?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The effects of 24-epibrassinolide (24-epiBL 10−8 M) were investigated on antioxidant enzymes activity, lipid peroxidation, and genes expression in 10-day-old Linum usitatissimum L. seedlings subjected to 6%, 12%, and 18% polyethylene glycol (PEG). Water deficit significantly enhanced the activity of CAT, POD, and SOD enzymes as well as elevation in protein, proline, and MDA contents whereas decreased activity of APOX and NR in a concentration-dependent manner. 24-epiBL seed priming improved flax adaptability due to the induction of antioxidant enzymes activity and protein and proline contents with a reduction in MDA content and antioxidant capacity. Gene expression studies showed that Mn-SOD, POD1, POD3, ERF, and WRKY 40 transcript levels were generally upregulated. Application of 24-epiBL caused induction in mRNA abundance of Mn-SOD, POD3, KRP2, and WRKY 40 under at least one level of PEG imposition. In conclusion, 24-epiBL seed priming could be implemented in order to counteract consequences of water deficit in flax.
Introduction
Flax (linseed), an annual herb, is one of the industrial oil seed crops grown in temperate climates. The seed oil of flax is enriched in α-linolenic acid (ALA) (18:3cisΔ9, 12, 15) which makes it useful for a variety of industrial products. In addition, ω-3-enriched flaxseed oil is important in livestock feed and aqua-feed applications. The linseed oil is also a good source of ALA in the human diet (Czemplik and Szopa Citation2009).
Drought is one of the abiotic stress factors, which significantly contributes to yield reduction worldwide. Water deficit induces oxidative damage and increases membrane lipid peroxidation (Abdul Jaleel et al. Citation2008). Plants mitigate abiotic stress-mediated consequences via adaptive capacity, mostly governed by the efficiency of the antioxidant defense system. This system includes ROS scavenging enzymes (such as superoxide dismutase, SOD; catalase, CAT; peroxidase, POD; and ascorbate peroxidase, APOX) and non-enzymatic components such as proline (Vardhini and Anjum Citation2015).
Brassinosteroids (BRs) are a class of polyhydroxylated steroidal phytohormones in plants. They are defined as the sixth plant hormone after auxin, gibberellins, cytokinin, abscisic acid, and ethylene (Tang et al. Citation2016). BRs play crucial roles in seed germination, immunity, reproduction, cell elongation, cell division, root growth, photo-morphogenesis, stomatal and vascular differentiation (Wei and Li Citation2016), radical oxidation, ethylene synthesis, and root gravitropic response. Moreover, they mediate plant responses to stresses such as nutrient deficiency, drought, salinity, freezing, heat, and disease (Ashraf et al. Citation2010).
One strategy to overcome water deficit is generation of drought tolerant crops through advanced molecular breeding techniques and biotechnological approaches. The prerequisite for this strategy is the identification of responsive genes in order to manipulate them. Transcription factors (TFs) are key players in the regulation of plant responses to abiotic stresses. They are activated or suppressed by protein kinases or phosphatases and interact with specific cis-elements in the promoter region of targeted genes (Danquah et al. Citation2014). ERFs belong to the large APETALA2 (AP2)/ERF TF superfamily and are involved in abiotic stress and disease resistance responses in plants (Trujillo et al. Citation2008). KRP2 gene plays a critical role in growth inhibition in response to drought and cold stress in plants (Sonju and Horvath Citation2005). WRKYs are also involved in gene regulation under multiple responses at the same time. They regulate stress and developmental responses as well as specialized metabolic pathways (Phukan et al. Citation2016). The MYB genes have been identified for their involvement in the regulation of abiotic stress responses (Li et al. Citation2015).
To the best knowledge of authors based on literature review, no research has been conducted on ameliorative effects of BR on deleterious impacts of drought stress at the seedling stage in flax, so far. This paper focuses on some triggered biochemical and molecular aspects under three severe levels of polyethylene glycol (PEG) treatments and 24-epiBL seed priming in flax.
Materials and methods
Hormone preparation and seed treatment
The 24-epiBL was purchased from Sigma, Alderich, and prepared in 10−8 M concentration by dilution of stock solution in distilled water (Hasan et al. Citation2014). Seeds of Linum usitatissimum L., TN-97-1 cultivar were obtained from the Agriculture Research and Natural Resources of West Azerbaijan, Urmia, Iran. The cultivar had been identified as cold sensitive variety (Ghoreishi et al. Citation2017). Based on preliminary experiments on some morphological and physiological parameters, the variety was also categorized as salt sensitive among 30 examined flax varieties (unpublished data). Seeds were surface sterilized with ethanol (70%) for 2 min, then with sodium hypochlorite (50%) solution for 4 min and later washed four times with sterilized water thoroughly. The seeds were soaked in sterilized water and kept in the refrigerator for 3 days. Then, seeds were soaked either in (i) sterilized water or in (ii) 24-epiBL (10−8 M) at 27 ± 2°C for 24 h under dark conditions. Afterwards, seeds were transferred to two sheets of sterile filter paper moistened with solutions including sterilized water, PEG (6%), PEG (12%), and PEG (18%) in sterile bottles and allowed to germinate in the dark at 27 ± 2°C for 3 days (Özdemir et al. Citation2004). After 3 days, bottles were retransferred to light conditions for 7 days. The temperature of the growth chamber was maintained at 27 ± 2°C and the light intensity was 350 µmol m−2 s−1. Daytime humidity was between 60% and 70%. Seedlings were harvested on day 7 and stored at an −80°C freezer for further analyses.
Enzyme extraction and protein determination
Flax samples (0.1 g) were ground in 2 ml of 50 mM Tris-HCL buffer (pH 7.5). Then, the extract was centrifuged at 10,000×g for 10 min at 4°C. The supernatant was used for the measurement of antioxidant enzymes activity. Soluble protein concentration was determined according to Bradford (Citation1976), using bovine serum albumin as a standard.
Antioxidant enzymes
The activity of ascorbate peroxidase enzyme (APOX, EC 1.11.1.1) was measured using the method of Behnamnia, Kalantari, and Ziaie (Citation2009). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbic acid, 0.1 mM EDTA, 0.15 mM H2O2, and 50 µl enzyme extract (supernatant). Oxidation of ascorbic acid was considered as a decrease in the absorbance at 290 nm, 1 min after the start of the reaction (€ = 2.6 mM−1 cm−1).
Catalase (CAT, EC 1.11.1.6) activity was assayed spectrophotometrically by monitoring a decrease in the absorbance of H2O2 at 240 nm using the method of Behnamnia, Kalantari, and Ziaie (Citation2009). The assay solution contained 50 mM potassium phosphate buffer (pH 7.0) and 15 mM H2O2. The reaction was started by addition of 60 µl enzyme extract to the reaction mixture. The change in absorbance was followed 1 min after the start of the reaction (€ = 43.6 mM−1 cm−1).
Peroxidase activity was determined using the guaiacol test (Behnamnia, Kalantari, and Ziaie Citation2009). The tetraguaiacol, formed in the reaction, has a maximum absorption at 470 nm and thus the reaction can be readily followed spectrophotometrically. The enzyme was assayed in a solution containing 50 mM phosphate buffer (pH 7.0), H2O2 (0.3%) and guaiacol (1%). The reaction started by the addition of 40 µl enzyme extract at 25°C (€ = 25.5 mM−1 cm−1).
Superoxide dismutase (SOD) reaction solution (3 ml) contained 50 µM NBT, 1.3 µM riboflavin, 13 mM methionine, 75 nM EDTA, 50 mM phosphate buffer (pH 7.8), and 20–50 µl of the enzyme extract. Test tubes containing the reaction solution were irradiated under light at 78 µmol m−2 s−1 for 15 min. The absorbance of the irradiated solution was read at 560 nm using a spectrophotometer (APEL PD 303 UV–Vis Spectrophotometer, Japanese). One unit of SOD activity was defined as the amount of enzyme that inhibited 50% ρ-nitro blue tetrazolium chloride (NBT) photoreduction (Abdoli Nejad and Shekafandeh Citation2014).
Nitrate reductase
The in vivo assay of nitrate reductase (NR) activity was done according to the procedure of Jaworski (Citation1971) with slight modifications. Fresh tissue of Linum usitatissimum L. was cut into 2 mm slices and placed in ice-cold incubation medium containing 1.5 ml of potassium phosphate buffer (100 mM, pH 7.2) and 1 ml of KNO3 (0.2 M) and 2 ml of isopropanol (5%). The sample tubes were centrifuged at 15,000×g for 15 min at 4°C. One milliliter of the supernatant was taken (i) and sample tubes were incubated in a water bath at 35°C for 60 min and then kept in boiling water for 5 min to stop the enzyme activity. One milliliter of the upper phase was again taken (ii). One milliliter of each sulfanilamide (1.0%) in 1N-HCl and N-(1-Napthyl)-ethylene diammonium dichloride (NEDD) (0.025%) in double distilled water were added to (i) and (ii) tubes. The pink color due to diazotization was allowed to develop for 30 min under dark conditions. The absorbance was read at 540 nm for both (i) and (ii), using the UV–Vis spectrophotometer.
Proline content
Free proline content was extracted from 0.1 g of flax samples in 3% (w/v) sulfosalicylic acid and estimated by using the ninhydrin reagent. The absorbance of fraction with toluene aspired from the liquid phase was read at 520 nm. Proline concentration was determined using a calibration curve and expressed as mmol proline g−1 FW (Özdemir et al. Citation2004).
Lipid peroxidation assay
Lipid peroxidation in samples was determined by measuring malondialdehyde (MDA), a major thiobarbituric acid reactive species and the product of lipid peroxidation (Heath and Packer Citation1968). The tissues (0.1 g) were ground in 1 ml of trichloroacetic acid (20%, w/v) and homogenates centrifuged at 15,000×g for 10 min at 4°C. An equal volume of supernatant and thiobarbituric acid (TBA) (5%) were added to TCA (20%), followed by heating at 96°C for 30 min, and thereafter cooled on ice for 5 min. The absorbance was read at 532 and 600 nm (€ = 156 mM−1 cm−1).
Ferric reducing antioxidant power assay
Measurement of ferric reducing antioxidant power (FRAP) of the herbal extract was carried out based on the procedure of Benzie and Strain (Citation1996). Firstly, sodium acetate buffer (300 mmol l−1, pH 3.6), 10 mmol l−1 TPTZ solution (40 mmol l−1 HCl as solvent), and 20 mmol l−1 iron (III) chloride solution were mixed in a volume ratio of 10:1:1 to generate FRAP reaction solution, which should be prepared fresh daily and be warmed to 37°C in a water bath before use. Then 150 µl methanolic extract of Linum usitatissimum L. was added to 3 ml of the FRAP reaction solution. After 4 min, the absorbance of the reaction mixture was recorded at 593 nm. The standard curve was constructed using FeSO4 solution, and results were expressed as µmol Fe(II) g−1 fresh weight of herbal material.
DPPH assay
In 3 ml of each diluted methalonic extract, 1 ml of methanol solution of DPPH (0.1 mmol l−1) was added. The mixture was kept in the dark at room temperature for 30 min and the absorbance measured at 517 nm against a blank. The following equation was used to determine the percentage of radical scavenging activity of each extract (Mensor et al. Citation2001).
RNA extraction and semi-quantitative RT–PCR
RNA samples were extracted by the CTAB method with slight modification (Gambino et al. Citation2008). First-strand cDNA was synthesized in a 12 µl reaction system containing 1 µl oligo dT, 2 µl total RNA, and 9 µl DEPS water at 65°C for 5 min. This was followed by addition of 2 µl dNTP, 1 µl reverse transcriptase, 1 µl RNase inhibitor, and 4 µl reaction buffer at 42°C for 1 h and 70°C for 5 min. RT–PCR reaction was performed according to the manufacturer’s instructions, with gene-specific primers (). PCR reaction was conducted in 25 µl volumes containing 12.5 µl master mix, 1 µl cDNA, 0.75 µM of each of the primers, and 10 µl H2O. The reactions were initiated at 95°C for 3 min, followed by 28–30 cycles of 95°C for 25 s, 58–62°C for 20 s, 72°C for 25 s, and a final extension at 72°C for 7 min. The intensity of PCR amplified bands was visualized under the UV and measured using a gel documentation system (Ingenius3, Syngene, UK).
Table 1. List of primers used in RT-PCR study.
Results
Antioxidant enzymes
Seedlings exposed to the PEG exhibited a significant decrease in APOX activity under 6% PEG (32%), 12% PEG (61%), and 18% PEG (76%) compared with the control group ((A)). The highest activity was observed in BR (22.05 U mg−1 protein) and the lowest detected under 18% PEG (3.97 U mg−1 protein). However, seed priming with BR enhanced the APOX activity to 18.3%, 31%, and 44% in BR + PEG (6%), BR + PEG (12%) and BR + PEG (18%) treated plants, respectively, compared to the PEG plants under the same PEG exposure. 24-epiBL seed priming increased (25%) the enzyme activity under the BR treatment compared to the control group.
Figure 1. (A) APOX enzymatic activity (U mg−1 protein), (B) CAT enzymatic activity (U mg−1 protein), (C) POD enzymatic activity (U mg−1 protein), (D) SOD enzymatic activity (U mg−1 protein), and (E) NR enzymatic activity (µmol g−1 h−1 NO−2) in 10 days old Linum usitatissimum L. seedlings under PEG-induced drought stress at 6%, 12%, and 18% with or without 24-epiBL(BR) application. C: control. Values are mean ± SE based on three replicates (n = 6).
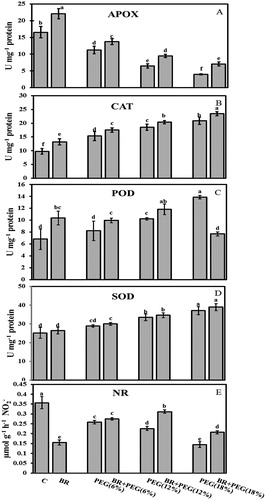
PEG-induced drought stress significantly elevated CAT activity to 15.3, 18.5, and 20.8 U mg−1 protein at 6%, 12%, and 18%, respectively, compared to the control group (9.7 U mg−1 protein) ((B)). However, 24-epiBL seed priming enhanced CAT enzymatic activity to 17.4 U mg−1 protein in BR + PEG (6%), 20.3 U mg−1 protein in BR + PEG (12%), and 23.4 U mg−1 protein in BR + PEG (18%) seedlings.
POD activity increased to 8.2, 10.2, and 13.8 U mg−1 protein in 6%, 12%, and 18% PEG-induced water deficit plants, respectively, compared to the controls (6.8 U mg−1 protein) ((C)). Seed priming with 24-epiBL enhanced POD activity to 9.9 and 11.8 U mg−1 protein in BR + PEG (6%) and BR + PEG (12%) plants, respectively, but reduced its activity to 7.6 U mg−1 protein under BR + PEG (18%) treatment.
Water deficit induced by PEG increased SOD activity to 28.85, 33.51, and 37.11 U mg−1 protein in seedlings exposed to PEG (6%), (12%), and (18%), respectively, compared to the control group (25.16 U mg−1 protein) ((D)). However, 24-epiBL application did not impose any significant change on SOD enzymatic activity under BR + PEG (6%) (30.00 U mg−1 protein), BR + PEG (12%) (34.62 U mg−1 protein), and BR + PEG (18%) (38.95 U mg−1 protein).
Nitrate reductase
The NR activity of 10-day-old flax seedlings significantly declined under PEG exposure ((E)). This reduction was reported 28.6% (PEG 6%), 37% (PEG 12%), and 60% (PEG 18%) compared to the control group (0.35 µmol g− h−1 NO−2), whereas 24-epiBL application enhanced NR activity to 7.5%, 29%, and 30.5% in 6%, 12%, and 18% PEG-treated seedlings, respectively.
Soluble protein content
The soluble protein content of 10-day-old flax seedlings significantly increased under PEG imposition ((A)). This enhancement was detected 2-, 3-, and 9-fold higher than control at PEG (6%), PEG (12%), and PEG (18%), respectively. The highest protein content was detected in the BR + PEG (18%) treated plants (7.68 mg g−1 FW).
Figure 2. (A) Soluble protein content (mg g−1 FW), (B) proline content (μmol g−1 FW), (C) MDA content (nmol g−1 FW) in 10 days old Linum usitatissimum L. seedlings under PEG-induced drought stress at 6%, 12%, and 18% with or without 24-epiBL(BR) application. C: control. Values are mean ± SE based on six replicates (n = 6).
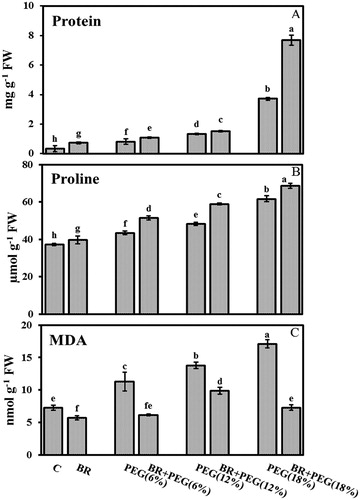
Proline content
PEG-induced drought stress caused elevation in the proline content ((B)). This induction was recorded 43.46, 48.33, and 61.72 µmol g−1 FW at exposures to 6%, 12%, and 18% PEG, respectively, compared to the control plants (37.29 µmol g−1 FW). 24-epiBL application revealed more elevation in the proline content and raised it to 51.47 µmol mg−1 FW in BR + PEG (6%), 58.97 µmol mg−1 FW in BR + PEG (12%), and 68.6 µmol mg−1 FW in BR + PEG (18%).
Lipid peroxidation
Seedlings grown under drought stress possessed comparatively higher MDA level compared to the control seedlings ((C)). This value was detected 1.5-, 1.8-, and 2.4-fold higher under PEG (6%), PEG (12%), and PEG (18%), respectively, compared to the control group. However, 24-epiBL reduced MDA content to almost the control level. There was no significant difference between BR + PEG (6%) (6.1 nmol g−1 FW) and BR + PEG (18%) (7 nmol g−1 FW) treated plants regarding the MDA content. The highest MDA content was identified in the PEG (18%)-treated plants (17 nmol g−1 FW) indicating more oxidative damage occurrence.
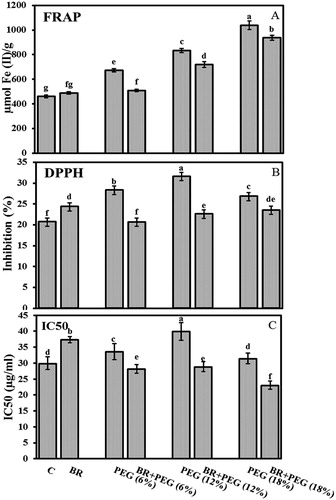
FRAP assay
The 10-day-old flax seedlings exhibited higher antioxidant activity of 673.51, 834.25, and 1037.96 µmol Fe (II) g−1 under imposition of PEG (6%), (12%), and (18%), respectively, compared to the control group (461.29 µmol Fe (II) g−1) ((A)). 24-epiBL seed priming significantly reduced the antioxidant capacity of BR + PEG (6%) to 509.6, BR + PEG (12%) to 719.4 and BR + PEG (18%) to 935.7 µmol Fe (II) g−1.
DPPH radical scavenging activity
Water deficit enhanced DPPH radical scavenging activity to 28.2%, 31.5%, and 26.7% in seedlings exposed to PEG (6%), (12%), and (18%), respectively, compared to the control seedlings (20.6%). Pre-sowing seed priming with 24-epiBL reduced DPPH radical scavenging activity to 20.6%, 22.5%, and 23.5% under BR + PEG (6%), BR + PEG (12%), and BR + PEG (18%) as compared with non-BR treated seedlings under the same PEG concentrations ((B)).
The IC50 values increased with PEG exposures: 33.5 µg ml−1 under 6% PEG, 39.9 µg ml−1 under 12% PEG, and 31.5 µg ml−1 under 18% PEG compared to the control group (29.9 µg ml−1). 24-epiBL application lowered IC50 values under water deficit to 28, 28.9, and 23 µg ml−1) in BR + PEG (6%), BR + PEG (12%), and BR + PEG (18%) seedlings, respectively ((C)).
Gene expression studies
Seedlings grown under 6% PEG possessed comparatively highest Mn-SOD transcript level compared to other PEG-treated seedlings. The gene mRNA abundance declined under PEG (12%) and (18%) exposures, compared to the control. 24-epiBL seed priming upregulated Mn-SOD expression under BR + PEG (12%) and BR + PEG (18%) treatments and returned it to almost control seedlings range ( and (A)).
Figure 4. RT–PCR gene expressions of Mn-SOD, POD1, POD3, ERF, WRKY, MYB, and KRP2 in Linum usitatissimum L. seedlings under PEG-induced drought stress at 6%, 12%, and 18% with or without 24-epiBL(BR) application.
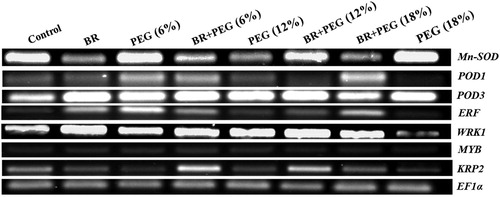
Figure 5. Relative mRNA levels of (A) Mn-SOD, (B) POD1, and (C) POD3 in Linum usitatissimum L. seedlings under PEG-induced drought stress at 6%, 12%, and 18% with or without 24-epiBL(BR) application.
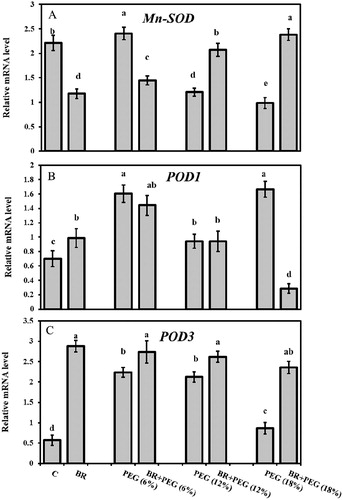
The POD1 gene expression was significantly elevated under PEG-induced water deficit, compared to the control seedlings, while priming with 24-epiBL down-regulated POD1 mRNA level ( and (B)). Similar to POD1, POD3 transcript also increased under PEG conditions. This induction was recorded 3.9-, 3.75-, and 1.5-fold higher under the imposition of PEG 6%, 12%, and 18%, respectively, in comparison to control plants ( and (C)). In response to BR signaling, two members of the POD gene family acted differently under PEG (18%) with POD1 tended to be repressed (82.5%) and POD3 upregulated (2.7-fold), compared to PEG (18%). However, under 24-epiBL treatments, POD enzymatic activity was more consistent with the expression of POD1gene.
The ERF mRNA was upregulated under PEG conditions, but most of all by PEG (18%) (3.5-fold), compared to the control. Upon 24-epiBL application, ERF transcript increased under BR + PEG (12%) but down-regulated in BR + PEG (6%) and BR + PEG (18%) seedlings, compared to the same PEG concentrations ( and (A)). Water deficit induced by PEG decreased KRP2 expression, 59%, 45%, and 26% under the imposition of 6%, 12%, and 18% PEG, respectively, compared to the control group. However, 24-epiBL seed priming resulted in the elevation in KRP2 transcript with 3.8- and 3-fold under 6% and 12% PEG treatments while decreasing its mRNA level (60%), compared to PEG (18%) ( and (B)).
Figure 6. Relative mRNA levels of (A) ERF, (B) KRP2, (C) WRKY40, (D) MYB in Linum usitatissimum L. seedlings under PEG-induced drought stress at 6%, 12%, and 18% with or without 24-epiBL(BR) application.
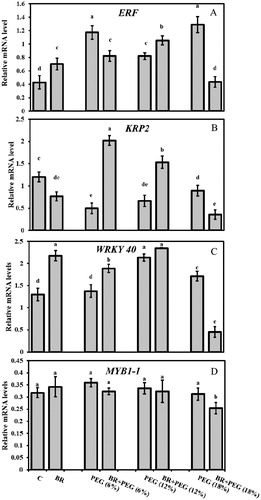
The transcript level of WRKY 40 significantly increased under PEG (12%) and PEG (18%) with maximum induction of 1.65-fold at PEG (12%), compared to the control. 24-epiBL application upregulated WRKY 40 mRNA level (1.37-fold) under BR + PEG (6%) in comparison to PEG (6%) exposed seedlings ( and (C)). Application of BR + PEG (18%) decreased the accumulation of WRKY 40 transcript (76%) compared to the PEG (18%).
The MYB1-1 transcript abundance was not influenced by PEG-induced drought stress in flax seedlings and expressed at almost constant level. However, the gene expression was significantly down-regulated under application of BR + PEG (18%) ( and (D)).
Discussion
24-epiBL induction of antioxidant enzymes activity under drought stress
In the present study, PEG-induced drought stress significantly affected the activity of antioxidant enzymes. The APOX is recognized as the most important peroxidase in H2O2 detoxification (Michalak Citation2006). In this study, APOX activity decreased under PEG-induced water deficit while increased with BR seed priming under drought stress indicating the efficient scavenging of H2O2, thereby preventing the H2O2-mediated cell damage (Mahesh et al. Citation2013). In accordance with our data, Behnamnia, Kalantari, and Ziaie (Citation2009) reported a decrease in APOX activity under water deficit. Similarly, Bajguz (Citation2010) observed that exogenous application of 24-epiBL increased the activity of APOX in Chlorella cultures under heavy metal stress. The higher APOX enzyme activity induced by BR indicates better protection capacity against oxidative damage.
Water stress induced by PEG led to an elevation in the activity of CAT in linseed seedlings and BR seed priming resulted in further elevation of CAT activity under PEG imposition. Consistent with our results, Mahesh et al. (Citation2013) reported an increase in CAT activity in radish seedlings treated with BRs under water stress. Induction in CAT activity could explain the oxidation of harmful substances, leading to the restoration of growth under water deficit. Cao et al. (Citation2005) suggested that BRs enhance the oxidative stress resistance in Arabidopsis by inducing transcript level of the CAT gene. These results are in agreement with Behnamnia, Kalantari, and Ziaie (Citation2009), who reported that 24-epiBL increases CAT activity under drought stress in Lycopersicon esculentum. Our data indicate that exogenous BR application protects flax seedlings against drought stress by elevation in CAT activity.
Peroxidases are members of oxidoreductase family catalyzing the hydrogen peroxide-dependent oxidation reaction. They have numerous physiological and biochemical functions in living organisms such as physical damage protection of tissues, defense against pathogens, and general stress responses (Ajila and Prasada Citation2009). In the present study, peroxidase activity increased in water stressed seedlings compared with the control. Increase in peroxidase activity has been reported as a mechanism of plant tolerance to water deficit (Zoz et al. Citation2013). Behnamnia, Kalantari, and Ziaie (Citation2009) and Behnamnia, Kalantari, and Rezanejad (Citation2009) showed an elevation in POD enzyme activity due to BRs application under drought imposition, which is in accordance with our findings at 6% and 12% PEG, showing alleviation of oxidation damage. However, the 24-epiBL reduced POD activity in BR + PEG (18%) seedlings to the level of control which shows minimum oxidative damage to cellular components through a well-coordinated antioxidant system response consisting of several enzymes (MDA results also confirm this conclusion). In agreement with our data, reduction of POD enzymatic activity was reported in heavy metal stressed radish seedlings under application of BR (Anuradha and Rao Citation2007).
SOD enzyme constitutes the first line of defense against ROS in plants, catalyzing the detoxification of O2− to H2O2 and O2 (Alscher et al. Citation2002). In the present study, increase in SOD activity was observed in water stressed Linum usitatissimum L. seedlings and BR seed priming did not significantly alter SOD activity under both normal and water stress conditions. A significant elevation in SOD activity has been reported for water stressed radish seedlings under exogenous application of BRs (Mahesh et al. Citation2013) which is not supported by our data. It seems SOD enzymatic activity is not influenced by BR in drought stressed flax seedlings.
24-epiBL enhances NR activity under water deficit
Nitrate has been widely distributed in diverse tissues of plants and NR is a key enzyme for nitrogen acquisition. Estimation of NR activity can be considered as a stress index where nitrate is the main form of N available for plants grown in soils (Caravaca et al. Citation2003). In this study, total NR activity decreased as a result of water stress. In agreement with our findings, lower NR activity has been shown in barely (Krček et al. Citation2008) and winter wheat (Xu and Yu Citation2006) plants exposed to drought stress compared to the plants under optimal water regime. Based on our data, NR activity increased with the application of 24-epiBL hormone indicating better nitrate acquisition in drought stressed seedlings under BR application.
24-epiBL elevates protein content in drought stressed seedlings
Proteins are involved in almost every biological functions. In the present work, soluble protein content increased under PEG imposition. In accordance with our findings, Perveen et al. (Citation2016) observed an enhancement in the protein level of plants exposed to drought stress. The 24-epiBL application displayed more elevation in protein content revealing that BR alleviates the detrimental effects of drought in flax by enhancing the content of protein. According to Balaraju et al. (Citation2015), exogenous application of BRs also improved soluble protein content in radish seedlings.
24-epiBL increases proline content under drought imposition
Proline can accumulate to high concentration without damaging cellular macromolecules. Therefore, it acts as a compatible osmolyte. Importantly, proline provides protection against membrane damage and protein denaturation during severe drought stress (Ain-Lhout et al. Citation2001). In this study, proline was progressively accumulated in flax seedlings as drought levels increased. Manuchehri and Salehi (Citation2014) reported elevation in the proline content with increasing salt or drought stress in bermudagrass (Cynodon dactylon L.). Based on our observations, BR seed priming enhanced the proline content under water deficit indicating more alleviation of drought stress. In accordance with our findings, there is a report revealing an increase in the proline content following application of BRs (28-HBL and 24-EBL) in sorghum plants exposed to osmotic stress (Vardhini and Rao Citation2003).
24-epiBL reduces MDA content in water stressed seedlings
Peroxidation of cell membranes is closely related to damages caused by some environmental stresses (Abdul Jaleel et al. Citation2008). In this work, the lipid peroxidation level significantly increased under drought stress and decreased with BR application. Based on our results for MDA assay, it is very possible that BR effectively protects cell membranes by enhancing the activity of antioxidant enzymes. Our data correspond with the induction of the lipid peroxidation level triggered by NaCl, which was significantly reduced in the BR-treated rice seedlings under salt stress (Özdemir et al. Citation2004).
24-epiBL decreases antioxidant capacity in PEG-induced drought stressed seedlings
The FRAP method was used to determine antioxidant activity due to the reduction of ferric ion to the ferrous form in the presence of antioxidant compounds (Benzie and Strain Citation1996). The results showed that the antioxidative capacity of flax seedlings increased during the stress period. In accordance with our observation, Astghik (Citation2016) reported induction in antioxidant capacity under drought stress based on the FRAP method. Exogenous application of BR exhibited a decrease in antioxidant capacity of flax seedlings grown under water deficit conditions demonstrating lower oxidative status.
The reactivity of flax seedlings was analyzed by DPPH, a stable free radical, under different treatments. As DPPH picks up one electron in the presence of a free radical scavenger, the absorption decreases and the resulting discoloration is stoichiometrically correlated to the number of electrons gained (Saeed et al. Citation2012). The results of the DPPH assay revealed that DPPH radical scavenging activity of samples increased significantly under water deficit conditions whereas BR seed priming resulted in lowering antioxidant levels demonstrating better oxidative stress management in flax seedlings. The induction of oxidative burst followed by the activation of the antioxidative system has also been shown in the previous studies (Habibi et al. Citation2004; Saleh and Plieth Citation2009).
24-epiBL enhances Mn-SOD, POD3, KRP2, and WRKY 40 genes expression under water deficit
SODs are found in different cellular compartments and classified into three different types depending on metal co-factors: iron (Fe-SOD), copper–zinc (Cu/Zn-SOD), and manganese (Mn-SOD) (Fukai and Ushio-Fukai Citation2011). A study indicates that the drought tolerant genotype has higher SOD expression than the drought-sensitive genotype (Shiriga et al. Citation2014). In the present study, Mn-SOD expression decreased under moderate and high PEG treatments indicating the role of Mn-SOD as an oxidative stress response gene. However, the application of 24-epiBL upregulated the gene transcript level revealing Mn-SOD association of improved oxidative stress tolerance. The transcript abundance of the Mn-SOD gene did not accompany the activity of SOD enzyme under some of PEG and 24-epiBL treatments. This inconsistency could be due to the fact that at the enzymatic activity level, all three types of SOD enzymes are measured together.
POD catalyzes the reduction of H2O2 and its activity has been induced under drought in leaves of Ramonda serbica (Veljovic-Jovanovic et al. Citation2006) and Medicago sativa (Wang et al. Citation2009). In our study, the elevation in POD1 or POD3 transcripts abundance was accompanied with higher POD enzymatic activity in water stressed plants. BRs can induce expression of some antioxidant genes and enhance the activity of antioxidant enzymes such as POD (Ogweno et al. Citation2008). According to the results, POD1 and POD3 genes are represented as BR-responsive genes indicating the regulatory role of BR in their transcription. However, POD1 and POD3 regulated by BR showed the opposite response in BR + PEG (18%) seedlings. This phenomenon makes the BR as a positive regulator of POD3 and a negative regulator of POD1 under the imposition of severe drought condition.
TFs help plants to withstand unfavorable drought conditions. These genes are potential genomic candidates due to their wide application in crop breeding (Joshi et al. Citation2016). TFs regulate gene expression through binding to cis-regulatory elements in the promoter region of stress-related genes. Hence, expression modification of these regulatory genes can highly influence plant stress tolerance (Joshi et al. Citation2016).
The AP2/ERF TF superfamily constitutes one of the biggest gene families in plants (Wessler Citation2005 ) performing diverse roles in biological processes, such as cell proliferation, vegetative and reproductive development, plant hormone, and abiotic/biotic stress responses (Sharoni et al. Citation2011; Xu et al. Citation2011). In this study, expression of ERF gene was upregulated in PEG exposed flax seedlings as stress alleviative. In agreement with our results, overexpression of ERF genes enhanced drought and salt tolerance in Arabidopsis (Hinz et al. Citation2010). However, BR declined ERF transcript to the pre-stress level as an adaptation response.
The KRP genes play a central role in the regulation of cell cycle differentiation through modulation of cyclin-dependent kinases (Himanen et al. Citation2002). They have also been identified to play a role in reprogramming cell proliferation and cell expansion under drought stress (Claeys and Inze Citation2013; Guan et al. Citation2014). In this study, expression of KRP2 gene decreased under drought stress revealing the gene as drought stress responsive gene. Application of 24-epiBL enhanced KRP2 transcript accumulation, making the gene, in part, responsible for drought tolerance in BR-treated plants.
WRKY, one of the largest and important TF families in plants, are involved in stress response (Yamasaki et al. Citation2013) and tolerance against various abiotic stresses such as drought stress (Karkute et al. Citation2015). In Arabidopsis, WRKY21 is highly expressed under drought stress and functions as an activator of the ABA signaling pathway. Elevated expression of WRKY57 also improved drought tolerance of Arabidopsis by elevation of the ABA level (Jiang et al. Citation2012). In this study, expression of WRKY 40 increased under PEG treatments in flax seedlings and even more induced with 24-epiBL application demonstrating tolerance improvement and helping the plant to withstand against deleterious effects of imposed stress.
MYBs, a large family of TFs in plants, regulate plant specific processes and biological functions such as phenylpropanoid metabolism (Hichri et al. Citation2011), biotic and abiotic stresses, and plant defense reactions (Segarra et al. Citation2009). In this work, the MYB1-1 mRNA level was not influenced by drought stress and expressed at a constant level. Hence, other MYB family members may contribute to develop an adaptive response. Katiyar et al. (Citation2012) showed upregulation of 51% and downregulation of 41% of AtMYB genes under drought condition.
Conclusion
The environmental stresses are the primary cause of crop loss worldwide and their adverse impacts are getting more serious in the past few decades. Thus, our understanding of mechanisms of drought stress tolerance and developing varieties that are more resilient must be improved. The present study revealed better protection mechanism of flax against drought stress under BR seed priming. BR application ameliorated the negative impacts of water deficit by reducing membrane lipid peroxidation and antioxidant capacity due to the enhancement in the activity of APOX, CAT, POD, and SOD enzymes. Moreover, BR application increased the accumulation of proline and protein and elevated NR enzymatic activity in PEG-induced drought stressed seedlings. At the molecular level, application of 24-epiBL caused induction in mRNA abundance of Mn-SOD, POD3, KRP2, and WRKY 40 genes under at least one level of PEG treatment, helping flax plant to withstand against deleterious effects of imposed stress. Since water deficit is a global problem, BR seed priming seems to be a good solution to reduce the harmful effects of drought stress.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Parastoo Aghaee
Mrs Parastoo Aghaee: MS in Plant Physiology.
Fatemeh Rahmani
Dr. Fatemeh Rahmani: PhD, Associate Professor in Biotechnology.
References
- Abdoli Nejad R, Shekafandeh A. 2014. Salt stress-induced changes in leaf antioxidant activity, proline and protein content in ‘Shah Anjir’ and ‘Anjir Sabz’ fig seedlings. J Hortic Sci Technol. 1(2):121–129.
- Abdul Jaleel C, Sankar B, Murali PV, Gomathinayagam M, Lakshmanan GM, Panneerselvam R. 2008. Water deficit stress effects on reactive oxygen metabolism in Catharanthus roseus; impacts on ajmalicine accumulation. Colloids Surf. 62:105–111.
- Ain-Lhout F, Zunzunegui M, Barradas MCD, Tirado R, Clavijo A, Novo FG. 2001. Comparison of proline accumulation in two Mediterranean shrubs subjected to natural and experimental water deficit. Plant Soil. 230:175–183.
- Ajila CM, Prasada R. 2009. Purification and characterization of black gram (Vigna mungo) husk peroxidase. J Mol Catal B Enzym. 60(1-2):36–44.
- Alscher RG, Erturk N, Heath LS. 2002. Role of super-oxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot. 53(372):1331–1341.
- Anuradha S, Rao SSR. 2007. The effect of brassinosteroids on radish (Raphanus sativus L.) seedlings growing under cadmium stress. Plant Soil Environ. 53(11):465–472.
- Ashraf M, Akram NA, Arteca RN, Foolad MR. 2010. The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Crit Rev Plant Sci. 29:162–190.
- Astghik S. 2016. Antioxidant capacity of maize corn under drought stress from the different zones of growing. Int J Biol Biomol Agric Food Biotechnol Eng. 10:8.
- Bajguz A. 2010. An enhancing effect of exogenous brassinolide on the growth and antioxidant activity in Chlorella vulgaris cultures under heavy metals stress. Environ Exp Bot. 68:175–179.
- Balaraju P, Ayodhya-Ramulu C, Venkateshwarlu M, Ugandhar T. 2015. Influence of PEG imposed water stress and exogenous application of brassinosteroids on metabolites in radish. Asian J Sci Tech. 6(1):951–955.
- Behnamnia M, Kalantari K, Rezanejad F. 2009. Exogenous application of brassinosteroid alleviates drought-induced oxidativestress in Lycopersicon esculentum L. Gen Appl Plant Physiol. 35(1–2):22–34.
- Behnamnia M, Kalantari K, Ziaie J. 2009. The effects of brassinosteroid on the induction of biochemical changes in Lycopersicon esculentum L. under drought stress. Turk J Bot. 33:417–428.
- Benzie IFF, Strain JJ. 1996. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal Biochem. 239:70–76.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254.
- Cao DN, Hussain A, Cheng H, Peng JR. 2005. Loss of function of four DELLA genes leads to light- and gibberellin-independent seed germination in Arabidopsis. Planta. 223:105–113.
- Caravaca F, Alguacil MM, Diaz G, Roldan A. 2003. Use of nitrste reductase activity for assessing the effectiveness of mycorrhizal symbiosis in Dorycnium pentsphyllum under induced water deficit. Comm Soil Sci Plant Anal. 34:2291–2302.
- Claeys H, Inze D. 2013. The agony of choice: how plants balance growth and survival under water-limiting conditions. Plant Physiol. 162:1768–1779.
- Czemplik M, Szopa J. 2009. Optimizing biomedical and industrial products development based on flax. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour. 4(062):1–10.
- Danquah A, de Zelicourt A, Colcombet J, Hirt H. 2014. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv. 32:40–52.
- Dmitriev AA, Kudryavtseva AV, Krasnov GS, Koroban NV, Speranskaya AS, Krinitsina AA. 2016. Gene expression profiling of flax (Linum usitatissimum L.) under edaphic stress. BMC Plant Biol. 16(3):927.
- Fukai T, Ushio-Fukai M. 2011. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 15(6):1583–1606.
- Gambino S, Perrone I, Gribauto I. 2008. A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem Anal. 19:520–525.
- Ghoreishi M, Rahmani F, Abdollahi Mandoulakani B, Hassanzadeh A. 2017. Impact of variety on resistance to cold stress at physiological levels in Linum usitatissimum. POJ. 10:269–276.
- Guan P, Wang R, Nacry P, Breton G, Kay SA, Pruneda-Paz JL, Davani A, Crawford NM. 2014. Nitrate foraging by Arabidopsis roots is mediated by the transcription factor TCP20 through the systemic signaling pathway. Proc Natl Acad Sci USA. 111:15267–15272.
- Habibi D, Boojar MMA, Mahmoudi A, Ardakani A, Taleghani D. 2004. Antioxidative enzymes in sunflower subjected to droughy stress. Proceedings of 4Th International Crop Science Congress; Sep 26–Oct 1; Brisbane, Australia.
- Hasan SA, Irfan M, Hayat S. 2014. Response of tomato cultivars on yield and quality attributes applied with two different modes of BR analogues: A comparative study. International Conference on Advances in Agricultural, Biological & Environmental Sciences; (AABES-2014) Oct 15–16, Dubai (UAE).
- Heath RL, Packer L. 1968. Photo peroxidation in isolated chloroplasts. Arch Biochem Biophys. 125:850–857.
- Hichri I, Barrieu F, Bogs J, Kappel C, Delrot S, Lauvergeat V. 2011. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J Exp Bot. 62:2465–2483.
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T. 2002. Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell. 14:2339–2351.
- Hinz M, Wilson IW, Yang J, Buerstenbinder K, Llewellyn D. 2010. Arabidopsis RAP2.2: an ethylene response transcription factor that is important for hypoxia survival. Plant Physiol. 153:757–772.
- Huis R, Hawkins S, Neutelings G. 2010. Selection of reference genes for quantitative gene expression normalization in flax (Linum usitatissimum L.). BMC Plant Biol. 10:71.
- Jaworski EK. 1971. Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Comm. 43:1274–1279.
- Jiang Y, Liang G, Yu D. 2012. Activated expression of WRKY57 confers drought tolerance in Arabidopsis. Mol Plant. 5:1375–1388.
- Joshi R, Wani SH, Singh B, Bohra A, Dar ZA, Lone AA, Pareek A, Singla-Pareek SL. 2016. Transcription factors and plants response to drought stress: Current understanding and future directions. Front Plant Sci. 7:1029.
- Karkute SG, Easwaran M, Gujjar RS, Piramanayagam S, Singh M. 2015. Protein modeling and molecular dynamics simulation of SlWRKY4 protein cloned from drought tolerant tomato (Solanum habrochaites) line EC520061. J Mol Model. 21:255.
- Katiyar A, Smita S, Lenka SK, Rajwanshi R. 2012. Genome-wide classification and expression analysis of MYB transcription factor families in rice and Arabidopsis. BMC Genomics. 13:544.
- Krček M, Slamka P, Olšovská K, Brestič M, Benčíková M. 2008. Reduction of drought stress effect in spring barley (Hordeum vulgare L.) by nitrogen fertilization. Plant Soil Environ. 54(1):7–13.
- Li C, Ng CKY, Fan LM. 2015. MYB transcription factors, active players in abiotic stress signaling. Environ Exp Bot. 114:80–91.
- Mahesh B, Parshavaneni B, Ramakrishna B, Rao SSR. 2013. Effect of brassinosteroids on germination and seedling growth of radish (Raphanus sativus L.) under PEG-6000 induced water stress. Am J Plant Sci. 4:2305–2313.
- Manuchehri R, Salehi H. 2014. Physiological and biochemical changes of common bermudagrass (Cynodon dactylon [L.] Pers.) under combined salinity and deficit irrigation stresses. S Afr J Bot. 92:83–88.
- Mensor LL, Menezes FS, Leitao GG, Reis AS, Dos-Santos TC, Coube CS. 2001. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 15:127–130.
- Michalak A. 2006. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud. 15(4):523–530.
- Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH. 2008. Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul. 27:49–57.
- Özdemir F, Bor M, Demiral T, Turkan I. 2004. Effect of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidant system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regul. 41:1–9.
- Paynel F, Schaumann A, Arkoun M, Douchiche O, Morvan C. 2009. Temporal regulation of cell-wall pectin methylesterase and peroxidase isoforms in cadmium-treated flax hypocotyl. Ann Bot. 104:1363–1372.
- Perveen SH, Iqbal M, Nawaz A, Parveen A, Mahmood S. 2016. Induction of drought tolerance in Zea mays l. by foliar application of triacontanol. Pak J Bot. 48(3):907–915.
- Phukan UJ, Jeena GS, Shukla RK. 2016. WRKY transcription factors: molecular regulation and stress responses in plants. Front Plant Sci. 7:760.
- Saeed N, Khan MR, Shabbir M. 2012. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 12:221.
- Saleh L, Plieth C. 2009. Fingerprinting antioxidative activities in plants. Plant Methods. 5:2.
- Segarra G, Van der ES, Trillas I, Pieterse CMJ. 2009. MYB72, a node of convergence in induced systemic resistance triggered by a fungal and a bacterial beneficial Microbe. Plant Biol. 11:90–96.
- Sharoni AM, Nuruzzaman M, Satoh K, Shimizu T, Kondoh H, Sasaya T, Choi IR, Omura T, Kikuchi S. 2011. Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol. 52:344–360.
- Shiriga K, Sharma R, Kumar K, Yadav S, Hossain F, Thirunavukkarasu N. 2014. Expression pattern of superoxide dismutase under drought stress in maize. Int J Innovative Res Sci Eng Technol. 3:11333–11337.
- Sonju R, Horvath DP. 2005. Cloning and expression of KRP genes from adventitious buds of the perennial weed leafy spurge. (Abstract) Midwestern Society of the American Society of Plant Biology.
- Tang J, Han ZH, Chai J. 2016. Q&A: what are brassinosteroids and how do they act in plants? BMC Biol. 14:113.
- Trujillo LE, Sotolongo M, Menéndez C, Ochogavía ME, Coll Y, Hernández I, BorrásHidalgo O, Thomma BPHJ, Vera P, Hernández L. 2008. SodERF3, a novel sugarcane ethylene responsive factor (ERF), enhances salt and drought tolerance when overexpressed in tobacco plants. Plant Cell Physiol. 49(4):512–525.
- Vardhini BV, Anjum NA. 2015. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front Environ Sci. 2:67.
- Vardhini BV, Rao SSR. 2003. Amelioration of water stress by brassinosteroids on seed germination and seedling growth of three varieties of sorghum. Plant Growth Regul. 41:25–31.
- Veljovic-Jovanovic S, Kukavica B, Stevanovic B, Navari-Izzo F. 2006. Senescence- and drought-related changes in peroxidase and superoxide dismutase isoforms in leaves of Ramonda serbica. J Exp Bot. 57:1759–1768.
- Wang WB, Kim YH, Lee HS, Kim KY, Deng XP, Kwak SS. 2009. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Biochem. 47:570–577.
- Wei Z, Li J. 2016. Brassinosteroids regulate root growth, development, and symbiosis. Mol Plant. 9:86–100.
- Wessler SR. 2005. Homing into the origin of the AP2 DNA binding domain. Trends Plant Sci. 10:54–56.
- Xu ZS, Chen M, Li LC, Ma YZ. 2011. Functions and application of the AP2/ERF transcription factor family in crop improvement. J Integr Plant Biol. 53:570–585.
- Xu ZZ, Yu ZW. 2006. Nitrogen metabolism in flag leaf and grain of wheat in response to irrigation regimes. J Plant Nutr Soil Sci. 169:118–126.
- Yamasaki K, Kigawa T, Seki M, Shinozaki K, Yokoyama S. 2013. DNA-binding domains of plant-specific transcription factors: structure, function, and evolution. Trends Plant Sci. 18(5):267–276.
- Zoz T, Steiner F, Guimaraes VF, Castagnara DD, Meinerz CC, Fey R. 2013. Peroxidase activity as an indicator of water deficit tolerance in soybean cultivars. Biosci J. 29:1664–1671.