al. (2015) reported that inoculation of PGPR resulted in enhanced production of plant growth regulators (PGR) in the presence of precursor. The PGR production ability both in soil and in culture was enhanced several times as a result of the addition of precursor in it (Raheem et al. Citation2017). Ahemad and Kibret (Citation2014) reported that water use efficiency and availability of nutrients to plant were enhanced by the synthesis of IAA in Canola.
Application of auxin-producing Pseudomonas enhanced the growth of Triticum aestivum (Iqbal et al. Citation2013). Improvement of plant growth and yield of wheat by the application of PGPR and l-tryptophan under salt stress has been demonstrated by Tamoor and Bano (Citation2014). Under stress conditions, environmental signals trigger abscisic acid (ABA) synthesis in plants which is responsible for stress adaptation by altering various physiological and biochemical processes (Lim et al. Citation2015). Besides ABA, the role of auxin under drought stress tolerance has been studied particularly in the modulation of reactive oxygen species as well as ABA content in the plant (Sharma et al. Citation2015).
The present study was aimed to evaluate the potential of PGPR isolated from rhizosphere of rice and maize from arid, semi-arid and irrigated zones on the production of IAA, gibberellic acid (GA) and ABA in culture, their transport to plant leaves when used as a bioinoculant and their release in rhizosphere soil in the presence and absence of l-tryptophan under induced drought stress.
Materials and methods
PGPR used in the study
Five PGPR strains were isolated and identified by 16S rDNA sequence analysis as, Pseudomonas sp. (1) (Accession no. KF307196) (from rhizosphere soil of maize from irrigated fields of National Agricultural Research Centre (NARC), Islamabad, Pakistan, Bacillus cereus (Accession no. KF307197) (from rhizospheric soil of maize grown in the semi-arid region of Kahuta, Punjab, Pakistan), Bacillus pumilus (Accession no. KF307203) (from rhizospheric soil of maize grown in the arid region of Jhang, Punjab, Pakistan), Proteus sp. (Accession no. KF307200) (from rhizospheric soil of rice grown in irrigated fields of Kalashahkaku, Punjab, Pakistan) and Pseudomonas sp. (2) (Accession no. KF307201) (from rhizospheric soil of rice grown in raised bed of Kalashahkaku, Punjab, Pakistan) were used in the present study.
Identification of PGPR strains by 16S ribosomal RNA sequences analysis
The nucleotide sequence of all 16S rRNA amplicons was determined by direct sequencing using the f27 and r1492 primers. The 16S rDNA PCR was used to amplify 16S rRNA genes for the identification of PGPR isolates using primers f27 (5′-AGAGTTTGATCMTGGCTCAGTAC-3′) and r1492 (5′-GGYTACCTTGTTACGACTT-3′) (Weisburg et al. Citation1991). The cycling conditions were: one cycle of 94°C for 5 min, 94°C for 30 s, 60°C for 30 s, 35 cycles of 72°C for 1 min 30 s and one cycle of 72°C for 5 min. PCR (500 ng) product was purified using the Isolate PCR and gel Kit (Bioline UK) according to the manufacturer’s instructions. The purified DNA was then sequenced directly at AGRF (Australian Genome Research Facility).
Sequence analysis
All sequences were compiled and the sequence traces checked using the BioEdit software package (Hall Citation2001). Multiple sequence alignments were generated using BioEdit software package (Tom Citation2007). Then, nucleotide sequences were compared with nucleotide bank (NCBI).
Production and quantification of phytohormones (IAA, GA and ABA) by PGPR isolates
The five PGPR isolates were analyzed for ABA, IAA and GA production in liquid culture. The selected PGPR isolates were grown in LB broth. Three replicate cultures were prepared. l-tryptophan (100 mg/L) was added in LB medium which was inoculated with 24-h-old bacterial cultures and placed on a shaker (EXCELLA E24, New Brunswick Scientific, USA) at 100 g, for 7 days. Thereafter, cultures were centrifuged at 10,000 rpm for 15 min and the supernatant was used for the extraction of growth hormones excreted in the growth medium. The pH of the supernatant was adjusted to 2.8 with 1 N HCl. Phytohormones were extracted with an equal volume of ethyl acetate, as described by Tien et al. (Citation1979). The ethyl acetate extract was dried using a rotary evaporator and the residue was dissolved in 1500 μL of pure methanol (Sigma Chemical Co). The samples were analyzed by HPLC (Agilent 1100, Germany) using a UV detector and C18 column (39 × 300 mm).
For identification of hormones, samples filtered through 0.45-µ-millipore filters were injected into the HPLC column. Methanol, acetic acid and water (30:1:70) were used as a mobile phase. The wavelength used for the detection of IAA was 280 nm (Sarwar et al. Citation1992) whereas for GA analysis it was adjusted at 254 nm (Li et al. Citation1994). For ABA, the injected sample was eluted with 0.1% acetic acid and methanol (30–70% methanol, linear gradient over 30 min) at 254 nm wavelength. These growth hormones are identified on the basis of retention time and peak area of the standards. Pure IAA, GA3 and ABA were used as standards for identification and quantification of plant hormones.
Pot experiment
A pot experiment was conducted in the greenhouse of Quaid-i-Azam University, Islamabad (latitude, 33o44′ N, longitude 73o08′E and altitude 2021 ft) to analyze capabilities of PGPR isolates alone and with l-tryptophan application in soil for enhancing the growth of maize under drought stress conditions.
The soil and sand thoroughly mixed in a 3:1 ratio; passed through 2 mm sieve was sterilized. The sterilization was done by autoclaving at 121°C, 15 psi for 15 min three times with the difference of 48 h. l-tryptophan (2.3 × 10−3 mg/kg) was mixed with the soil in pots on the same day before seed sowing.
Fresh cultures (24 h) were inoculated in 100 mL of LB broth and kept on a shaker for 48 h, then centrifuged for 10 min at 10,000 rpm. The supernatant was discarded and the pellet was dissolved in the autoclaved distilled water up to 100 mL. The optical density of the bacterial solution was adjusted to one at 600 nm with a spectrophotometer.
Maize seeds variety ‘Islamabad White (Sawan 3)’ were collected from NARC, Islamabad. Seeds were surface sterilized with 95% ethanol for 3 min followed by treating with 10% sodium hypochlorite for 3 min with gentle shaking and successively washed with sterilized water. Thereafter, sterilized seeds were soaked for 2–4 h in bacterial culture (108 cells/g); subsequently, the seeds were sown in plastic pots (8.5 inches (length) × 8 inches (width)) containing sterilized soil and sand in a 3:1 ratio.
Following treatments were employed and all the treatments were replicated three times.
Soil moisture (12.5% of dry weight of soil) was maintained constant during the experiment by daily sprinkling with sterile distilled water. After 15 days of germination, drought stress was induced by withholding water supply. Water-stressed seedlings and their corresponding unstressed controls were harvested after 7 days of exposure to drought for physiological and biochemical analysis and soil samples were collected for soil moisture content analysis.
Relative water content of leaves
Relative water content (RWC) of flag leaves was measured after 7 days of induction of water stress (soil moisture 1.32%), by the method of Weatherley (Citation1950). Fresh weight of the leaves was recorded. The leaves were then immersed in distilled water in beakers and left for 24 h. Thereafter, fully turgid leaves were weighed again. Then, leaves were dried in an oven for 72 h at 70°C, until a constant weight of leaves was obtained. RWC was calculated by the following formula:where RWC is the relative water content, DW is the dry weight, FW is the fresh weight and FTW is the fully turgid weight.
Extraction and purification of phytohormones from leaves (IAA, GA and ABA)
The flag leaves (1 g) were ground in 80% methanol, at 4°C with butylated hydroxyl toluene, used as an antioxidant. The extraction and purification was done following the method of Kettner et al. (Citation1995). The extraction was done at 4°C till 72 h in dark with a subsequent change of solvent at each 24 h. The extracted sample was centrifuged at 10,000 rpm and the supernatant was reduced to the aqueous phase using a rotary thin film evaporator at 35°C. The pH of the aqueous phase was adjusted to 2.5–3.0 with 0.1 N HCl and partitioned four times with one-half volume of ethyl acetate. The ethyl acetate was dried down completely using a rotary thin film evaporator. The dried samples were re-dissolved in 1 mL of methanol (100%) and were analyzed for the presence of IAA, GA and ABA on HPLC (Agilent 1100, Germany) using a UV detector and C-18 column (39 × 300 mm) as described previously.
Analysis of phytohormone (IAA and GA) in rhizospheric soil
The GA and IAA contents of soil were analyzed by the method of Hartung et al. (Citation2002). Soil samples were extracted in threefold excess of 1 mM CaCl2 for 1 h. The pH was adjusted to 2.5–3.0 and partitioned three times with one-third volume of ethyl acetate. The ethyl acetate phase was dried down completely using a rotary thin film evaporator. The dried samples were dissolved in 1 mL of methanol (100%) and analyzed for the presence of GA and IAA using HPLC as described previously.
Proline content
The proline content of flag leaves was estimated according to the method of Bates et al. (Citation1973). Fresh plant material (0.1 g) was homogenized with 4 mL sulfosalicylic acid (3.0%) in a mortar and placed overnight at 5°C. The suspension was centrifuged at room temperature at 3000 rpm for 5 min. The supernatant was mixed with 4 mL of acidic ninhydrin reagent. The reaction mixture was mechanically shaken; and contents in the tubes were heated in boiling water bath for 1 h. Thereafter, the content in the tubes was cooled and the mixture was extracted with 4 mL of toluene in a separating funnel. The absorbance of toluene layer was recorded at 520 nm. The concentration of the unknown sample was calculated with reference to the standard curve.
Total proteins
The protein content of the leaves was determined following the method of Lowry et al. (Citation1951) using bovine serum albumin (BSA) as a standard. Fresh leaves (0.1 g) were ground in 1 mL of phosphate buffer (pH 7.5) with a mortar and pestle. Centrifugation of the homogenate was done at 3000 rpm for 10 min at room temperature. The supernatant was transferred to test tubes and distilled water was added to make a total volume of 1 mL. Alkaline copper sulfate reagent (1 mL) was added and shaked for 10 min. In this mixture, 0.1 mL of the Folin’s reagent was added and incubated for 30 min. The absorbance of each sample was recorded at 650 nm against a blank (1.0 mL of 0.5 M sodium hydroxide). The concentration of soluble protein was determined with reference to a standard curve using BSA.
Superoxide dismutase activity
The superoxide dismutase (SOD) was determined following the method of Beauchamp and Frodovich (Citation1971). Fresh leaves (0.2 g) were ground in 4 mL of phosphate buffer (pH 7.8), containing 1% (w/v) PVP (at 4°C). The centrifugation of the plant material was done at 4°C. The supernatant was collected in a fresh test tube and recentrifugation was carried out. The supernatant was collected again and the final volume was raised to 0.8 mL. The reaction mixture contained riboflavin (1.17 × 10−6 M), methionine (0.1 M), potassium cyanide (2 × 10−5M) and nitroblue tetrazolium (NBT) (5.6 × 10−5 M) salt dissolved in 3 mL of 0.05 M sodium phosphate buffer (pH 7.8). The reaction mixture (3 mL) was added to 1 mL of enzyme extract. One set of the mixture was kept in light to initiate the reaction at 30°C for 1 h. The other identical set was kept under dark, served as a blank. The absorbance was recorded at 560 nm against the blank.
Peroxidase activity
The peroxidase (POD) activity was measured by the strategy for Vetter et al. (Citation1958) as altered by Gorin and Heidema (Citation1976). The test blend contained 50 µL of sample, 675 µL of 100 mM MES buffer (pH 5.5), 300 µL of 0.05% H2O2 and 100 µL of 0.1% phenylenediamine. Changes in the absorbance were recorded at 485 nm for 3 min. The POD activity was introduced as a change in OD 485 nm/min/mg of protein.
Catalase activity
Catalase (CAT) activity was done by the technique for Kumar et al. (Citation2010) with few alterations. 100 µL of 3% H2O2, 500 µL of 0.05 M phosphate support (pH 7) and 200 µL of sample were added to make the reaction mixture, and its absorbance was measured at 240 nm for 0 and 3 min.
Osmotic potential
The osmotic potential of the cell sap was measured from flag leaves according to the method of Capell and Doerffling (Citation1993). Leaf material was enclosed in a 2-mL plastic syringe and stored at 20°C to obtain the cell sap (50 µL). Subsequently, it was pressed to ooze out the sap from the thawed leaf. Reading was taken from a freezing point osmometer (Gonotech GmbH model OSMOMAT 010) as mosmol/kg and was converted to –Mpa.
Photosynthetic pigments (chl a, chl b, carotenoids)
Photosynthetic pigments (chlorophyll a, chlorophyll b, carotenoids) were determined by following the method of Hiscox and Tsraelstam (Citation1979). Leaf (0.05 g) of each sample was soaked in an aliquot of 2.5 mL of DMSO in test tubes. Then, it was left for 3 days. A spectrophotometer was used to record the absorbance of samples at 663, 645 and 470 nm for chlorophyll a, chlorophyll b and carotenoid contents, respectively.
Statistical analyses
The data were analyzed statistically by the analysis of variance technique and comparison among means was made by Statistix 8.1. Comparison between mean values of treatments was made by least significant difference to test significant differences at p = .05 (Gomez et al. Citation1984).
Results
Identification of strains
The isolates obtained from rhizosphere of maize grown in the arid (HYJW), semi-arid (HY9 K) and irrigated (HY8N) region have the sequences length of 1410, 1297 and 1296 base pairs, respectively. The comparison of the nucleotide sequence with data nucleotide bank showed the highest sequence similarity with 1281/1283 and 99% with that of Bacillus pumilus strain (ACC no: KT 003271.1), 1269/1279 and 99% with that of Bacillus cereus strain (ACC no: DQ289055.1) and 1263/1263 and 100% with that of Pseudomonas sp. (ACC no: KJ191560.1), respectively.
The isolates obtained from rhizosphere of rice grown in raised (HY13KR) and irrigated bed (HY 13KI) conditions have a total sequence length of 1301 and 1297 nucleotide base pairs, respectively. The comparison of the nucleotide sequence with data nucleotide bank showed the highest 1267/1268 and 99% sequence similarity with Proteus sp. (ACC no: KM016981.1) and 1270/1270 and 100% with that of Pseudomonas sp. (ACC no: KJ191560.1), respectively.
Phytohormone (IAA, GA and ABA) production by PGPR isolates
Results in (a) showed that all the PGPR isolates produced IAA, GA and ABA in culture media. The higher amount of IAA, GA and ABA was produced by Proteus sp. (7.5 µg/mL), Pseudomonas sp. (1) (55.8 µg/mL) and Pseudomonas sp. (2) (17.6 µg/mL), respectively.
Figure 1. (a,b) Production of phytohormones (IAA, GA and ABA) by PGPR isolates in the presence of l-tryptophan in culture media. Whereas B. pumilus = B. pumilus isolated from rhizosphere of maize grown at the arid region, Ps. sp. (1) = Pseudomonas sp. (1) isolated from rhizosphere of maize grown at irrigated fields, B. cereus = Bacillus cereus isolated from rhizosphere of maize grown at the semi-arid region. Proteus sp. = Proteus sp. isolated from rhizosphere of rice grown at irrigated fields. Ps. sp. (2) = Pseudomonas sp. (2) isolated from rhizosphere of rice grown at raised bed condition. All such means which share a common English letter are similar, otherwise they differ significantly (p = .05).
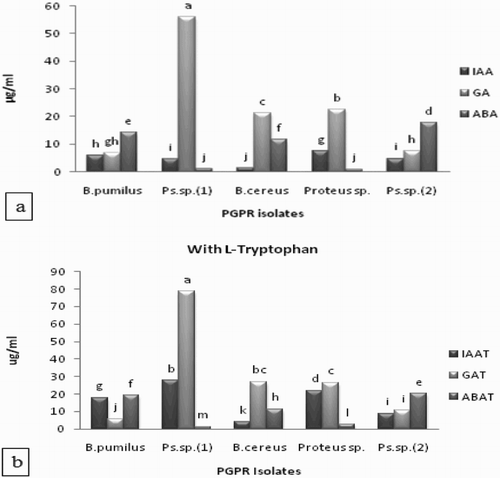
Addition of l-tryptophan in culture media increased IAA, GA and ABA production by all isolates ((b)). Pseudomonas sp. (1) induced l-tryptophan conversion to IAA which were 64% and 14% higher as compared to Bacillus pumilus and Bacillus cereus, respectively.
Bacillus pumilus increased the ABA content (36%) by the addition of l-tryptophan over that of control. The ability of Bacillus pumilus to produce ABA (14.07 µg/mL) in culture media was 14× greater than Pseudomonas sp. (1). The quantity of ABA produced by Bacillus pumilus was 19.05 µg/mL in culture media which was 19× greater than Pseudomonas sp. (1) (1.19 µg/mL) following the addition of l-tryptophan. Pseudomonas sp. (2) showed a 15% increase in ABA production over the control (without l-tryptophan), which was 17× higher than Proteus sp. Addition of l-tryptophan significantly increased GA production in Pseudomonas sp. (1) (79 µg/mL) over the control (56 µg/mL) which was 7% higher than Bacillus pumilus. Higher GA (3×) was produced by Proteus sp. as compared to Pseudomonas sp. (2) in culture media while the addition of l-tryptophan enhanced GA production by 18% over control.
Pot experiment
Effects of l-tryptophan and PGPR on maize under drought stress
Phytohormones (IAA, ABA and GA) of leaves
Effect of drought on maize plant was evident after withholding supply of water for 7 days. Constant decreases in seedling vigor and enhanced leaf senescence were observed. Inoculated plants showed better tolerance to reduced soil moisture (1.3%) than uninoculated plants. All the strains modulated the IAA, GA and ABA contents of the leaves of inoculated maize plants significantly over uninoculated control both under unstressed and drought-stressed conditions ((a–c)). Under unstressed condition, Pseudomonas sp. (1) from rhizosphere of maize grown at irrigated fields was more efficient in increasing IAA (470%) and GA (270%) while the ABA content of maize leaves was decreased (36%) over uninoculated control as well as over that of Bacillus pumilus and Bacillus cereus isolated from the arid and semi-arid region, respectively. Under unstressed conditions, Proteus sp. (from irrigated fields of rice) efficiently enhanced IAA (410%) and GA (300%) while ABA content of maize leaves was decreased by 45% as compared to that of Pseudomonas sp. (2) (from raised bed fields of rice).
Figure 2. Effect of l-tryptophan and PGPR on phytohormones content (a) IAA, (b) GA and (c) ABA of maize leaf under drought-stressed conditions. All such means which share a common English letter are similar, otherwise they differ significantly (p = .05). Detail of treatments as in .
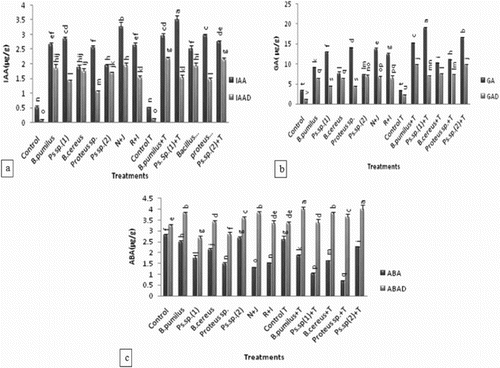
The co-inoculation treatment of Pseudomonas sp. (1) and Bacillus pumilus (N + J) increased the IAA and GA content (22% and 50%, respectively) of the plants grown under well-watered condition while the ABA content was decreased (48%) over single application of Bacillus pumilus but under drought stress, the % increase in IAA, GA and ABA was greater over that of Pseudomonas sp. (1). Similar was the response when Proteus sp. was co-inoculated with Pseudomonas sp. (2) (R + I), the increases in IAA and GA contents were higher (35% and 67%) in the leaves of inoculated plants grown under well-watered condition while the ABA content was decreased (43%) over single application of Pseudomonas sp. (2). But under drought stress, the magnitude of increase in IAA, GA and ABA was higher over that of Proteus sp. The addition of l-tryptophan in the rhizosphere further augmented the effect of the isolates.
Phytohormones (IAA, GA and ABA) of maize rhizospheric soil
All the strains elevated the IAA, GA and ABA contents of the rhizosphere soil of inoculated maize plants significantly over uninoculated control both under unstressed and drought-stressed conditions ((a–c)). Under unstressed condition, Pseudomonas sp. (1) efficiently increased IAA (11×) and GA (5×) while the ABA content of the rhizospheric soil was decreased (3×) over uninoculated control as well as over that of Bacillus pumilus and Bacillus cereus. Tryptophan augmented IAA production greater than 2× in Pseudomonas (1) and Proteus sp. inoculated rhizospheric soil.
Figure 3. Effect of l-tryptophan and PGPR on phytohormones content (a) IAA, (b) GA and (c) ABA of rhizosphere soil of maize under drought-stressed conditions. All such means which share a common English letter are similar, otherwise they differ significantly (p = .05). Detail of treatments as in .
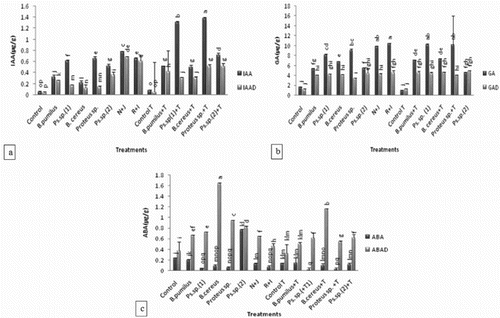
Under unstressed conditions, Proteus sp. efficiently enhanced IAA (12×) and GA (5.6×) while the ABA content of the rhizospheric soil was decreased (2×) over uninoculated control as well as to that of Pseudomonas sp. (2). Co-inoculation (treatment N + J) of Pseudomonas sp. (1) with Bacillus pumilus increased IAA and GA contents in the leaves of inoculated plants by 139% and 84%, respectively, while the ABA content was decreased by 46% over that of single application of Bacillus pumilus but under drought stress, the % increase in IAA, GA and ABA was higher over that of Pseudomonas sp. (1).
Similar was the effect with co-inoculation of Proteus sp. + Pseudomonas sp. (2) (R + I) which increased IAA and GA contents in leaves by 27% and 89%, respectively, while the ABA content of the rhizospheric soil was decreased by 73% over single application of Pseudomonas sp. (2). But under drought stress, % increase in IAA, GA and ABA was higher over that of Proteus sp.
RWC of maize leaves
(a) revealed that all inocula increased RWC of maize leaves significantly over uninoculated control both under unstressed and drought-stressed conditions. Under unstressed conditions, Bacillus pumilus and Pseudomonas sp. (1) showed a maximum enhancement in RWC of maize leaves as compared with Bacillus cereus.
Figure 4. Effect of l-tryptophan and PGPR inoculation on (a) relative water content of maize, (b) shoot length, (c) shoot dry weight, (d) root length, (e) root dry weight, (f) proline content under drought-stressed conditions. All such means which share a common English letter are similar, otherwise they differ significantly (p = .05), Detail of treatments as in .
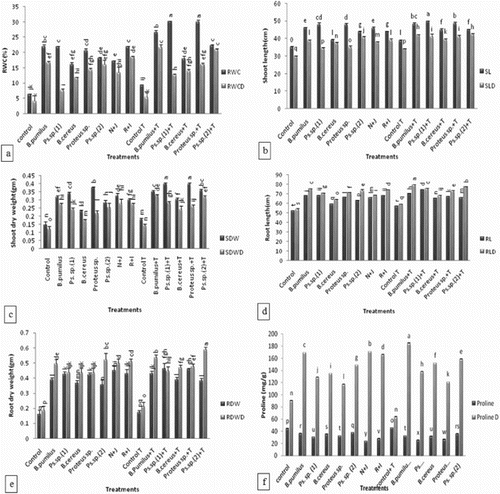
The combined treatment of Pseudomonas sp. (1) and Bacillus pumilus (N + J) showed 20% decrease of RWC over that of Bacillus pumilus and Pseudomonas sp. (1) under irrigated conditions. However, under drought stress, N + J treatment showed a significantly higher RWC over Pseudomonas sp. (1) alone. Under unstressed conditions, Proteus sp. efficiently enhanced RWC of maize leaves as compared to Pseudomonas sp. (1). The co-inoculation treatment R + I with Proteus sp. and Pseudomonas sp. (2) significantly increased RWC over that of Pseudomonas sp. (2) alone under irrigated condition. Under drought stress condition, inoculation with Pseudomonas sp. (2) showed a significant increase in RWC over that of Proteus sp.
Inoculation with Pseudomonas sp. (1) in the presence of l-tryptophan increased the RWC by 28% over that of Pseudomonas sp. (1) without l-tryptophan. Whereas, under unstressed condition, Proteus sp. and Pseudomonas sp. (2) inoculated plants exhibited a significant increase in RWC of leaves in the presence of l-tryptophan. Under induced drought stress, the addition of l-tryptophan significantly enhanced the RWC of leaves in Pseudomonas sp. (2) inoculated plants.
Shoot and root length and dry weight
The strains increased the shoot and root length and dry weight of maize plants significantly over uninoculated control both under unstressed and drought-stressed conditions ((b,c)). Under unstressed condition, Pseudomonas sp. (1) was more efficient in increasing the shoot length (38%) and dry weight (132%) while Proteus sp. efficiently enhanced the root length (38%) and dry weight (156%) over uninoculated control. When Pseudomonas sp. (1) was co-inoculated with Bacillus pumilus (N + J), the increase in root dry weight was higher (40%) over single application of Bacillus pumilus but under drought stress % increase in root dry weight was higher over that of Pseudomonas sp. (1). When Proteus sp. was co-inoculated with Pseudomonas sp. (2) (R + I), the increase in shoot and root dry weight was higher (8% and 20%, respectively) over single application of Pseudomonas sp. (2) but under drought stress, % increase in shoot and root length, and dry weight was higher over that of Proteus sp.
Addition of l-tryptophan in soil enhanced the shoot and root length and dry weight. Under unstressed conditions, shoot length (15%) and dry weight (32%) were increased by Bacillus cereus as compared to the control (without l-tryptophan addition). An increase in root length (40%) and dry weight (12%) of maize plants inoculated with Pseudomonas sp. (1) and Bacillus pumilus along with l-tryptophan addition was higher as compared to control (without l-tryptophan addition) under unstressed conditions. Under drought stress, root length (12%) was increased by the Bacillus pumilus inoculation along with l-tryptophan addition as compared to the control.
Proline content
Results showed that all inocula decreased the proline content of leaves of maize significantly over the control under non-stressed conditions but an increased amount of proline was observed under drought stress ((e)). Under unstressed condition, Pseudomonas sp. (1) was more efficient in reducing the proline content of maize by 31% over the uninoculated control as well as over that of Bacillus pumilus and Bacillus cereus isolated from an arid and a semi-arid region, respectively.
Under unstressed conditions, Proteus sp. efficiently reduced the proline content (27%) over the uninoculated control as well as to that of Pseudomonas sp. (2). The co-inoculation of Pseudomonas sp. (1) with Bacillus pumilus (N + J) decreased the proline content (35%) over single application of Bacillus pumilus but under drought stress % increase in the proline content was higher over Pseudomonas sp. (1). When the isolate from the irrigated field of rice Proteus sp. was co-inoculated with Pseudomonas sp. (2) (R + I), the decrease in the proline content was higher (26%) over the single application of Pseudomonas sp. (2) but under drought stress % increase in the proline content was higher over Proteus sp.
Total protein
Total protein content of maize plants significantly enhanced with bacterial inoculation under non-stressed and induced drought-stressed conditions ((a)). The highest rise in the protein content was shown by Bacillus pumilus and Pseudomonas sp. (1) inoculation under drought stress conditions (85% and 87%), respectively, over the uninoculated control. The co-inoculation of Pseudomonas sp. (1) with Bacillus pumilus (N + J) showed a higher increase in the protein content over uninoculated control (96%) as well as over their single application under drought stress condition.
Figure 5. Effect of l-tryptophan and PGPR inoculation on (a) protein content and SOD activity, (b) POD and CAT activity under drought-stressed conditions.
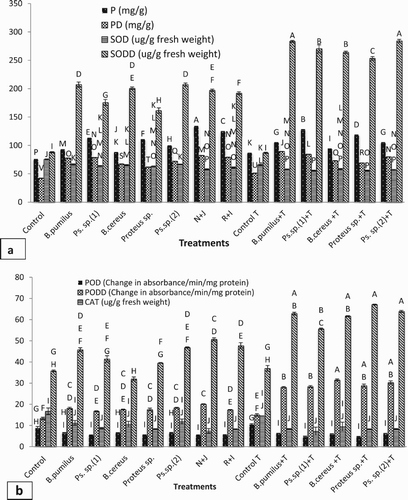
Addition of l-tryptophan in soil and bacterial inoculation increased the protein content under unstressed condition and drought stressed. Under drought stress conditions, l-tryptophan addition along with Bacillus pumilus inoculation showed the highest rise in osmotic potential by 112%. Proteus sp. and Pseudomonas sp. (2) augmented the protein content by 12% as compared to without l-tryptophan control.
Antioxidant enzymes (SOD, POD and CAT) activity
Bacterial inoculation decreased antioxidant enzymes SOD, POD and CAT activity in leaves of maize significantly over the control under non-stressed conditions, but an increased amount of these enzymes was observed under drought stress ((a,b)).
Under drought-stressed conditions, Bacillus pumilus, Proteus sp. and Pseudomonas sp. (2) efficiently increased the SOD content by 135%, 128% and 135%, respectively, over the uninoculated control. The co-inoculation of Pseudomonas sp. (1) with Bacillus pumilus (N + J) and Proteus sp. with Pseudomonas sp. (2) (R + I) increased the SOD content over their single application.
Addition of l-tryptophan in soil decreased antioxidant enzymes activity of leaves of maize significantly over the control under non-stressed conditions, but an increased amount of these enzymes was observed under drought stress. l-tryptophan augments all bacterial inoculants performance by enhancing antioxidant enzymes activity under drought stress. l-tryptophan addition in soil along with Bacillus pumilus and Pseudomonas sp. (2) inoculation showed a higher SOD activity; however, all bacterial inoculants showed a similar rise in POD and CAT activity as compared to uninoculated control under drought unstressed conditions.
Osmotic potential
Results showed that all inocula enhanced the osmotic potential of leaves of maize significantly under non-stressed and induced drought-stressed conditions over the control ((a)).
Figure 6. Effect of l-tryptophan and PGPR inoculation on (a) osmotic potential, Chl a and Chl b, (b) carotenoids activity under drought-stressed conditions.
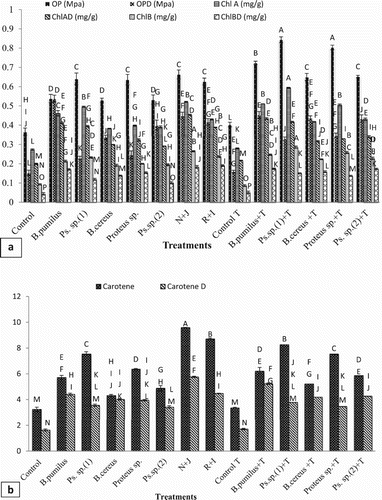
Under drought-stressed conditions, among all the inoculated strains the maximum increase in osmotic potential was shown by Bacillus pumilus over the uninoculated control. The co-inoculation of Pseudomonas sp. (1) with Bacillus pumilus (N + J) and Proteus sp. with Pseudomonas sp. (2) (R + I) showed a higher increase in osmotic potential over their single application under drought stress condition.
l-tryptophan augments all bacterial inoculants performance by enhancing the osmotic potential under drought stress and unstressed control. l-tryptophan addition in soil along with Bacillus pumilus, Bacillus cereus and Pseudomonas sp. (2) inoculation showed a higher osmotic potential as compared to uninoculated control under drought unstressed conditions.
Photosynthetic pigments
Photosynthetic pigments (chl a, b and carotenoids) were significantly enhanced with bacterial inoculation under non-stressed and induced drought-stressed conditions ((a,b)).
A maximum decline in photosynthetic pigments was observed under drought stress condition even after l-tryptophan addition in soil. All the inoculated treatments showed a similar enhancement in chl b ((a)) and carotenoid ((b)) contents under drought stress. The highest rise in chl a was shown by Bacillus pumilus and Pseudomonas sp. (1) under drought stress conditions (106% and 96%), respectively, over the uninoculated control.
The co-inoculation of Pseudomonas sp. (1) with Bacillus pumilus (N + J) showed a higher increase in chlorophyll a and carotenoid contents over their single application under drought stress condition. However, the co-inoculation of Pseudomonas sp. (2) with proteus sp. (R + I) showed a higher increase in the chlorophyll b content.
Addition of l-tryptophan in soil and bacterial inoculation increased the photosynthetic pigments and Pseudomonas sp. (1) was more effective under normal condition. Under drought stress, l-tryptophan with Bacillus pumilus and with Pseudomonas sp. (1) augmented chl a by 106% and 97%, respectively, as compared to uninoculated control. However, in same conditions, Bacillus pumilus showed the highest increase in carotenoid contents.
Discussion
It is evident from the result that l-tryptophan addition increased plant IAA, GA and ABA not only under normal condition but also under drought stress. These studies are partly in line with the findings that tryptophan may involve in the production of IAA (Raheem et al. Citation2017).The PGPR isolates B. pumilus and Pseudomonas sp. (2) both from moisture-deficit area produce less IAA and GA but more ABA in culture. The ability of Bacillus pumilus to produce ABA in culture media was 5× more than Pseudomonas sp. (1) with l-tryptophan addition. The presence of l-tryptophan in culture medium may stimulate the expression of genes involved in ABA biosynthetic pathway. Increased ABA production in response to l-tryptophan may be due to a change in phytohormones ratio (Daszkowska Citation2011). The magnitude of ABA production by the PGPR isolates due to l-tryptophan increased successively with a decrease in soil moisture content of the rhizosphere soil from where isolation of PGPR was made (arid > semi-arid > irrigated). Similarly, Pseudomonas sp. (1) and Proteus sp. had more IAA/ABA and GA/ABA than that of Bacillus cereus and Bacillus pumilus and Pseudomonas sp. (2) in culture with and without the addition of l-tryptophan.
The l-tryptophan addition further increased IAA and ABA in B. pumilus such that it maintained IAA/ABA ratio 1, but in Pseudomonas sp. the percentage increase in IAA was lower than that of B. pumilus and ratio of IAA/ABA was less than 1, i.e. 0.5 this was in contrast to the isolates from irrigated areas which always have more GA and IAA and less ABA, besides l-tryptophan addition in these isolates emphasized more on IAA and GA. These traits of PGPR isolates demonstrated that the PGPR has to maintain higher ABA or ABA equal to IAA to combat drought stress similar to higher plants. Secondly, the adaptive mechanism to combat moisture stress differs with different PGPR species.
When used as a bioinoculant these PGPR also modulated the endogenous level of phytohormone and the ratio of promoter to inhibitor varied in the plant in correspondence with the moisture status of the growing condition of the plant. Plants inoculated with B. pumilus had IAA slightly lower than those of Pseudomonas (1) or Proteus sp. from irrigated area; and on induction of drought stress, these inoculated plants also exhibited IAA along with ABA higher than those of the plants inoculated with isolates from irrigated area. Russo et al. (Citation2010) reported that ABA is involved in the regulation of stress signaling during plant growth; therefore, its biosynthesis can be affected by the presence of rhizobacteria in abiotic stress. These results are also conferred by Russo et al. (Citation2010) who found that Arabidopsis plants inoculated with A. brasilense Sp245 showed more ABA content than uninoculated plants under drought stress condition. Sharp et al. (Citation2002) demonstrated that ABA contents in plants were increased under water stress and declined as the plants experienced lesser stress. The same strategy was implicated by the PGPR, e.g. B. pumilus during the present investigation. These isolates from moisture-deficit area when used as a bioinoculant maintained higher IAA, GA and ABA contents than that of isolates from irrigated areas and the addition of l-tryptophan further assisted them to maintain this ratio of phytohormones under drought stress.
The plants inoculated with isolates from moisture-deficit condition showed the least decrease in RWC on induction of drought stress and tryptophan ameliorated the effects of drought stress by stimulating RWC in plants inoculated with B. pumilus or Pseudomonas (2) similar to that of isolates from irrigated area. This strategy of the conservation of water budget by tryptophan in association with PGPR from the stressed area was reflected in higher root and shoot dry weight of the inoculated plants. It was evident from the result that the phytohormones of maize leaves were modulated significantly by the PGPR which was used as a bioinoculant and the pattern of changes incurred due to their inoculation was a function of the IAA, GA and ABA produced by the isolates in culture.
In our study, inoculation of PGPR strains native to the stressed area, under drought stress conditions, increased the photosynthetic pigments (Chl a, b and carotenoids), accumulation of proline and antioxidant enzymes (SOD, POD and catalase). These findings are in accordance with those of Armada et al. (Citation2014) and Gusain et al. (Citation2015), who reported an increased accumulation of compatible solutes like proline, which is an important adaptation mechanism for metabolic adjustment of plants grown under drought stress. Moreover, PGPR inoculation have been reported to increase plant biomass and photosynthetic pigments (Chl a, b and carotenoids) under drought stress as compared to uninoculated control (Vurukonda et al. Citation2016).
Noteworthy, on co-inoculation, the two PGPR isolates belonging to two different moisture regime behaved differently under two different conditions of growth, e.g. the consortium of Bacillus pumilus (from the arid region of maize rhizosphere) with Pseudomonas (1) from rhizosphere of maize grown in irrigated region showed a predominating effect of Bacillus pumilus under drought stress whereas under irrigated condition the Pseudomonas (1) predominated. This is a special type of synergism and indicated a precise signal exchange between the host and the symbiont.
Hence, it was observed that inoculation with PGPR strains alleviated the plant drought stress, increasing plant growth and yield, and different strains performed differently (Sarma et al. Citation2012; Vurukonda et al. Citation2016). It was also evident from the result that IAA production was stimulated by 5–6× in plants as compared to rhizosphere soil when inoculated with Bacillus pumilus and Bacillus cereus, in the presence of l-tryptophan under induced drought stress. Similarly, Pseudomonas sp. (2) inoculation increased the IAA content of leaves by 4× greater than soil in the presence of l-tryptophan under induced drought stress. The isolates from water scarce environment may use IAA along with ABA as a direct mechanism to reduce the negative effects produced by drought in plants. Induction of LEA (late-embryogenesis abundant) genes by TLD1/OsGH3.13 encoding IAA amidosynthetase demonstrated a direct role of IAA in drought tolerance (Battacharyya Citation2012).
Growth potential appears to be correlated with the IAA, GA and ABA production in leaves and soil. The correlation between IAA hormone in soil with and without l-tryptophan application in rhizosphere soil was positive and highly significant with that of microbes and plants (r= 1.00). The correlation between IAA in microbes in the presence of l-tryptophan in culture media was also positively and highly correlated with that IAA in soil and plants with and without l-tryptophan application(r = 0.97) ().
Table 1. Correlation coefficient of IAA production by microbes, plants and soil with and without l-tryptophan application.
Conclusion
It can be concluded from the present study that IAA could play a direct role to combat drought stress in plants in addition to ABA which is a known stress hormone. Application of l-tryptophan in moisture-deficient soil along with PGPR inoculum could be an effective method to combat drought stress in arid and semi-arid areas. Tryptophan addition increased IAA and GA production in isolates from irrigated areas and further augmented ABA production in isolates from moisture-deficit areas, indicating that tryptophan addition may be beneficial for inducing drought tolerance ability but cannot assist much to the isolates from irrigated area. It is the intrinsic ability of the isolates which is important to combat drought stress. Stress-specific microbe–microbe interactions modulate the effect of microbial associations on plant physiology (Peleg et al. Citation2011).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahemad M, Kibret M. 2014. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci. 26:1–20. doi: 10.1016/j.jksus.2013.05.001
- Ahmed ZI, Ansar M, Tariq M, Anjum MS. 2008. Effect of different Rhizobium inoculation methods on performance of lentil in Pothowar region. Int J Agric Biol. 10:85–88.
- Armada E, Roldan A, Azcon R. 2014. Differential activity of autochthonous bacteria in controlling drought stress in native Lavandula and Salvia plants species under drought conditions in natural arid soil. Microb Ecol. 67:410–420. doi: 10.1007/s00248-013-0326-9
- Arshad M, Shaharoona B, Mahmood T. 2008. Inoculation with plant growth promoting rhizobacteria containing ACC-deaminase partially eliminates the effects of water stress on growth, yield and ripening of Pisum sativum L. Pedosphere. 18:611–620. doi: 10.1016/S1002-0160(08)60055-7
- Bates LS, Waldren RP, Teare ID. 1973. Rapid determination of free proline for water-stress studies. Plant Soil. 39:205–207. doi:10.1007/BF00018060.
- Beauchamp C, Fridovich I. 1971. Superoxide dismutase improved assays and an assay applicable to acrylamide gel. Anal Biochem. 44:276–287. doi: 10.1016/0003-2697(71)90370-8
- Bhattacharyya PN, Jha DK. 2012. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 28:1327–1350. doi:10.1007/s11274-011-0979-9.
- Capell B, Doerffling K. 1993. Genotypic specific differences in chilling tolerance of maize to chilling induced changes in water status and abscisic acid accumulation. Physiol Plant. 88:683–694. doi: 10.1111/j.1399-3054.1993.tb01383.x
- Daszkowska-Golec A. 2011. Arabidopsis seed germination under abiotic stress as a concert of action of phytohormones. Omics-A J Integr Biol. 15(11):763–774. doi:10.1089/omi.2011.0082.
- De_Souza R, Ambrosini A, Passaglia LMP. Growth-promoting bacteria as inoculants in agricultural soils. Gene Mol Biol. 38(4):S1415–475738420150053.
- Figueiredo MVB, Burity HA, Martinez CR, Chanway CP. 2008. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl Soil Ecol. 40:182–188. doi: 10.1016/j.apsoil.2008.04.005
- Gomez KA, Gomez AA. 1984. Statistical procedures for agricultural research, 2nd ed. London: John Wiley and sons, Inc. p. 13–175.
- Gorin N, Heidema FT. 1976. Peroxidase activity in Golden Delicious apples as a possible parameter of ripening and senescence. J Agric Food Chem. 24:200–01. doi: 10.1021/jf60203a043
- Gusain YS, Singh US, Sharma AK. 2015. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryza sativa L.) Afr. J. Biotechnol. 14(2015):764–773.
- Hall T. 2001. Bioedit version 5.0.6. Carlsbad (CA): Ibis Therapeutics.
- Hartung W, Sauter A, Hose E. 2002. Abscisic acid in the xylem: where does it come from, where does it go to? J Exp Bot. 53(366):27–32. doi: 10.1093/jexbot/53.366.27
- Hiscox JD., Israelstam GF. 1979. A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot. 57:1332–1334. doi: 10.1139/b79-163
- Iqbal A, Hasnain S. 2013. Auxin producing Pseudomonas strains: biological candidates to modulate the growth of Triticum aestivum beneficially. Am J Plant Sci. 4:1693–1700. doi: 10.4236/ajps.2013.49206
- Kettner J, Doerffling K. 1995. Biosynthesis and metabolism of abscisic acid in tomato leaves infected with Botrytis cinerea. Planta. 196:627–634. doi: 10.1007/BF01106753
- Khalid A, Arshad M, Zahir ZA. 2006. Phytohormones: microbial production and applications. In: Uphoff N., editor. Biological approaches to sustainable soil systems. Boco Raton (FL): A.S.CRC Press; p. 207–220.
- Kumar M, Kumari P, Gupta V, Anisha PA., Reddy CR., Jha B. 2010. Differential responses to cadmium induced oxidative stress in marine macroalga Ulva lactuca (Ulvales, Chlorophyta). BioMetals. 23:315–325. doi: 10.1007/s10534-010-9290-8
- Lee S, Ka JO, Song HG. 2012. Growth promotion of Xanthium italicum by application of rhizobacterial isolates of Bacillus aryabhattai in microcosm soil. J Microbiol. 50(1):45–49. doi: 10.1007/s12275-012-1415-z
- Li JC, Shi J, Zhao XL, Wang G, Yu FH, Ren YJ, Fenxi H. 1994. Separation and determination of three kinds of plant hormones by high performance liquid chromatography. Fenxi-Hauxan. 22:801–804.
- Lim CW, Baek W, Jung J, Jung-Hyun K, Lee SC. 2015. Function of ABA in stomatal defense against biotic and drought stresses. Int J Mol Sci. 16:15251–15270. doi:10.3390/ijms160715251.
- Lowry OH., Rosebrough NJ., Farr AL., Randall RJ. 1951. Protein measurement with the folin phenol reagent. J Biol Chem. 193:265–275.
- Moe LA. 2013. Amino acids in the rhizosphere: from plants to microbes. Am J Bot. 100(9):1692–1705. doi: 10.3732/ajb.1300033
- Peleg Z, Blumwald E. 2011. Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol. 14(3):290–295. doi: 10.1016/j.pbi.2011.02.001
- Quiroz V, Hernández SNZ, Luna-Romero I, Amora-Lazcano E, Rodríguez-Dorantes A. 2012. Assessment of plant growth promotion by rhizobacteria supplied with tryptophan as phytohormone production elicitor on Axonopu saffinis. Agri Sci Res J. 2(11):574–580.
- Raheem A, Shaposhnikov A, Andrey A, Belimov IC, Dodd AB. 2017. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch Agron Soil Sci. doi:10.1080/03650340.2017.1362105.
- Riaz A, Younis A, Taj AR, Karim A, Tariq U, Munir S, Riaz S. 2013. Effect of drought stress on growth and flowering of marigold (Tagetes Erecta L.). Pak J Bot. 45(S1):123–131.
- Russo A, Carrozza GP, Vettori L, Felici C, Cinelli F, Toffanin A. 2010. Plant beneficial microbes and their application in plant biotechnology. In: Agbo E.D., editor. Innovations in biotechnology. Rijeka: InTech; p. 57–72. 978-953-51-0096-6.
- Sandhya VSKZ, Grover M, Reddy G, Venkatswarlu B. 2010. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of Zea mays under drought stress. Plant Growth Regul. 62:21–30. doi: 10.1007/s10725-010-9479-4
- Sarma BK, Yadav SK, Singh DP, Singh HB. 2012. Rhizobacteria mediated induced systemic tolerance in plants: prospects for abiotic stress management. Bacteria in agrobiology. In: Maheshwaried D.K., editor. Stress management. Berlin: Springer; p. 225–238.
- Sarwar M, Arshad M, Martens DA, Frankenberger WTJr. 1992. Tryptophan dependent biosynthesis of auxins in soil. Plant Soil. 147:207–215. doi: 10.1007/BF00029072
- Sharma E, Sharma R, Borah P, Jain M, Khorana J. 2015. Emerging roles of auxin in abiotic stress responses. In: G. K. Pandey, editor. Elucidation of abiotic stress signaling in plants SE – 11. New York (NY): Springer; p. 299–328. doi:10.1007/978-1-4939-2211-6_11.
- Sharp REW, Voetberg GS, Saab IN, Le Noble ME. 2002. Confirmation that abscisic acid accumulation is required for Zea mays primary root elongation at low water potentials. J Exp Bot. 45:1743–1751. doi: 10.1093/jxb/45.Special_Issue.1743
- Swain P, Raman A, Singh SP, Kumar A. 2017. Breeding drought tolerant rice for shallow rainfed ecosystem of eastern India. Field Crop Res. 209:168–178. doi: 10.1016/j.fcr.2017.05.007
- Tamoor UH, Bano A. 2014. Role of plant growth promoting rhizobacteria and L-tryptophan on improvement of growth, nutrient availability and yield of wheat (Triticum aestivum) under salt stress. IJAAR. 4(2):30–39.
- Tien TM, Gaskins MH, Hubbell DH. 1979. Plant growth substances produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.). Appl Environ Microbiol. 37:1016–1024.
- Tom H. 2007. Bioedit: an important software for molecular biology. GERF Bull Biosci. 2(1):60–61.
- Torres RO, Henry A. Forthcoming 2016. Yield stability of selected rice breeding lines and donors across conditions of mild to moderately severe drought stress. Field Crop Res. https://doi.org/10.1016/j.fcr.2016.09.011.
- Vetter JL, Steinberg M, Nelson AI. 1958. Quantitative determination of peroxidase in sweet corn. J Agric Food Chem. 6:39–41. doi: 10.1021/jf60083a006
- Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A. 2016. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res. 184(Supplement C):13–24. doi:10.1016/j.micres.2015.12.003.
- Weatherley PE. 1950. Studies in water relations of cotton plants. The field measurement of water deficit in leaves. New Phytol. 49:81–87. doi: 10.1111/j.1469-8137.1950.tb05146.x
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S-ribosomal DNA amplification for phylogenetic study. J Bacteriol. 173:697–703. doi: 10.1128/jb.173.2.697-703.1991
