ABSTRACT
Heavy metal chromium (Cr) is considered to be a serious environmental contaminant due to its toxic effect on living organisms. To mitigate and reduce the negative impacts of Cr in rice plant, the effect of exogenous supplementary calcium was evaluated as it functions as a signaling molecule at cellular level. In this study, growth parameters, protein content, and membrane stability were found to be restored due to calcium under Cr stress. Further, Atomic absorption spectrophotometric analysis revealed that calcium inhibits Cr translocation from root to shoot in rice seedlings. This event was addressed by the enhanced accumulation of phytochelatin that leads to vacuolar sequestration of Cr in roots. Furthermore, increased activity of Catalase, Peroxidase, and Glutathione reductase along with elevated glutathione also assures that calcium enhances antioxidant defense mechanism to cope with Cr toxicity.
KEYWORDS:
Introduction
Chromium toxicity has become a serious problem for plants and animals over the past few decades (Shanker et al. Citation2005; Gill et al. Citation2015) and received highlighted attention. The stable forms of Cr are the trivalent Cr(III) and the hexavalent Cr(VI), although there are various other valence states which are unstable in biological systems. Among these two forms, Cr(VI) is considered the most toxic, which usually forms chromate (CrO42–) or dichromate (Cr2O72–) oxyanions with oxygen. Stunting plant growth, chlorosis in new leaves, wilting of tips, impaired photosynthesis, damage of roots, and finally plant death are the common phenomenon of Cr toxicity (Sharma et al. Citation2003; Scoccianti et al. Citation2006). Moreover, reactive oxygen species (ROS) produced by Cr can harm the biomolecule (such as lipids, proteins, and nucleic acids) synthesis, thereby, interrupting both mitochondrial respiration and carbohydrate metabolism (Gill and Tuteja Citation2010; Gill et al. Citation2015).
To cope with the toxicity of heavy metal like Cr, plants make grow different mechanisms such as prevention of heavy metals (HM) uptake into root cells by confining HM ions to the apoplast, binding them to the cell wall, storage into the cell vacuoles, activation of oxidative stress defense mechanisms and the synthesis of stress-related proteins and signaling molecules.
The plasma membrane prevents the uptake of metals into the cell by active efflux pumping outside the cell. Active efflux systems are more vernacular and are used to control heavy metal accumulation inside the cell. This heavy metal efflux pumps in plants are the P1B-ATPases and the CDF families of transporters. P1B-type ATPases is a member of P-type ATPase super family and apply energy from ATP hydrolysis to translocate diverse metal cations across biological membranes (Axelsen and Palmgren Citation2001).
Several physiological studies pointed out the role of PCs in the homeostasis and detoxification of Cr and other metals in plants (Shanker et al. Citation2005; Singh et al. Citation2013). Plant removes the toxic effect of Cr by reducing Cr (VI) to Cr (III), followed by complexation of Cr (III) with PC and then this PC-Cr complexes transported to vacuoles (Wu et al. Citation2013). In addition to PC, metallothioneins (MTs), cysteine-rich low molecular weight proteins, also act in Cr detoxification in plants (Shanker et al. Citation2004; Panda and Choudhury Citation2005).
In order to remove the injurious effect of ROS induced oxidative stress, plants have developed a complex ROS scavenging enzymatic mechanism comprising of catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), and Glutathione reductase (GR) (Maiti et al. Citation2012; Pourrut et al. Citation2013). The activation or suppression of antioxidants in plants against metal-induced oxidative damage depends upon plant and ROS type (Shahid et al. Citation2014). Chromium-induced increase in CAT, POD, SOD, and GR activities has been reported in Gossypium hirsutum (Daud et al. Citation2014; Farooq et al. Citation2016). Moreover, lower molecular weight glutathione provides defense against Cr-mediated oxidative damage by taking part in various physiological and biochemical processes such as modulation of thiol-disulphide status, reduction of peroxides, and free radical scavenging (Foyer and Noctor Citation2005).
Calcium ions play vital roles as a second messenger in coupling physiological responses to external and developmental signals (Reddy and Reddy Citation2004). Changes in cytosolic free calcium ion concentration are authenticated during transduction of abiotic stimulants including light, low and high temperature, touch, hyperosmotic and oxidative stresses as well as in the case of biotic stimuli like fungal elicitors and nodulation (Nod) factors (Rudd and Franklin-Tong Citation2001; Sanders et al. Citation2002). It is also uptaken by the plant as a nutrient has a well-defined role in the growth and developmental regulation of plants and in heavy metal detoxification (Suzuki Citation2005). Maintenance of antioxidant enzyme activities, reduction of lipid peroxidation of cell membranes, improvement of physiological and biochemical processes are the key function of Ca2+ under heavy metal stress (Khan et al. Citation2010; Siddiqui et al. Citation2012; Ahmad et al. Citation2015). Restricting of Cd uptake in root due to Ca2+ application is also reported in maize grown under Cd stress (Kurtyka et al. Citation2008).
However, the possible role of Ca2+ on acquired Cr tolerance mechanism in rice is still unclear. Therefore, a number of biochemical analyses were executed to explore the role of calcium in alleviating of Cr toxicity in rice.
Materials and methods
Plant cultivation
Seeds of rice (BRRI 51) were washed thoroughly and then sterilized with 95% (v/v) ethanol for 10 min. Seeds were then germinated in Petri Dishes containing moist filter paper for 2–3 days in the dark at room temperature. Only uniform germinated seedlings were transferred to the hydrophonic solution (Hogland and Arnon Citation1950) containing the following nutrient concentrations (µM): KNO3 (16000), Ca(NO3)2.4H2O (6000), NH4H2PO4 (4000), MgSO4.7H2O (2000), KCl (50), H3BO3 (25), Fe-EDTA (25), MnSO4.4H2O (2), ZnSO4 (2), Na2MoO4.2H2O (0.5), and CuSO4.5H2O (0.5). The nutrient solution was supplemented with 0 or 100 µM K2Cr2O7 and 0 or 100 µM CaCl2 as mentioned previously (Greger et al. Citation2016; Kabir Citation2016; Mahmud et al. Citation2018). The pH of the hydrophonic nutrient was adjusted to 6.0. The nutrient media were continuously aerated and incubated in the growth chamber under 10-h light and 14-h dark (550–560 mmol s-1 per mA). Plants were harvested for the experiment after 7 days of treatment.
Measurement of morphological feature s
To determine Morphological growth parameters, such as root length, root dry weight, shoot height, and shoot dry weight roots and leaves of 1-week-old plants were separated manually and then dried in an oven at 80°C for 2 days before taking the dry weight.
Estimation of relative water content (RWC)
Leaf RWC was measured as described previously (Barrs and Weatherley Citation1962). Fresh roots and leaves were weighed as FW and then dried at 80°C for 48 h. After drying, again weighed as DW. RWC was calculated applying the formula RWC (%) = [(FW− DW)/(TW− DW)] × 100. (Here, FW = Fresh weight, DW = Dried weight).
Determination of chlorophyll and carotenoids concentration
Leaf Chlorophyll and carotenoid content were determined according to the well-established method described by Lichtenthaler and Wellburn (Citation1985) using 90% (v/v)acetone and calculated as FW basis.
Determination of Cr and Fe by atomic absorption spectroscopy
After harvesting, roots and shoots were washed with CaSO4 and deionized water and then dried in oven at 80°C for 3 days. Then dried samples were digested in 3 ml HNO3 with 1 ml H2O2 and were heated at 109°C for 15 min. These digested samples were then analysed for Cr and Fe concentration by flame atomic absorption spectroscopy outfitted with ASC- 6100 autosampler and air–acetylene atomization gas mixture system (Model No. AA-6800, Shimadzu).
Determination of total soluble proteins
Total soluble proteins of root and shoot were extracted according to the procedure described by, Guy et al. (Citation1992) with some modification. Amount of soluble proteins were determined according to Bradford (Citation1976) method. A calibration curve prepared with different concentrations of bovine serum albumin (BSA) was used.
Measurement of electrolyte leakage
Electrolyte leakage was determined by an electrical conductivity meter as previously described, with some modifications (Lutts et al. Citation1996). Shortly, seedlings were washed with deionized water, weighed, and kept in individual vials containing 20 ml deionized water and then incubated at 25°C on a shaker (100 rpm) for 2 h. Electrical conductivity of the solution was then measured after incubation.
Estimation of lipid peroxidation
Malondialdehyde (MDA) content as a marker of lipid peroxidation was determined according to Heath and Packer (Citation1968). Root samples (0.5 g) were homogenized in 5% (w/v) trichloroacetic acid (TCA), and then centrifuged at 11,500×g for 15 min. A mixture of the supernatant with thiobarbituric acid (TBA) was heated at 95°C for 30 min in a water bath. Absorbance was read at 532 nm after cooling the supernatant. MDA content was calculated on FW basis by using extinction coefficient 155 mM–1 cm–1 and expressed as nmol of MDA mg–1 FW.
Enzymatic analysis
CAT (EC. 1.11.1.6), POD (EC. 1.11.1.7), SOD (EC. 1.15.1.1), and GR (EC. 1.6.4.2) enzymes were extracted in the roots of one-week-old plants according to Goud and Kachole (Citation2012) with slight modifications. Shortly, root tissues were ground in phosphate buffer (100 mM) and then centrifuged for 10 min at 13,000×g. For CAT(EC. 1.11.1.6) analysis, the reaction mixture (2 ml) comprised of 100 mM potassium phosphate buffer (pH 7.0), 400 µl of 6% (v/v) H2O2, and 100 µl root extract. Once root extract was added, the decrease in absorbance was read at 240 nm (extinction coefficient of 0.036 mM−1 cm−1) in a UV spectrophotometer at 30-s intervals up to 1 min. CAT activity is expressed as mmol of H2O2 oxidized min −1 (mg protein−1). Reaction mixture (2 ml) of POD (EC. 1.11.1.7) analysis carried out in 100 mM potassium phosphate buffer (pH 6.5), 1 ml of 0.05 M pyrogallol solution, 400 µl of 200 mM H2O2, and 100 µl root extract. Similarly, the differences of absorbance were read at 430 nm (extinction coefficient 12 mM −1 cm −1) in a spectrophotometer from 30 s up to 1.5 min. The specific activity of POD is expressed as mmol pyrogallol oxidized min −1(mg protein−1). Moreover, SOD (EC. 1.15.1.1) assay mixture comprised of 50 mM sodium carbonate/bicarbonate buffer (pH 9.8), 0.1 mM EDTA, 0.6 mM epinephrine, and enzyme (Sun and Zigman Citation1978). Adrenochrome formation for 4 min was then recorded at 475 nm in a UV-Vis spectrophotometer once epinephrine is supplied. Per unit SOD activity is expressed as the amount of enzyme needed for 50% inhibition of epinephrine oxidation. For GR (EC. 1.6.4.2) analysis, the reaction mixture was prepared containing 1 ml of 0.2 M phosphate buffer (pH 7.0), 1 mM EDTA, 0.75 ml distilled water, 0.1 ml of 20 mM oxidized glutathione (GSSG), and 0.1 ml of 2 mM NADPH and 0.1 ml root extract. Oxidation of NADPH by GR was then recorded at 340 nm. The rate of GR activity (nmol min −1) was then counted using the extinction coefficient of 6.12 mM −1 cm −1 (Halliwell and Foyer Citation1978).
The hydrogen peroxide
The hydrogenperoxide (H2O2) concentration was measured in roots as previously described (Alexieva et al. Citation2001). Briefly, tissues were grinded in 0.1% (w/v) trichloroacetic acid (TCA) and centrifuged at 10,000×g for 15 min. The supernatant was kept in dark for 1 h before adding K-phosphate buffer (10 mM, pH7.0) and potassium iodide (M). The absorbance of the mixture was read at 390 nm. The amount of hydrogen peroxide was calculated applying a standard curve prepared with known concentrations of H2O2.
Estimation of phytochelatin (PC) content
Phytochelatin content was determined by subtracting the total glutathione (GSH) content from the total non-protein thiol content. Ellman’s assay mixture was used to measure non-protein thiol content after homogenizing leaves in 3% (w/v) sulfosalicylic acid and recorded spectrophotometrically at 412 nm (Ellman Citation1959).
Estimation of proline (pro) content
Leaf and root samples were homogenized in 3% (w/v) sulfosalicylic acid and centrifuged at 11,500×g for 12 min. Reaction mixture was prepared by using 100 μl of 3% (w/v) sulfosalicylic acid, 200 μl glacial acetic acid, 200 μl acidic ninhydrin and 100 μl supernatant of the plant extract and incubated the tubes at 96°C for 60 min and then cooled on ice. Readings were taken immediately at a wavelength of 520 nm in spectrophotometer (Bates et al. Citation1973). The standard curve was used to determine proline concentration and calculated on a FW basis.
Estimation of glutathione content
Glutathione extraction followed the protocol of Anderson et al. (Citation1992). Total glutathione was measured after the reduction of GSSG to GSH. The GSSG reduction was performed by applying the root extract to a mixture consisting of 130 mM sodium phosphate buffer (pH 7.4) containing one unit of GR for 10 min. at 30°C. Thereafter, NADPH at 50 mM and sodium phosphate buffer at 7 mM (pH 6.8) containing 6 mM of 5,5- dithiobis (2- nitrobenzoic acid) (DTNB) were added and the reaction mixture was maintained at 30°C for 10 min. The absorbance was read at 412 nm and GSH were estimated using GSH as a standard (Griffith Citation1980).
Statistical analysis
All experiments were performed in a completely randomized block design with four independent replications for each sample. Statistical significance was set at p ≤ .05 by one-way ANOVA followed by Duncan’s multiple range test (DMRT) using SPSS Statistics 20 Software. Moreover, the graphical presentation was developed using Graph Pad Prism 8.
Results
Morphological growth features
Root length, shoot height, root dry weight, and shoot dry weight significantly decreased under the treatment with Cr compared to non-treated plants. Application of calcium along with chromium in the culture medium, root length, shoot height, root dry weight, and shoot dry weight were significantly increased likened to chromium stressed plants. Addition of calcium chloride in the absence of chromium did not show any significant change in both root and shoot compared to control plants. However, no significant change in total chlorophyll content (A &B) was observed among these treatments. But carotenoid synthesis was significantly increased under chromium stress compared to control plants. In addition, the rate of water retention was reduced in both root and shoot of chromium treated plants compared to control. But the presence of calcium with chromium enhanced this rate in both root and shoot ().
Table 1. Root length, root dry weight, shoot height, shoot dry weight, level of photosynthetic pigment (chl a and chl b) in leaves and water retention rate of root and shoot of Oryza sativa seedlings. Means (±SD) were calculated from four replications (n = 4) for each treatment. Values with different letters are significantly different at ≤ .05 applying test.
Cr and Fe concentration
The concentration of chromium significantly increased in both roots and shoots in chromium stressed plants compared to non-treated plants. However, the application of calcium with chromium could reduce the amount of chromium significantly in the shoot but not in root. However, Cr concentration both in root and shoot was as like as that of non-treated control due to calcium treatment without Cr. Translocation rate of chromium was reduced from 41% to 26% due to treated with calcium under chromium stress compared with chromium treated plants ().
Figure 1. Chromium and iron concentration in the roots and shoots and their translocation rate (%) from root to shoot of 7 days old rice plants grown under different growth conditions of Cr and Ca+. Different letters in each column indicates significant differences between means ± SD of treatments (n = 4) at a p < .05 significance level.
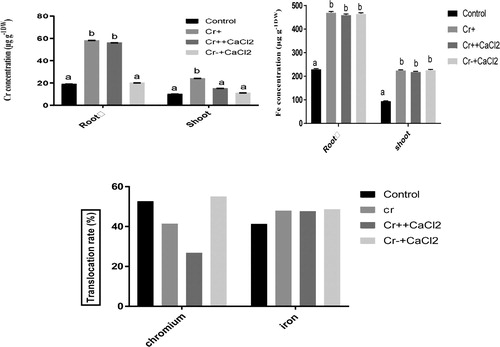
Furthermore, Iron content in both root and shoot of rice grown under chromium stress is significantly higher than that of non-treated control plant (). Due to the application of calcium along with and without chromium in media, iron content showed no significant change in both roots and shoots compared to Cr stressed plants.
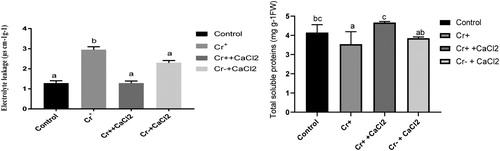
Measurement of electrolyte leakage and total soluble protein
As the initial investigation reveals that calcium-mediated chromium detoxification is root based mechanism, Electrolyte leakage and total soluble protein of root were examined. Electrolyte leakage was significantly increased under chromium stress compared to control plant. However, the addition of calcium chloride in the presence of chromium did significantly decreased compared to chromium stressed plant.
Furthermore, chromium stress caused a significant reduction in total soluble protein content compared to non-treated control plants. However, it was significantly increased in root compared to chromium forced when calcium was applied along with chromium. Action of calcium in the absence of chromium was not considerable compared to the control plant ().
Figure 2. Total soluble protein content and electrolytic leakage in the roots of 7 d old rice plants grown under different growth conditions of chromium and calcium. Different letters in each column indicate significant differences between means ± SD of treatments (n = 4) at p < .05 significance level.
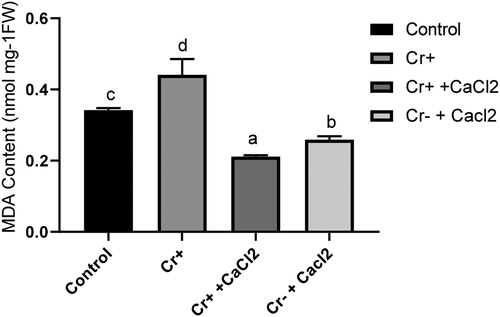
Lipid peroxidation
Cr in growth medium enhanced the accumulation of MDA in root compared to the control. However, the application of calcium in combination with Cr significantly reduced the accumulation of MDA compared with both treated and non-treated plant. When calcium was applied solely, it also decreased the MDA content compared with non-treated control plants ().
Changes in enzymatic activity
CAT, POD, and GR enzyme activities in root were significantly decreased under chromium stress compared with control plants. However, the application of calcium along with chromium CAT, POD, and GR activities increased significantly compared with chromium stressed plant. Moreover, the application of calcium solely GR activity was decreased significantly compared with non-treated control plants. But in the case of SOD activity, no significant differences were found among the treatments (). Furthermore, the amount of H2O2 in root raised under chromium stress. Supplementation of calcium with chromium reduced this amount significantly compared with non-treated control as well as chromium stressed plants.
Table 2. Enzymatic activities and hydrogen peroxide content in roots of rice seedlings, cultivated for 7 days in nutrient medium with calcium in presence or absence of chromium. Different letters in each column indicated significant differences between means ± SD of treatments (n = 4) at p ≤ .05 significant level.
Changes in phytochelatin, glutathione, and proline in root
The level of glutathione in chromium stressed plants was similar to that of non-treated plants in roots. However, after the application of calcium under chromium stress, the level of glutathione significantly increased. Further, Phytochelatin content in root was significantly increased due to calcium addition with chromium compared to treated and non-treated plants. Calcium without Cr also increased this level compared to control plants. In addition, proline content showed no significant changes in roots due to Ca+2 applications under Cr stress in comparison with Cr-stressed plants ().
Table 3. The level of glutathione, phytochelatin and proline concentrations in roots of 7-day-old seedling grown in nutrient medium with calcium in presence or absence of chromium. Different letters in each column indicate significant differences between means ± SD of treatments (n = 4) at p ≤.05 significant level.
Discussion
In plants, chromium toxicity is associated with the increasable production of ROS and oxidative stress development as well as with inhibition of pigment synthesis and modification of virtually all cellular components (Farooq et al. Citation2016; Jabeen et al. Citation2016; Ahmad et al. Citation2017; Uliana et al. Citation2018; Yang-ErChena et al. Citation2018). These metabolic changes result in seedling development, cell death, reduction of plant biomass and crop yield (Antoniadis et al. Citation2017; Yang-ErChena et al. Citation2018) reduction of soluble protein content (Singh et al. Citation2012; Das et al. Citation2014; Jabeen et al. Citation2016) and membrane stability (Begum et al. Citation2016). Plants subjected to chromium also showed reduced growth in this study. In the present experiment Ca+2 was applied alone as well as in combination with Cr to restore the altered plant growth in rice as Calcium protects plants from deleterious impacts of stress by acting in signaling pathways and regulating calmodulin like proteins to promote several growth mechanisms in plants (Sarwat et al. Citation2013). The present study also indicates that the presence of Ca+2 during cultivation with Cr restored the growth features.
Biochemical analyses also indicate that the supplementation of Ca+2 under Cr stress restored the growth features, membrane stability and the total soluble protein content. These findings are in agreement with the previous reports shown in mustard (Ahmad Citation2015; Ahmad et al. Citation2015), wheat, almond, and sunflower plants under cadmium stress (Elloumi et al. Citation2014; Abd_Allah et al. Citation2015). The increased soluble proteins (SP) content might be due to the efficient working of gas exchange and photosystem of plants which was regulated by the addition of Ca+2 under Cr stress (Aderholt et al. Citation2017). Moreover, Carotenoids is the key pigment known to be involved in protecting plant organs from stresses and its production may be enhanced depending on the metal type, concentration, and the plant species (Sinha et al. Citation2003). In this investigation, the concentration of carotenoid was significantly higher compared with non-treated control plant as well as plants were treated with calcium in combination with chromium indicating that Ca alleviate chromium stress in rice plant.
In the present study, we found that Cr and Fe concentration both in root and shoot increased significantly under chromium stress indicating that Cr uptake is associated with iron transportation system which was established in rice previously (Kabir Citation2016). Calcium treatment also enhances Fe uptake and its translocation. However, in case of chromium, calcium could not inhibit its uptake but restrict its translocation from root to shoot. This accumulating evidence indicates the compartmentation of excess Cr in the root which is a mechanism of metal detoxification in plants (Dragisic Maksimovi et al. Citation2007; Adrees et al. Citation2015). Plant cell vacuoles are pivotal organelle functioning in the storage of metabolites, mineral nutrients, and toxicants in higher plants. This Vacuolar sequestration primarily controlled by cytosolic metal chelators and tonoplast-localized transporters, or the interaction between them under HM stress (Mendoza-Cozatl et al. Citation2011; Peng and Gong Citation2014). To do that plants often increase the synthesis of PC and metallothioneins (Lee et al. Citation2004; Roosens et al. Citation2004; Peng and Gong Citation2014; Nahar et al. Citation2016; Mahmud et al. Citation2018).
In this study, phytochelatin accumulation was found to enhance due to calcium treatment under chromium stress that was involved with vacuolar sequestration of Cr. Increased PC inside cells due to varying levels of HMs, binds to HM via sulfhydryl and carboxyl groups (Cobbett Citation2000; Emamverdian et al. Citation2015). However, PC found to facilitate Cd storage in wheat, which varies with tissue type (Marentes and Rauser Citation2007).
In addition, GSH is an antioxidant known to play critical roles in scavenging ROS (Cobbett and Goldsbrough Citation2002) as well as precursor of PC were increased when Ca+2 was applied along with Cr. These findings suggest that Ca+2 interact with cellular mechanisms associated with vacuolar sequestration upon Cr exposure through PC synthesis regulation in the roots of rice plants to withstand Cr toxicity. Similar type of result was also found in rice after applying silicon and salicylic acid under chromium stress (Huda et al. Citation2016, Citation2017).
In the present investigation, CAT, POD, and GR enzyme activities were found to be increased and H2O2 to be reduced significantly due to Ca+2 supplementation with Cr, pointing that Ca+2 interacts with ROS signal pathway for scavenging Cr-induced oxidative stress in rice plants. Previous investigation also supports the interaction of Ca+2 with ROS signal pathway and induce defense mechanisms by keeping the H2O2 and O2 at a constant level (Thounaojam et al. Citation2012). Similarly, Cd-induced oxidative damage was minimized by modifying the antioxidant defense system in sesame due to Ca+2 applications (Abd_Allah et al. Citation2017).
Furthermore, outcomes of this study established that S-containing metabolite, proline showed no significant increase in roots due to Ca+2 application under Cr stress in comparison with Cr-stressed plants, suggesting that metabolites having antioxidant properties (Yadav Citation2010; Nahar et al. Citation2016) are not linked with Ca+2 mediated tolerance to Cr stress in rice plants.
Conclusion
In the present study, morpho-physiological findings suggest that the exogenous supplementation of Ca alleviates the detrimental effects of Cr in rice. This mitigation is due to effectively stores excess Cr in roots through phytochelatin mediated vacuolar sequestration leading to reduced translocation in shoots. Moreover, regulation of antioxidant defense by modulating CAT, POD, GR as well as glutathione in roots provides partial protection from Cr induced oxidative damage. Findings of this study not only advance our understanding of chromium stress tolerance in rice plant but also point to potential areas of improvement for the alleviation of heavy metal toxicity. These improvements ultimately diminish the Cr contamination in crops and food materials.
Acknowledgements
The authors are grateful to NafiNano scientific for supplying all reagents on time.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Rumana Haque Mukta
Rumana Haque Mukta received her BSc (Hons) and MSc degree in Biotechnology and Genetic Engineering from Islamic University, Kushtia, Bangladesh in 2015 and 2016, respectively. Currently she is involved with a research project.
Mossammad Rima Khatun
Mossammad Rima Khatun received her BSc (Hons) and MSc degree in Biotechnology and Genetic Engineering from Islamic University, Kushtia, Bangladesh in 2015 and 2016, respectively. Currently she is involved with a research project.
A. K. M. Nazmul Huda
A. K. M. Nazmul Huda is an Associate Professor at the Department of Biotechnology and Genetic Engineering, Islamic University, Kushtia, Bangladesh. He received his BSc (Hons) and MSc in Genetics and Breeding from University of Rajshahi in 2002 and 2003, respectively. He also received his PhD degree from Islamic University, Bangladesh in 2018. His research interests encompass the effect of heavy metals on plants and its remediation and have published several papers in world-class journals. He is also responsible to supervise masters and doctoral research work. He is also working as a Laboratory biosafety auditor as well as a resource person on Biosafety and Biosecurity implementation program in Bangladesh launched by iccdrb, Bangladesh and CDC, Atlanta, USA jointly.
References
- Abd_Allah EF, Abeer Hashem C, Alqarawi AA, Wirth S, Egamberdiev D. 2017. Calcium application enhances growth and alleviates the damaging effects induced by Cd stress in sesame (Sesamum indicum L.). J Plant Interact. 12(1):237–243.
- Abd_Allah EF, Hashem A, Alqarawi AA, Alwathnani HA. 2015. Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscular mycorrhizal fungi. Pak J Bot. 47(2):785–795.
- Aderholt M, Vogelien DL, Koether M, Greipsson S. 2017. Phytoextraction of con- taminated urban soils by Panicum virgatum L. enhanced with application of a plant growth regulator (BAP) and citric acid. Chemosphere. 175:85–96.
- Adrees M, Ali S, Rizwan M, Zia-Ur-Rehman M, Ibrahim M, Abbas F, Farid M, Qayyum MF, Irshad MK. 2015. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Saf. 119:186–197.
- Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, Tran LS, Zhang JS. 2015. Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & coss.) by calcium application involves various physiological and biochemical strategies. PLoS One. 10(1):e0114571. doi:10.1371/ journal.pone.0114571
- Ahmad R. 2015. Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol Environ Saf. 120:310–317.
- Ahmad R, Ali S, Hannan F, Rizwan M, Iqbal M, Hassan Z, Akram NA, Maqbool S, Abbas F. 2017. Promotive role of 5-aminolevulinic acid on chromium-induced morphological, photosynthetic, and oxidative changes in cauliflower (Brassica oleracea botrytis L.). Environ Sci Pollut Res. 24:8814–8824.
- Alexieva VA, Sergiev I, Mapelli S, Karanov E. 2001. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 24:1337–1344.
- Anderson JS, Lall SP, Anderson DM, Chandrasoma J. 1992. Apparent and true availability of amino acids from common feed ingredients for Atlantic salmon (Salmo salar) reared in sea water. Aquaculture. 108(1–2):111–124.
- Antoniadis V, Shaheen SM, Boersch J, Frohne T, Du Laing G, Rinklebe J. 2017. Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany. J Environ Manag. 186:192–200.
- Axelsen KB, Palmgren MG. 2001. Inventory of the Superfamily of P-type Ion pumps in Arabidopsis. Plant Physiol. 126:696–706.
- Barrs HD, Weatherley PE. 1962. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci. 15:413–428. doi: 10.1071/BI9620413
- Bates LS, Waldren RP, Teari D. 1973. Rapid determination of free proline for water stress studies. Plant Soil. 39:205–207.
- Begum MC, Islam MS, Islam M, Amin R, Pavez MS, Kabir AH. 2016. Biochemical and molecular responses underlying differential arsenic tolerance in rice (Oryza sativa L.). Plant Physiol Biochem. 104:266–277.
- Bradford MM. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein- dye binding. Anal Biochem. 72:248–254.
- Cobbett C, Goldsbrough P. 2002. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol. 53:159–182.
- Cobbett CS. 2000. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 123(3):825–832.
- Das BC, Panda A, Sahoo PK, Jena S, Padhi P. 2014. Effect of chromium (VI) on wheat seedlings and the role of chelating agents. Curr Sci. 106:1387–1395.
- Daud M, Mei L, Variath M, Ali S, Li C, Rafi q M, Zhu S. 2014. Chromium (VI) uptake and tolerance potential in cotton cultivars: effect on their root physiology, ultramorphology, and oxidative metabolism. BioMed Res Int. 2014:e975946. doi: 10.1155/2014/975946
- Dragisic Maksimovi CJ, Bogdanovi CJ, Maksimovi CV, Nikolic M. 2007. Silicon modulates the metabolism and utilization of phenolic compounds in cucumber (Cucumis sativus L.) grown at excess manganese. J Plant Nutr Soil Sci. 170:739–744.
- Ellman G. 1959. Tissue sulfhydryl groups. Arch Biochem Biophys. 32:70–77.
- Elloumi N, Zouari M, Chaari L, Jomni C, Marzouk B, Elloumi FBA. 2014. Effects of cadmium on lipids of almond seedlings (Prunus dulcis). Bot Stud. 55(61):1–9.
- Emamverdian A, Ding Y, Mokhberdoran FX. 2015. Heavy metal stress and some mechanisms of plant defense response. Sci World J. 2015:e756120. doi:10.1155/2015/756120.
- Farooq M, Ali S, Hameed A, Bharwana S, Rizwan M, Ishaque W, Farid M, Mahmood K, Iqbal Z. 2016. Cadmium stress in cotton seedlings: physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. S Afr J Bot. 104:61–68.
- Foyer CH, Noctor G. 2005. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 28:1056–1071.
- Gill RA, Zang L, Ali B, et al. 2015. Chromium-induced physiochemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere. 120:154–164.
- Gill SS, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 48:909–930.
- Goud PB, Kachole MS. 2012. Antioxidant enzyme changes in neem, pigeonpea and mulberry leaves in two stages of maturity. Plant Sig Behav. 7:1258–1262.
- Greger M, Kabir AH, Landberg T, Maity PJ, Lindberg S. 2016. Silicon reduces cadmium uptake into cells of wheat. Environ Pollut. 211:90–97.
- Griffith. 1980. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 106(1):207–212.
- Guy C, Haskell D, Neven L, Klein P, Smelser C. 1992. Hydration-state responsive protein link cold and drought stress in spinach. Planta. 188:265–270.
- Halliwell B, Foyer CH. 1978. Properties and physiological function of a glutathion reductase purified from spinach leaves by affinity chromatography. Planta. 139:9–17.
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 125:189–198.
- Hogland DR, Arnon DI. 1950. The water culture method for growing plants without soil. Calif Agric Exp Stn Circ. 347:1950.
- Huda AKM, Swaraz NAM, Abu Reza M, Haque MA, Kabir AH. 2016. Remediation of chromium toxicity through exogenous salicylic acid in rice (Oryza sativa L.). Water Air Soil Pollut. 227(2016):e278. doi: 10.1007/s11270-016-2985-x
- Huda AKMN, Haque MA, Reshma Zaman S, M A, Kabir AH. 2017. Silicon ameliorates chromium toxicity through phytochelatin-mediated vacuolar sequestration in the roots of (Oryza sativa L.). Int J Phytoremediation. 19(3):246–253. doi:10.1080/15226514.2016.1211986.
- Jabeen N, Abbas Z, Iqbal M, Rizwan M, Jabbar A, Farid M, Ali S, Ibrahim M, Abbas F. 2016. Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch Agron Soil Sci. 62:648–662.
- Kabir AH. 2016. Biochemical and molecular changes in rice seedlings (Oryza sativa L.) to cope with chromium stress. Plant Biol. 18(4):710–719.
- Khan MN, Siddiqui MH, Mohammad F, Naeem M, Khan MMA. 2010. Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol Plant. 32:121–132.
- Kurtyka R, Ma łkowski E, Kita A, Karcz W. 2008. Effect of calcium and cadmium on growth and accumulation of cadmium, calcium, potassium and sodium in maize seedlings. Polish J Environ Stud. 17:51–56.
- Lee J, Shim D, Song WY, Hwang I, Lee Y. 2004. Arabidopsis metallothioneins 2a and 3 enhance resistance to cadmium when expressed in Vicia faba guard cells. Plant Mol Biol. 54:805–815.
- Lichtenthaler HK, Wellburn AR. 1985. Determination of total carotenoids and chlorophyll a and b of leaf extract in different solvents. Biochem Soc Trans. 11:591–592.
- Lutts S, Kinet JM, Bouharmont J. 1996. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivar differing in salinity resistance. Ann Bot. 78:389–398.
- Mahmud JA, Hasanuzzaman M, Nahar K, Borhannuddin Bhuyan MH, Masayuki MF. 2018. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol Environ Saf. 147:990–1001.
- Maiti S, Ghosh N, Mandal C, Das K, Dey N, Adak MK. 2012. Responses of the maize plant to chromium stress with reference to antioxidation activity. Braz J Plant Physiol. 24:203–212.
- Marentes E, Rauser WE. 2007. Different proportions of cadmium occur as Cd-binding phytochelatin complexes in plants. Plant Physiol. 131:291–301.
- Mendoza-Cozatl DG, Jobe TO, Hauser F, Schroeder JI. 2011. Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr Opin Plant Biol. 14:554–562.
- Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M. 2016. Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense, and methylglyoxal detoxification systems. Ecotoxicol Environ Saf. 126:245–255.
- Panda S, Choudhury S. 2005. Chromium stress in plants. Braz J Plant Physiol. 17:95–102.
- Peng J, Gong J. 2014. Vacuolar sequestration capacity and long-distance metal transport in plants. Front Plant Sci. 5:19.
- Pourrut B, Shahid M, Douay F, Dumat C, Pinelli E. 2013. Molecular mechanisms involved in lead uptake, toxicity and detoxification in higher plants. In: Gupta D, Corpas F, Palma J, editors. Heavy metal stress in plants. Berlin: Springer; p. 121–147.
- Reddy VS, Reddy ASN. 2004. Proteomics of calcium-signaling components in plants. Phytochemistry. 65:1745–1776.
- Roosens NH, Bernard C, Leplae R, Verbruggen N. 2004. Evidence for copper homeostasis function of metallothionein (MT3) in the hyperaccumulator Thlaspi caerulescens. FEBS Lett. 577:9–16.
- Rudd JJ, Franklin-Tong VE. 2001. Unravelling response-specificity in Ca2+ signalling pathways in plant cells. New Phytol. 151:7–33.
- Sanders D, Pelloux J, Brownlee C, Harper JF. 2002. Calcium at the crossroads of signaling. Plant Cell. 14:S401–S417.
- Sarwat M, Ahmad P, Nabi G, Hu X. 2013. Ca2+ signals: the versatile decoders of environmental cues. Crit Rev Biotechnol. 33:97–109.
- Scoccianti V, Crinelli R, Tirillini B, Mancinelli V, Speranza A. 2006. Uptake and toxicity of Cr (III) in celery seedlings. Chemosphere. 64:1695–1703.
- Shahid M, Pourrut B, Dumat C, Nadeem M, Aslam M, Pinelli E. 2014. Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. Rev Environ Contam Toxicol. 232:1–44.
- Shanker A, Djanaguiraman M, Sudhagar R, Jayaram K, Pathmanabhan G. 2004. Expression of metallothionein 3-like protein mRNA in sorghum cultivars under chromium (VI) stress. Curr Sci. 86:901–902.
- Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S. 2005. Chromium toxicity in plants. Environ Int. 31:739–753.
- Sharma DC, Sharma CP, Tripathi RD. 2003. Phytotoxic lesions of chromium in maize. Chemosphere. 5:63–68.
- Siddiqui MH, Al-Whaibi MH, Sakran AM, Basalah MO, Ali HM. 2012. Effect of calcium and potassium on antioxidant system of Vicia faba L. under cadmium stress. Int J Mol Sci. 13:6604–6619.
- Singh D, Gupta R, Tiwari A. 2012. Potential of duckweed (Lemna minor) for removal of lead from wastewater by phytoremediation. J Pharm Res. 5:1578–1582.
- Singh HP, Mahajan P, Kaur S, Batish DR, Kohli RK. 2013. Chromium toxicity and tolerance in plants. Environ Chem Lett. 11:229–254.
- Sinha S, Bhatt K, Pandey K, Singh S, Saxena R. 2003. Interactive metal accumulation and its toxic effects under repeated exposure in submerged plant Najas indica. Cham. Bull Environ Contam Toxicol. 70:696–704.
- Sun M, Zigman S. 1978. An improved Spectrophotomeric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem. 90:81–89.
- Suzuki N. 2005. Alleviation by calcium of cadmium-induced root growth inhibition in Arabidopsis seedlings. Plant Biotech. 22:19–25.
- Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD, Sahoo L, Panda SK. 2012. Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem. 53:33–39.
- Uliana YA, Stambulska M, Bayliak M, Volodymyr IL. 2018. Chromium(IV) toxicity in legume plant: modulation effect of Rhizobial symbiosis. BioMed Res Int. 2018:e 8031213.
- Wu Z, McGrouther K, Chen D, Wu W, Wang H. 2013. Subcellular distribution of metals within Brassica chinensis L. in response to elevated lead and chromium stress. J Agric Food Chem. 61:4715–4722.
- Yadav SK. 2010. Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot. 76:167–179.
- Yang-ErChena H-TM, Ma J, Wu N, Zhang C-M, Su Y, Zhang Z-W, Yuan M, Zhang H-Y, Xian-Yin Zeng S. 2018. Biomonitoring chromium III or IV solublepollution by moss chrlorophyll fluorescence. Chemosphere. 194:220–228.