ABSTRACT
It has been shown that salicylic acid (SA) acts as an endogenous signal molecule responsible for inducing stress tolerance. The aim of the present work is to investigate the effect of sodium chloride (0, 100, and 200 mM) and exogenous SA (1 mM) on some biochemical and molecular responses of safflower. Results revealed that K+, Ca2+, indol-3-acetic acid (IAA), and gibberellic acid (GA) contents decreased under salinity however, Na+ content, and SOS1 and NHX1 genes expression increased. Further, palmitic and oleic acids contents decreased, while stearic, linoleic, and linolenic acids content increased under salinity. Exogenous SA had a positive effect on K+, Ca2+, IAA, and GA contents, but decreased Na+ content. In addition, SA induced expression of SOS1 and NHX1 genes in all plants. Our data indicate that SA helps safflower to better cope with salinity. The results provide new insights to mechanisms that help regulate salinity resistance in safflower. SA may be considered as a foliar application to ameliorate salinity effects, due to its low price and availability.
Highlights
SA helps safflower plants to better cope with saline conditions by the expression of SOS1 and NHX1
SA regulates various aspects of plant responses to salt stress through signaling cross-talk with other hormones
Exogenous SA in salt-stressed safflower showed a large increase in desaturation of fatty acids in membrane
Introduction
Climate change is one of the major challenges of our time and the socio-economic consequences are alarming. It is predicted that global climate change will alter environmental parameters such as rainfall distribution with e.g. less rainfall in some regions, which in turn may increase the salinity of soils. As a consequence of increased soil salinity, plant nutrient availability reduces, and therefore, growth and productivity decreases (Zahedi et al. Citation2012). Salinity is also a significant problem in safflower (Carthamus tinctorius L.) productivity in arid and semi-arid areas. Safflower is a multipurpose crop grown for its flowers and seeds, which have numerous biological properties (Bowles et al. Citation2010). Further, safflower is known as a salt-tolerant plant. However, its growth and yield decrease as the salinity level increases (Bassil and Kaffka Citation2002).
The homeostasis of Na+ to K+ ions concentration in the cytosol is fundamental in salt stress conditions. It is also important for the activities of cytosolic enzymes and maintaining membrane potential. Thus, plant cells employ some transporter proteins to maintain an optimal Na+/K+ ratio. To prevent growth cessation, excessive Na+ has to be extruded to the apoplast or compartmentalized into the vacuoles (Hasegawa et al. Citation2000). Na+ extrusion from the cytosol is carried out by transporter proteins such as Na+/H+ exchanger-1 (NHX1) and salt overly sensitive-1 (SOS1), which have an important role in plant adaptations to salinity (Silva et al. Citation2010).
To mitigate environmental stresses, plants also induce production of some key hormones such as auxin (Indole-3-acetic acid: IAA) and gibberellic acid (GA), as well as desaturation of fatty acids in cells. Plant hormones play central roles in adaptation to changing environments, by mediating nutrient allocation, growth, and development. Further, hormonal cross-talk results in synergetic or antagonistic interactions that play crucial roles in plant response to stress.
The ability to respond to environmental stimuli is among the most fundamental processes that enable plants to survive. There are many agents in cells which act as signal transducers in response to stress conditions. Salicylic acid (SA), a well-known signaling messenger, is able to reduce symptoms of environmental stresses in plants (Hayat et al. Citation2010). SA regulates various aspects of plant responses to stress through extensive signaling cross-talk with other hormones (Jayakannan et al. Citation2013). The ability of exogenous SA to enhance antioxidant protection, increase the accumulation of osmolytes, and maintain optimum Na+/K+ ratio under saline conditions has been suggested as potential mechanisms of salt tolerance in plants (Ashraf et al. Citation2010; Hayat et al. Citation2010). These qualities make SA an ideal chemical to increase resistance to salt stress in plants.
Our previous works indicated that SA minimizes the negative effects of salt stress by improving growth parameters, accumulation of compatible solutes, and increasing antioxidant activity, and therefore, could be used for partial amelioration of salt stress in safflower (Shaki et al. Citation2017, Citation2018). To the best of our knowledge, there is no information available so far about the effect of SA on ions homeostasis, hormonal cross-talk and fatty acids compositions in salt-treated safflower plants. Thus, the working hypothesis for this study was that beneficial effects of SA during salt stress may be related to up-regulation of sodium/proton transporters genes and consequent effects on intracellular ionic homeostasis of Na+ and K+, as well as desaturation of fatty acids in membrane. Consequently, the aim of this work was to investigate the impact of SA on some key physiological parameters in safflower, as well as genes expression. Revealing the mechanisms underlying salt tolerance of safflower, which is mediated by SA, might provide a basis to improve safflower growth and productivity in saline areas.
Materials and methods
Plant cultivation and chemical treatments
Seeds from safflower plants were sown in Tref peat in a greenhouse with a 15 h light/9 h dark photoperiod. Seedlings were transferred to plastic pots (15 cm in diameter, 15 cm deep) filled with perlite, 4 weeks after sowing. Then pots were divided to six groups and the plants were treated with different salinity concentrations (0, 100, 200 mM NaCl) with or without salicylic acid (SA) (1 mM) for 21 days. Half-strength Hoagland’s nutrient solution (pH 6.8–7) used as a nutrient media (Hoagland and Arnon Citation1950). The SA was dissolved in distilled water and approximately 3 ml of the solution was sprayed (three times a week for 3 weeks) on the vegetative stage of plants.
Sampling for gene expression was done 24 and 48 h after the last treatment and leaves were immediately frozen in liquid nitrogen before being stored at −70°C. The final harvest was performed after 21 days of treatment for biochemical analysis and leaves were sampled, fresh leaf samples from each plant were stored at −70°C until performing biochemical analysis.
Determination of Na+ content
The oven-dried leaves (0.5 g) were digested with 2 ml of H2SO4 according to the method of Wolf (Citation1982). Sodium in the digests was determined with a flame photometer (JENWAY PEP 7).
Determination of K+ and Ca2+ contents
Samples were finely ground, and oven-dried leaves (0.1 g) were digested with 5 ml H2SO4 in digestal system (Nyomora et al. Citation1997). The concentrations of K+ and Ca2+ were analyzed by ICP-ODS (Vista-MPX).
RNA extraction
Frozen tissue was ground to a fine powder in liquid nitrogen using a mortar and pestle. Then, total RNA was extracted from leaf tissues (0.1 g) with the Spectrum Plant Total RNA Kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instruction. The quality and concentration of RNA samples were examined by EB-stained agarose gel electrophoresis and UV spectrophotometer (UV-160, Shimadzu and Tokyo, Japan). Total RNA was treated with DNaseI (Fermentase, Germany) to remove DNA contamination before cDNA synthesis according to the manufacturer’s instructions.
cDNA synthesis
Three micrograms of total RNA was reverse transcribed into complementary DNA (cDNA) using Revert AidTM Reverse Transcriptase (Fermentas, Germany), oligo dT18 and random hexamer primers (MWG, Germany) in a total volume of 20 µl reaction mixture, according to the manufacturer’s instructions.
Primer design
The primer pairs for NHX1 and SOS1 genes were designed using PRIMER EXPRESS software (Applied Biosystems). The house keeping gene actin was used as the standard for checking the quantity and quality of cDNA and/or RNA templates. Primers used for qRT-PCR are listed in .
Table 1. Primer sequences used for RT-qPCR in this study.
RT-qPCR analysis
The relative expression level was quantified in comparison with the house keeping gene b-actin as an internal control (Buyuk et al. Citation2016). Quantitative real-time PCR was performed using Applied Biosystems 7500 Real-Time PCR System (Applied Biosystem/MDS SCIEX, Foster City, CA, USA), with 10 ng cDNA, 10 µl of SYBR Green I master mix (Takara, Shiga, Japan), and 200 nM of forward and reverse primers up to final reaction volumes of 20 µl. The PCR was performed by an initial denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The specificity of the PCR products was examined by melting curve analysis, restriction endonuclease digestion followed by 12% polyacrylamide gel electrophoresis. The genes expression level was expressed relative to the appropriate control. Serial dilutions of cDNA were examined to obtain a standard curve for each primer pair.
Determination of indol-3-acetic acid (IAA)
The method for determination of IAA production was described by Malik and Singh (Citation1980). Fresh leaf tissue (0.1 g) was extracted in 3 ml ethanol 96%. The IAA concentration was determined using UV–Vis spectrophotometer at 535 nm.
Determination of gibberellic acid (GA)
Determination of GA was based on the method described by Berríos et al. (Citation2004). Fresh leaf tissue (0.1 g) was extracted in 3 ml ethanol 96%. The absorbance of the solution was measured by spectrophotometer at 254 nm. The concentration of GA in the sample was determined using a linear regression equation of the standard graph.
Extraction and analysis of fatty acids
Samples (1 g) were extracted with chloroform: methanol (2:1 v/v) following the modified procedure of Bligh and Dyer (Citation1959). Gas chromatography (GC-17A Shamadzu) with DB-Wax column (30 m long, 0.25 mm diameter, and flame ionization detector) was used for fatty acid profile determination. To evaluate the efficiency of the desaturation pathway during salt treatment, the desaturation ratios from oleic to linoleic (ODR: oleic desaturation ratio) and from linoleic to linolenic acid (LDR: linoleic desaturation ratio) were calculated as follows: ODR = [(% C18:2 + % C18:3) / (% C18:1 + % C18:2 + % C18:3)] × 100 LDR = [(% C18:3) / (% C18:2 + % C18:3)] × 100.
The magnitude of desaturation ratios represents the amount of substrate which is successfully desaturated from C18:1 to C18:2 and C18:3, and thus measure the desaturating enzymes’ activities (Mondal et al. Citation2010).
Statistical analysis
The experiment was laid out in a completely randomized design (CRD) with three replications. Each data point was the mean of three replicates (n = 3) in each group. Statistical calculations were performed with SPSS (version 18). Tests for significant differences were conducted using analysis of variance (ANOVA) with Duncan’s multiple range tests at the 0.05 level of confidence. The principal component analysis (PCA) and hierarchical cluster analysis (HCA) were performed using the XLSTAT (version 2018.7) and CIMminner, respectively.
Results
The effects of increasing level of NaCl on some biochemical and molecular parameters of safflower were determined at 21 days after the start of treatments. Na+ accumulation in safflower plants increased when the plants were exposed to salt stress (). Exogenous SA on salt-stressed plants reduced Na+ content about 37.56% in comparison with controls. Further, Na+ stress reduced K+ and Ca2+ contents in plants. However, exogenous SA treated plants had higher K+ content under saline and non-saline conditions. In salt-stressed plants, Ca2+ contents were increased in SA treated plants when compared to controls.
Table 2. Effects of NaCl (0, 100, 200 mM) and salicylic acid on ions content at 21 days after the start of treatments in safflower.
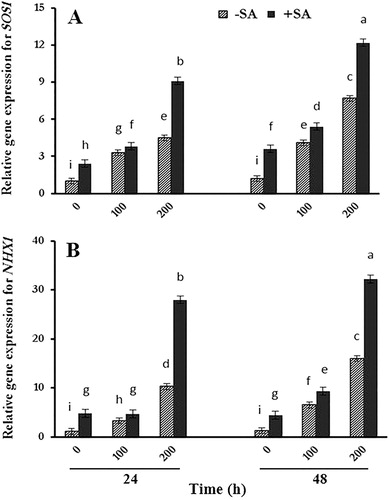
To better understand the underlying mechanisms of resistance against salinity, SOS1, and NHX1 genes expression were investigated as key transporters in reducing toxic Na+ in the cytosol. SOS1 gene expression increased in salt-treated plants ((a)). Our results indicated that following exogenous SA treatment, SOS1 gene expression was dramatically induced in both salt-treated and untreated plants in comparison with controls. The induction of SOS1 gene expression in salt-treated plants was greater after 48 h. NHX1 gene expression also increased in plants under salt stress ((b)) and SA treatment enhanced the amount of expression in all plants. This increase was greater 48 h after the last treatment in salt-treated safflower plants. There was no significant difference between control plants after 24 and 48 h.
Figure 1. RT-qPCR analyses of A SOS1 and B NHX1 genes transcript of safflower plants treated with salinity and SA after 24 and 48 h. The groups are −SA (plants with no SA treatment) and +SA (plants sprayed with 1 mM SA every other day). B-Actin was used as an endogenous control to normalize the data for input RNA difference between the various samples. Columns indicate mean ± SE.
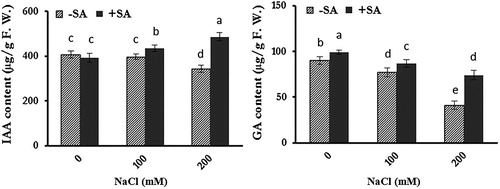
Salt stress reduced IAA content in the leaves of safflower in 200 mM NaCl-treated plants when compared with controls (). Exogenously applied SA increased IAA content in only salt-stressed plants. The highest amount of IAA content was detected at 200 mM NaCl-treated plants under SA application. Salinity also reduced GA content in salt-treated plants. Exogenous SA increased GA content in both salt-stressed and unstressed plants. This increase was most pronounced in 200 mM NaCl-treated plants, which was almost 2-fold higher than the amount in leaves without SA application.
Figure 2. Effects of salinity and exogenous SA on content of auxin (IAA) and gibberellic acid (GA) in safflower plants at 21 days after the start of treatments. The groups are −SA (plants with no SA treatment) and +SA (plants sprayed with 1 mM SA every other day). Columns indicate mean ± SE.
The fatty acid profiles displayed great quantitative differences in safflower plants (). Plants showed a remarkable increase in stearic acid (18:0), followed by linoleic (C18:2) and linolenic acids (18:3), but decrease in palmitic (C16:0) and oleic acids (C18:1) under salinity. In terms of saturated fatty acids, exogenous SA increased palmitic acid content in only 100 mM NaCl-treated plants, and decreased stearic acid content in all plants in comparison with controls. Also, in terms of unsaturated fatty acids, exogenous SA increased oleic acid content in all salt-treated plants. However, SA application decreased linoleic acid content in both untreated and salt-treated plants, as well as linolenic acid content in only 200 mM NaCl-treated plants.
Table 3. Effects of NaCl (0, 100, 200 mM) and salicylic acid on fatty acids content at 21 days after the start of treatments in safflower.
In general, NaCl treatment resulted in a remarkable increase of unsaturated fatty acids in safflower. The same pattern was also evidenced by the increase of ODR in salt-treated plants, which reflects the higher efficiency of the desaturation system from linoleic to linolenic acid.
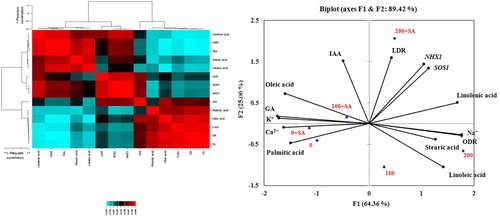
Principal component analysis indicated that principal component 1 (F1) described 64.36% of total variation and principal component 2 (F2) described 25.06% () with a cumulative percentage of 89.42%. PCA allowed for easy visualization of the complex data. The contributors to the principal component of F1 and F2 were compared. LDR, NHX1, SOS1, and linolenic acid were grouped together with positive loading on the right upper side of the biplot. Further, Na+, ODR, stearic acid, and linoleic acid were observed on the right lower side of the biplot. Also, Ca2+ and palmitic acid were found on the left lower part, whereas K+, GA, oleic acid, and IAA were grouped on the left upper part of the biplot. In addition, HCA which is a method of cluster analysis, indicated a hierarchy of clusters (). This suggests that these parameters had a positive correlation among themselves, and SA had a positive effect on parameters on the upper side of the biplot.
Discussion
Our previous work indicated that growth parameters which were followed by measuring fresh and dry weight of safflower plants were remarkably inhibited under different NaCl concentrations. However, the application of SA improved the negative effect of salinity by increasing plant growth especially in 200 mM NaCl-treated plants (Shaki et al. Citation2017). In the current study, some biochemical and molecular parameters were investigated to better understand the effects of exogenous application of SA on safflower plants in saline conditions. In this investigation, the accumulation of Na+ increased in plants due to salt stress, whereas the levels of K+ and Ca2+ decreased. However, the application of SA lowered the accumulation of Na+ content accompanied by an enhanced accumulation of K+ and Ca2+ in plants (). However, the mechanism by which SA accomplished this decline in Na+ and enhanced K+ and Ca2+ accumulation needs further research. It is well established that salt tolerance is commonly characterized to enhance Na+ exclusion and increase absorption of K+ to maintain optimum K+/Na+ ratio (Malekzadeh Citation2015). As Ashraf and Harris (Citation2004) assumed, this ratio might be a valid selection criterion for assessing the salinity resistance of different species of plants. Accordingly, potassium acquisition from soil is a critical process for salt tolerance of plants. Our results can be explained in the light of some previous reports in which it has been found that salt stress increased the accumulation of Na+ and reduces that of K+ and Ca2+ in some plant species (Habib et al. Citation2012; Malekzadeh Citation2015). Other findings on Arabidopsis indicated an increase in the electrolyte leakage, which is mainly related to K+ efflux from plant cells, under osmotic stress. However, reduction of that was observed by the application of SA on plants (Jayakannan et al. Citation2013). It seems that the ability of SA to ameliorate the negative effects of salt stress on growth may be due to a decrease of electrolyte leakage and the increase of accumulation of ions in plants.
Maintenance of ion homeostasis in the cytosol is important for plants in stress conditions. Salinity has been reported to increase the activity and gene expression of Na+ transporters such as SOS1 and NHX1 in cells (Chen et al. Citation2010). Previous studies indicated that salt acclimation in other plant species occurred with Na+ transportation into the apoplast and/or its sequestration into the vacuoles, which may further modulate the ion homeostasis in the cytosol (Chen et al. Citation2010; Katschnig et al. Citation2015). According to our results, it seems like SA functions in defense system by reducing toxic Na+ in cytosol following the increase in SOS1 and NHX1 genes expression. Overall, our results provide the first evidence, to our knowledge, that SA plays a role in enhancing salt tolerance of safflower by increasing salt secretion through increased expression of the SOS1 gene. Moreover, SA could induce increased Na+ sequestration into the vacuoles via increasing the expression of NHX1 gene. SA-modulated activity of Na+ transporters is closely correlated with the salt tolerance of safflower.
There are some evidence on the role of endogenous auxin in salt stress but, much more information is needed on salt-induced changes in the synthesis and metabolism of auxin. In this experiment, the IAA content decreased at a severe concentration of NaCl (). Supporting our results, Naqvi et al. (Citation1986) reported a decrease in IAA content in etiolated Zea mays coleoptiles under salinity. Similarly, IAA content was found to decrease with decreasing water potential in Triticum aestivum plants (Rubin et al. Citation2002). Further, SA resulted in accumulation of IAA in safflower plants. IAA is known to increase cell wall extensibility (Cleland Citation1981), and therefore, enhanced levels of IAA under SA treatment may increase leaf cell extensibility, which is involved in maintaining growth under conditions of transiently reduced hydration due to salt stress. Supporting this idea, Li et al. (Citation2003) reported that increased levels of IAA due to exogenous gibberellic acid delayed the biosynthesis of lignin and induced more growth in Myrica rubra plants.
Our results clearly indicate that overall gibberellin levels in safflower plants were affected by NaCl stress. Similarly, it has been proved that desiccating excised lettuce leaves indicated a rapid decline in gibberellin-like activity (Aharoni et al. Citation1977). Further, exogenous application of GA reduced the adverse effects of salinity on the growth and productivity of rice (Prakash and Prathapasenan Citation1990), Sorghum (Amzallag et al. Citation1992), and soybean (Hamayun et al. Citation2010). It is also reported that SA may have a role in some of the physiological processes associated with GA, since the exogenous application of SA was able to improve seed germination in Arabidopsis thaliana under salt stress conditions (Alonso-Ramírez et al. Citation2009). These data support the idea that SA can have an important role in GA biosynthesis and action and that some of the physiological effects of this hormone may be mediated by GA. In summary, our results show the existence of cross-talk between these two hormones in stress conditions, showing another junction in the complex mechanism of hormone interactions.
Salinity also modified fatty acids composition in safflower, which is considered to be very critical in stress tolerance (Azachi et al. Citation2002). In this experiment, increased content of linoleic and linolenic acids was observed under salinity. Further, a redirection of the lipidic metabolism towards the synthesis of unsaturated fatty acids was obtained (). Thus, such increase in unsaturated fatty acids contents could be related to the importance of maintaining a high degree of unsaturation to control membrane fluidity crucial for proper functions of the plasma membrane (Azachi et al. Citation2002).
Supporting our results, López-Pérez et al. (Citation2009) reported increased unsaturation index in Brassica oleracea plants under salt stress. Similarly, increased level of unsaturation was observed in A. thaliana under drought stress (Gigon et al. Citation2004). They showed that drought stress caused an increase in linolenic acid content, which could result from the activation of desaturase activities. In addition, salt-induced harmful effects on fatty acids composition were alleviated by SA. Exogenous SA caused considerable change in key saturated and unsaturated fatty acids of safflower. It can be assumed that SA, to some extent, modulates stress impacts on fatty acids compositions of safflower. Similarly, the role of SA in amelioration of stress effects on fatty acids profile of sunflower (Helianthus annuus L.) was investigated by other researchers, which can support our results (Noreen and Ashraf Citation2010; Ebrahimian and Bybordi Citation2012).
The PCA and HCA grouping allows certain parameters to be identified as those responsible for plant behavior changes under stress conditions. PCA is used to extract the important information from a multivariate data table and to express this information as a set of few new variables called principal components. The information in a given data set corresponds to the total variation it contains. To investigate the contributors to the principal component, the loadings in F1 and F2 were compared (). It is suggested that parameters with vectors in the same directions had a positive correlation among themselves, and a negative correlation with other parameters, indicating that impacts of salinity on these parameters may be different from the effects on the other measured parameters. Further, it was observed that SA had a positive effect on most measured parameters under 100 and 200 mM NaCl treatments.
Conclusion
Taken together, our data revealed that SA, to some extent, helps safflower plants to cope with saline conditions. It is supported by the expression of the key genes responsible in ion homeostasis, such as SOS1 and NHX1, as well as the desaturation of fatty acids in membrane. Further, the results indicate the existence of cross-talk between plant hormones, showing another junction in the complex mechanism of hormone interactions. Our results provide new insights towards identifying the underlying mechanisms of salt tolerance in safflower. Further, SA may be considered as a foliar application to ameliorate salinity effects, due to its low price and availability.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Fatemeh Shaki
Fatemeh Shaki is a Plant Physiologist at the University of Tehran in the Department of Plant Biology, College of Science. Her research examines the effects of salinity and also plant growth regulators (e.g. salicylic acid) on safflower plants. Her research aims to understand how safflower plants respond to stress conditions and how they maintain homeostasis in the face of salt stress.
Hasan Ebrahimzadeh Maboud
Hasan Ebrahimzadeh Maboud is Professor Emeritus at the University of Tehran in the Department of Plant Biology, College of Science. His research examines the effects of biotic and abiotic stress conditions on crops growth and productivity, as well as important metabolites productions in plant cells. His research aims to understand the underlying mechanisms of stress tolerance in plants.
Vahid Niknam
Vahid Niknam is Professor at the University of Tehran in the Department of Plant Biology, College of Science. His research examines the effects environmental stress conditions on crops growth and productivity, as well as important metabolites productions in plants to identify the underlying mechanisms of stress tolerance. His research aims to understand how plants respond to stress conditions and how they maintain homeostasis in the face of environmental stress.
References
- Aharoni N, Blumenfeld A, Richmond AE. 1977. Hormonal activity in detached lettuce leaves as affected by leaf water content. Plant Physiol. 59(6):1169–1173.
- Alonso-Ramírez A, Rodríguez D, Reyes D, Jiménez JA, Nicolás G, López-Climent M, Gómez-Cadenas A, Nicolás C. 2009. Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol. 150(3):1335–1344.
- Amzallag G, Lerner H, Poljakoff-Mayber A. 1992. Interaction between mineral nutrients, cytokinin and gibberellic acid during growth of Sorghum at high NaCI salinity. J Exp Bot. 43(1):81–87.
- Ashraf M, Akram N, Arteca R, Foolad M. 2010. The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Critic Rev Plant Sci. 29(3):162–190.
- Ashraf M, Harris P. 2004. Potential biochemical indicators of salinity tolerance in plants. J Plant Sci. 166(1):3–16.
- Azachi M, Sadka A, Fisher M, Goldshlag P, Gokhman I, Zamir A. 2002. Salt induction of fatty acid elongase and membrane lipid modifications in the extreme halotolerant alga Dunaliella salina. Plant Physiol. 129(3):1320–1329.
- Bassil ES, Kaffka SR. 2002. Response of safflower (Carthamus tinctorius L.) to saline soils and irrigation: I. consumptive water use. Agric Water Manag. 54(1):67–80.
- Berríos J, Illanes A, Aroca G. 2004. Spectrophotometric method for determining gibberellic acid in fermentation broths. Biotechnol Lett. 26(1):67–70.
- Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 37(8):911–917.
- Bowles VG, Mayerhofer R, Davis C, Good AG, Hall JC. 2010. A phylogenetic investigation of Carthamus combining sequence and microsatellite data. Plant Syst Evol. 287(1–2):85–97.
- Buyuk I, Bolukbasi E, Aras S. 2016. Expression of ctFAD2 gene for early selection in safflower oleic/linoleic oil content. J Anim Plant Sci. 26(5):1383–1388.
- Chen J, Xiao Q, Wu F, Dong X, He J, Pei Z, Zheng H. 2010. Nitric oxide enhances salt secretion and Na+ sequestration in a mangrove plant, Avicennia marina, through increasing the expression of H+-ATPase and Na+/H+ antiporter under high salinity. Tree Physiol. 30(12):1570–1585.
- Cleland R. 1981. Wall extensibility: hormones and wall extension. In: Tanner W, Loewus FA, editors. Plant carbohydrates II. Encyclopedia of plant physiology (new series). Vol. 13/B. Berlin: Springer; p. 255–273.
- Ebrahimian E, Bybordi A. 2012. Effect of salinity, salicylic acid, silicium and ascorbic acid on lipid peroxidation, antioxidant enzyme activity and fatty acid content of sunflower. Afr J Agric Res. 7(25):3685–3694.
- Gigon A, Matos AR, Laffray D, Zuily-Fodil Y, Pham-Thi AT. 2004. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (ecotype Columbia). Ann Bot. 94(3):345–351.
- Habib N, Ashraf M, Ali Q, Perveen R. 2012. Response of salt stressed okra (Abelmoschus esculentus Moench) plants to foliar-applied glycine betaine and glycine betaine containing sugarbeet extract. S Afr J Bot. 83:151–158.
- Hamayun M, Khan SA, Khan AL, Shin JH, Ahmad B, Shin DH, Lee IJ. 2010. Exogenous gibberellic acid reprograms soybean to higher growth and salt stress tolerance. J Agric Food Chem. 58(12):7226–7232.
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. 2000. Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol. 51(1):463–499.
- Hayat Q, Hayat S, Irfan M, Ahmad A. 2010. Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot. 68(1):14–25.
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. Circ Calif Agric Exp Stn. 347(2nd ed.):1–32.
- Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S. 2013. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J Exp Bot. 64(8):2255–2268.
- Katschnig D, Bliek T, Rozema J, Schat H. 2015. Constitutive high-level SOS1 expression and absence of HKT1; 1 expression in the salt-accumulating halophyte Salicornia dolichostachya. Plant Sci. 234:144–154.
- Li X, Li S, Lin J. 2003. Effect of GA3 spraying on lignin and auxin contents and the correlated enzyme activities in bayberry (Myrica rubra Bieb.) during flower-bud induction. Plant Sci. 164(4):549–556.
- López-Pérez L, del Carmen Martínez-Ballesta M, Maurel C, Carvajal M. 2009. Changes in plasma membrane lipids, aquaporins and proton pump of broccoli roots, as an adaptation mechanism to salinity. Phytochemistry. 70(4):492–500.
- Malekzadeh P. 2015. Influence of exogenous application of glycinebetaine on antioxidative system and growth of salt-stressed soybean seedlings (Glycine max L.). Physiol Mol Biol Plants. 21(2):225–232.
- Malik CP, Singh MB. 1980. Plant enzymology and histoenzymology. New Delhi: Kalyani. 51–53.
- Mondal N, Bhat K, Srivastava P. 2010. Variation in fatty acid composition in Indian germplasm of sesame. J Am Oil Chem Soc. 87(11):1263–1269.
- Naqvi S, Ansari R, Hanif M. 1986. Auxin physiology in Zea mays L. under saline growth conditions. In: Ahmed R, Pietro AS, editors. Prospects for biosaline research. Karachi: University of Karachi; p. 307–313.
- Noreen S, Ashraf M. 2010. Modulation of salt (NaCl)-induced effects on oil composition and fatty acid profile of sunflower (Helianthus annuus L.) by exogenous application of salicylic acid. J Sci Food Agric. 90(15):2608–2616.
- Nyomora A, Sah R, Brown P, Miller R. 1997. Boron determination in biological materials by inductively coupled plasma atomic emission and mass spectrometry: effects of sample dissolution methods. Fresenius J Anal Chem. 357(8):1185–1191.
- Prakash L, Prathapasenan G. 1990. NaCl-and gibberellic acid-induced changes in the content of auxin and the activities of cellulase and pectin lyase during leaf growth in rice (Oryza sativa). Ann Bot. 65(3):251–257.
- Rubin N, Carman JG, Salisbury FB. 2002. Water stress, CO2 and photoperiod influence hormone levels in wheat. J Plant Physiol. 159(3):307–312.
- Shaki F, Ebrahimzadeh Maboud H, Niknam V. 2017. Central role of salicylic acid in resistance of safflower (Carthamus tinctorius L.) against salinity. J Plant Interact. 12(1):414–420.
- Shaki F, Maboud HE, Niknam V. 2018. Growth enhancement and salt tolerance of safflower (Carthamus tinctorius L.), by salicylic acid. Curr Plant Biol. 13:16–22.
- Silva P, Façanha AR, Tavares RM, Gerós H. 2010. Role of tonoplast proton pumps and Na+/H+ antiport system in salt tolerance of Populus euphratica oliv. J Plant Growth Regul. 29:23–34.
- Wolf B. 1982. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun Soil Sci Plant Anal. 13(12):1035–1059.
- Zahedi AM, Fazeli I, Zavareh M, Dorry H, Gerayeli N. 2012. Evaluation of the sensitive components in seedling growth of common bean (Phaseolus vulgaris L.) affected by salinity. Asian J Crop Sci. 4(4):159–164.