ABSTRACT
Research on nanotechnology as an emerging discipline has advanced several branches of technology. Although iron is considered as an essential element for plant growth, its role in mitigating abiotic stresses has not been studied widely. Therefore, in this research, it has been attempted to investigate the effect of magnetic Fe3o4 nanoparticles on tomato plants under cadmium stress using 5 levels of magnetic (Fe3O4) nanoparticles (nano-Fe3O4) (0, 10, 20, 50, 100 mg/L) and 3 levels of CdCl2 (0, 100, 200 μM). Cadmium reduced growth and photosynthesis parameters as well as nutritional elements and increased the content of MDA, H2O2, and proline in tomato plants. Meanwhile 20 mg/L nano-Fe3o4 was able to improve cadmium toxicity by reduction in cadmium accumulation and increase in nutrient intake. However, 20 mg/L nano-Fe3o4 is potentially useful for plant growth and may motivate the variations of plants defense mechanisms in response to cadmium toxicity.
1. Introduction
The occurrence of high levels of heavy metals like copper (Cu), nickel (Ni), cadmium (Cd), and cobalt (Co) in the environment is a major potential threat to ecosystems and also an important worldwide environmental concern (Chen et al. Citation2017).
Among all toxic heavy metals, cadmium leads to a high level of damage to plants as well as human health. Cadmium, which is not an essential element for plants, can be easily absorbed and distributed to different parts of plant and then transferred to human body through the food chain (Gill et al. Citation2013; Kao Citation2014). Due to its hydrophilic nature, this element is readily absorbed and accumulated in plants (Hasan et al. Citation2011). Cadmium causes changes in several vital growth processes, including mineral nutrition transpiration, enzyme activities that are related to metabolism and biosynthesis of chlorophyll and nucleic acids (Ouariti et al. Citation1997; Gill et al. Citation2013), resulting in invisible damage symptoms to plants such as chlorosis, necrosis, browning of root tip, and eventually death (Gill et al. Citation2013). The mechanism of Cd toxicity has not been fully understood, and a likely mechanism is Cd binding to sulfhydryl and/or carboxyl groups or replacement of essential co-factors such as Zn with Cd (Hasan et al. Citation2011).
Nanotechnology, which is an evolving field of twenty-first century, has had a crucial impact on peoples’ lives and has enhanced the quality of life via applications in several fields, including agriculture and food technology (Tripathi et al. Citation2017). Nanoparticles have important characteristic such as high surface-to-volume ratio, optical, thermal, and electrical characteristics, which have prominent physical, chemical, and biological properties with regard to uptake and activity, and the higher surface area to volume ratio of nanoparticles causes them to be better than their counterparts (Rastogi et al. Citation2017).
Iron is an essential microelement associated with many physiological reactions and is the fourth in terms of importance among abundant elements, but its amount is low or insufficient for plant requirements (Askary et al. Citation2016; Askary et al. Citation2017). Fe is mostly found in insoluble Fe3+ form, especially in high-pH as well as aerobic soils; hence, such soils are usually deficient in Fe2+ form of iron (Rui et al. Citation2016). Because of low solubility of minerals containing iron in many places of the world, one solution to address iron deficiency is the use of nanoparticles (Askary et al. Citation2017).
Iron nanoparticles that interact at molecular level within living cells have the potential to improve the capacity of plants to absorb nutrients (Hasan et al. Citation2011). Iron oxide nanoparticles are smaller than typical iron oxide molecules and create more complexes providing higher iron levels to plants. Enhancement growth, facilitation of transfer of materials, and exploitation of new Fe fertilizers with nanotechnology as well as nanomaterials may provide alternate methods of eliminating symptoms of Fe chlorosis (Sheykhbaglou et al. Citation2018). Iron oxide nanoparticles are among the most important oxides in the field of nanomaterials, which are also present in nature as nano-sized crystals in the form of both maghemite (Fe2O3) and magnetite (Fe3O4) (Bombin et al. Citation2015; Rui et al. Citation2016), and the latter nanoparticles have been successfully used to absorb heavy metal ions such as cadmium (Konate et al. Citation2017).
Tomato (Solanum lycopersicum L.) ranks as the second plant with the highest level of commercial consumption after potato in planting area and production. Also it uses as a model plant in experiments (Hashem et al. Citation2015). There is little information on magnetite (Fe3O4) nanoparticle on regulation of abiotic stress and associated mechanisms. Hence, in this study, our objectives is to investigate comparative impact of magnetite (Fe3O4) nanoparticles under Cd stress on tomato plants by analyzing growth, photosynthesis pigments, oxidative stress, and mineral elements’ regulation.
2. Materials and methods
2.1. Exposure of tomato plants
Tomato (cv. Calj-N3) seeds were procured from National Seed Corporation, Tehran. Before being rinsed three times with deionized water, the tomato seeds were sterilized by soaking in 10% sodium hypochlorite solution for 10 min. The seeds (10 seed per group) were then set on a wet filter paper in 100 × 15 mm2 Petri dishes and just deionized water was added to Petri dishes. The dishes were covered and the seeds were left to produce radicals over 72 h at 25°C in 75% relative humidity and a dark growth chamber. After 4 days seed germination, plants were transferred to plastic pots. The germinated seeds were sown in plastic pots (20 × 30 cm2) and grown under a photon flux density (PFD) of 230 µmol photons m−2 s−1 and relative humidity of 55–65% with a light and dark periods 16/8 h at 23 ± 2°C for 14 days in a growth chamber and nutritioned with 0.5 strength Hoagland's solution.
2.2. Preparing Fe3O4 magnetic nanoparticles
A chemical co-precipitation method was used to prepare magnetic nanoparticles of Fe3O4, so that under nitrogenous atmosphere, Fe3+ chloride hexahydrate (4.6 g, 0.017 M) as well as Fe2+ chloride tetrahydrate (2.2 g, 0.011 M) were dissolved in 100 mL of deionized water at 60°C and vortexed. Afterwards, 10.0 mL of 25% NH4OH was quickly injected into the reaction mixture, which was incubated for another 60 min, after which the mixture was cooled up to ambient temperature. Subsequently, the ensuing magnetic particles were subject to external magnetic separation and then several times washed using deionized water. Finally, the product was dehydrated under room temperature.
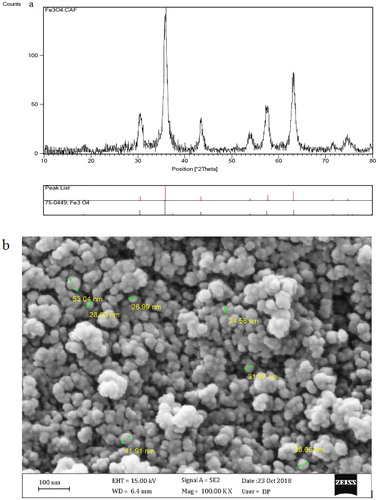
The presence of magnetite nanoparticles was indicated by XRD (a) and the size and shape of nanoparticles were determined using transmission electron microscope (Day Petronic Co. Tehran, Iran). SEM micrograph obviously illustrated the size variation of magnetite nanoparticles from 24.56 to 53.04 nm (b).
2.3. Preparation of nano-Fe3O4 and treatment with cadmium
We used a hydroponic culture system to evaluate the following: effects of nano-Fe3O4 on tomato plants, impact of cadmium stress on plant growth as well as that of nano-Fe3O4 on reduction of this stress, especially the oxidative stress induced by cadmium. This testing with three replications was conducted as a randomized complete block design. The plant growth was investigated at 0, 10, 20, 50 and 100 mg/L Fe3O4 nanoparticles with 0, 100, or 200 µM CdCl2. Nano-Fe3O4 was directly suspended in deionized water, after which ultrasonic vibration was applied for 40 min to have uniformly dispersed stable nano-Fe3O4 suspensions in the mentioned concentrations. After seedling transplantation, nano-Fe3O4 was sprayed on 14-day-old plants, once per day up to run off for 5 days. Then, Cd was applied on plants for 14 days. The following parameters were measured at the end of experiment both shoot and root: fresh weight, dry weight, length and leaf area. Aluminum foil was used to cover fresh samples, which were frozen in liquid nitrogen and stored at −80°C until physiological and biochemical analyses such as chlorophyll, malondialdehyde (MDA), hydrogen peroxide (H2O2), proline, reduced sugar, soluble sugar, total protein, free amino acid (FAA) content, and atomic absorption.
2.4. Estimation of physio-biochemical characteristics
2.4.1. Growth parameters and photosynthetic pigments
Shoot and root fresh weight, dry weight, length and leaf area of treated and untreated tomato plants were systematically measured as described. Shoot and root dry weight was determined by drying samples in an oven for 24 h at 75°C until a constant weight was achieved. The leaf surface area of treated and untreated plant was determined using equation Y = (X − -0.001)/0.007 here Y is Leaf area (cm2) and X is Paper Weight (g). To measure the photosynthetic pigments, 20 mg of fresh leaves from control and treated seedlings was crushed in 80% acetone; the pigments were then extracted and centrifuged. The absorbance of extract was read at 663, 646, and 470 nm using UV–visible spectrophotometer (Model 1700, Shimadzu, Japan). The total quantity of chlorophyll was calculated according to the Lichtenthaler method (Citation1987).
2.4.2. Oxidative stress markers: malondialdehyde, hydrogen peroxide and proline
MDA content was determined according to the Heath and Packer method. The leaves were weighted, and homogenates containing 10% trichloroacetic acid and 0.65% 2-thiobarbituric acid were heated at 95°C for 60 min, which were cooled to room temperature and centrifuged at 10,000 g for 10 min. The absorbance of supernatant was read at 532 and 600 nm versus a reagent blank. For estimating hydrogen peroxide level, fresh root and shoot samples (50 mg) from control and treated seedlings were crushed in 0.1% (w/v) trichloroacetic acid to estimate hydrogen peroxide (H2O2) levels. The reaction mixture (2 mL) contained tissue extract (0.5 mL), 10 mM potassium phosphate buffer (pH 7.0), and 1 M potassium iodide solution. The absorbance of reaction mixture at 390 nm was recorded. H2O2 concentration was calculated using a standard curve of H2O2 based on the Velikova et al. method (Citation2000). The ninhydrin method (Bates et al. Citation1975) was used to determine proline concentration spectrophotometrically. First, 300 mg of fresh leaf sample was homogenized in 3% sulfosalicylic acid, after which 2 mL each of ninhydrin and glacial acetic acid were added and the sample heated up to 100°C. The mixture was subsequently extracted using toluene, and free toluene level was quantified at 520 nm.
2.4.3. Biochemical parameters: soluble sugars, reducing sugars, total protein, and free α-amino acids
Soluble sugar was extracted following the Fales method (Citation1951) with some modification. A 100 mg sample from plant tissue was ground in 2 mL of 80% ethanol and then heated (80°C) in water bath for 30 min. After cooling to room temperature, the extracts were centrifuged, and total soluble sugar content was spectrophotometrically determined (λ = 625 nm, single beam mode, Perkin-Elmer, Uberlinger, Germany) as well as using Somogyi procedure at λ = 600 nm (Citation1952) for estimation of reducing sugars. In both cases, sugar content was quantified against a standard glucose calibration curve. The treated and untreated plants were used to isolate total protein, which was estimated using the Bradford method (Citation1976). Bovine serum albumin (BSA) in different concentrations was used to draw a standard curve. Free α-amino acid content was assayed using the ninhydrin colorimetric method, and glycine was used to plot the standard curve (Hwang and Ederer Citation1975).
2.4.4. Element analysis using ICP and OES
Shoot and root samples were oven dried at 70°C for 72 h. After determination of dry biomass, 0.5 g of samples was dissolved in 10 mL of 65% (w/v) nitric acid (supra pure, Merck). After digestion, the volume of each sample was adjusted to 50 mL using double deionized water. Total concentrations of Cd2+, Ca2+, Fe2+, Mg2+ and K+ were measured by inductively coupled plasma atomic emission spectroscopy (ICP, OES, Varian CO) (Sagner et al. Citation1998). The stability of the device was evaluated every 10 samples via examination of the internal standard. Reagent blanks were used to find potential contamination during digestion and analytical procedure. The samples were then analyzed in triplicate. Moreover, we added standards with final Cd2+, Ca2+, Fe2+, Mg2+ and K + concentrations that were in the range of plants in the analyzed solution for quality control (Sagner et al. Citation1998).
2.5. Statistical Analysis
All treatments were done in triplicate, and the results were presented as mean ± SD (standard deviation). Statistical differences were assessed by Microsoft Excel and Two-Way ANOVA, followed by Duncan's new multiple range test. P < 0.05 was considered to show significant difference.
3. Results
3.1. Impact of nano-Fe3O4 on growth and photosynthetic pigments under Cd stress
The results showed that 200 μM CdCl2 reduced fresh weight by 101.7% and 160%, dry weight by 127.2% and 190.4%, length by 111% and 132.5% in shoot and root and leaf surface area by 112.3% in comparison with control. Also chlorophyll a, b, total chlorophyll contents and carotenoids were negatively affected by cadmium, which showed 132.7%, 152.6%, 143%, and 322% decrease, respectively (P < 0.05) () but the addition of 20 mg/L nano-Fe3o4 improved all these parameters. Combined treatment with nano-Fe3o4 and CdCl2 significantly mitigated the effect of cadmium in reducing the fresh weight by 29.3% and 10.2%, dry weight by 56.2 and 79.4%, length by 64.1% and 47.6% in shoot and root and leaf surface area by 33.8%, chlorophyll a 91.5, chlorophyll b 7.2, total chlorophyll 33.9 and carotenoids 63.9 in comparison with control (P < 0.05).
Table 1. Effects of different concentrations of CdCl2 and nano-Fe3O4 on shoot and root fresh weight, dry weight, length, leaf area, Chla, Chlb, Chlt and Car.
3.2. Impact of nano-Fe3O4 on oxidative stress markers under CdCl2 stress
To study the role of nano-Fe3o4 in the regulation of oxidative stress in tomato plants under cadmium stress, specific oxidative stress markers such as MDA, aldehydes, H2O2, and proline were measured in both shoot and root. Cadmium treatment increased MDA content by 421.4% and 409%, aldehydes by 650% and 73.3%, H2O2 by 202.2% and 184.4%, proline by 89.9% and 88.2% both in stem and root compared to control; nonetheless, nano-Fe3o4 treatment improved all stress markers, so that combined nano-Fe3o4 and cadmium treatment caused increase by 271.4% and 193.3% in MDA content, 125.7% and 116.8% in H2O2 content, and 37.5% and 52.7% in proline content in both shoot and root in comparison to control ().
Table 2. Effects of different concentrations of CdCl2 and nano-Fe3O4 on shoot and root MDA, aldehydes, H2O2 and proline.
3.3. Impact of nano-Fe3o4 on biochemical parameters (soluble sugar, reducing sugar, protein, and FAA)
The results indicated that cadmium stress significantly increased soluble and reducing sugar contents by 121% and 103.4%, protein by 228.1% and 292.9%, FAA by 172.6% and 315.2% in both shoot and root relative to control. In contrast, soluble and reducing sugar in root were reduced by 404.9% and 97.8%. The addition of 20 mg/L nano-Fe3o4 improved all these parameters. After combined treatment of nano-Fe3o4 and CdCl2, shoot and root soluble sugar and shoot reduced sugar decreased by 119.5%, 182.7%, and 236.5%, respectively; nevertheless, shoot reducing sugar, shoot and root protein, and FAA of shoot and root increased by 57%, 120%, 156.3% and 103.6%, 185.5%, respectively (P < 0.05) ().
Table 3. Effects of different concentrations of CdCl2 and nano-Fe3O4 on shoot and root soluble sugar, shoot reduced sugar, protein and FAA.
3.4. Impact of nano-Fe3O4 on the element contents under CdCl2 stress
To determine how nano-Fe3o4 affects the uptake of other elements and how it reduces cadmium stress, the absorption of some elements such as Cd, Fe, K, Mg, and Ca in shoot and root was measured. The results showed that cadmium stress by alone significantly decreased Fe content by 123.3% and 110.5%, K content by 132.2% and 217.5%, Mg content by 182.3% and 518.7%, Ca content by 246.5% and 252.3% in both shoot and root (P < 0.05), but increased Cd content by 650.9% and 524.1% in shoot and root, respectively. Although the addition of nano-Fe3o4 increased the content of nutrient elements in shoot and root, combined treatment with nano-Fe3o4 and CdCl2 significantly mitigated the effect of cadmium in reducing the absorb of nutrient elements, so that the decrease in Fe content was only 26.4% and 29%, K content 44.4% and 90.8%, Mg content 21.1% and 77.5%, Ca content 37.4% and 54.3% in shoot and root, respectively (P < 0.05) ().
Table 4. Effects of different concentrations of CdCl2 and nano-Fe3O4 on shoot and root Cd2+, Fe2+, K+, Mg2+, and Ca2+.
4. Discussion
In this study, cadmium stress had a negative impact on growth parameters of tomato plant. Reduction in growth parameters is one of the most important characteristics of cadmium toxicity in plants such as tomato (Ünyayar et al. Citation2006), cosmos (Liu et al. Citation2017), wheat (Ci et al. Citation2010), Cucumis sativus (Feng et al. Citation2010). Under cadmium stress, the decrease in growth may be due to stronger bonds between pectin molecules in the cell wall, which is associated with a reduction in the size of intercellular space. On the other hand, injection of lignin into the cell wall under cadmium stress leads to hardening and decreasing expansion of the wall (Pál et al. Citation2006). This stress also leads to increasing the production of ROS, which is followed by damage to the cell membrane and macromolecules (Gonçalves et al. Citation2009) as well as changes in photosynthesis process and absorption of food elements (Pál et al. Citation2006). In addition, PSII reaction center is damaged by cadmium stress, which can contribute to further reduction in the rate of photosynthesis (Ci et al. Citation2010). The study of Liu et al. (Citation2017) clearly showed the mechanisms involved in detoxification of cadmium at low and high concentrations. Accumulation in soluble fraction is the main mechanism of cadmium toxicity at low concentrations. Increasing concentration of cadmium reduces its accumulation in soluble fraction; however, cadmium binding to cell wall components (e.g. glucose semi-cellulose, pectate, and proteins) is significantly increased, suggesting that both mechanisms work together to detoxify cadmium at high concentrations (Liu et al. Citation2017). Nanotechnology has been claimed to have a high potential for reducing the toxicity of heavy metals from the environment, which has been confirmed in the study of Konate et al. (Citation2017). Chemical composition, size, surface coating, reactivity, and, most importantly, concentration are factors that determine the performance of nanoparticles. Studies have shown that nanomaterials may play a dual role in plants as toxic and signaling molecules. Although nanoparticles can eliminate ROS, they induce ROS generation at high concentrations; therefore, it can be suggested that nanoparticles have toxic effects on plants at high concentrations, while their use at low concentrations reduces the destructive effects of a biotic stresses and enhances the performance of plants. Nanomaterials may play a key role in protecting against environmental stresses by increasing the activity of antioxidant enzymes, accumulation of osmolytes, free amino acids, and nutrients (Farhangi-Abriz and Torabian Citation2018). Since iron is an essential element for growth and photosynthesis, iron deficiency can reduce plant growth. If applied in a suitable dose, nano ferric oxide can significantly increase the amount of chlorophyll. The advantage of nano ferric oxide is that it is more stable in the soil (Wang et al. Citation2015). In this study, the reduction in growth parameters of tomato plant was evident under cadmium treatment. On the contrary, the addition of nano-Fe3O4 together with cadmium significantly (P < 0.05) improved the harmful effects on growth parameters, which was associated with decreased cadmium accumulation. It seems that nano-Fe3O4 does this by increasing the synthesis of organic compounds such as proteins and chlorophyll, which leads to increased absorption and transport of nutrients elements (Jalali et al. Citation2016). Sheykhbaglou et al. (Citation2018) reported that nano ferric oxide increased the chlorophyll a, b, and total chlorophyll in soybean. Iannone et al. (Citation2016) also showed that the application of 20 mg/L nano-Fe3O4 in wheat increased the growth parameters. Shankramma et al. (Citation2015) also reported that the use of nano iron oxide increased the length and fresh and dry weight of shoot and root in tomato plant.
It is well documented that heavy metals stress in plants increases the production of ROS, causing oxidative stress and damaging macromolecules such as lipids, proteins, and nucleic acids (Gratão et al. Citation2015). MDA is a lipid peroxidation product, the concentration of which reflects the intensity of oxidative stress (Liu et al. Citation2017). Liu et al. (Citation2017) study in cosmos plant demonstrated that rising cadmium concentrations increased MDA level. In this study, it was found that MDA content, H2O2, and proline were significantly increased under cadmium stress (P < 0.05). Our results clearly show the protective role of nano-Fe3O4 against oxidative stress. Similarly, Askary et al. (Citation2017) have reported the protective role of nano-Fe3O4 against oxidative stress induced by NaCl in Mentha piperita L. Konate et al. (Citation2017) also showed the protective role of nano-Fe3O4 against cadmium-induced oxidative stress in wheat. Moreover, Jalali et al. (Citation2016) reported that nano-Fe3O4 use leads to an increase in antioxidant capacity and improves growth in corn, which is due to an increase in the content of chlorophyll, sugar, total protein, and nutrients elements as well as a decrease in MDA content that confirms our results as a whole. Nano ferric oxide treatment increased protein content in watermelon in the study of Wang et al. (Citation2015) and in soybean in Sheykhbaglou et al. study (Citation2018), which was consistent with our results.
Decreasing absorption of nutrients can lead to iron deficiency, which has unintended consequences on root and stem growth. As a divalent cation, cadmium can compete with Fe2+, Mg2+, and Ca2+ in transfer across cell membrane (Nazar et al. Citation2012). For example, both Ca2+ and Cd2+ compete for calcium channels in different plants, so that cadmium toxicity can be affected by the interaction between Ca2+ and Cd2+. Moreover, nutrients cause cadmium distribution in plant partitions via the production of phytoclatin and thus contribute to the reduction of cadmium toxicity (Sarwar et al. Citation2010), so that the decrease in P, S, Mg, Fe, and Mn contents has been observed under cadmium stress. Liu et al. (Citation2017) indicated that increasing concentrations of cadmium in cosmos plant decreased the absorption of Ca and Zn. The oxidative stress caused by cadmium toxicity in plant cells is alleviated with the provision of potassium and magnesium. Similar to potassium, magnesium also reduces the oxidative stress but its protective role is mainly due to an increase in the antioxidant system rather than cadmium absorption inhibition. On the other hand, Fe reduces the absorption and transfer of Cd, increasing the plant growth and aggregation of photosynthetic pigments followed by mitigation of cadmium stress because of a stronger light phase (Nazar et al. Citation2012). Results showed that cadmium stress reduced the nutrients element content in shoot and roots of tomato plant, while the addition of iron nanoparticles together with CdCl2 significantly (P < 0.05) improved cadmium-induced stress (). In agreement with our results, Sheykhbaglou et al. (Citation2018) reported that the application of nano iron oxide increased the contents of Fe, Ca, and Mg in soybean. Askary et al. (Citation2017) also reported that the use of nano iron oxide increased the content of K, Fe, and Ca. Jalali et al. (Citation2016) reported that nano-Fe3O4 use increased the absorption of Fe and Ca in Zea mays L. plant. Li et al. (Citation2016) reported that nano iron oxide increased Fe content in Zea mays L. Several mechanisms have been proposed on the effects of nutrients element on toxicity of heavy metals in plants. In this regard, provision of food elements reduces the toxicity of heavy metals in plant. It has also been shown that increasing biomass eases the heavy metal toxicity due to dilution in plant structure, which is known as the dilution effect (Tripathi et al. Citation2015).
5. Conclusion
The results of this study indicate that pre-treatment with 20 mg/L was useful in protecting tomato plants against cadmium stress. The findings of this study indicate three possible mechanisms for the reduction of cadmium stress by nanoparticle: (i) decreasing accumulation of cadmium in the shoot and root, (ii) reducing oxidative stress due to decreased Cd accumulation, (iii) regulating the absorption of nutrients element that plays a protective role against the oxidative stress. This study may provide information on the role of nano-Fe3O4 in easing the cadmium stress. However, further research on biochemical and molecular levels is needed for a deeper insight in order to understand the nature of nano-Fe3O4 and cadmium interaction in plants.
Acknowledgment
This study has been conducted in Kerman Graduate University of Advanced Technology, which is funded by Ministry of Science, Research and Technology of Iran.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Notes on contributors
Razieh Rahmatizadeh
Razieh Rahmati Zadeh, Phd student in plant physiology, Her research focuses on the effects of plant growth regulators (SA) and nanoparticles on plants, abiotic tress such as heat and heavy metals (Cd) and skilled in tissue culture.
Seyyed Mohammad Javad Arvin
Seyyed Mohammad Javad Arvin, Professor in horticulture, His research focuses on plant growth regulators and abiotic stresses such as heavy methal, salt, cold and drought stress on plants.
Rashid Jamei
Rashid Jamei, Associate Professor in plant physiology, His research focuses on the effects of nanoparticles and nanotubes on plants and abiotic stresses.
Hossein Mozaffari
Hossein Mozaffari, Assistant Professor in plant physiology, His research focuses on the effects of abiotic stresses.
Farkondeh Reza Nejhad
Farkhondeh Reza Nejad, Professor in plant development, Her research focuses on the effects of some factor such as air pollution, cold, heavy metal and hormones on anatomical, molecular, cellular properties of plants.
References
- Askary M, Talebi SM, Amini F, Dousti Balout Bangan A. 2016. Effect of NaCl and iron oxide nanoparticles on Mentha piperita essential oil composition. Environ Exp Bot. 14:27–32. doi: 10.22364/eeb.14.05
- Askary M, Talebi SM, Amini F, Dousti Balout Bangan A. 2017. Effects of iron nanoparticles on Mentha piperita L. under salinity stress. Biologija. 63(1):65–67. doi: 10.6001/biologija.v63i1.3476
- Bates LS, Waldren RP, Tears ID. 1975. Rapid determination of free proline in water stress studies. Plant Soil. 39:205–207. doi: 10.1007/BF00018060
- Bombin S, LeFebvre M, Sherwood J, Xu Y, Bao Y, Ramonell KM. 2015. Developmental and reproductive effects of iron oxide nanoparticles in Arabidopsis thaliana. Int J Mol Sci. 16:24174–24193. doi: 10.3390/ijms161024174
- Bradford MM. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. doi: 10.1016/0003-2697(76)90527-3
- Chen R, Zhang C, ZhaoY Huang., Liu Z.. 2017. Foliar application with nano-silicon reduced cadmium accumulation in grains by inhibiting cadmium translocation in rice plants. Environ Sci Pollitr. 25(3):2361–2368. doi: 10.1007/s11356-017-0681-z
- Ci D, Jiang D, Wollenweber B, Dai T, Jing Q, Cao W. 2010. Cadmium stress in wheat seedlings: growth, cadmium accumulation and photosynthesis. Acta Physiol Plant. 32:365–373. doi: 10.1007/s11738-009-0414-0
- Fales FW. 1951. The assimilation and degradation of carbohydrates by yeast cells. J Biol Chem. 193:113–124.
- Farhangi-Abriz S, Torabian SH. 2018. Nano-silicon alters antioxidant activities of soybean seedlings under salt toxicity. Protoplasma. 255(3):953–962. doi: 10.1007/s00709-017-1202-0
- Feng J, Shi Q, Wang X, Wei M, Yang F, Xu H. 2010. Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Sci Hort. 123:521–530. doi: 10.1016/j.scienta.2009.10.013
- Gill SS, Hasanuzzaman M, Nahar K, Macovei A, Tuteja N. 2013. Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol Biochem. 63:254–261. doi: 10.1016/j.plaphy.2012.12.001
- Gonçalves GF, Antes FG, Maldaner J, Pereira LB, Tabaldi LA, Rauber R, Rossato LV, Bisognin DA, Dressler VL, Flores EMDM, Nicoloso FT. 2009. Cadmium and mineral nutrient accumulation in potato plantlets grown under cadmium stress in two different experimental culture conditions. Plant Physiol Biochem. 47:814–821. doi: 10.1016/j.plaphy.2009.04.002
- Gratão PL, Monteiro CC, Tezotto T, Carvalho RF, Alves LR, Peters LP, Azevedo RA. 2015. Cadmium stress antioxidant responses and root- to- shoot communication in grafted tomato plants. Bio Metals. 28(5):803–816.
- Hasan SA, Hayat SH, Ahmad A. 2011. Brassino steroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere. 84:1446–1451. doi: 10.1016/j.chemosphere.2011.04.047
- Hashem A, Abd_Allah EF, Alqarawi AA, Al Huqail Asma A, Egamberdieva D, Wirth S. 2015. Alleviation of cadmium stress in Solanum lycopersicum L.by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J Biol Sci. 23(2):272–281. doi: 10.1016/j.sjbs.2015.11.002
- Hwang M, Ederer GM. 1975. Rapid hippurate hydrolysis method for presumptive identification of group B streptococci. J Clin Microbiol. 1:114–117.
- Iannone MF, Groppa MD, Sousa ME, Raap MBFV, Benavides MP. 2016. Impact of magnetite iron oxide nanoparticles on wheat (Triticum aestivum L.) development: Evaluation of oxidative damage. Environ Exper Bot. 131:77–88. doi: 10.1016/j.envexpbot.2016.07.004
- Jalali M, Ghanati F, Modarres-Sanavi AM. 2016. Effect of Fe3O4 nanoparticles and iron chelate on the antioxidant capacity and nutritional value of soil-cultivated maize (Zea mays) plants. Crop Pasture Sci. 67:621–628. doi: 10.1071/CP15271
- Kao CH. 2014. Cadmium stress in rice plants: influence of essential elements. Crop Environ Bioinfo. 11:113–118.
- Konate A, He X, Zhang Z, Ma Y, Zhang P, Alugongo GM, Rui Y. 2017. Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability. 9(5):790. doi: 10.3390/su9050790
- Li J, Hu J, Ma C, Wang Y, Wu C, Huang J, Xing B. 2016. Uptake, translocation and physiological effects of magnetic iron oxide (g-Fe2O3) nanoparticles in corn (Zea mays L.). Chemosphere. 159:326–334. doi: 10.1016/j.chemosphere.2016.05.083
- Lichtenthaler H. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148:350–382. doi: 10.1016/0076-6879(87)48036-1
- Liu Y, Yu X, Feng Y, Zhang C, Wang C, Zeng J, Huang Z, Kang H, Fan X, Sha L, et al. 2017. Physiological and transcriptome response to cadmium in cosmos (Cosmos bipinnatus Cav.) seedlings. Sci. Rep. 7(1):14691. doi: 10.1038/s41598-017-14407-8
- Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NA. 2012. Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci. 3:1476–1489. doi: 10.4236/ajps.2012.310178
- Ouariti O, Boussama N, Zarrouk M, Cherif A, Gorbal MH. 1997. Cadmium – and copper – induced changes in tomato membrane lipids. Phytochemistry. 45:1343–1350. doi: 10.1016/S0031-9422(97)00159-3
- Pál M, Horváth E, Janda T, Páldi E, Szalai G. 2006. Physiological changes and defense mechanisms induced by cadmium stress in maize. J Plant Nutr Soil Sci. 169:239–246. doi: 10.1002/jpln.200520573
- Rastogi A, Zivcak M, Sytar O, Kalaji HM, He X, Mbarki S, Brestic M. 2017. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front Chem. 5:78. doi: 10.3389/fchem.2017.00078
- Rui M, Ma C, Hao Y, Guo J, Rui Y, Tang X, Zhao Q, Fan X, Zhang Z, Hou T, Zhu S. 2016. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front Plant Sci. 7:815. doi: 10.3389/fpls.2016.00815
- Sagner S, Kneer R, Wanner G, Cosson JP, Deus-Neumann B, Zenk MH. 1998. Hyperaccumulation, complexation and distribution of nickel in Sebertia acuminate. Phytochemistry. 47:339–347. doi: 10.1016/S0031-9422(97)00593-1
- Sarwar N, Saifullah, Malhi SS, Zia MH, Naeem A, Bibi S, Farid GH. 2010. Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric. 90:925–937.
- Shankramma K, Yallappa S, Shivanna MB, Manjanna J. 2015. Fe2O3 magnetic nanoparticles to enhance S. lycopersicum (tomato) plant growth and their biomineralization. Appl Nanosci. 6(7):983–990. doi: 10.1007/s13204-015-0510-y
- Sheykhbaglou R, Sedghi M, Fathi-Achachlouie B. 2018. The effect of ferrous nano-oxide particles on physiological traits and nutritional compounds of soybean (glycine max L.) seed. An Acad Bras Cienc. 90(1):485–494. doi: 10.1590/0001-3765201820160251
- Somogy M. 1952. Notes on sugar determination. J. Biol Chem. 195:19–29.
- Tripathi DK., Singh VP., Prasad SM., Chauhan DK., Dubey NK.. 2015. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol Bioch. 96:189–198. doi: 10.1016/j.plaphy.2015.07.026
- Tripathi DK, Singh S, Singh VP, Prasad SM, Dubey NK, Chauhan DK. 2017. Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol Biochem. 110:70–81. doi: 10.1016/j.plaphy.2016.06.026
- Ünyayar S, Keleş Y, Çekiç FÖ. 2006. The antioxidative response of two tomato species with different drought tolerances as a result of drought and cadmium stress combinations. Plant Soil Environ. 51(2):57–64. doi: 10.17221/3556-PSE
- Velikova V, Yordanov I, Edreva A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 151:59–66. doi: 10.1016/S0168-9452(99)00197-1
- Wang M, Liu X, Hu J, Li J, Huang J. 2015. Nano-ferric oxide promotes watermelon growth. J Biomater Nanobiotechnol. 6:160–167. doi: 10.4236/jbnb.2015.63016