ABSTRACT
In field, plants are exposed to multiple biological stresses simultaneously; for example, insect damage and pathogenic virus infection. In this study, we constructed a simple tripartite experimental system using tomato plant, their insect pest Bemisia tabaci (whitefly), and infectious tomato mosaic virus (ToMV). In the choice test, whiteflies laid more eggs on uninfected plant than on ToMV-infected plant. Further, whiteflies hatched more and grew better on the uninfected plant. Salicylic acid (SA) accumulated in infected plants, and the expression of genes involved in SA production and SA-related response was significantly induced. These plant responses against ToMV infection were similar to those against whitefly attack or SA-treated plants. To examine the importance of plant endogenous SA for whitefly preference, we repeated the choice test using the NahG transgenic tomato that cannot accumulate SA. Whiteflies did not show oviposition preference between NahG plants with and without ToMV infection or whitefly infestation. Overall, whiteflies prefer healthy plants, and SA accumulation in response to biological stresses is involved in their preference.
Introduction
Plants under field condition experience stress by pathogen and herbivores simultaneously. Infection by pathogens and attack by herbivores are serious biological stresses to plants, accounting for approximately 40% loss of crop and vegetable production (Oerke et al. Citation1994; Gottula and Fuchs Citation2009). Therefore, the elucidation of defense system mechanisms underlying the plant–insect–microbe interaction is important to reduce crop loss. Plant endogenous signal molecules, particularly salicylic acid (SA), jasmonate (JA), and ethylene, play important roles in inducing defense response against biotic stresses (Alazem and Lin Citation2015; Caarls et al. Citation2015; Okada et al. Citation2015). Generally, the infection by biotrophic pathogens and viruses, and attack by sucking insects induce SA accumulation and SA-related response in plant, whereas, the infection by necrotrophic pathogens and damage by chewing insects induce the JA accumulation and JA-related response (Hayat et al. Citation2010; Mauck et al. Citation2010; Nguyen et al. Citation2016; Verma et al. Citation2016).
Bemisia tabaci (whitefly) is a serious global agricultural pest that causes severe damage through feeding and by acting as a vector to plant viruses (Gottlieb et al. Citation2006). It is a sucking-type insect, and its attack induces SA signal transduction in plants (Leitner et al. Citation2005; Kawazu et al. Citation2012a; Park and Ryu Citation2015; Zhang et al. Citation2015). In addition, whiteflies act as a vector for the disease-causing tomato yellow leaf curl virus (TYLCV) (Cohen and Harpaz Citation1964). It is known that some whitefly populations carrying TYLCV prefer to colonize the plants infected with TYLCV and other populations poorly spreading TYLCV prefer healthy plant (Shi et al. Citation2016). It is thought that some viruses can alter the preference of vector insects by interacting with the insects themselves or with their endogenous microorganisms (Su et al. Citation2013; Guo et al. Citation2014; Wang et al. Citation2016). However, it is not clear how whiteflies discriminate infected plants from healthy plants.
Tobamoviruses are the most studied viral pathogen, and they infect plants belonging to several genera; however, they are not vectored by whiteflies. These viruses induce resistance response pathways mediated by SA in tomato plant (Hayat et al. Citation2010; Mauck et al. Citation2010). Similar to those of TYLCV-infected plants, tomato plants infected with tomato mosaic virus (ToMV) exhibit severe growth suppression as well as reduced yield and commercial value of its fruits (Sanders et al. Citation1992).
In the present study, we constructed a plant–insect–virus tripartite experiment system consisting of tomato plant, whitefly, and ToMV. They were selected to simplify the experimental system, as whitefly is not a vector of ToMV. Given the advantages of tomato model plants, which are rich in genetic information, and the ease of handling of transgenic plants, we examined the oviposition preference of whiteflies for virus-infected, whitefly-infested, and healthy plants and their performance on virus-infected plants (Shikata et al. Citation2016; Yamamoto et al. Citation2018). We aimed to acquire information on the mechanisms underlying the preference and performance of whiteflies.
Materials and methods
Plant and insect materials and growth conditions
The seeds of tomato plants (Solanum lycopersicum (L.) ‘Micro-Tom’ wild type and transgenic plants) were sown on 1:1 mixture of commercial culture soil (Sakata super mix A; Sakata Seed, Yokohama, Japan) and vermiculite. The plants were grown under white fluorescent light (2.4 klx) with a 16:8 h light/dark photoperiod and 40–50% humidity (RH) at 25°C for 4 weeks. Whiteflies (B. tabaci) were purchased from Sumika Technoservice Co. (Takarazuka, Japan) and were mass-reared on potted Micro-Tom plants in an acrylic box maintained in a glass room (25°C, 40–50% RH).
Production of transgenic plants
A binary vector expressing bacterial gene encoding salicylate hydrase (NahG) under the control of an enhanced 35S promoter was constructed by Kobayashi et al. (Citation2010). The construct was used to transform tomato (S. lycopersicum (L.) Micro-Tom) by the leaf disc co-cultivation method with Agrobacterium tumefaciens LBA4404 (Horsch et al. Citation1985). A plant transformed with empty vector was also prepared as control.
Inoculation with tomato mosaic virus
The fourth leaf from the bottom of intact potted plant was gently rubbed with carborundum and ToMV at a concentration of 0.4 μg mL−1 suspended in 10 mM phosphate buffer (pH 7.0) or carborundum and buffer as mock treatment. After rinsing under a gentle stream of tap water, the treated plants were incubated under the same condition of growth. After 7 days, when the symptoms of ToMV infection appeared in the systemic leaves (those above the inoculated leaf), the inoculated plants were used for the choice and performance tests with whiteflies. To extract phytohormones and total RNA, the upper systemic leaves were sampled, frozen in liquid nitrogen, and immediately stored at −80°C.
Infection with whiteflies
Infection with whiteflies was performed as described by Kawazu et al. (Citation2012b) with some modifications. On the fourth leaf of the potted plants, five mated females were introduced and gently covered with a small cage (25 mm diameter × 15 mm height) of infested leaf. After 3 days, the whole infested plants were subjected to the choice test, with the small cage containing whiteflies, to discriminate newly laid eggs in the choice test. An intact plant with empty cage covering the same position was used as uninfested control. To extract phytohormones and total RNA, the upper systemic leaves were stored at −80°C.
Treatment with SA
The fourth leaf of intact potted plant was sprayed with about 600 µL of 1 mM SA aqueous solution or sterilized distilled water (SDW) as control. After 2 days, the plants were used for the choice test.
Quantification of phytohormones
The extraction and quantification of SA, SA glucoside (SAG), and JA in the frozen leaf samples were performed as previously described using a gas chromatograph equipped with a quadrupole mass spectrometer (Hewlett-Packard, Wilmington, DE, USA) (Seo et al. Citation1995; Baldwin et al. Citation1997). The phytohormones were extracted from five independent samples.
RNA extraction and quantitative RT–PCR
Frozen leaf samples were ground with liquid nitrogen using a motor and pestle, and the total RNA from each sample was extracted with TRIzol® reagent (Invitrogen, San Diego, CA, USA) as recommended by the supplier. The quantitative reverse transcriptase PCR (qRT-PCR) was performed with 1 μg of total RNA using the iScript™ cDNA Synthesis Kit (BIO-RAD, Hercules, CA, USA) and iQ™ SYBR® Green supermix (BIO-RAD) as recommended by the supplier. To study the expression of disease-related genes—SlPIN2 (HQ127077), SlPR4 (FJ151171), and SlSOD (AK246715)—specific primers were designed as shown in . Control reactions to normalize qRT-PCR amplification were run using the Actin (SlACT) gene (FJ532351). The PCR was carried out using the Real Time PCR System CFX96 (BIO-RAD). The thermal cycling program was as follows: 95°C for 60 s; 45 cycles of 95°C for 15 s; and 60°C for 30 s.
Table 1. Gene specific primers for quantitative RT-PCR.
Choice test
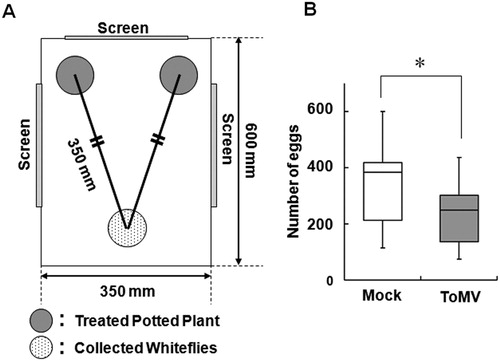
The oviposition preference of whiteflies was tested in an acrylic box (600 mm × 350 mm × 300 mm; with three nylon-gauze-covered windows and two doors). In the box with two potted Micro-Tom plants subjected to different treatments, 25 whiteflies collected in a 1.5-mL tube were released at equidistance from the two plants (A). The plants and whiteflies in the box were maintained under conditions of a climate-controlled room at 25 ± 2°C, 40–50% RH, and fluorescent light (2.4 klx) with a 16:8 h light/dark photoperiod. After 7 days, the number of eggs laid on each plant was counted.
Figure 1. Choice test of whitefly on ToMV-inoculated and mock-treated healthy plants. A, Experimental system of choice test. Each potted plant was placed at the two corners of the acrylic box (gray circles). Whiteflies set at equidistant from the two plants (dotted circle). B, Preference of whiteflies for ToMV-inoculated (gray box) and mock-treated plants (white box). Box plot explanation: upper horizontal line of box, 75th percentile; lower horizontal line of box, 25th percentile; horizontal bar within box, median; upper and lower lines outside the boxes, minimum and maximum values (error bars). Experiments were repeated independently (n = 12). Asterisk indicates that the data compared were significantly different (P < 0.05, Wilcoxon signed-rank test).
Performance of whitefly
To assess the performance of whiteflies on the systemic leaves of ToMV-inoculated and mock-treated plants, the egg hatching and adult emergence rates were examined. The egg hatching test was performed as described by Liu et al. (Citation2014) with some modifications. In a small cage (25 mm diameter × 15 mm height), two mated female whiteflies were inoculated on the upper systemic leaves detached from the treated plants. The cut end of the detached leaves was covered with wet cotton wool during the tests. To test the rate of adult emergence, in an acrylic cage (160 mm × 120 mm × 260 mm; with a nylon-gauze-covered window), two mated females were directly introduced onto the upper systemic leaves of potted plants and enclosed by a nylon-mesh with the leaves. After 1 day, in both the tests, mated females were removed and the detached leaves or the potted plants were maintained at 25°C in each cage for 20 or 30 days. Subsequently, the number of freshly hatched larvae and emerged adults was counted every day.
Statistical analyses
The choice test data were analyzed by Wilcoxon signed-rank test. The hatching rate of whiteflies was analyzed by the log-rank test. The data of emergence test, qRT-PCR, and phytohormone quantification were subjected to the analysis of variance (ANOVA), followed by t test. These data were subjected to statistical analyses using the statistical software package R version 3.5.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Whiteflies preferred to lay eggs on healthy plants than on virus-infected plants
The ToMV-inoculated and mock-treated plants were prepared and the preference of whiteflies to the plants was analyzed at 7 days after inoculation when the symptoms of ToMV are appeared in the systemic leaves. The spread of ToMV was confirmed by normalizing the viral genomic RNA content to tomato actin (SlACT) mRNA level (Figure S1). The number of eggs on mock-treated plants was higher than that on ToMV-inoculated plants (B). The result of 12 independent assays revealed that the whiteflies preferred mock-treated plants over ToMV-infected plants.
Performance of whiteflies on diseased and healthy plants
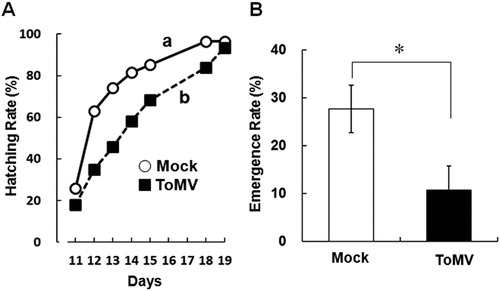
To verify whether the growth performance of whiteflies on healthy plants was better than that on diseased plant, the hatching rate of eggs and emergence rate of adults on each treated plant were compared. The eggs on diseased leaves hatched more slowly than those on healthy leaves (A). Approximately 80% of eggs on mock-treated leaves hatched until 2 weeks, whereas, only 60% of eggs on ToMV-inoculated leaf hatched during the same period. The emergence rate of adult whitefly on mock-treated plant was also significantly higher than that on ToMV-inoculated plants (B).
Figure 2. Performance of whiteflies on ToMV-inoculated or mock-treated plants. A, Hatching rate of whiteflies on the systemically infected leaf of ToMV-inoculated and mock-treated plants. Open circle with solid line indicates the hatching rate on the leaf of mock-treated plant. Closed square with dash line indicates the hatching rate on the leaf of ToMV-infected plants. The same experiments were repeated twice and the average values are indicated. The total number of eggs was 239 on ToMV-infected leaves and 181 on mock-treated leaves. Different letters indicate that the data compared were statistically significantly different (P < 0.05, log-rank test). B, The emergence rate of whiteflies on ToMV-inoculated (black) and mock-treated plants (white). Bar graph indicates the mean ± standard error of the mean of repeated experiments on mock-treated plant (n = 5) and on ToMV-inoculated plant (n = 6). Asterisk indicates that the data compared were statistically significantly different (P < 0.05, t-test).
Response of tomato plant against ToMV infection
Although whiteflies preferred healthy plants over diseased plants and presented faster growth on healthy plants, the nutritional value of mock-treated leaves and ToMV-inoculated leaves appeared to be similar as there was no difference between their carbon and nitrate content (Figure S2).
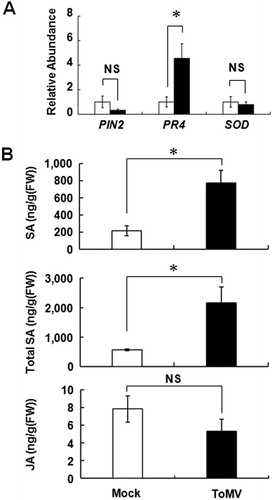
As typical stress response genes, the mRNA level of PIN2, PR4, and SOD was evaluated at 7 days after inoculation. Among the evaluated genes, the mRNA level of PR4, a typical marker gene for SA, was significantly increased upon ToMV inoculation. On the contrary, the expression level of mRNA of PIN2, a typical marker gene for wound response, and SOD, a typical marker gene for oxidative stress, was not different between each treatment (A). Further, free SA and total SA levels (SA + SAG) significantly accumulated in the leaves of ToMV-inoculated plants (B). Contrarily, the content of JA was not altered by ToMV inoculation.
Figure 3. Molecular response of Micro-Tom against ToMV infection. A, Expression profile of the typical stress-inducible genes in the systemic leaf after ToMV inoculation. The total RNA was extracted 7 days after ToMV inoculation and mRNA of PIN2, PR4 and SOD genes was quantified by the qRT-PCR. B, Accumulation of SA, total SA (SA + SAG), and JA in the systemic leaf after ToMV inoculation. White indicates mock treatment and black indicates ToMV infection. Each bar indicates the mean ± standard error of the mean of repeated experiments (A: n = 3; B: n = 4). Asterisk indicates that the data compared were statistically significantly different (P < 0.05, t-test). NS indicates no significance.
Whitefly avoids SA-accumulated tomato plants as oviposition site
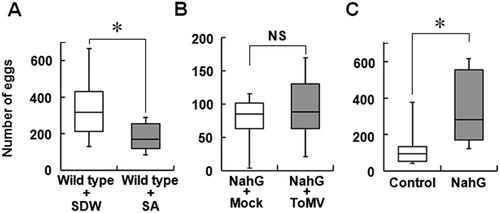
Based on the above results, we focused on the effects of SA accumulation in tomato plants on whitefly preference. The preference of whitefly for SA-treated plants over control plants was assayed. Whiteflies significantly preferred SDW-treated plant over SA-treated plant (A). SA-deficient transgenic tomato plants were produced by the introduction of a construct expressing NahG (Kobayashi et al. Citation2010). The suppression of SA accumulation was confirmed in the second generation of NahG transgenic plants (Figure S3). The preference of whitefly for ToMV-inoculated and mock-treated NahG tomato plants was investigated. Whitefly did not exhibit preference for healthy or diseased plants, indicating SA accumulation in ToMV-infected tomato plants induces disfavor in whitefly (B).
Figure 4. Effect of SA on the preference of whitefly for tomato plants. A, Whitefly preference for SA-treated and SDW-treated plants. Wild type Micro-Tom plant was sprayed with 1 mM SA or SDW, and 2 days after each treatment, the plants were set in acryl box (A) and used in the choice test. The number of eggs laid on each plant was counted at 7 days after inoculation. B, Preference of whiteflies for mock-treated and ToMV-infected NahG plant. NahG tomato plants were ToMV inoculated or mock treated, and the preference of whitefly was assessed at 7 days after each treatment. C, Preference of whiteflies for intact NahG and control plants. Box plot explanation: Upper horizontal line of box, 75th percentile; lower horizontal line of box, 25th percentile; horizontal bar within box, median; upper and lower lines outside the boxes, minimum and maximum values (error bars). Each test was repeated independently (A: n = 6; B: n = 6; C: n = 8). Asterisk indicates that the data compared were significantly different (P < 0.05, Wilcoxon signed-rank test). NS indicates no significance.
Response of tomato plant to sucking infestation by whiteflies and its effect on the preference of whitefly
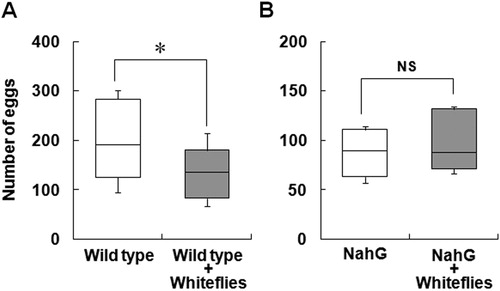
Whiteflies significantly preferred the leaves of NahG plant over those of control plant without ToMV inoculation (C). Whitefly infestation increased the mRNA level of SA inducible gene, PR4, as well as SA accumulation, whereas the expression of mRNA of PIN2 was not different between the plants. On the contrary, the expression of mRNA of SOD was significantly increased upon whitefly infestation (A). Furthermore, free SA and total SA levels (SA + SAG) significantly accumulated in the leaves of whitefly-infested plants (B). To confirm whether SA accumulation induced by whitefly infestation affects the preference of whiteflies, whitefly preference was tested by the local infestation of whitefly on tomato leaves clipped with a small cage. Whiteflies significantly preferred uninfested plants over infested wild-type tomato plant (A). However, this difference in preference was not observed when NahG plants were used (B). These results indicate that whitefly select healthy plant over whitefly-infested plant, and SA accumulation induced by whitefly infestation is responsible for the disfavor of whitefly.
Figure 5. Micro-Tom response to whitefly infection. A, Expression pattern of the typical stress-inducible genes in wild type Micro-Tom after infestation with five whiteflies. The total RNA was extracted 3 days after infestation with whiteflies. White indicates intact plant and black indicates whitefly-infected plant. B, Accumulation of SA, total SA, and JA in control plant (transformed with empty vector) after the infestation with whiteflies. White indicates intact plant and black indicates whitefly-infested plant. Each bar represents the mean ± standard error of the mean of repeated experiments (A: n = 5; B: n = 4). Asterisk indicates that the data compared were statistically significantly different (P < 0.05, t-test). NS indicates no significance.
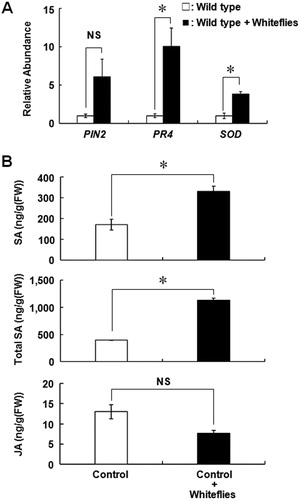
Figure 6. Effect of inoculation of tomato plants with whitefly on the preference of whitefly. The preference of whitefly for infested and uninfested plants using wild type tomato (A) and NahG transgenic tomato (B). Tomato plants were locally infested with five whiteflies using a small cage and uninfested with empty cage treatment for 3 days. After treatment, each plant was set in acrylic box (A), and 25 whiteflies were released in the box. Numbers of eggs laid were counted after 7 days. Box plot explanation: upper horizontal line of box, 75th percentile; lower horizontal line of box, 25th percentile; horizontal bar within box, median; upper and lower lines outside the boxes, minimum and maximum values (error bars). Each test was repeated independently (A: n = 8; B: n = 6). Asterisk indicates that the data compared were significantly different (P < 0.05, Wilcoxon signed-rank test). NS indicates no significance.
Discussion
In the constructed experimental system, whiteflies selected and laid more eggs on healthy plant than on ToMV-infected plant (B). As the nutritional value of plants might not be reduced by the infection stress (Figure S2), the responses of tomato plant against ToMV infection could have affected the preference of whitefly. The infection of TYLCV has been reported to alter the palatability of vector insects by interacting with the insects or their endogenous microorganisms (Su et al. Citation2013; Wang et al. Citation2016). It has been known that there are different biotypes of whitefly, with their endogenous microorganisms determining their palatability (Guo et al. Citation2014). Q biotype whiteflies carrying TYLCV prefer landing and colonizing the plants infected with TYLCV. Contrarily, B biotype whiteflies without TYLCV (poorly spreading TYLCV) prefer healthy plant (Shi et al. Citation2013, Citation2016). In the present study, we observed that the whitefly, which is not affected by virus, prefer healthy plants over virus-infected plants. It appears that plant defense response against ToMV is effective to reduce further attacks by virus-free whiteflies, but that the response against TYLCV is not effective for TYLCV-carrying Q biotype whiteflies. The TYLCV-carrying whiteflies could counteract the effects of plant viral responses or shift their oviposition preference after the viral infection. The components of plants associated with the preference of whiteflies should be determined by comparing the virus-mediated and non-mediated systems.
Whiteflies avoided oviposition on the exogenously SA applied plants (A). Further, when NahG tomato plants, which cannot accumulate SA, were used in the choice test for oviposition, whiteflies did not show preference between healthy plant and diseased plant (B). Thus we conclude that SA accumulation determines the preference of whitefly for healthy and infected plants.
It has been suggested that herbivores with similar feeding type antagonize the growth performance of each other when feeding simultaneously on the same plant as a result of induced plant defenses (Soler et al. Citation2013; Schimmel et al. Citation2017). The present study confirmed that whitefly infestation induces accumulation of SA and expression of SA-related genes (), as previously reported by Zhao et al. (Citation2015). In addition, the results that whiteflies avoid locally infested plant (A) also suggest whiteflies seek for foods of better quality and avoid plants that can be disadvantageous to the growth of offspring in competition with other whiteflies. However, such behavior was not observed toward NahG tomato that is deficient in SA accumulation (B).
Previous studies reported preference of herbivore to healthy or stressed plants in various plant–herbivore interactions, but it was not clarified what plant mechanisms determine the preference. The results presented herein indicate that SA is the key molecule for the negative preference of whitefly for oviposition on ToMV-infected or whitefly-infested tomato plants. On the other hand, it is likely adaptive for whiteflies to select plants other than these stressed plants. It is of interest to understand whether whiteflies recognize SA-accumulated plant by means of taste, smell, or other factors. Furthermore, it should be clarified whether SA has similar effect on other insect–plant interactions including whitefly with or without vectoring virus, or insect with other feeding mode.
Supplemental Material
Download MS Word (114.1 KB)Acknowledgements
We thank Dr S. Seo for SA and JA analysis, and technical advice.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alazem M, Lin NS. 2015. Roles of plant hormones in the regulation of host–virus interactions. Mol Plant Pathol. 16(5):529–540. doi: 10.1111/mpp.12204
- Baldwin IT, Zhang ZP, Diab N, Ohnmeiss TE, McCloud ES, Lynds GY, Schmelz EA. 1997. Quantification, correlations and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta. 201(4):397–404. doi: 10.1007/s004250050082
- Caarls L, Pieterse CM, Van Wees SC. 2015. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front Plant Sci. 6:170. doi: 10.3389/fpls.2015.00170
- Cohen S, Harpaz I. 1964. Periodic, rather than continual acquisition of a new tomato virus by its vector, the tobacco whitefly (Bemisia tabaci gennadius). Entomol Exp Appl. 7(2):155–166. doi: 10.1111/j.1570-7458.1964.tb02435.x
- Gottlieb Y, Ghanim M, Chiel E, Gerling D, Portnoy V, Steinberg S, Tzuri G, Horowitz AR, Belausov E, Mozes-Daube N, et al. 2006. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl Environ Microbiol. 72(5):3646–3652. doi: 10.1128/AEM.72.5.3646-3652.2006
- Gottula J, Fuchs M. 2009. Toward a quarter century of pathogen-derived resistance and practical approaches to plant virus disease control. Adv Virus Res. 75:161–183. doi: 10.1016/S0065-3527(09)07505-8
- Guo H, Qu Y, Liu X, Zhong W, Fang J. 2014. Female-biased symbionts and tomato yellow leaf curl virus infections in Bemisia tabaci. PLoS One. 9(1):e84538. doi: 10.1371/journal.pone.0084538
- Hayat Q, Hayat S, Irfan M, Ahmad A. 2010. Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot. 68(1):14–25. doi: 10.1016/j.envexpbot.2009.08.005
- Horsch RB, Rogers SG, Fraley RT. 1985. Transgenic plants. Cold Spring Harb Symp Quant Biol. 50:433–437. doi: 10.1101/SQB.1985.050.01.054
- Kawazu K, Mochizuki A, Sato Y, Sugeno W, Murata M, Seo S, Mitsuhara I. 2012a. Different expression profiles of jasmonic acid and salicylic acid inducible genes in the tomato plant against herbivores with various feeding modes. Arthropod Plant Interact. 6(2):221–230. doi: 10.1007/s11829-011-9174-z
- Kawazu K, Wasano N, Konno K, Ohashi Y, Mochizuki A, Mitsuhara I. 2012b. Evaluation of anti-herbivory genes using an Agrobacterium-mediated transient expression system. Plant Biotechnol. 29(5):495–499. doi: 10.5511/plantbiotechnology.12.0711a
- Kobayashi M, Seo S, Hirai K, Yamamoto-Katou A, Katou S, Seto H, Meshi T, Mitsuhara I, Ohashi Y. 2010. Silencing of WIPK and SIPK mitogen-activated protein kinases reduces tobacco mosaic virus accumulation but permits systemic viral movement in tobacco possessing the N resistance gene. Mol Plant Microbe Interact. 23(8):1032–1041. doi: 10.1094/MPMI-23-8-1032
- Leitner M, Boland W, Mithofer A. 2005. Direct and indirect defences induced by piercing–sucking and chewing herbivores in Medicago truncatula. New Phytol. 167(2):597–606. doi: 10.1111/j.1469-8137.2005.01426.x
- Liu X, Xiang W, Jiao X, Zhang Y, Xie W, Wu Q, Zhou X, Wang S. 2014. Effects of plant virus and its insect vector on Encarsia formosa, a biocontrol agent of whiteflies. Sci Rep. 4:5926. doi: 10.1038/srep05926
- Mauck KE, De Moraes CM, Mescher MC. 2010. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc Natl Acad Sci USA. 107(8):3600–3605. doi: 10.1073/pnas.0907191107
- Nguyen D, Rieu I, Mariani C, van Dam NM. 2016. How plants handle multiple stresses: hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol Biol. 91(6):727–740. doi: 10.1007/s11103-016-0481-8
- Oerke EC, Dehne HW, Schönbeck F, Weber A. 1994. Crop production and crop protection-estimated losses in major food and cash crops. Amsterdam: Elsevier Science.
- Okada K, Abe H, Arimura G. 2015. Jasmonates induce both defense responses and communication in monocotyledonous and dicotyledonous plants. Plant Cell Physiol. 56(1):16–27. doi: 10.1093/pcp/pcu158
- Park YS, Ryu CM. 2015. Inter-organ defense networking: leaf whitefly sucking elicits plant immunity to crown gall disease caused by Agrobacterium tumefaciens. Plant Signal Behav. 10(11):e1081325. doi: 10.1080/15592324.2015.1081325
- Sanders PR, Sammons B, Kaniewski W, Haley L, Layton J, LaVallee BJ, Delannay X, Tumer NE. 1992. Field resistance of transgenic tomatoes expressing the tobacco mosaic virus or tomato mosaic virus coat protein genes. Mol Plant Pathol. 82(6):683–690.
- Schimmel BCJ, Ataide LMS, Chafi R, Villarroel CA, Alba JM, Schuurink RC, Kant MR. 2017. Overcompensation of herbivore reproduction through hyper-suppression of plant defenses in response to competition. New Phytol. 214(4):1688–1701. doi: 10.1111/nph.14543
- Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. 1995. Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science. 270(5244):1988–1992. doi: 10.1126/science.270.5244.1988
- Shi X, Chen G, Tian L, Peng Z, Xie W, Wu Q, Wang S, Zhou X, Zhang Y. 2016. The salicylic acid-mediated release of plant volatiles affects the host choice of Bemisia tabaci. Int J Mol Sci. 17(7):1048. doi: 10.3390/ijms17071048
- Shi X, Pan H, Xie W, Wu Q, Wang S, Liu Y, Fang Y, Chen G, Gao X, Zhang Y. 2013. Plant virus differentially alters the plant's defense response to its closely related vectors. PLoS One. 8(12):e83520. doi: 10.1371/journal.pone.0083520
- Shikata M, Hoshikawa K, Ariizumi K, Fukuda N, Yamazaki Y, Ezura H. 2016. TOMATOMA update: phenotypic and metabolite information in the micro-tom mutant resource. Plant Cell Physiol. 57(1):e11. doi: 10.1093/pcp/pcv194
- Soler R, Erb M, Kaplan I. 2013. Long distance root–shoot signalling in plant–insect community interactions. Trends Plant Sci. 18(3):149–156. doi: 10.1016/j.tplants.2012.08.010
- Su Q, Pan H, Liu B, Chu D, Xie W, Wu Q, Wang S, Xu B, Zhang Y. 2013. Insect symbiont facilitates vector acquisition, retention, and transmission of plant virus. Sci Rep. 3:1367. doi: 10.1038/srep01367
- Verma V, Ravindran P, Kumar PP. 2016. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 16:86. doi: 10.1186/s12870-016-0771-y
- Wang ZZ, Shi M, Huang YC, Wang XW, Stanley D, Chen XX. 2016. A peptidoglycan recognition protein acts in whitefly (Bemisia tabaci) immunity and involves in Begomovirus acquisition. Sci Rep. 6:37806. doi: 10.1038/srep37806
- Yamamoto T, Kashojiya S, Kamimura S, Kameyama T, Ariizumi T, Ezura H, Miura K. 2018. Application and development of genome editing technologies to the Solanaceae plants. Plant Physiol Biochem. 131:37–46. doi: 10.1016/j.plaphy.2018.02.019
- Zhang X, Xue M, Zhao H. 2015. Species-specific effects on salicylic acid content and subsequent Myzus persicae (Sulzer) performance by three phloem-sucking insects infesting Nicotiana tabacum L. Arthropod Plant Interact. 9(4):383–391. doi: 10.1007/s11829-015-9385-9
- Zhao H, Zhang X, Xue M, Zhang X. 2015. Feeding of whitefly on tobacco decreases aphid performance via increased salicylate signaling. PLoS One. 10(9):e0138584. doi: 10.1371/journal.pone.0138584
