ABSTRACT
The wood-boring wasp, Sirex noctilio, is an invasive pest of numerous species of pine trees worldwide. The female S. noctilio wasps selectively lay eggs on different pine trees. The relationship between host volatile organic compounds and the wood wasps remains elusive. Here, the behaviors of the wood towards the host volatiles wasps were investigated. In Junde Forest Farm, three tree species, Pinus sylvestris var. mongolica, Larix gmelinii (Rupr.) Kuzen., and P. koraiensis Sieb.et Zucc., were girdled. Four treatments of trees, un-girdled, girdled, dying, and dead tree, were randomly selected as sample trees. Lindgren 12-funnel trap was placed on sample tree. The traps were checked once a week and the number of S. noctilio was recorded. The relationship between volatiles and S. noctilio attraction was analyzed by collecting volatiles from different tree species and treatments. trans-β-Ocimene, terpinolene, α-pinene oxide, and longifolene were present only in girdled P. sylvestris var. mongolica. Girdling the host tree can make released more volatiles, which was conducive to attracting S. noctilio. Effect of a single compound on S. noctilio is limited, but a mixture of multiple components formed a specific chemical signature to female S. noctilio. It could be useful in the monitoring and effective management of wood wasps.
Introduction
The European woodwasps Sirex noctilio F. (Hymenoptera: Siricidae) is a wood-boring insect native to Eurasia and North Africa, which is known to attack dead and dying Pinus species in these regions, where the woodwasp is not generally considered to be a major pest of pine trees (Spradbery and Kirk Citation1978; Haugen Citation2007). It was accidentally introduced to several countries, including New Zealand (first record 1900), Australia (1952), Uruguay (1980), Argentina (1985), Brazil (1988), South Africa (1994), Chile (2001), and United States (2004) (Rawlings Citation1948; Iede et al. Citation1988; Madden Citation1988; Tribe Citation1995; Hoebeke et al. Citation2005; Slippers et al. Citation2015). In these countries, S. noctilio has caused severe damage to pine plantations and is considered a major pest of pine.
At present, there is no evidence that S. noctilio has a long-range sex pheromone (Crook et al. Citation2008). Volatile components are important in the attraction of S. noctilio to host trees and attractants are present in the cambium-phloem (Madden Citation1971). The major components of all volatile collections were monoterpene hydrocarbons and constituted over 95% of the total volatiles from felled Pinus radiate. Females S. noctilio primarily attacked on stressed trees (Madden Citation1968). Madden (Citation1968, Citation1971) showed that the attractiveness of trees to S. noctilio increased after felling or ringing, reaching a maximum after some days or weeks, eg 5–7 days after felling. For alien invasive forest insect species, traps baited with tree stress volatiles are among the most effective tools for monitoring spread and establishment (Brockerhoff et al. Citation2006; Witzgall et al. Citation2010; Nadel et al. Citation2012). A key component of S. noctilio detection and management plans in the world where S. noctilio has become established is girdled trap trees.
The widely used commercial Sirex lures comprise of 70% α-pinene and 30% β-pinene (Alpha Scents Inc., OR, USA), and was developed based on EAG response to host volatiles (Morgan and Stewart Citation1972; Simpson Citation1976; Simpson and McQuilkin Citation1976; Hurley et al. Citation2007; Bashford Citation2008; Bashford and Madden Citation2012). While current monitoring tools used in many invaded regions rely on traps lured with combinations of pine volatiles, these techniques do not yet offer an ideal solution to detect the pest at low densities (Bashford and Madden Citation2012; Hurley et al. Citation2015). Evidence continues to suggest that additional volatile compounds may be involved in signaling since girdled and/or herbicide-treated trap trees nearly always outperform artificial traps baited with the recommended mixed-pinene lure (Madden Citation1971; Morgan and Stewart Citation1972; Simpson Citation1976; Simpson and McQuilkin Citation1976; Neumann et al. Citation1982; Crook et al. Citation2012).
In August 2013, S. noctilio was detected as a pest of Pinus sylvestris var. mongolica in the Duerbote Mongolian Autonomous County, Daqing City, Heilongjiang Province, China (N 46°37′47″, E 124°25′51″) (Li et al. Citation2015). Subsequently, S. noctilio was also found in Junde Forest Farm, Hegang City, Heilongjiang Province (N 47°12′11″, E 130°17′47″). There were three tree species, P. sylvestris var. mongolica, Larix gmelinii (Rupr.) Kuzen., and P. koraiensis Sieb.et Zucc., and S. noctilio only harm P. sylvestris var. mongolica in Junde. The factors affecting susceptibility of different pine species toward wasp attack is unclear. Junde provides suitable conditions for this study. Field environment, temperature, soil, and precipitation are similar on management. Comparing the terpenoid volatile compositions of three tree species may help narrow the categories of important terpenoid volatiles in pines attracted by S. noctilio. In our study, we girdled real alive tree trunks and selected trees of different vitality. Then, their volatiles were collected and analyzed. We believe that the results of this study could improve the monitoring, trapping, and control of woodwasps in the future.
Materials and methods
Tree materials and treatments
Three tree species, P. sylvestris var. mongolica, L. gmelinii, and P. koraiensis, were distributed in Junde Forest Farm, Hegang City, Heilongjiang Province. Sample trees were selected randomly in the farm (). No dead or dying L. gmelinii and P. koraiensis was found in Junde Forest Farm. The spacing between the sample trees was about 50 m. The girdling was made through the bark to the xylem at a height of 130–150 cm (about 2 cm in width).
Table 1. Details of sample trees from Junde Forest Farm.
Field trapping
Lindgren 12-funnel trap (Geruibiyuan Technology Co., Ltd.) without chemical was placed on each sample tree at a height of 2.50 m in August. The traps were checked once a week and the number of S. noctilio was recorded. The experiment was postponed from 29th August to 3rd September because of the rain.
Tree trunks volatile collections
We girdled tree trunk in the last week of July. Volatiles were collected from 9 to 11 am and from 2 to 4 pm in the first week of August. The girdled day and the collection day are 5–7 days apart. We used a push–pull system to collect headspace volatile organic compounds (VOCs) from tree trunk. Tree trunks were placed in a clean roasting bag (Reynolds, Richmond, VA, USA). The bag was sealed around the tree trunk with a sealing strip about 130–150 cm above the ground. Humidified, charcoal-filtered air was pushed into the bag with a pump (QC-1S, Beijing Institute of Labor Instruments, China) at 200 mL/min, while the air mixed with volatiles was pulled out from the bag with a pump and passed over to an adsorbent cartridge. The adsorbent cartridge was a 3 × 100 mm glass column containing 200 mg of Porapak Q (80/100 mesh, CNW Technologies GmbH, Germany) between plugs of glass wool. Each sample was aerated for 100 min. Volatiles were eluted from the adsorbent cartridge with 500 μL redistilled hexane at room temperature. The final extracts were concentrated to 50 μL using a slow streamed of nitrogen and then subjected to gas chromatography-mass spectrometry (GC-MS). If not used immediately, extracts were stored in glass vials at – 20°C until use.
Gas chromatography-mass spectrometry (GC-MS)
Headspace volatiles were analyzed with gas chromatography-mass spectrometry (GC-MS; model: GCMS-QP2010 Ultra; Shimadzu Corporation). A SH-Rtx-5 chromatographic column was used (30 m long; internal diameter: 0.25 mm; film thickness: 0.25 μm; Shimadzu Corporation). The carrier gas was He (99.999%, 1 ml/min). The initial temperature of 40°C was maintained for 2 min. Next, the temperature was increased to 180°C at a rate of 6°C/min before heating to 270°C in 15°C/min steps. The electrons were from an EI source of 70 ev; the scanned mass-to-charge ratio range was 40–400 amu and the ion source temperature was 230°C.
The chromatograms were analyzed using the GC-MS solution (software version 4.11, 1999–2013; Shimadzu Corporation). Based on the gas chromatographs, retention time and MS data were verified and compared with the National Institute of Standards and Technology (NIST) database. We removed impurities (silicon compounds) from control groups and air. Using total ion current peak area normalization, the relative amount of each identified component was calculated.
Statistical analysis
Mean numbers of females captured in traps with different tree species (P. sylvestris var. mongolica, L. gmelinii, P. koraiensis) and treatments (un-girdled, girdled, dying, dead) in the field were analyzed by one-way ANOVA. The means were separated by Tukey's multiple comparison tests (P < 0.05). Multivariate analysis of variance (MANOVA) was performed to identify statistically significant differences between terpene volatiles (dependent variables) emitted by different tree species and treatments. If significant, one-way ANOVA and Tukey's multiple comparison tests were conducted to test for proportional differences of individual and total headspace terpene volatiles emitted by different tree species and treatments. Different tree species and emitted terpene volatiles were grouped using principal component analysis (PCA) and cluster analysis. Among the VOCs emitted by the different tree species and treatments, only terpenoid compounds were subjected to PCA and cluster analysis. Terpenoid compounds were selected based on two principles: (i) these compounds accounted for a relatively high percentage of tree species or treatments, and their mean proportion in total VOCs was not less than 0.01%; (ii) the amounts of selected VOCs were significantly different between tree species or treatments (ANOVA: P < 0.05). PCA was used to create a two-dimensional display of the multivariate dataset and to graphically determine whether clustering of different tree species and treatments occurred on the base of their terpene volatiles profiles. Hierarchical cluster analysis of each sample was carried out using the between-group linkage method and Euclidean distance. All data were analyzed with the IBM SPSS Statistics version 22.0 for Windows (Chicago, IL, USA).
Results
Forest trap
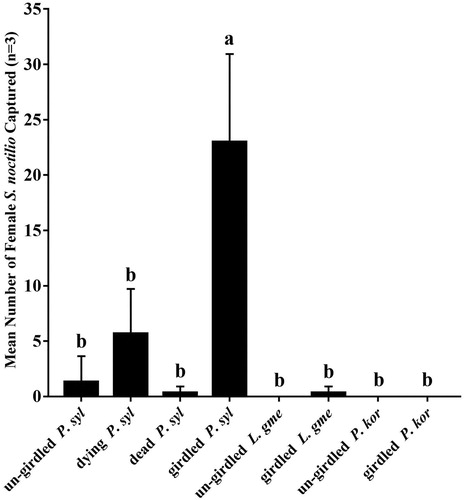
The numbers of S. noctilio captured by traps were counted once a week in August. The average number of females S. noctilio captured were the highest and 23.00 ± 7.94 (n = 3) per trap on the girdled tree of P. sylvestris var. mongolica (). The number of females S. noctilio captured were significantly higher than those of the other three tree conditions (un-girdled, died, dying) and those under the conditions of others tree species (F = 17.90, df = 7, P < 0.05). The number of females S. noctilio captured was 5.67 ± 4.04 (n = 3), 1.33 ± 2.31 (n = 3) and 0.33 ± 0.58 (n = 3) on the dying, un-girdled and dead P. sylvestris var. mongolica, respectively. The girdled L. gmelinii captured very low S. noctilio female of 0.33 ± 0.58 (n = 3), while S. noctilio were not captured on un-girdled trees of L. gmelinii. Similarly, no S. noctilio was captured on un-girdled P. koraiensis and girdling P. koraiensis trees.
Figure 1. The mean number of female Sirex noctilio caught per treatment in Junde. Small bars represent standard deviation. Different letters on bars indicate significant differences (one-way ANOVA followed by Tukey's multiple comparison test, P < 0.05).
Note: P. syl = Pinus sylvestris var. mongolica, L. gme = Larix gmelinii, P. kor = Pinus koraiensis.
Chemical analysis of tree trunk volatiles of three tree species
A total of 13 terpene compounds was detected from un-girdled and girdled tree trunks of P. sylvestris var. mongolica, P. koraiensis and L. gmelinii () under our experimental conditions. The relative amount of each identified compound was based on peak area normalization of total ion current.
Table 2. Relative TIC-peak areas of VOCs collected in the headspace of the tree trunks from three tree species un-girdled and girdled.
Four terpene compounds were found from un-girdled P. sylvestris var. mongolica, L. gmelinnii, and P. koraiensis tree trunks. The major volatile components of the un-girdled tree trunks were α-pinene (68.91 ± 1.48%, 55.26 ± 2.25% and 63.15 ± 0.41%, respectively) and β-pinene (24.51 ± 1.06%, 37.37 ± 3.49% and 24.88 ± 0.20%, respectively), which accounted for 93.42%, 92.63% and 88.03% of the total VOCs amount.
More volatiles were released by girdled tree trunks with 12, 8 and 9 terpene compounds detected from P. sylvestris var. mongolica, L. gmelinii and P. koraiensis, respectively. The primary components of terpene included α-pinene (57.20 ± 1.36% from P. sylvestris var. mongolica, 55.25 ± 4.82% from L.gmelinii and 66.13 ± 2.86% from P. koraiensis), β-pinene (36.85 ± 0.99%, 35.09 ± 5.96% and 28.64 ± 2.45%, respectively). In addition to these two compounds, limonene was detected from P. sylvestris var. mongolica (2.26 ± 0.25%) and L. gmelinii (7.81 ± 0.62%), which accounted for 96.31% and 98.15% in two tree trunks. Furthermore, girdled P. koraiensis also released sabinene (2.38 ± 0.31%), which was significantly higher than those from other girdled tree trunks (F = 164.43, df = 5, P < 0.05).
PCA and hierarchical cluster analysis
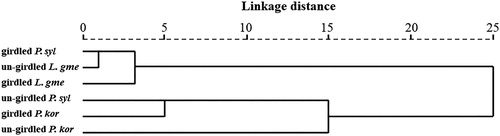
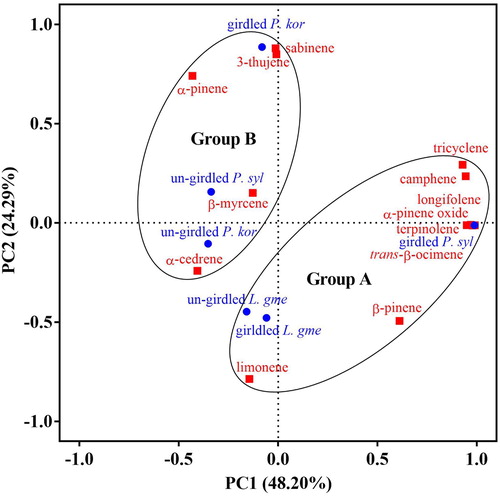
The PCA clearly divided the VOCs into significantly different two groups. The PCA horizontal axis explained 48.20% of the total variance and the vertical axis explained a further 24.29% (). The VOCs from girdled and un-girdled P. sylvestris var. mongolica were separated into Group A and B, respectively. However, both girdled and un-girdled L. gmelinii grouped together in Group A, and both girdled and un-girdled P. koraiensis grouped together in Group B. These are supported by the hierarchical cluster analysis between-groups linkage (Squared Euclidean distance) at a distance >15 and <25 (). The common characteristics of un-girdled L. gmelinii, girdled L. gmelinii and girdled P. sylvestris var. mongolica were the relatively high percentage of β-pinene (37.37 ± 3.49% to 35.09%±5.96%) and the relatively low percentage of α-pinene (55.25%±4.82% to 57.20%±1.36%).
Figure 2. Principal component analysis of volatile organic compounds produced by un-girdled and girdled trees of P. sylvestris var. mongolica, P. koraiensis, and L. gmelinii. Blue circles represent the three tree species un-girdled or girdled. Black circles represent the classification of three trees. Red squares represents 13 different compounds, based on the PCA of the component matrix.
Note: P. syl = Pinus sylvestris var. mongolica, L. gme = Larix gmelinii, P. kor = Pinus koraiensis.
Chemical identification of VOCs from P. sylvestris var. mongolica trunk
The VOCs from P. sylvestris var. mongolica tree trunk were further analyzed under girdled, un-girdled, dying and dead conditions (). A total of 12 terpene compounds were identified. Same compounds were detected from un-girdled and girded P. sylvestris var. mongolica between and . Six terpene compounds were detected in the volatiles of dying P. sylvestris var. mongolica. The main volatile components of dying P. sylvestris var. mongolica included α-pinene (65.99 ± 2.10%), α-pinene (25.41 ± 1.17%) and limonene (5.39 ± 0.85%), accounting for 96.79% of the total VOCs amount. Only two terpene compounds, α-pinene (75.08 ± 1.00%) and β-pinene (24.92 ± 1.00%), were identified from dead P. sylvestris var. mongolica. In comparison with other three conditions (un-girdled, girdled, and dying) of P. sylvestris var. mongolica, α-pinene was most abundant in dead P. sylvestris var. mongolica. The PCA separated the girdled tree trunk from un-girdled, dying and dead tree trunks as a single group namely Group B, which clearly released significantly different volatiles ( and ). The PCA horizontal axis explained 74.28% of the total variance and the vertical axis explained a further 15.97% (). The characteristics of Group B were the high percentage of β-pinene (36.85% ± 0.99%) and the low percentage of α-pinene (57.20% ± 1.36%). Furthermore, the VOCs of girdled P. sylvestris var. mongolica contained trace amounts (<0.31%) of 3-thujene, sabinene, trans-β-Ocimene, terpinolene, α-pinene oxide, and longifolene, which were not detected in un-girdled, dying, and dead P. sylvestris var. mongolica tree trunks.
Figure 4. Principal component analysis of volatile organic compounds produced by four treatments, un-girdled, dying, dead and girdled of P. sylvestris var. mongolica. Blue circles represent four treatments of P. sylvestris var. mongolica. Black circles represent the classification of four treatments. Red squares represents 12 different compounds, based on the PCA of the component matrix.
Note: P. syl = Pinus sylvestris var. mongolica, L. gme = Larix gmelinii, P. kor = Pinus koraiensis.
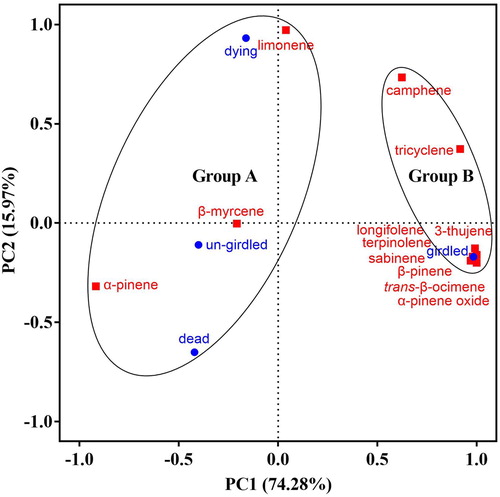
Figure 5. Phylogenetic tree of these volatiles derived from P. sylvestris var. mongolica, un-girdled, dying, dead and girdled based on Squared Euclidean distance, at a distance > 10 and < 25.
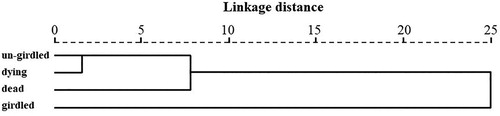
Table 3. Relative TIC-peak areas of VOCs collected in the headspace of the tree trunks from P. sylvestris var. mongolica.
Discussion
Because of the lack of effective attractants, baits and traps have not been successfully used as important tools for monitoring and controlling wood wasps (Hurley et al. Citation2015; Slippers et al. Citation2015). Previously published study found that higher numbers of S. noctilio attracted to its native host P. sylvestris compared with P. resinosa (Zylstra et al. Citation2010). The results of our filed study showed that girdled P. sylvestris var. mongolica attracts more adult female wood wasps and this attractive effect was stronger than that of L. gmelinii and P. koraiensis (). It is possible that S. noctilio chosen to harm the host according to host's VOCs when there were several species of trees. The VOCs from wasp hosts have good prospects in attracting S. noctilio. The relative changes in the volatile contents of suitable host trees may affect the behavior of female wasps.
Some studies mainly focused on α-pinene and β-pinene. The lures with just β-pinene had no success in attracting S. noctilio, and the lure with just α-pinene showed only limited success (Bashford Citation2008). These studies had shown that single compounds had a limited effect on female S. noctilio. In and , α-pinene and β-pinene are the main components. However, in three tree species and different conditions of P. sylvestris var. mongolica, α-pinene and β-pinene from girdled P. sylvestris var. mongolica are significantly different with those in other trees. shows that the relative content of α-pinene in girdled P. sylvestris var. mongolica is significantly reduced, while the relative content of α-pinene in girdled L. gmelinii and P. koraiensis are almost unchanged or increasing. In , α-pinene from girdled P. sylvestris var. mongolica is significantly less than the others, and β-pinene is significantly more. The results show that when α-pinene and β-pinene are used in the lure, their proportion is important for the attraction of S. noctilio.
When a tree is subjected to artificial pressure, such as a girdling (removing the bark around the whole circumference of the tree), the osmotic pressure decreases. This leads to changes in bark permeability, resulting in the release of more volatiles from the phloem and cambial sap (Madden Citation1968, Citation1971, Citation1988). shows that the terpene volatiles of the three tree species are all four components before girdling. After girdling, the types of terpenoids in the three tree species increased, and the relative contents also changed. The girdled P. sylvestris var. mongolica has the largest variety of terpenoids. The most attractive tree trunk was girdled P. sylvestris var. mongolica, and proportion of camphene, tricyclene, trans-β-Ocimene, terpinolene, α-pinene oxide and longifolene released are significantly different from those of other trees. Trans-β-Ocimene, terpinolene, α-pinene oxide, and longifolene were present only in girdled P. sylvestris var. mongolica. Similar findings show that there are also some minor monoterpenes, that is, namely thujene, sabinene, γ-terpinene, and terpinolene. These monoterpenoid are detected in P. sylvestris var. mongolica and P. strobus, and good target compounds for behavioral analysis and may still be important components of an attractive blend (Böröczky et al. Citation2012). These results suggest that it is important to mix these minor components with α-pinene and/or β-pinene to improve the attractiveness of wood wasp trap. In addition, oxygenated constituents could be more attractive to S. noctilio than hydrocarbon monoterpenes (Simpson and McQuilkin Citation1976). It is required for further study to determine whether these components have any effect on the behaviors of the wood wasps in the field.
Based on the comprehensive consideration of the results of PCA, cluster analysis and ANOVA of terpenoid compounds, mixtures containing camphene, tricyclene, trans-β-Ocimene, terpinolene, α-pinene oxide, longifolene, and α-pinene are indispensable for girdled P. sylvestris var. mongolica to attract S. noctilio.
Our study can also be determined that the chemical differences in the extracts from different tree species in Junde Forest Farm were genetically driven because the growth environment of different trees was the same. This discovery was significant for understanding the host selection behavior of S. noctilio, because invasive pests spread to new host groups. These differences were genetically, so the damage can be reduced by planting trees that are not easily accepted by wood wasps. In areas with large populations of wood wasps, volatiles of L. gmelinii and P. koraiensis can be used as repellents to reduce potential wood wasp outbreaks.
Acknowledgements
We thank Feng Zhou for his assistance with sample collection, and workers of the Forestry Bureau in Hegang city for their assistance with fieldwork. The authors also thank Lili Ren, Muhammad Akram, Xiaobo Liu, Lixiang Wang and Min Bao (Beijing Forestry University, Beijing, China) for their help in laboratory assays. Prof Jing-Jiang Zhou (Beijing Forestry University, Beijing, China) for critical editing and suggestions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Notes on contributors
Qiang Xu
Qiang Xu is a graduate student at the College of Forestry of Beijing Forestry University.
Xue-Ting Sun
Xue-Ting Sun is a graduate student at the College of Forestry of Beijing Forestry University.
Peng-Fei Lu
Peng-Fei Lu is an associate professor at the College of Forestry of Beijing Forestry University, Key Laboratory of Beijing for the Control of Forest Pests, and Laboratory of Forest Boring Insects, and Sino-France Joint Laboratory for Invasive Forests Pests in Eurasia.
You-Qing Luo
You-Qing Luo is a professor at the College of Forestry of Beijing Forestry University, Key Laboratory of Beijing for the Control of Forest Pests, and Laboratory of Forest Boring Insects, and Sino-France Joint Laboratory for Invasive Forests Pests in Eurasia.
Juan Shi
Juan Shi is a professor at the College of Forestry of Beijing Forestry University, Key Laboratory of Beijing for the Control of Forest Pests, and Laboratory of Forest Boring Insects, and Sino-France Joint Laboratory for Invasive Forests Pests in Eurasia.
References
- Bashford R. 2008. The development of static trapping systems to monitor for wood-boring insects in forestry plantations. Aust For. 71:236–241. doi: 10.1080/00049158.2008.10675041
- Bashford R, Madden J. 2012. The use of kairomone lures for the detection of Sirex noctilio in susceptible Pinus radiata plantations in Australia. In: Slippers B, de Groot P, Wingfield MJ, editors. The Sirex woodwasp and its Fungal Symbiont. Dordrecht: Springer; p. 159–166.
- Böröczky K, Zylstra KE, McCartney NB, Mastro VC, Tumlinson JH. 2012. Volatile profile differences and the associated Sirex noctilio activity in two host tree species in the northeastern United States. J Chem Ecol. 38(2):213–221. doi: 10.1007/s10886-012-0077-y
- Brockerhoff EG, Liebhold AM, Jactel H. 2006. The ecology of forest insect invasions and advances in their management. Can J For Res. 36:263–268. doi: 10.1139/x06-013
- Crook DJ, Böröczky K, Zylstra KE, Mastro VC, Tumlinson JH. 2012. The chemical ecology of Sirex noctilio. In: Slippers B, de Groot P, Wingfield, MJ, editors. The Sirex woodwasp and its Fungal Symbiont. Dordrecht: Springer; p. 149–158.
- Crook DJ, Kerr LM, Mastro VC. 2008. Sensilla on the antennal flagellum of Sirex noctilio (Hymenoptera: Siricidae). Ann Entomol Soc Am. 101(6):1094–1102. doi: 10.1603/0013-8746-101.6.1094
- Haugen DA. 2007. Sirex woodwasp: biology, ecology and management.
- Hoebeke ER, Haugen DA, Haack RA. 2005. Sirex noctilo: discovery of a Palearctic Siricid woodwasp in New York. Newsl Mich Entomo Soc. 50:24–25.
- Hurley BP, Garnas J, Cooperband MF. 2015. Assessing trap and lure effectiveness for the monitoring of Sirex noctilio. Agr For Entomol. 17:64–70. doi: 10.1111/afe.12081
- Hurley BP, Slippers B, Wingfield MJ. 2007. A comparison of control results for the alien invasive woodwasp, Sirex noctilio, in the southern hemisphere. Agr For Entomol. 9:159–171. doi: 10.1111/j.1461-9563.2007.00340.x
- Iede ET, Penteado SRC, Bisol JC. 1988. Primeiro registro de ataque de Sirex noctilio em Pinus taeda no Brasil. Circular Técnica EMBRAPA-CNPF n. 20. Circular Técnica EMBRAPA-CNPF n. 20. EMBRAPA, Brazil.
- Li DP, Shi J, Lu M, Ren LL, Zhen CA, Luo YQ. 2015. Detection and identification of the invasive Sirex noctilio (Hymenoptera: Siricidae) Fungal Symbiont, Amylostereum areolatum (Russulales: Amylostereacea), in China and the stimulating effect of insect Venom on Laccase production by A. areolatum YQL03. J Econ Entomol. 108(3):1136–1147. doi: 10.1093/jee/tov072
- Madden J. 1968. Physiological aspects of host tree favourability for the woodwasp, Sirex noctilio F. Proc Ecol Soc Aust. 3:147–149.
- Madden JL. 1971. Some treatments which render Monterey pine (Pinus radiata) attractive to the wood wasp Sirex noctilio F. Bull Entomol Res. 60:467–472. doi: 10.1017/S0007485300040414
- Madden JL. 1988. Sirex in Australasia. In: Berryman AA, editor. Dynamics of forest insect populations. New York (NY): Springer; p. 407–429.
- Morgan FD, Stewart NC. 1972. Developing and testing a lure-trap for the woodwasp Sirex noctilio F. Aust For. 36(1):38–46. doi: 10.1080/00049158.1972.10675568
- Nadel RL, Wingfield MJ, Scholes MC, Lawson SA, Slippers B. 2012. The potential for monitoring and control of insect pests in southern hemisphere forestry plantations using semiochemicals. Ann For Sci. 69(7):757–767. doi: 10.1007/s13595-012-0200-9
- Neumann FG, Harris JA, Kassaby FY, Minko G. 1982. An improved technique for early detection and control of the Sirex wood wasp in radiata pine plantations Sirex noctilio, Pinus radiata, Australia. Aust For. 45:117–124. doi: 10.1080/00049158.1982.10674342
- Rawlings GB. 1948. Recent observations on the Sirex noctilio population in Pinus radiata forest in New Zealand. NZ J For Sci. 5:411–421.
- Simpson R, McQuilkin R. 1976. Identification of volatiles from felled Pinus radiata and the electroantennograms they elicit from Sirex noctilio. Entomol Exp Appl. 19:205–213. doi: 10.1111/j.1570-7458.1976.tb02599.x
- Simpson RF. 1976. Bioassay of pine oil components as attractants for Sirex noctilio (Hymenoptera: Siricidae) using electroanthennogram techniques. Entomol Exp Appl. 19(1):11–18. doi: 10.1111/j.1570-7458.1976.tb02576.x
- Slippers B, Hurley BP, Wingfield MJ. 2015. Sirex woodwasp: a model for evolving management paradigms of invasive forest pests. Annu Rev Entomol. 60:601–619. doi: 10.1146/annurev-ento-010814-021118
- Spradbery J, Kirk A. 1978. Aspects of the ecology of siricid woodwasps (Hymenoptera: Siricidae) in Europe, North Africa and Turkey with special reference to the biological control of Sirex noctilio F. in Australia. Bull Entomol Res. 68:341–359. doi: 10.1017/S0007485300009330
- Tribe GD. 1995. The woodwasp Sirex noctilio Fabricius (Hymenoptera: Siricidae), a pest of Pinus species, now established in South Africa. Afr Entomol. 3:215–217.
- Witzgall P, Kirsch P, Cork A. 2010. Sex pheromones and their impact on pest management. J Chem Ecol. 36(1):80–100. doi: 10.1007/s10886-009-9737-y
- Zylstra KE, Dodds KJ, Francese JA, Mastro V. 2010. Sirex noctilio in North America: the effect of stem-injection timing on the attractiveness and suitability of trap trees. Agr For Entomol. 12:243–250.