ABSTRACT
Ulvan, carrageenan, alginate and laminarin were tested in olive trees’ twigs to elicit phenolic metabolism and control verticillium wilt of olive (VWO) caused by Verticillium dahliae. The elicitation effect was determined through phenylalanine ammonia-lyase activity, total polyphenol content and lignin content. VWO was assessed in twigs previously elicited (24 h) and maintained in a solution containing bio-elicitors (2 g/L) and conidial suspension (106 conidia/mL). Our results showed stimulation of the phenolic metabolism and the decline of wilt symptoms. Ulvan reduced significantly the area under the disease progress curve for severity to 39.9% and the final incidence to 28.9%. Ulvan and alginate produced significant inhibitory rates on mycelial growth of the fungus in vitro. Seaweed polysaccharides might help to overcome VWO by strengthening the host defense metabolism and restricting the pathogen’s growth.
Introduction
Olive trees (Olea europaea L.) represent a significant socio-economic interest for Morocco. Olive cultivation covers an area of 784 000 ha in the country. The olive groves consist overwhelmingly of a single variety: 96% of the trees are of the Picholine Marocaine variety. However, Picholine Marocaine has proven to be susceptible to fungal diseases, in particular verticillium wilt of olive (VWO), caused by Verticillium dahliae Kleb (V. dahliae). This is considered one of the most serious diseases that attack olive trees (Jiménez-Díaz et al. Citation2012) and the most challenging to control because of the fast fungal dissemination, the persistence of its microsclerotia in the soil for years and its wide host range (López-Escudero and Mercado-Blanco Citation2011). It has been reported that in 60% of inspected olive orchards, V. dahliae affected 10% to 30% of trees in Morocco (Serrhini and Zeroual Citation1995). In the last decades, stimulation of natural plant defenses has been considered as an innovative approach to control pests. The induction of plant defense mechanisms using polysaccharide or oligosaccharide represents a promising protection strategy (Benhamou and Rey Citation2012). Seaweeds are considered a natural resource of bioelicitors because of their high polysaccharide content. Their involvement in early signaling processes through the activation of secondary metabolic pathways and the mobilization of signal molecules has been suggested (Paulert et al. Citation2009; Sharma et al. Citation2014). The use of seaweed polysaccharides to protect plants against pathogens has been widely reported. For instance, laminarin (Jayaraj et al. Citation2008; Cosse et al. Citation2009), carrageenan (Mercier et al. Citation2001; Sangha et al. Citation2015), alginate (Chandía et al. Citation2004; An et al. Citation2009) and ulvan (de Freitas and Stadnik Citation2012; El Modafar et al. Citation2012; Abouraicha et al. Citation2015) have shown effective stimulating properties of plant defense mechanisms. Although the elicitor effect of these substances has also been confirmed in non-woody plants such as arabidopsis, tobacco, tomato, wheat, rice, etc (Chandía et al. Citation2004; El Modafar et al. Citation2012; Vera et al. Citation2012; Abouraicha et al. Citation2015, Citation2017; Zhang et al. Citation2015), their effectiveness has never been tested on the olive tree/verticillium pathosystem. Because of seaweeds’ high eliciting potential and great abundance in the Moroccan coastal areas, our study aimed to test alginate and laminarin (brown algae), carrageenan (red algae) and ulvan (green algae) against VWO. We investigated the phenolic metabolism and examined their potential in controlling wilting symptoms in Picholine Marocaine twigs, using a simple, fast and non-destructive method.
Materials and methods
Effect of seaweed polysaccharides on induction of the phenolic metabolism
Plant material
The study was conducted on twigs of 10 cm in length with 16 leaves, collected from the same Picholine Marocaine mother tree.
Extraction and purification of ulvan polysaccharide
Polysaccharide extraction from pre-treated powder of green algae (Ulva lactuca) was carried out according to the protocol described by Ray and Lahaye (Citation1995). The algae cell-wall residue (100 g) was suspended in 2 L of 50 mM sodium oxalate solution at pH 6.0 and incubated for 3 h at 100°C under stirring. The resulting solution was supplemented with two volumes of sodium oxalate solution and centrifuged at 10 000 g for 15 min. The supernatant was ultrafiltered and added to three volumes of isopropanol. The precipitate was then collected and dissolved in 50 mL osmosed water prior to being freeze-dried. The yield was 20% of crude polysaccharides/algae dry weight.
Ulvan purification was carried out according to the method of El Boutachfaiti et al. (Citation2009). Crude extract of Ulva lactuca polysaccharides was dissolved in distilled water (10 g/L). The crude extract was adjusted to pH 2.0 using 4 N HCl and centrifuged for 15 min at 10,000 g. Ulvan purification was carried out on the supernatant. The preparation was adjusted at pH 7.0 using 4 N NaOH and then dialyzed. Isopropanol (3 V) was added to the preparation and the precipitate was collected after centrifugation at 10,000 g for 15 min, then dissolved in 50 mL osmosed water prior to being freeze-dried. The yield was 17.5% of ulvan/algae dry weight.
Elicitation test
Four polysaccharide extracts were used in this study. Three commercial extracts of alginate, carrageenan and laminarin were purchased from Sigma–Aldrich Chimie S.a.r.l. and St-Quentin Fallavier (France). The fourth, ulvan, was extracted from the green algae Ulva lactuca, as described earlier. The four polysaccharide extracts were used at a randomly chosen concentration of 2 g/L (w/v) as a preliminary test. All extracts were prepared with the nutrient solution of Hoagland. Twigs were soaked in the polysaccharide solution and placed in a growth chamber for a photoperiod of 16/8 h (day/night) and cool-white light (240 µmol m−2 s−1) at a temperature of 25 ± 2°C. Twigs soaked in extract-free Hoagland solution were used as control.
The effect of the elicitors on the induction of the phenolic metabolism was evaluated in a 1 cm fragment of the stem above the elicitation site (stem base), which is the area in direct contact with the elicitor solution, where most defense reactions may occur. Samples were collected every 24 h for five days. Each point of the obtained curves represents the average of six repetitions, for both the elicited and control plants.
Extraction and determination of phenylalanine ammonia-lyase activity
Determination of phenylalanine ammonia-lyase (PAL) activity was carried out according to the modified protocol of Liu et al. (Citation2005). It consists in measuring spectrophotometrically, at 290 nm, the amount of cinnamic acid produced as a result of the reaction of PAL with its substrate, phenylalanine. The twig segment (100 mg) was crushed in the presence of liquid nitrogen, in 2 mL of 100 mM sodium borate buffer, pH 8.8, containing 5% (w/v) insoluble PVP and 1 mM ethylene diamine tetra-acetic acid and then centrifuged for 30 min at 10,000 g. The supernatant was collected and used as the enzymatic extract. The reaction medium, containing 600 µL of the enzyme extract and 250 µL of 20 mM L-phenylalanine, was incubated in the presence of 2 mL of 100 mM sodium borate buffer, pH 8.8, at 30°C for 1 h. The reaction was stopped by the addition of 100 µL of HCl (6 N) and the assay was performed at 290 nm. In the blank tube the phenylalanine solution was replaced with sodium borate buffer. The results were expressed in percentage relative to the control’s values.
Extraction and determination of total polyphenol content
Samples of 100 mg were crushed in the presence of liquid nitrogen, homogenized in 2 mL of 80% (v/v) methanol and placed in an ultrasound bath for 1 h. After centrifugation at 15,000 g for 10 min, the supernatant (phenol extract) was tested in the absence and in the presence of 1% (w/v) of polyvinylpolypyrrolidine (PVPP), which specifically adsorbs polyphenols (Bridi et al. Citation2014). The total polyphenol content was determined using the colorimetric method of Folin–Ciocalteu (Singleton and Rossi Citation1965). 100 µL of Folin reagent was added to 75 µL of phenolic extracts. After stirring, the mixture was added to 200 µL of sodium carbonate (NaCO3), then incubated in darkness for 30 min at 37°C. The absorbance values were read at 760 nm and were corrected by the difference between the optical density values obtained with and without PVPP. Phenol contents were calculated by reference to a standard range established with gallic acid and reported in milligrams of gallic acid equivalent per gram of dry matter (EqGA mg/g DM). Results were expressed in percentage relative to the control’s values.
Extraction and determination of lignin content
Lignin content was determined by the method of El Modafar and El Boustani (Citation2001), with some modifications. This technique is based on the solubilization of the lignin in an alkaline medium, following its reaction with thioglycolic acid. Crushed samples of 100 mg were homogenized in 2 mL of ethanol and then centrifuged at 7000 g for 20 min. The pellet, dried overnight at 30°C and weighed (25 mg), was added to 0.5 mL of thioglycolic acid and 2.5 mL of HCl (2 M). The sample was heated at 100°C for 8 h, then cooled and centrifuged at 7000 g for 20 min. The pellet was washed three times with distilled water, then suspended in 5 mL of NaOH (1 M) and centrifuged at 7000 g for 20 min, after being stirred for 18 h. The supernatant was added to 1 mL of HCl to precipitate the complex thioglycolic acid-lignin during 4 h at 4°C. After centrifugation, for 20 min at 7000 g, the pellet was dissolved in 1 mL of NaOH (1 M). The absorbance was measured at 280 nm and the results were expressed in percentage relative to the control’s values.
Determination of total protein
Proteins were measured at 595 nm using the colorimetric method of Bradford (Citation1976). 100 µL of enzyme extract was added to 2 mL of Bradford reagent, stirred and then incubated for 5 min. The protein content of samples was determined by reference to a standard range of bovine serum albumin (BSA).
Effect of algal polysaccharides on controlling verticillium wilt of olive
Pathogen and plant materials
V. dahliae isolates were preliminarily isolated from symptomatic wood of olive trees (Olea europaea). The pathogenicity was tested by a bioassay on olive. Isolate with the defoliating pathotype was selected for the rest of experiments. Disks of 1 cm in diameter from one-week-old cultures of V. dahliae developed on potato dextrose agar (PDA) were grown in 250 mL of Czapek medium previously autoclaved for 20 min at 121°C. After two weeks of stirring on a shaker running at 120 rpm in the dark, the concentration of the conidial suspension was adjusted to 106 conidia/mL.
The study was conducted on twigs of 10 cm in length with 16 leaves, in a completely randomized block of 10 replicates of Picholine Marocaine twigs coming from the same tree.
Elicitation and inoculation test
Olive twigs previously elicited during 24 h in 2 g/L of the polysaccharide solutions (alginate, carrageenan, laminarin and ulvan) were soaked in a solution containing both the V. dahliae conidial suspension and the seaweed polysaccharide solution at a concentration of 106 conidia/mL and 2 g/L respectively. The twigs were incubated in a culture chamber at a 25 ± 2°C, under cool-white light (240 µmol m−2 s−1) and a photoperiod of 16/8 h (day/night). Twigs dipped only in the conidial suspension were taken as control. For each saccharide/pathogen combination, two blocks with five samples were used. The approach of eliciting before inoculation was based on the effectiveness of ulvan shown by the results of Abouraicha et al. (Citation2015) when used in apple against Penicillium expansum and Botrytis cinerea. Daily observations were done for a month starting from the fifth day after inoculation.
Symptom assessment
The pathogenesis of V. dahliae was estimated by calculating per each twig: the percentage of the area under the disease progress curve (AUDPC) for severity, AUDPC for incidence, final incidence and final severity and evaluating the vascular browning and the mortality. A score ranging from 0 to 4 was assigned to each twig, following a scale as described in . The disease severity was determined according to the modified formula of El Said et al. (Citation2012): Disease severity = [n*(A*0) + n*(B*1) + n*(C*2) + n*(D*3) + n*(E*4)]/Total number of leaves, where n: number of leaves in a category; A, B, C, D, E: categories of symptoms. The final severity, one month after inoculation, was calculated. The final incidence was the mean of defoliation incidence, dieback incidence and twisting incidence, determined by the number of wilted leaves and divided by the total number of leaves*100.
Table 1. Scale of wilt symptoms in olive tree twig.
The AUDPC combines all the daily observations and shows the effectiveness of the algal polysaccharides. The AUDPC was calculated using the formula of Campbell and Madden (Citation1990), AUDPC = [(t/2)*(y2 + 2*y3+ … .+2yi−1 + yi)/4n]*100, where t = time between two observations, yi: final mean severity or final incidence, yi−1: disease severity or incidence of the observation i − 1, 4: maximum disease score and n: number of observations.
The vascular browning and mortality were checked at the end of the experiment. The mortality of the twigs was determined as the number of dead twigs and divided by the total number of twigs*100. The vascular browning was measured in the longitudinal sections of the treated and non-treated samples.
Pathogen re-isolation
In order to confirm that the observed symptoms were due to V. dahliae, we attempt to re-isolated it from twigs soaked for a month in a solution containing the conidial suspension (106 conidia/mL) and the polysaccharide solution (2 g/L). Fragments of the twigs were washed with tap water, cleaned for 2 min with commercial bleach (sodium hypochlorite), cut into small pieces and placed under aseptic conditions on Petri dishes containing PDA medium. Fungi re-isolation was similarly conducted from control twigs. After one week of incubation at 25 ± 2°C in darkness, the V. dahliae colonies were microscopically checked.
Effect of algal polysaccharides on mycelial growth of V. dahliae in vitro
Mycelial growth of V. dahliae was evaluated by using the method of Huang (Citation1993). Potato dextrose agar was supplemented with algal polysaccharides at a final concentration of 2 g/L. 1-cm disk from one-week-old cultures of V. dahliae was aseptically transferred to the center of each Petri plate. Plates were incubated at 25° ± 2°C in the dark. The non-treated plates served as the control. The colony diameter was determined one and two weeks after incubation. Six replicates per extract were used. The effect of the algal polysaccharides on the mycelial growth of V. dahliae was calculated according to the following formula: Inhibitory rate on mycelial growth (%) = [colony diameter of the control−colony diameter of treatment/(colony diameter of the control − 1 cm)] × 100.
Statistical analysis
Data were expressed as mean and standard deviation. PAL, polyphenol and lignin contents results were tested by one-way analysis of variance (ANOVA) using Tukey test at P < 0.05 for multiple comparisons. The inhibition rate of mycelial growth of V.dahliae, AUDPC and vascular browning were tested according to Dunnett t-tests at P < 0.05. All statistical analyses were performed using SPSS software Version 10.0.
Results
Effect of seaweed polysaccharides on induction of the phenolic metabolism
Phenylalanine ammonia-lyase response to elicitor treatment
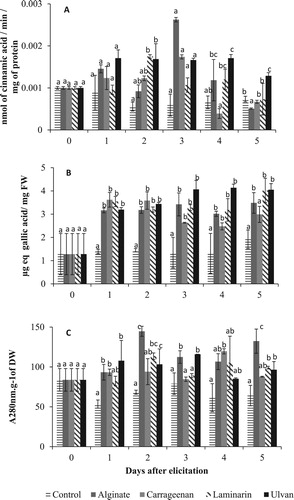
Treatment of Picholine Marocaine twigs by seaweed polysaccharides increased the enzymatic activity of PAL in the stem. The application of the four seaweed polysaccharides caused differential response of the PAL activity (a). PAL activity increased significantly on the second day in twigs treated with ulvan and laminarin. Ulvan was the only treatment stimulating significant activity on the fourth day. The PAL activity in twigs declined thereafter to reach, on the fifth day, values similar to those of the control, except for ulvan and alginate. However, with ulvan treatment, PAL activity was almost doubled (at P < 0.05). The precocity and the intensity of the increase in PAL were variable, according to the elicitor type.
Figure 1. Kinetics of induction of phenylalanine ammonialyase activity (A), total polyphenol content (B) and lignin content (C) in the stem of the Picholine Marocaine’s twigs in response to treatment by alginate, carrageenan, laminarin and ulvan at 2 g/L. Each value is the mean of six repetitions ± standard deviation. At the same day and for each elicitor treatment, the values followed by a common letter do not differ significantly at P < 0.05 according to Tukey test.
Total polyphenol accumulation in response to elicitor treatment
Elicitation of olive twigs caused a significant (P < 0.05) rapid and intense increase in total polyphenol content, of about 2.5 times higher than in the control from the first day (b). The polyphenol contents decreased subsequently from the second day for alginate, carrageenan and laminarin treatments. However, the decline was observed only on the fourth day for ulvan. Despite that, the values were significantly (P < 0.05) higher than in the control. The highest accumulation was noticed in twigs treated with ulvan. It was three times the control value on the fourth day. Ulvan, alginate and laminarin had a significant effect in the fifth day. Our results showed that the polyphenols accumulation followed almost the same kinetic regardless of the type of elicitor.
Lignin content accumulation in response to elicitor treatment
After the seaweed polysaccharide treatments, lignin content increased significantly from the first day in twigs treated by alginate, carrageenan or ulvan (c). The maximum values were obtained on the first day in response to ulvan treatment and later with alginate treatment after the second day, carrageenan and ulvan on the third day. On the fourth day the lignification process was significantly stimulated in twigs treated with carrageenan. On the fifth day the lignin content decreased in the twigs elicited with carrageenan, unlike laminarin, alginate and ulvan, where values were significantly higher than in the control.
Effect of algal polysaccharides on controlling verticillium wilt of olive
Disease assessment
Symptoms such as early decline and curved leaves began to appear a week after inoculation in control twigs. In the second week, the color of the leaves turned to faded green, twisting intensity increased and leaves started to fall at the slightest touch. In the third week, the leaf color became grayish, twisting and defoliation increased and the stems started to dry out. After four weeks, the drying and browning were generalized in all stems and almost 99% of the leaves had fallen.
Among the four tested bio-elicitors, ulvan proved to be the most effective treatment and reduced significantly the development of wilt symptoms. The final severity, final incidence and AUDPC values were significantly low in comparison to the control and to twigs treated with other seaweeds; it was followed by carrageenan, alginate and laminarin ().
Table 2. Effect of algal polysaccharides on controlling verticillium wilt of olive in Picholine Marocaine twigs.
Longitudinal sections taken from control stems after a month of inoculation with the conidial suspension of V. dahliae showed advanced vascular browning. However, twigs inoculated and elicited with the algal polysaccharides showed variable levels of browning, depending on the polysaccharide type. The vascular browning in treated twigs was significantly lower than the controls. Vascular browning reached 2.17, 2.25 and 2.97 cm in twigs stimulated by alginate, laminarin and ulvan respectively ().
The percentage of mortality decreased as well. Ulvan effected a remarkable reduction in mortality (60%) whereas the control twigs reached 100% ().
Pathogen re-isolation
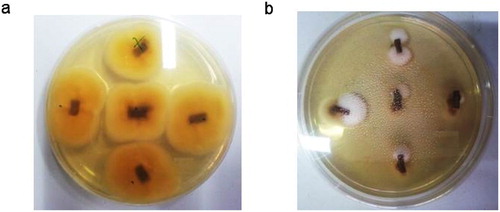
To check whether the symptoms observed in Picholine Marocaine twigs were due to the inoculation, re-isolation of the pathogen from all twigs was undertaken. Samples were taken from inoculated twigs with and without the elicitor. Microscopic observations proved the presence of V. dahliae (). However, growth was reduced in twigs treated by the elicitors.
Figure 2. One week old colonies of Verticillium dahliae re-isolated on PDA medium from fragments of Picholine Marocaine’s control twig (a) and from twigs treated with alginate (b). Samples were elicited 24 h before being soaked, for a month, in a solution containing the pathogen suspension (106 conidia/mL) and the algal polysaccharide (2 g/L). Controls were inoculated only with the conidial suspension.
Effect of algal polysaccharides on mycelial growth of V. dahliae in vitro
Ulvan and alginate significantly inhibited the mycelial growth of V. dahliae in vitro (). This inhibition was reduced over time. The inhibition rate of ulvan was 93.31% and 71.52% after one and two weeks of treatment, respectively. Alginate had a moderate inhibiting effect on mycelial growth of V. dahliae. The inhibitory rates of alginate were 53.17% and 51.39%. Carrageenan had low antifungal activity, with an inhibition rate under 10%. However, laminarin markedly increased mycelial growth compared with the control.
Table 3. Effect of algal polysaccharides on the mycelial growth of Verticillium dahliae in vitro.
Discussion
The three main components associated with the phenolic metabolism and the plant defense systems: PAL, polyphenol and lignin contents, were investigated. PAL is considered one of the key enzymes in resistance mechanisms; it is correlated with cell wall lignification and phenolic compound accumulation. Treatment of olive trees’ twigs with seaweed polysaccharides increased the enzymatic activity of PAL in the stem. Similar results have been reported when oligoalginate was sprayed on rice (Zhang et al. Citation2015) or in post-elicitation of wheat and tobacco by polymannuronic fraction (Poly-Ma) of alginic acid and λ oligocarrageenan respectively (Chandia et al. Citation2004; Vera et al. Citation2012). Our results indicated that the strongest and most significant PAL reaction was obtained in response to elicitation with ulvan. El Modafar et al. (Citation2012), revealed that the application of ulvan in tomato induced a significant increase in PAL activity in comparison with laminarin and carrageenan. Other than PAL, the algal polysaccharides application stimulated the total polyphenols and lignin content, mainly when using ulvan. The intensity of accumulation of phenol compounds, precursors of lignin, depended on the induction of the PAL activity, since the inhibition of this enzyme by aminooxy-acetic acid reduced to 50% the lignin and polyphenol content and made Eucalyptus calophylla susceptible to Phytophthora cinnamomi (Cahill and McComb Citation1992). Our results corroborated those of Abouraicha et al. (Citation2015): ulvan treatment (5 g/L) of apples produced an increase of phenolic compounds and lignin amounts. Similarly, in tomato plants, the application of 3 g/L of ulvan raised the amount of phenolic compounds (El Modafar et al. Citation2012).
The efficiency of the algal polysaccharides was proved not only by inducing olive trees’ defense arsenal, but also through delaying senescence symptoms. A significant decrease in vascular browning and defoliation was achieved after elicitation. According to our results, about 50% of vascular browning was reduced in twigs treated with alginate, laminarin and ulvan. The observed vascular browning was a plant response to the pathogen. V. dahliae’s invasion of xylem vessels caused callose accumulation, which generated the vessels’ obstruction (Robb et al. Citation2012). Overall, ulvan was the ultimate protector against VWO with the lowest AUDPC, final severity and final incidence values. Ulvan treatment at 2 g/L significantly reduced the AUDPC for severity to 39.9%. This result was in agreement with several studies. El Modafar et al. (Citation2012) injected ulvan at the internodes and noticed a significant reduction in wilting symptoms of tomato plants inoculated with Fusarium oxysporum f. sp. Lycopersii. de Freitas and Stadnik (Citation2012) revealed that bean plants treated with ulvan at 10 g/L caused an important decrease in disease severity, locally (60%) and systemically (40%), ensuring protection against Colletotrichum lindemuthianum. Similarly, ulvan (3–6 g/L) reduced the disease severity and protected beans, grapes and cucumber against the agents of powdery mildew (Jaulneau et al. Citation2011). Delgado et al. (Citation2013) reported as well that spraying ulvan at 1 g/L reduced up to 80% of the disease severity of bean rust and up to 45% and 24% respectively of AUDPC and the disease severity of angular leaf spot in bean plants. The protective effect of algae, mainly ulvan, may be owing to its efficiency in stimulating the phenolic metabolism. In fact, bio-elicitors trigger the defense responses in plants prior to a possible pathogen infection by mimicking the pathogen aggression (Hahn Citation1996). Moreover, the accumulation of phenolic compounds at the invasion spot can restrict or slow down pathogen proliferation (El Modafar and El Boustani Citation2005). Besides, lignification makes the cell wall more resistant to mechanical pressure applied during fungal penetration (Bechinger et al. Citation1999) and stronger against enzymatic degradation due to the diffusion of enzymes and toxins from the fungus to the host plant (Vance et al. Citation1980). The algal polysaccharide’s distinctive eliciting potentials could be due to their structural characteristics. Indeed, Menard et al. (Citation2005) demonstrated that polysaccharides’ sulfation modified their affinity to receptors located in cell walls, which made laminarin a stronger elicitor. Moreover, the low degree of polymerization and sulfation of laminarin activated the signaling pathway depending to salicylic acid and provided to tobacco plants full protection against the tobacco mosaic virus (Ménard et al. Citation2004), whereas unsulfated laminarin induced low resistance (Trouvelot et al. Citation2008). Similarly, Vera et al. (Citation2012) reported that the application of oligocarrageenans (λ) with a high degree of sulfation induces long-term protection against viral, fungal and bacterial infections, compared to other oligomers. In addition, El Modafar et al. (Citation2012) reported that ulvan desulfation reduced PAL activity in tomato seedlings. Apart from that, ulvan’s high rhamnose content might be involved in elicitor ability, as revealed in various bacterial lipopolysaccharides (Dow et al. Citation2000; Courtois Citation2009). The saccharide chain length may also be a determinant factor, since it affects the viscosity and so the diffusibility of molecules into host tissues. Numerous studies proved its involvement (Aziz et al. Citation2007; Fu et al. Citation2011; Abouraicha et al. Citation2015, Citation2017). Oligoulvans with a low degree of polymerization displayed the highest defense response and better protection against Penicillium expansum and Botrytis cinerea in apple than ulvan (Abouraicha et al. Citation2015).
Our experiments suggest that algal polysaccharide treatments might have two effects. On the one hand it might enhance the defense responses in olive tree twigs. On the other hand, the treatments might limit the spread of the pathogen. Alginate and ulvan showed direct fungitoxicity on V. dahliae and significantly inhibited the mycelial growth of the fungus in vitro (). This important potential of alginate to suppress verticillium wilt development was also proven when associated with Carbendazim + BHT or Topsin-M + BHT (Khaskheli et al. Citation2014). Carrageenan had a slight inhibitory effect on the mycelial growth of V. dahliae. This result is very similar to that reported by Reeslev and Kjøller (Citation1995), who observed that the growth of Penicillium commune, Aureobasidium pullulans and Paecilomyces farinosus on Carrageenan X-4910 was generally the same as that on agar. However, ulvan directly inhibited mycelial growth of V. dahliae in vitro. Ahmed et al. (Citation2016) found that water extract of Ulva lactuca exhibited a strong antifungal effect against Phytophthora infestan, the causal agent of potato late blight disease. In contrast, the mycelial growth of both Penicillium expansum and Botrytis cinerea were not affected by ulvan in vitro (Abouraicha et al. Citation2015). The fungicidal activities of Ulva lactuca seem to depend to the sensitivity of the fungus. Thus, further studies are necessary to better understand algal polysaccharides’ fungitoxicity on V. dahliae.
Conclusion
Stimulation of the natural defensive system of plants is currently one of the alternative strategies being promoted for crop protection, taking into account environmental considerations. The effectiveness of the inoculation/elicitation method was highlighted. Phenolic metabolism was stimulated 24 h after elicitation and VWO’s symptoms started to appear only 15 days after inoculation. This method reduced the incubation period needed for the infection to occur and for the host plant to react. This technique can also be described as simple and non-destructive, since it requires only twigs and does not affect the mother tree. Twigs provided a single spot of inoculation or elicitation and are thus an easy model in comparison to the complicated rhizosphere system. The resistance induced by the algal polysaccharides reduced the senescence symptoms. Ulvan’s stimulating activity at only 2 g/L might be a beneficial approach to protect Picholine Marocaine from VWO. However, further research is needed to understand exactly how these algal polysaccharides interact to regulate V. dahliae invasion.
Acknowledgment
This work was supported by the Académie Hassan II des Sciences et Techniques under the project ‘Rhizolive’ and the project ‘ArimNet Pestolive’.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abouraicha E, El Alaoui-Talibi Z, El Boutachfaiti R, Petit E, Courtois B, Courtois J, El Modafar C. 2015. Induction of natural defence and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bio-elicitors isolated from green algae. Sci Hortic. 181:121–128. doi: 10.1016/j.scienta.2014.11.002
- Abouraicha E, El Alaoui-Talibi Z, Tadlaoui-Ouafi A, El Boutachfaiti R, Petit E, Douira A, Courtois B, Courtois J, El Modafar C. 2017. Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. J Appl Phycol. 29:471–480. doi: 10.1007/s10811-016-0926-0
- Ahmed SM, El-Zemity SR, Selim RE, Kassem FA. 2016. A potential elicitor of green alga (Ulva lactuca) and commercial algae products against late blight disease of Solanum tuberosum L. Asian J Agric Food Sci. 4:2321–1571.
- An QD, Zhang GL, Wu HT, Zhang ZC, Zheng GS, Luan L, Murata Y, Li X. 2009. Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sp. LXA and its potential application in protection against pathogens. J Appl Microbiol. 106:161–170. doi: 10.1111/j.1365-2672.2008.03988.x
- Aziz A, Gauthier A, Bezier A, Poinssot B, Joubert JM, Pugin A, Heyraud A, Baillieul F. 2007. Elicitor and resistance-inducing activities of β-1,4 cellodextrins in grapevine, comparison with β-1,3 glucans and α-1,4 oligogalacturonides. J Exp Bot. 24:1–10.
- Bechinger C, Giebel KF, Schnell M, Leiderer P, Deising HB, Bastmeyer M. 1999. Optical measurements of invasive forces exerted by appressoria of a plant pathogenic fungus. Science. 285:1896–1899. doi: 10.1126/science.285.5435.1896
- Benhamou N, Rey P. 2012. Stimulators of natural plant defenses: a new phytosanitary strategy in the context of sustainable ecoproduction: II. Interest of the SND in crop protection. Phytoprotection. 92:24–35. doi: 10.7202/1013299ar
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. doi: 10.1016/0003-2697(76)90527-3
- Bridi R, Troncoso MJ, Folch-Cano C, Fuentes J, Speisky H, López-Alarcón C. 2014. A Polyvinylpolypyrrolidone (PVPP) assisted Folin-Ciocalteu assay to assess total phenol content of commercial beverages. Food Anal Methods. 7:2075–2083. doi: 10.1007/s12161-014-9856-0
- Cahill DM, McComb JA. 1992. A comparison of changes in phenylalanine ammonia-lyase activity, lignin and phenolic synthesis in the roots of Eucalyptus calophylla (field resistant) and Eucalyptus marginata (susceptible) when infected with Phytophthora cinnamomi. Physiol Mol Plant Path. 40:315–332. doi: 10.1016/0885-5765(92)90014-M
- Campbell CL, Madden LV. 1990. Introduction to plant disease epidemiology. New York: Wiley-Interscience.
- Chandía NP, Matsuhiro B, Mejías E, Moenne A. 2004. Alginic acids in Lessonia vadosa: partial hydrolysis and elicitor properties of the polymannuronic acid fraction. J Appl Phycol. 16:127–133. doi: 10.1023/B:JAPH.0000044778.44193.a8
- Cosse A, Potin P, Leblanc C. 2009. Patterns of gene expression induced by oligoguluronates reveal conserved and environment-specific molecular defence responses in the brown alga Laminariadigitata. New Phytol. 182:239–250. doi: 10.1111/j.1469-8137.2008.02745.x
- Courtois J. 2009. Oligosaccharides from land plants and algae: production and applications in therapeutics and biotechnology. Curr Opin Microbiol. 12:261–273. doi: 10.1016/j.mib.2009.04.007
- de Freitas MB, Stadnik MJ. 2012. Race-specific and ulvan-induced defense responses in bean (Phaseolus vulgaris) against Colletotrichum lindemuthianum. Physiol Mol Plant Path. 78:8–13. doi: 10.1016/j.pmpp.2011.12.004
- Delgado DZ, de Freitas MB, Stadnik MJ. 2013. Effectiveness of saccharin and ulvan as resistance inducers against rust and angular leaf spot in bean plants (Phaseolus vulgaris). Crop Prot. 47:67–73. doi: 10.1016/j.cropro.2013.01.003
- Dow M, Newman MA, Von Roepenack E. 2000. The induction and modulation of plant defence responses by bacterial lipopolysaccharides. Annu Rev Phytopathol. 38:241–261. doi: 10.1146/annurev.phyto.38.1.241
- El Boutachfaiti R, Delattre C, Petit E, El Gadda M, Courtois B, Michaud P, El Modafar C, Courtois J. 2009. Improved isolation of glucuronan from algae and the production of glucuronic acid oligosaccharides using a glucuronan lyase. Carbohydr Res. 344:1670–1675. doi: 10.1016/j.carres.2009.05.031
- El Modafar C, El Boustani E. 2001. Cell wall-bound phenolic acid and lignin contents in date palm as related to its resistance to Fusarium oxysporum. Biol Plantarum. 44:125–130. doi: 10.1023/A:1017942927058
- El Modafar C, El Boustani E. 2005. The role of phenolics in plant defence mechanisms. In: Regnault-Roger C, Philogène BJR, Vincent C, editor. Biopesticides of plant origin. Andover (UK): Springer-Verlag, Intercept; p. 157–172.
- El Modafar C, Elgadda M, El Boutachfaiti R, Abouraicha E, Zehhar N, Petit E, Courtois B, Courtois J. 2012. Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bio-elicitors isolated from green algae. Sci Hortic. 138:55–63. doi: 10.1016/j.scienta.2012.02.011
- El Said SH, Hegazi AA, Allatif AMA. 2012. Resistance of some olive cultivars to verticillium wilt. J Appl Sci Res. 8:2758–2765.
- Fu Y, Yin H, Wang W, Wang M, Zhang H, Zhao X, Du Y. 2011. β-1,3-Glucan with different degree of polymerization induced different defense responses in tobacco. Carbohydr Polym. 86:774–782. doi: 10.1016/j.carbpol.2011.05.022
- Hahn MG. 1996. Microbial elicitors and their receptors in plants. Annu Rev Phytopathol. 34:387–412. doi: 10.1146/annurev.phyto.34.1.387
- Huang ZX. 1993. Experimental guide of plant chemical protection. Beijing: Agricultural Publications.
- Jaulneau V, Lafitte C, Corio-Costet MF, Stadnik MJ, Salamagne S, Briand X, Esquerré-Tugayé MT, Dumas B. 2011. An Ulva armoricana extract protects plants against three powdery mildew pathogens. Eur J Plant Pathol. 131:393–401. doi: 10.1007/s10658-011-9816-0
- Jayaraj J, Wan A, Rahman M, Punja ZK. 2008. Seaweed extract reduces foliar fungal diseases on carrot. Crop Prot. 27:1360–1366. doi: 10.1016/j.cropro.2008.05.005
- Jiménez-Díaz RM, Cirulli M, Bubici G, del Mar Jiménez-Gasco M, Antoniou PP, Tjamos EC. 2012. Verticillium wilt, a major threat to olive production: current status and future prospects for its management. Plant Dis. 96:304–329. doi: 10.1094/PDIS-06-11-0496
- Khaskheli IM, Ling Sun J, Pu He S, Qin Zhu H, Ming Du X. 2014. The elite use of butylated hydroxytoluene (BHT) and sodium alginate (SA) for the suppression of verticillium wilt of cotton. Int J Dev Research. 4:1667–1678.
- Liu H, Jianga W, Bi Y, Luo Y. 2005. Postharvest BTH treatment induces resistance of peach (Prunuspersica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol Technol. 35:263–269. doi: 10.1016/j.postharvbio.2004.08.006
- López-Escudero FJ, Mercado-Blanco J. 2011. Verticillium wilt of olive: a case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil. 344:1–50. doi: 10.1007/s11104-010-0629-2
- Ménard R, Alban S, de Ruffray P, Jamois F, Franz G, Fritig B, Yvin JC, Kauffmann S. 2004. β-1,3 glucan sulfate, but not β-1,3 glucan, induces the salicylic acid signaling pathway in tobacco and Arabidopsis. Plant Cell. 16:3020–3032. doi: 10.1105/tpc.104.024968
- Ménard R, de Ruffray P, Fritig B, Yvin JC, Kauffmann S. 2005. Defense and resistance-inducing activities in tobacco of the sulfated β-1,3 glucan PS3 and its synergistic activities with the unsulfated molecule. Plant Cell Physiol. 46:1964–1972. doi: 10.1093/pcp/pci212
- Mercier L, Lafitte C, Borderies G, Briand X, Esquerré-Tugayé MT, Fournier J. 2001. The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phythol. 149:43–51. doi: 10.1046/j.1469-8137.2001.00011.x
- Paulert R, Talamini V, Cassolato JEF, Duarte MER, Noseda MD, SmaniaJrA, Stadnik MJ. 2009. Effects of sulfated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). J Plant Dis Prot. 116:263–270. doi: 10.1007/BF03356321
- Ray B, Lahaye M. 1995. Cell-wall polysaccharides from the marine green alga Ulva “rigida” (ulvales, chlorophyta). Extraction and chemical composition. Carbohydr Res. 274:251–261. doi: 10.1016/0008-6215(95)00138-J
- Reeslev M, Kjøller A. 1995. Comparison of biomass dry weights and radial growth rates of fungal colonies on media solidified with different gelling compounds. Appl Environ Microbiol. 61:4236–4239.
- Robb J, Shittu H, Soman KV, Kurosky A, Nazar RN. 2012. Arsenal of elevated defense proteins fails to protect tomato against Verticillium dahliae. Planta. 236:623–633. doi: 10.1007/s00425-012-1637-7
- Sangha JS, Kandasamy S, Khan W, Bahia NS, Singh RP, Critchley AT, Prithiviraj B. 2015. λ-carrageenan suppresses tomato chlorotic dwarf viroid (TCDVd) replication and symptom expression in tomatoes. Mar Drugs. 13:2875–2889. doi: 10.3390/md13052875
- Serrhini MN, Zeroual A. 1995. Verticillium wilt in Morocco. Olivae. 58:58–61.
- Sharma HS, Fleming C, Selby C, Rao J, Martin T. 2014. Plant biostimulants: a review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J Appl Phycol. 26:465–490. doi: 10.1007/s10811-013-0101-9
- Singleton VL, Rossi JA. 1965. Colorimetry of total phenolics with phosphomolybdic-phophotungstic acid reagents. Am J Enol Vitic. 16:144–158.
- Trouvelot S, Varnier AL, Allegre M, Mercier L, Baillieul F, Arnould C, Gianinazzi-Pearson V, Klarzynski O, Joubert JM, Pugin A, Daire X. 2008. A β-1,3 glucan sulfate induces resistance in grapevine against Plasmopara viticola through priming of defense responses, including HR-like cell death. Mol Plant Microbe Interact. 21:232–243. doi: 10.1094/MPMI-21-2-0232
- Vance CP, Kirk TK, Sherwood RT. 1980. Lignification as a mechanism of disease resistance. Annu Rev Phytopathol. 18:259–288. doi: 10.1146/annurev.py.18.090180.001355
- Vera J, Castro J, Contreras RA, González A, Moenne A. 2012. Oligo-carrageenans induce a long-term and broad-range protection against pathogens in tobacco plants (var. Xanthi). Physiol Mol Plant Pathol. 79:31–39. doi: 10.1016/j.pmpp.2012.03.005
- Zhang S, Tang W, Jiang L, Hou Y, Yang F, Chen W, Li X. 2015. Elicitor activity of algino-oligosaccharide and its potential application in protection of rice plant (Oryza saliva L.) against Magnaporthe grisea. Biotechnol Biotechnol Equip. 29:646–652. doi: 10.1080/13102818.2015.1039943
