ABSTRACT
It has been shown that penconazole (PEN) acts as an endogenous signal molecule responsible for inducing stress tolerance in plants. The effect of PEN (15 mg l–1) and sodium chloride (0, 100, and 200 mM NaCl) on some biochemical and molecular responses of safflower was studied. Results revealed that chlorophylls and total soluble protein contents decreased under salinity, however total carotenoid, anthocyanin, flavonoid, and carbohydrate contents increased as well as SOS1 and NHX1 genes expression. The exogenous PEN had a positive effect on chlorophylls, carotenoid, anthocyanin, flavonoid, soluble protein and carbohydrate contents. In addition, RT-qPCR analysis showed that the exogenous PEN induced expression of SOS1 and NHX1 genes in both salt-treated and untreated plants. Our data indicate that PEN helps safflower plants to better cope with salt stress. The results can provide new insights to better realizing the responsible mechanisms to regulate salinity resistance in safflower. PEN can be considered in order to ameliorate salinity effects, due to the low price and their availability.
KEYWORDS:
Introduction
Salt poses particular challenges to global agriculture as it already affects 20% of cultivated and 33% of irrigated agricultural lands (Shrivastava and Kumar, Citation2015), with some predictions that salinization could impact 50% of arable lands by 2050 (Jamil et al., Citation2011). Salinity is an environmental factor limiting plant growth and productivity in arid and semi-arid regions (Aftab et al., Citation2011). It is also a significant problem in safflower (Carthamus tinctorius L.) production in many areas in the world (Shaki et al., Citation2017). Like most of the cultivated plants, growth and yield of safflower decrease under salinity (Bassil and Kaffka Citation2002). Safflower is rated as a moderately salt tolerant plant and it can produce profitable crops on saline soils (Bassil and Kaffka, Citation2002; Kaya and Ipek, Citation2003). While generally regarded as tolerant, safflower yields also have been reduced by high levels of soil salts (Bassil and Kaffka, Citation2002). Safflower, kind of herbaceous plant, belongs to Asteraceae family and has been widely cultivated for its oil and flowers (Bassil and Kaffka Citation2002; Sadeghi et al. Citation2013).
Salinity could dramatically change leaf pigments such as chlorophylls and carotenoids in many plant species (Dhanapackiam and Ilyas, Citation2010). Among the antioxidant components present in the chloroplasts, carotenoids (Car) play an important role in the protection of photosynthetic components against harmful environmental factors (Ramel et al., Citation2012). They scavenge reactive oxygen species (ROS) formed in stress condition and moderate the effect of stress in plants (Moharekar et al., Citation2003).
Flavonoids are a group of secondary metabolites which are synthesized via the phenylpropanoid pathway. Flavonoids have a key role in stress protection in plants (Dkhil and Denden, Citation2012). These compounds act as antioxidant agents by scavenging ROS in cells. Their ability to act as antioxidants depends on the reduction potentials of the radicals (Chutipaijit et al., Citation2009). Some kinds of water-soluble pigments derived from flavonoids via the shikimic acid pathway are anthocyanins. Plants produce anthocyanin under stressful conditions in order to cell protection. These compounds accumulate in tissues under the influence of environmental stimuli (Chutipaijit et al., Citation2009).
Carbohydrates are also accumulated in some plant tissues under stress conditions. The major functions of carbohydrates are carbon storage, radical scavenging, and osmotic adjustment (Parida and Das, Citation2005). High carbohydrate contents under stress condition prevent oxidative damage in cells. They also contribute to the maintenance of the protein structure (Hassanpour et al., Citation2013).
Under salt stress, intracellular Na+ to K+ ions homeostasis is crucial for salt tolerance in plants (Chen et al., Citation2010; Sun et al., Citation2014). To maintain an optimal Na+ to K+ ratio in the cytosol, plants remove excess Na+ through Na+ extrusion to the apoplast or compartmentalization into the vacuoles (Chen et al., Citation2010; Katschnig et al., Citation2015; Yang et al., Citation2017). Active Na+ extrusion from the cytosol is carried out by transmembrane transport proteins such as plasma membrane Na+/H+ antiporter which is encoded by the salt overly sensitive-1 (SOS1) gene and vacuolar membrane-located Na+/H+ antiporter which is encoded by Sodium/proton exchanger-1 (NHX1). It has been reported that SOS1 and NHX1 genes expression is up-regulated in response to salt stress in many plant species (Adabnejad et al., Citation2015; Chen et al., Citation2010; Shi et al., Citation2000).
There are various compounds in plant cells which function mainly in response to stress conditions as signal transducers (Palma et al., Citation2009; Sorahinobar et al., Citation2016). Penconazole (PEN), a type of triazolic compound, induces salinity resistance in plants by regulating physiological processes through signaling. That causes several biochemical and morphological responses in plants (Hassanpour et al., Citation2012, Citation2013; Merati et al., Citation2014). This compound causes reduction in ROS damage, increase in antioxidant potential, and induction of growth in roots which indicate its significant role in increasing the plant resistance under stress conditions (Hassanpour et al., Citation2012; Jaleel et al., Citation2007; Manivannan et al., Citation2007; Merati et al., Citation2014).
Developing salt tolerant crops for the future will rely on identifying new mechanisms of tolerance. One of the most promising paths for identifying new mechanisms of salt tolerance is through studies of the genetic and physiological mechanism of plants (Jamil et al., Citation2011).
Numerous efforts have been made to identify mechanisms of resistance against salinity, but there is a little information available about the effects of triazolic compounds on plants (Fahad et al., Citation2015). To the best of our knowledge, there has been no previous determination of safflower salt tolerance reported under PEN treatment. Therefore, the purpose of the current study was to assess the impact of PEN on some physiological parameters in safflower plants as well as some genes expression. The knowledge of alterations in these processes mediated by PEN might provide a basis to enhance the growth and productivity of safflower in salt-affected areas.
Materials and methods
Plant cultivation and chemical treatments
Based on the agronomic traits, Goldasht is considered as a salt tolerant cultivar of safflower and is one of the most common cultivars used usually for cultivation in semi-arid regions of Iran where soil salinity is high. Therefore, we chose this cultivar for assessing its physiological responses to salt stress and possible ameliorating effects of PEN on salt stress tolerance.
Seeds from Goldasht were sown in Tref peat in a greenhouse with a 15 h light/9 h dark photoperiod at 27 ± 2°C temperature for seed germination and subsequent growth. Following germination, plants were transplanted to plastic pots with perlite. Each pot was considered as one replicate and there were four replicates of each treatment. Each pot was treated with different salinity concentration (0, 100, 200 mM NaCl) and penconazole (15 mg l) with 100 ml of half-strength Hoagland’s nutrient solution (PH 6.8–7), which served as a nutrient media at alternative days (Hoagland and Arnon, Citation1950). In preliminary experiments, the seedlings were treated with different concentrations (0, 10, 15, 20 mg l–1) of PEN to determine the optimum concentration desired. Among them, 15 mg l–1 of PEN increased the growth and also dry and fresh weight significantly. Hence, the 15 mg l–1 PEN was applied to this experiment. A foliar spray of PEN (once a week) (Hassanpour et al., Citation2012) was applied to the plants at vegetative stage. The final harvest was performed 21 days after the start of treatments. The two youngest pairs of fully expanded leaves were sampled from each plant. Leaves were immediately frozen in liquid nitrogen before being stored at –70°C.
Determination of chlorophyll and carotenoid contents
Leaf samples (0.1 g) extracted in 80% acetone/water (v/v) were used for the spectrophotometric determination of chlorophyll a, b and total carotenoids (LICHTENTHALER and Wellburn, Citation1983). The absorbance was determined at 470, 646.8, and 663.2 nm using UV–VIS spectrophotometer (UV-160, Shimadzu, Tokyo, Japan). The chlorophyll and carotenoid contents obtained using the following formulas.
Chla (mg/ml) = 12.25 A663.2–2.79 A646.8
Chlb (mg/ml) = 21.50 A646.8–5.10 A663.2
Tchl (mg/ml) = chla + chlb
Cx + c = (1000 A470–1.8 Ca – 85.02 Cb)/198
In these formulas Chla, Chlb, Tchl, and Cx + c are chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids (consist of Xanthophyll and carotene), respectively.
Determination of total flavonoid content
Flavonoid quantification was done using aluminum chloride colorimetric method (Pourmorad et al., Citation2006). Plant leaf tissue (0.1 g) was extracted in 2 ml of 80% methanol. The solution centrifuged at 8000g for 10 min. Plant extracts (0.5 ml) were mixed with 2 ml of methanol, 0.1 ml of 10% aluminum chloride (ALCL3), 0.1 ml of potassium acetate (1 M), and 2.8 ml of distilled water and kept at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm.
>Determination of anthocyanin content
For anthocyanin determination, plant leaf tissues (0.1 g) were homogenized in 10 ml of methanol (99% methanol, 1% HCl) (Wagner, Citation1979). The solution centrifuged at 6000g for 10 min. The absorbance of the samples was read at 550 nm. Anthocyanin was determined using the extinction coefficient 150 mM/cm.
Total carbohydrate content
Total carbohydrate content was determined using phenol sulphuric method (Dubois et al., Citation1956). Thus, 0.1 g of dry leaf powder was extracted using 3 ml of ethanol:distilled water (8:2; v/v), and supernatant was collected after centrifugation at 5000 g. Then, 0.5 ml phenol solution (5%) and 2.5 ml sulphuric acid (98%) were added to each sample and the absorbance was read at 485 nm.
Protein extraction and determination
Leaf tissues were homogenized at 4°C in 1 M Tris–HCl (pH = 6.8) to determine protein content. The Tris–HCl buffer contained 5 mM 1.4 dithiotheritol, 0.5 mM NaCl, and 5 mM ethylenediaminetetraacetic acid. The homogenate was centrifuged at 13000g (J2-21 M, Beckman, Palo Alto, USA) for 30 min at 4°C. The obtained supernatant was used for protein determination. Proteins were determined according to Bradford (Bradford, Citation1976), using bovine serum albumin (BSA) as standard. Five milliliters of the Bradford reagent and 50 μl of the each protein extract were mixed and then mixtures were incubated at room temperature for 20 min. The absorbance values were measured at 595 nm.
RNA extraction
Total RNA was extracted from leaf tissues with the Spectrum Plant Total RNA Kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instruction. The quality and quantity of RNA samples were examined by EB-stained agarose gel electrophoresis and Qubit analysis. Total RNA was treated with DNaseI (Fermentase, Germany) to remove DNA contamination before cDNA synthesis.
cDNA synthesis
Three micrograms of total RNA was reverse transcribed into complementary DNA (cDNA) using Revert AidTM Reverse Transcriptase (Fermentas, Germany), oligo dT18 and random hexamer primers (MWG, Germany) in a total volume of 20 µl reaction mixture, according to the manufacturer’s instructions.
Primer design
The primer pairs for PAL gene were designed using PRI-MER EXPRESS software (Applied Biosystems). The housekeeping gene actin was used as the standard for checking the quantity and quality of cDNA. Primers used for RT-qPCR are listed in .
Table 1 . Primer sequences used for RT-qPCR in this study.
RT-qPCR
The relative expression level was quantified in comparison with the house keeping gene b-actin as an internal control. Quantitative real-time PCR was performed using Applied Biosystems 7500 Real-Time PCR System (Applied Biosystem/MDS SCIEX, Foster City, CA, USA), with 10 ng cDNA, 10 µl of SYBR Green I master mix (Takara, Shiga, Japan), and 200 nM of forward and reverse primers up to final reaction volumes of 20 µl. The PCR was performed through the following instruction: an initial denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. A serial dilution of cDNA was examined to obtain a standard curve for each primer pairs.
Statistical analysis
Statistical analysis was performed with a randomized complete block design. Tests for significant differences among treatments were conducted using analysis of variance (ANOVA) using SPSS (version 21) with Duncan’s multiple range tests and P values ≤ .05 are considered to be significant. The graphs were drawn using GraphPad Prism 7.03 and principal component analysis (PCA) was performed using the XLSTAT program in windows 10.
Results
The effects of increasing level of NaCl on chlorophyll contents were determined 21 days after exposure to salinity (). It was found that chlorophyll contents were negatively affected by salinity, whereas PEN treatment significantly increased its contents in both salt-stressed and unstressed plants. In this study, the highest induction of chlorophyll production was in PEN-treated plants under 200 mM NaCl treatment. This increase was about threefold in these plants in comparison with controls.
Table 2 . Effects of PEN on pigment contents of C. tinctorious L. under different NaCl concentrations.
In our study, carotenoid content increased in safflower under salinity (). There was a significant difference in plants under 100 and 200 mM NaCl treatments. Exogenously applied PEN significantly increased carotenoid content in both unstressed and salt-stressed plants.
According to the data obtained from this experiment, flavonoid production increased dramatically as salinity levels increased ((a)). This effect was about threefold in plants under severe salt stress in comparison with controls. During the experimental period, exogenous application of PEN to both salt-stressed and unstressed plants caused a higher induction of this compound when compared to controls. Furthermore, this effect was more pronounced in 200 mM NaCl-treated plants. In our study, the higher induction of flavonoid content was related to PEN-treated plants under 200 mM NaCl treatment.
Figure 1. Effects of salinity (0, 100, 200 mM NaCl) and PEN on content of a flavonoid and b anthocyanin in safflower plants at 21 days after the start of treatments. The groups are –PEN (plants with no penconazole treatment) and +PEN (plants sprayed with 15 mgl–1 penconazole once a week). Columns indicate mean ± SE. Means with different letters indicate a significant difference at P ≤ .05 using Duncan multiple range test.
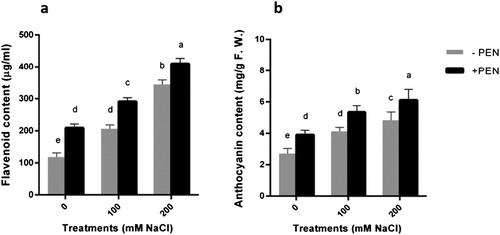
In this experiment, salt stress significantly increased anthocyanin content in salt-stressed plants in comparison with controls ((b)). This effect was more pronounced in plants under 200 mM NaCl treatment. Exogenously applied PEN in current work increased anthocyanin content in salt-stressed plants ((b)).
The increase of carbohydrate content found in salt-stressed safflower plants ((a)). PEN application in salt-stressed plants induced the higher amount of carbohydrates in comparison to without PEN. This effect was more pronounced in plants under 200 mM NaCl treatment.
Figure 2. Effects of salinity (0, 100, 200 mM NaCl) and PEN on content of a carbohydrate and b protein contents in safflower plants at 21 days after the start of treatments. The groups are – PEN (plants with no penconazole treatment) and +PEN (plants sprayed with 15 mgl–1 penconazole once a week). Columns indicate mean ± SE. Means with different letters indicate a significant difference at P ≤ .05 using Duncan multiple range test.
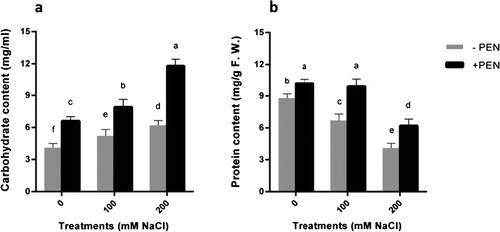
To have a more conclusive study, the amount of protein content in plants was investigated. Soluble protein contents of leaves decreased in response to salinity ((b)). This effect was greater at severe stress conditions. In our experiment, the protein contents remarkably changed by PEN application on plants. In PEN-treated plants under 200 mM NaCl treatment, the total soluble protein content was maximum ((b)).
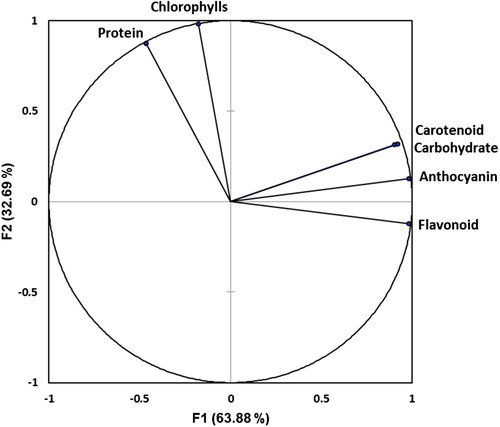
Loading plots of principal components 1 and 2 of the PCA results obtained from biochemical data of safflower plants subjected to salinity and exogenous PEN are illustrated in . In this study, principal component 1 (F1) explained 63.88% of the total variation and principal component 2 (F2) explained 32.69%. The cumulative percentage of F1 and F2 was 96.57%. PCA in this experiment allowed for easy visualization of complex data, and the biochemical parameters among plants were separated by F1 and F2. It was clear that the carotenoid, carbohydrate, and anthocyanin were grouped together with positive loading on the right upper side of the biplot and the flavonoid on the right lower side. However, chlorophylls and protein were observed on the left upper side of the biplot and almost on opposite directions.
Figure 3. Loading plots of principal components 1 and 2 of the PCA. Results obtained from biochemical data of safflower plants subjected to salinity and exogenous PEN.
To better understand the underlying mechanisms of resistance against salinity, SOS1 and NHX1 genes expression were investigated as key transporters in reducing toxic Na+ in cytosol. SOS1 gene expression significantly increased in salt-treated plants ((a)). Our results indicated that following exogenous PEN treatment, SOS1 gene expression was dramatically induced in both salt-treated and untreated plants in comparison with controls. This increase was greater 48 hours after the last treatment in salt-treated plants.
Figure 4. RT-qPCR analyses of a SOS1 and b NHX1 genes transcript of safflower plants treated with salinity and PEN after 24 and 48 hours. Salinity levels were 0, 100 and 200 mM NaCl. The groups are –PEN (plants with no PEN treatment) and +PEN (plants sprayed with 15 mg/l PEN once a week). B-Actin was used as an endogenous control to normalize the data for input RNA difference between the various samples. Columns indicate mean ± SE. Means with different letters indicate a significant difference at P ≤ .05.
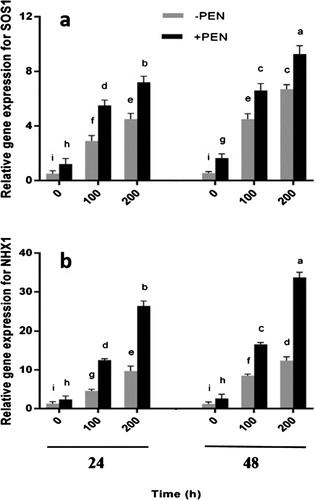
NHX1 gene expression also increased in plants under salt stress ((b)) and PEN treatment enhanced the amount of expression in all plants. This increase was greater 48 hours after the last treatment in salt-treated plants. There was no significant difference between control plants after 24 and 48 hours.
Discussion
In the current study, some biochemical and molecular parameters were investigated to better understand the effects of PEN on safflower plants under salinity condition. This study, for the first time, reports this effect in resistance of safflower against salinity.
In our experiment, chlorophylls content was dramatically inhibited under different NaCl concentrations and was severe at the highest concentration (). Salinity may change leaf pigments especially chlorophylls and carotenoids in many plant species subjected to salt stress (Dhanapackiam and Ilyas, Citation2010; Gengmao et al., Citation2015). Our results are in accordance with the other works which have shown that the total chlorophyll contents significantly decreased by different NaCl concentrations in safflower (ERDAL and ÇAKIRLAR, Citation2014; Gengmao et al., Citation2015). Furthermore, researchers evaluated 64 safflower genotypes under water deficit conditions and observed that cultivars with low seed yield were characterized by low chlorophyll values (Amini et al., Citation2013).
Generally, triazolic compounds are known to enhance the photosynthesis rate due to enhanced chlorophyll contents by an increase in chlorophyll biosynthesis and/or prevent of chlorophyll degradation (Hassanpour et al., Citation2013). Therefore, in the present study, it sounds like salt tolerance due to the exogenous application of PEN on safflower plants might be a reason for increased chlorophyll contents in the PEN-treated plants.
Among the antioxidants present in the chloroplasts, carotenoids play an important role in the mechanisms protecting the photosynthetic components against harmful environmental factors (Ramel et al., Citation2012). They scavenge ROS formed in stress condition and decrease the effect of stress in plants (Strzałka et al., Citation2003). In our experiment, carotenoid content increased under salinity and PEN treatment enhanced its amount more in plants. Our results are in accordance with other researches using other types of triazolic compounds in various plant species (Hassanpour et al., Citation2013; KHALIL, Citation1995).
A work on cereals has also demonstrated that Chlorophyll and Carotenoid Contents are affected by triazolic compounds such as paclobutrazol and its analogues, triadimefon and diclobutrazol (KHALIL, Citation1995). Greening effect which is related to the enhanced chlorophyll or carotenoid biosynthesis in the plants was associated with the growth compaction induced by these triazolic compounds.
As mentioned above, flavonoids which categorized as phenolic compounds in cells, protect plants against various environmental stresses and play an important role in the interaction between the plants and their environment (Samanta et al., Citation2011). In this experiment, flavonoid production increased as salinity levels increased. Our results are in accordance with the results on salt-stressed seedlings of rice (Chutipaijit et al., Citation2009). Flavonoids are frequently induced by stress (Chutipaijit et al., Citation2009; Dixon and Paiva, Citation1995; Grace and Logan, Citation2000). They accumulate in plant tissues and function as ROS scavenger because of the hydroxyl groups in their structure (Chutipaijit et al., Citation2009).
Exogenous PEN had a higher induction of flavonoid when compared to controls. It is demonstrated that various triazolic compounds could enhance the amount of phenolic compounds in plant cells (Hassanpour et al., Citation2013). Therefore, flavonoid enhancement in the PEN-treated plants in this work may probably be due to the enhancement in biosynthesis of phenolic compounds.
Salinity is yet an environmental factor known to trigger anthocyanin accumulation in a number of plant species (Matus et al., Citation2010). In this study, salinity significantly increased anthocyanin content in salt-stressed plants especially under 200 mM NaCl. The accumulation of anthocyanin in plants exposed to salinity has previously been reported (Kennedy and De Filippis, Citation1999; Eryılmaz, Citation2006; Dkhil and Denden, Citation2012). Anthocyanin accumulation that is known as a hallmark of plant stress was stimulated by increasing concentrations of NaCl in tomato and red cabbage (Dkhil and Denden, Citation2012).
Carbohydrate accumulation has also been observed in many plant species under salinity (Kerepesi and Galiba, Citation2000; Khatkar and Kuhad, Citation2000; Singh et al., Citation2000; Parida et al., Citation2002; Parida and Das, Citation2005). The ROS scavenging and osmotic adjustment are as important functions of the carbohydrates under stressful condition (Parida and Das, Citation2005). It has also been reported that they could maintain protein structures in stressful environmental conditions. The hydroxyl groups of these compounds may substitute for water to maintain hydrophilic interactions in protein structures and therefore, prevent protein denaturation (Hassanpour et al., Citation2013). In this experiment, the carbohydrate content of the safflower increased with increasing salinity level. This is in agreement with previously reported findings for safflower under salt stress conditions (Ashraf and Fatima, Citation1995; Karimi et al., Citation2014).
The greater amount of carbohydrate under PEN treatment in this work could be the result of induction of chlorophyll biosynthesis and probably photosynthesis rate in plants. Although photosynthetic rate was not evaluated in this experiment, it can be assumed that increase in chlorophyll contents as the main photosynthetic active pigments in cells could probably result in more carbohydrate production. There are some evidences about the triazolic compounds effect on various plant species (Fahad et al., Citation2015; Fletcher and Hofstra, Citation1990; Hajihashemi et al., Citation2007; Jaleel et al., Citation2008). Paclobutrazol (a triazolic compound) treatment in wheat accumulated more carbohydrates in stressful conditions (Hajihashemi et al., Citation2007). Application of propiconazole ameliorated the salt stress on Madagascar periwinkle plants by activities of enzymatic antioxidants and improving plant growth (Jaleel et al., Citation2008).
There are reports which prove the protein reduction under salinity (Agastian et al., Citation2000; Parida and Das, Citation2005). The reduction in protein content might be due to the decrease in protein synthesis, denaturation of protein structures, and/or denaturation of enzymes involved in protein synthesis. It is likely that greater protein content in PEN-treated plants could be the result of PEN influence on cytokinin phytohormone production (Fletcher et al., Citation2010). Therefore, our results reveal that PEN may alleviate the negative effect of salinity by inhibition of protein degradation.
The PCA grouping allows certain parameters to be identified as those responsible for plant behavior changes under stress condition. PCA is used to extract the important information from a multivariate data table and to express this information as a set of few new variables called principal components. The information in a given data set corresponds to the total variation it contains. To investigate the contributors to the principal component, the biochemical loadings in F1 and F2 were compared (). Our results suggest that PCA analysis is a useful tool for identification of parameters responsible for salt tolerance of safflower. In this experiment, it is suggesting that carotenoid, carbohydrate, and anthocyanin had a positive correlation among themselves, with the same directions while, they had a negative correlation with chlorophylls and protein, on the opposite directions, indicating that impacts of salt stress and PEN on chlorophylls and protein may be different from the effects on the other measured parameters. Furthermore, regarding to the results, there was a considerable overlap between the carotenoid and carbohydrate.
Maintenance of internal cellular ion homeostasis is important for all living organisms. Salt stress creates ion imbalances, causing inhibition of K+ uptake by plants and therefore changing the internal Na+/K+ ratio. In plants, this ratio can be restored either by pumping excess Na+ out of the cell by SOS1 antiporter or by sequestration of Na+ into the vacuole by NHX1antiporter. Besides detoxifying the cytosol, this accumulation can allow plants to drive water into the cells.
The force for maintaining an active sodium/proton antiporter in the plasma membrane is offered by the H+-ATPase protein (Chen et al., Citation2010). This protein energizes the plasma membrane by forming an electrochemical gradient of protons and pH difference across the membrane which is required for activity of ion transporters that move their substrates against a concentration gradient (Chen et al., Citation2010).
It has been reported that sodium/proton antiporters in the plasma membrane are critical for plant growth under high salt concentrations (Shi et al., Citation2003). These ion transporters remove the toxic Na+ from the cytosol to apoplast and/or compartmentalize them into the vacuole. The activity of Na+/H+ antiporters has a key role in plant adaptations to salinity. Salt stress has been reported to increase the activity of Na+/H+ antiporters (Chen et al., Citation2010).
The overexpression of SOS1 in transgenic plants significantly improved salt tolerance in Arabidopsis, and mutant plants were remarkably sensitive to salinity (Shi et al., Citation2003). The relative transcript abundance of SOS1 mRNA in Avicennia marina (Chen et al., Citation2010), Salicornia dolichostachya (Katschnig et al., Citation2015), and a number of glycophytes and halophytes (Katschnig et al., Citation2015) significantly increased after prolonged exposure to salt stress which presumably contributes to Na+ transport into the apoplast and may further modulate the ion homeostasis in cytosol.
In addition, vacuolar Na+ sequestration is an important strategy for osmotic adjustment that also reduces the Na+ concentration in the cytosol (Silva et al., Citation2010). Na+ sequestration into the vacuoles depends on the expression and activity of NHX1 antiporters in the tonoplast (Shi et al., Citation2002). Overexpression of NHX1 in Arabidopsis resulted in plants able to grow under salt stress (Silva et al., Citation2010). Previous studies showed that salt accumulation in several plant species such as Populus euphratica Oliv. (Silva et al., Citation2010), Avicennia marina (Chen et al., Citation2010), and Salicornia dolichostachya (Katschnig et al., Citation2015) occurred with Na+ sequestration into their vacuoles. In our experiment, there was no significant difference in control plants under PEN treatment in the expression of NHX1 gene (). It can be assumed that PEN might only induce NHX1 gene expression under stress conditions.
According to our results, it seems like PEN functions in defense system by reducing toxic Na+ in cytosol following the increase in SOS1 and NHX1 genes expression. Overall, our results provide the first evidence, to our knowledge, that PEN plays a role in enhancing salt tolerance of C. tinctorius by increasing salt secretion through increased expression of the SOS1 gene. Moreover, PEN could induce increased Na+ sequestration into the vacuoles via increasing the expression of NHX1. PEN-modulated activity of Na+/H+ antiporters is closely correlated with the salt resistance of the safflower plants.
Conclusion
Our results indicate that PEN helps safflower plants to better cope with salt stress. This fact is supported by the expression of the key genes in defense system, such as SOS1 and NHX1, as well as an increase of some antioxidant compounds observed in such conditions. The results can provide new insights to better realizing the responsible mechanisms to regulate salinity resistance in safflower. PEN can be considered in order to ameliorate salinity effects in safflower, due to the low price and their availability. Further investigations are required on the signaling systems and also understanding the potential mechanisms underlying PEN-modulated gene expression in controlling salt tolerance to obtain more information about how PEN ameliorate salinity effects in plants.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Reference
- Adabnejad H, Kavousi HR, Hamidi H, Tavassolian I. 2015. Assessment of the vacuolar Na+/H+ antiporter (NHX1) transcriptional changes in Leptochloa fusca L. in response to salt and cadmium stresses. Mol Biol Res Commun. 4:133.
- Aftab T, Khan MMA, da Silva JAT, Idrees M, Naeem M. 2011. Role of salicylic acid in promoting salt stress tolerance and enhanced artemisinin production in Artemisia annua L. J Plant Growth Regul. 30:425–435. doi: 10.1007/s00344-011-9205-0
- Agastian P, Kingsley S, Vivekanandan M. 2000. Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes. Photosynthetica. 38:287–290. doi: 10.1023/A:1007266932623
- Amini H, Arzani A, Bahrami F. 2013. Seed yield and some physiological traits of safflower as affected by water deficit stress. Int J Plant Prod. 7.
- Ashraf, M., and Fatima, H. 1995. Responses of salt-tolerant and salt-sensitive lines of safflower [Carthamus tinctorius L.] to salt stress. Acta Physiol Plant. 17:00–00.
- Bassil ES, Kaffka SR. 2002. Response of safflower (Carthamus tinctorius L.) to saline soils and irrigation: I. consumptive water use. Agric Water Manage. 54:67–80. doi: 10.1016/S0378-3774(01)00148-2
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. doi: 10.1016/0003-2697(76)90527-3
- Chen J, Xiao Q, Wu F, Dong X, He J, Pei Z, Zheng H. 2010. Nitric oxide enhances salt secretion and Na+ sequestration in a mangrove plant, Avicennia marina, through increasing the expression of H+-ATPase and Na+/H+ antiporter under high salinity. Tree Physiol. 30:1570–1585. doi: 10.1093/treephys/tpq086
- Chutipaijit S, Cha-Um S, Sompornpailin K. 2009. Differential accumulations of proline and flavonoids in indica rice varieties against salinity. Pak J Bot. 41:2497–2506.
- Dhanapackiam S, Ilyas MM. 2010. Effect of salinity on chlorophyll and carbohydrate contents of Sesbania grandiflora seedlings. Indian J Sci Technol. 3:64–66.
- Dixon RA, Paiva NL. 1995. Stress-induced phenylpropanoid metabolism. Plant Cell. 7:1085–1097. doi: 10.1105/tpc.7.7.1085
- Dkhil BB, Denden M. 2012. Effect of salt stress on growth, anthocyanins, membrane permeability and chlorophyll fluorescence of okra (Abelmoschus esculentus L.) seedlings.Amer J Plant Physiol. 7:174–183. doi: 10.3923/ajpp.2012.174.183
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith Fred. 1956. Calorimetric method for determination of sugars and related substances. Anal Chem. 28:350–356. doi: 10.1021/ac60111a017
- ERDAL ŞÇ, ÇAKIRLAR H. 2014. Impact of salt stress on photosystem II efficiency and antioxidant enzyme activities of safflower (Carthamus tinctorius L.) cultivars. Turkish J Biol. 38:549–560. doi: 10.3906/biy-1401-33
- Eryılmaz F. 2006. The relationships between salt stress and anthocyanin content in higher plants. BiotechnolBiotechnol Equip. 20:47–52. doi: 10.1080/13102818.2006.10817303
- Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S, Hassan S, Shan D, Khan F, Ullah N. 2015. Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul. 75:391–404. doi: 10.1007/s10725-014-0013-y
- Fletcher R, Hofstra G. 1990. Improvement of uniconazole-induced protection in wheat seedlings. J Plant Growth Regul. 9:207–212. doi: 10.1007/BF02041964
- Fletcher RA, Gilley A, Sankhla N, Davis TD. 2010. Triazoles as plant growth regulators and stress protectants. Hortic Rev. 24:55–138.
- Gengmao Z, Yu H, Xing S, Shihui L, Quanmei S, Changhai W. 2015. Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind Crops Prod. 64:175–181. doi: 10.1016/j.indcrop.2014.10.058
- Grace SC, Logan BA. 2000. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Phil Trans R Soc B. 355:1499–1510. doi: 10.1098/rstb.2000.0710
- Hajihashemi S, Kiarostami K, Saboora A, Enteshari S. 2007. Exogenously applied paclobutrazol modulates growth in salt-stressed wheat plants. Plant Growth Regul. 53:117–128. doi: 10.1007/s10725-007-9209-8
- Hassanpour H, Khavari-Nejad RA, Niknam V, Najafi F, Razavi K. 2012. Effects of penconazole and water deficit stress on physiological and antioxidative responses in pennyroyal (Mentha pulegium L.). Acta Physiol Plant. 34:1537–1549. doi: 10.1007/s11738-012-0952-8
- Hassanpour H, Khavari-Nejad RA, Niknam V, Najafi F, Razavi K. 2013. Penconazole induced changes in photosynthesis, ion acquisition and protein profile of Mentha pulegium L. under drought stress. Physiol Mol Biol Plants. 19:489–498. doi: 10.1007/s12298-013-0192-4
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. Circular. Califotherinfoornia Agricultural Experiment Station. 347.
- Hussain MI, Lyra D-A, Farooq M, Nikoloudakis N, Khalid N. 2016. Salt and drought stresses in safflower: a review. Agron Sust Dev. 36:245. doi: 10.1007/s13593-015-0344-8
- Jaleel CA, Gopi R, Kishorekumar A, Manivannan P, Sankar B, Panneerselvam R. 2008. Interactive effects of triadimefon and salt stress on antioxidative status and ajmalicine accumulation in Catharanthus roseus. Acta Physiol Plant. 30:287–292. doi: 10.1007/s11738-007-0119-1
- Jaleel CA, Gopi R, Manivannan P, Panneerselvam R. 2007. Responses of antioxidant defense system of Catharanthus roseus (L.) G. Don. to paclobutrazol treatment under salinity. Acta Physiol Plant. 29:205–209. doi: 10.1007/s11738-007-0025-6
- Jamil A, Riaz S, Ashraf M, Foolad M. 2011. Gene expression profiling of plants under salt stress. Crit Rev Plant Sci. 30:435–458. doi: 10.1080/07352689.2011.605739
- Karimi S, Arzani A, Saeidi G. 2014. Differential response of ion and osmolyte accumulation to salinity stress in salt-tolerant and salt-sensitive seedlings of safflower (Carthamus tinctorius L.). Res Crops. 15:802–800. doi: 10.5958/2348-7542.2014.01415.6
- Katschnig D, Bliek T, Rozema J, Schat H. 2015. Constitutive high-level SOS1 expression and absence of HKT1;1 expression in the salt-accumulating halophyte Salicornia dolichostachya. Plant Sci. 234:144–154. doi: 10.1016/j.plantsci.2015.02.011
- Kaya MD, Ipek A, A ÖZTÜRK. 2003. Effects of different soil salinity levels on germination and seedling growth of safflower (Carthamus tinctorius L.). Turkish J Agric For. 27:221–227.
- Kennedy B, De Filippis L. 1999. Physiological and oxidative response to NaCl of the salt tolerant Grevillea ilicifolia and the salt sensitive Grevillea arenaria. J Plant Physiol. 155:746–754. doi: 10.1016/S0176-1617(99)80092-3
- Kerepesi I, Galiba G. 2000. Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci. 40:482–487. doi: 10.2135/cropsci2000.402482x
- Khalil, I. A. 1995. Chlorophyll and carotenoid contents in cereals as affected by growth retardants of the triazole series. Cereal Res Commun. 0:183–189.
- Khatkar D, Kuhad M. 2000. Short-term salinity induced changes in two wheat cultivars at different growth stages. Biol Plant. 43:629–632. doi: 10.1023/A:1002868519779
- Lichtenthaler, H. K., and Wellburn, A. R. 1983. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Portland: Press Limited.
- Manivannan P, Jaleel CA, Kishorekumar A, Sankar B, Somasundaram R, Sridharan R, Panneerselvam R. 2007. Changes in antioxidant metabolism of Vigna unguiculata (L.) walp. by propiconazole under water deficit stress. Colloids Surf B. 57:69–74. doi: 10.1016/j.colsurfb.2007.01.004
- Matus J, Poupin M, Cañón P, Bordeu E, Alcalde J, Arce-Johnson P. 2010. Isolation of WDR and bHLH genes related to flavonoid synthesis in grapevine (Vitis vinifera L.). Plant Mol Biol. 72:607–620. doi: 10.1007/s11103-010-9597-4
- Merati MJ, Hassanpour H, Niknam V, Mirmasoumi M. 2014. Exogenous application of penconazole regulates plant growth and antioxidative responses in salt-stressed Mentha pulegium L.. J Plant Int. 9:791–801.
- Moharekar S, Lokhande S, Hara T, Tanaka R, Tanaka A, Chavan P. 2003. Effect of salicylic acid on chlorophyll and carotenoid contents of wheat and moong seedlings. Photosynthetica. 41:315–317. doi: 10.1023/B:PHOT.0000011970.62172.15
- Palma F, Lluch C, Iribarne C, García-Garrido JM, García NAT. 2009. Combined effect of salicylic acid and salinity on some antioxidant activities, oxidative stress and metabolite accumulation in phaseolus vulgaris. Plant Growth Regul. 58:307–316. doi: 10.1007/s10725-009-9380-1
- Parida A, Das AB, Das P. 2002. Nacl stress causes changes in photosynthetic pigments, proteins, and other metabolic components in the leaves of a true mangrove, Bruguiera parviflora, in hydroponic cultures. J Plant Biol. 45:28–36. doi: 10.1007/BF03030429
- Parida AK, Das AB. 2005. Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf. 60:324–349. doi: 10.1016/j.ecoenv.2004.06.010
- Pourmorad F, Hosseinimehr S, Shahabimajd N. 2006. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotechnol. 5.
- Ramel F, Birtic S, Ginies C, Soubigou-Taconnat L, Triantaphylidès C, Havaux M. 2012. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc Natl Acad Sci. 109:5535–5540. doi: 10.1073/pnas.1115982109
- Sadeghi M, Dehghan S, Fischer R, Wenzel U, Vilcinskas A, Kavousi H. R, Rahnamaeian M. 2013. Isolation and characterization of isochorismate synthase and cinnamate 4-hydroxylase during salinity stress, wounding, and salicylic acid treatment in Carthamus tinctorius. Plant Signal Behav. 8:e27335. doi: 10.4161/psb.27335
- Samanta A, Das G, Das SK. 2011. Roles of flavonoids in plants. Carbon N Y. 100:6.
- Shaki F, Ebrahimzadeh Maboud H, Niknam V. 2017. Central role of salicylic acid in resistance of safflower (Carthamus tinctorius L.) against salinity. J Plant Int. 12:414–420.
- Shi H, Ishitani M, Kim C, Zhu J-K. 2000. The arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. ProcNatl Acad Sci. 97:6896–6901. doi: 10.1073/pnas.120170197
- Shi, H, Lee B-H, Wu S-J, Zhu J-K. 2003. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nature Biotechnol. 21:81–85. doi: 10.1038/nbt766
- Shi H, Quintero FJ, Pardo JM, Zhu J-K. 2002. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell. 14:465–477. doi: 10.1105/tpc.010371
- Shrivastava P, Kumar R. 2015. Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci. 22:123–131. doi: 10.1016/j.sjbs.2014.12.001
- Silva P, Façanha AR, Tavares RM, Gerós H. 2010. Role of tonoplast proton pumps and Na+/H+ antiport system in salt tolerance of Populus euphratica oliv. J Plant Growth Regul. 29:23–34. doi: 10.1007/s00344-009-9110-y
- Singh, S, Sharma, H, Goswami, A, Datta, S, Singh, S. 2000. In vitro growth and leaf composition of grapevine cultivars as affected by sodium chloride. Biol Plant. 43:283–286. doi: 10.1023/A:1002720714781
- Sorahinobar M, Niknam V, Ebrahimzadeh H, Soltanloo H, Behmanesh M, Enferadi ST. 2016. Central role of salicylic acid in resistance of wheat against Fusarium graminearum. J Plant Growth Regul. 35:477–491. doi: 10.1007/s00344-015-9554-1
- Strzałka K, Kostecka-Gugała A, Latowski D. 2003. Carotenoids and environmental stress in plants: significance of carotenoid-mediated modulation of membrane physical properties. Russ J Plant Physiol. 50:168–173. doi: 10.1023/A:1022960828050
- Sun J, Zou D, Luan F, Zhao H, Wang J, Liu H, Xie D, Su D, Ma J, Liu Z. 2014. Dynamic QTL analysis of the Na+ content, K+ content, and Na+/K+ ratio in rice roots during the field growth under salt stress. Biol Plant. 58:689–696. doi: 10.1007/s10535-014-0445-2
- Wagner GJ. 1979. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 64:88–93. doi: 10.1104/pp.64.1.88
- Yang L, Liu H, Fu S, Ge H, Tang R, Yang Y, Wang H, Zhang H. 2017. Na+/H+ and K+/H+ antiporters AtNHX1 and AtNHX3 from arabidopsis improve salt and drought tolerance in transgenic poplar. Biol Plant. 61:641–650. doi: 10.1007/s10535-017-0724-9
