?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Melatonin (MT) can protect plants against abiotic stress. In order to explore whether melatonin can improve photosynthetic function under NaCl stress, Solanum lycopersicum L. cv. Liaoyuanduoli were exposed to 150 mmol L−1 NaCl stress with or without pretreatment with 150 μmol L−1 melatonin. The results showed that NaCl stress can lead to reduced chlorophyll content, lower photosynthetic function, increased reaction oxygen species (ROS) levels, and decreased PSII activity. These changes were mainly due to the reduction in oxygen-evolving complex (OEC) activity on the donor side of PSII and the blockage of electron transfer from QA to QB on receptor side of PSII. The donor side of PSII was more sensitive to NaCl stress relative to the receptor side of PSII. Interestingly, application of MT enhanced tomato NaCl tolerance. MT reduced the production of ROS by balancing the distribution of photosynthetic electron flux, facilitated the repair of PSII by maintaining the abundance of Psb O and D1, and promoting the ability of the donor and acceptor sides of PSII to deliver electrons. MT also enhanced the scavenging ability of ROS by stimulating the activity of enzymes involved in the AsA-GSH cycle.
Introduction
Soil salinity is a common abiotic stress that limits agricultural yield and productivity worldwide (Munns and Tester Citation2008). Salt stress results in ion imbalance, hyperosmotic stress, photosynthesis, oxidative damage, and other physiological disturbances in plants (Zhu Citation2002). In particular, salt stress can reduce the activities of photosystem II (PSII) and photosystem I (PSI), which leads to a decrease in electron receptor production (Takahashi and Murata Citation2008). The inhibition of photosynthesis electron transport (PET) may be caused by the degradation of D1 (Zhu Citation2002; Zhang et al. Citation2012). In general, electrons are transferred from the primary to secondary quinone electron acceptor of PSII, then go to PSI and interact with the electron acceptor NADP+ through ferredoxin (Fd). However, superfluous electrons induced by reduced OEC activity either on the donor or the receptor sides of PSII can be transferred either to oxygen in PSI or generate reactive oxygen species (ROS) through a Mehler reaction (Foyer and Noctor Citation2009). These excessive electrons can leak and attack free oxygen molecules. Thus, salt stress generates reactive oxygen species (ROS) and damages PSII reaction centers, further leading to the peroxidation and dissociation of the thylakoid membranes (Zhang et al. Citation2012). ROS production and scavenging balance is necessary for photosynthetic function. Salinity-induced photoinhibition and excessive ROS often underlie oxidative damage. Excessive ROS damages cells by reducing the function of membrane transporters and associated enzymes (Zhan et al. Citation2014; Zhang et al. Citation2015a). Exogenous substances can be used to enhance plant stress tolerance and improve the crop’s productivity under stress (Yang et al. Citation2015; Han et al. Citation2016). Melatonin (MT) is an effective exogenous substance that is widely used to ameliorate the effects of stress on crops (Galano et al. Citation2011; Zhang et al. Citation2015b).
MT is a natural antioxidant that can effectively scavenge ROS in animals and plants (Arnao and Hernández-Ruiz Citation2015). MT protects plants against various environmental stressors, including extreme temperatures (Lei et al. Citation2004; Kang et al. Citation2010; Shi and Chan Citation2014), heavy metals (Tan et al. Citation2007; Posmyk et al. Citation2008), UV radiation (Afreen et al. Citation2006), and salt stress (Tal et al. Citation2011; Li et al. Citation2012; Fan et al. Citation2014). Moreover, MT regulates plant development, including, seed germination (Tiryaki and Keles Citation2012; Zhang et al. Citation2013), leaf senescence (Wang et al. Citation2013a; Wang et al. Citation2013b; Wang et al. Citation2014), root development (Park and Back Citation2012; Pelagio-Flores et al. Citation2012; Zhang et al. Citation2013), and circadian rhythm (Kolář et al. Citation1997; Arnao and Hernández-Ruiz Citation2009). MT’s intermediate product, N1-acetyl-N2-formyl-5-methoxykynuramine (AMFK), can scavenge ROS in a direct and efficient manner (Tan et al. Citation2007; Manchester et al. Citation2015). Furthermore, MT can stimulate the activities of antioxidant enzymes involved in the ascorbate–glutathione (AsA–GSH) cycle to scavenge excess ROS in apple and cucumber (Wang et al. Citation2012; Li et al. Citation2015; Li et al. Citation2016a). Tomato (Solanum lycopersicum L. cv Liaoyuanduoli) is an important crop, and its growth and development can be hindered due to salt stress. Although, the salt tolerance response in tomato has been studied previously (Zheng et al. Citation2015; Zhou et al. Citation2016), the role of MT in regulating the distribution of the PSII electron transport system and ROS scavenging ability of the AsA-GSH cycle to enhance salt tolerance in tomato remains unclear. This study aims to provide insights into improving the quality and yield of tomato cultivation in soil with high levels of salt.
Here, we investigated whether MT can alleviate salt stress in tomato by regulating the distribution of electron fluxes and ROS balance. We determined the changes on the donor and acceptor sides of PSII using the polyphasic rise of fluorescence transients (OJIP fluorescence induction curve), which is the process that generally gives rise to increased ROS levels in high salt environments. In addition, we performed western blots to analyze the changes in the core proteins in the donor and acceptor sides. Moreover, we evaluated membrane integrity and activity of ROS-related antioxidative enzymes involved in the AsA–GSH cycle. This information is critical for improving our understanding of how MT may alleviate salt stress in tomato.
Materials and methods
Plant materials and NaCl treatment
Tomato (S. lycopersicum L. cv Liaoyuanduoli) seeds were germinated and grown hydroponically in a growth chamber. Plants were placed in Hoagland solution at 25°C under fluorescent light (300 μmol m−2 s−1, 13 h light/11 h dark) and 60% relative humidity. The nutrient solution was renewed every other day in order to provide a stable nutrient supply. To investigate the effects of MT in tomato seedlings in response to NaCl stress, the leaves of tomato seedlings at the four-leaf stage were pretreated with exogenous MT that was irrigated into the Hoagland solution at 0, 10, 50, 100, 150, 250 μM. Among these treatments, 150 μM MT was selected as most effective in relieving salinity stress (unpublished data). After three days of MT pretreatment, the control and NaCl stressed seedlings were treated with NaCl (150 mM) for seven days, where each treatment was conducted over 12 plants. The treatments were as follows: CK-Hoagland’s solution only as control; CK+MT-Hoagland’s solution with 150 μM MT; NaCl-Hoagland’s solution with 150 mM NaCl; and NaCl+MT-Hoagland’s solution with 150 μM MT and 150 mM NaCl. Leaves were harvested seven days after treatment and either used freshly or collected into liquid nitrogen and preserved at −80°C. At least three biological replicates were collected for each treatment.
Measurement of chlorophyll content
Chlorophyll a (Chla), chlorophyll b (Chlb) and total chlorophyll (Chls) contents were determined by acetone, anhydrous ethanol and distilled water (4.5:4.5:1,V:V:V) in the dark at 4°C until leaves turned completely white. The absorbance of the supernatant was measured at 450, 532, and 600 nm, and recorded as OD450, OD532 and OD600, respectively (Porra Citation2002).
Measurement of net CO2 assimilation rate
Photosynthesis parameters, such as net photosynthetic rates (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rates (Tr) of leaves were measured at 10:00–11:00 am using the portable photosynthesis system LI-6400 (LI-COR Biosciences, Lincoln, USA). The radiation that was used to activate photosynthesis was 600 μmol m−2 s−1 (saturation light). The air temperature and humidity were 25°C and 50–60 %, respectively (Zhang et al. Citation2018). Concentration of CO2 was changed at 3 min intervals in a sequence of 1600, 1200, 1000, 800, 600, 400, 300, 200, 150, 100, and 0 μmol mol−1. Irradiance and CO2 concentration were controlled by the system. CE was obtained from the slope of the Pn-Ci response curve (Cheng et al. Citation2016).
Membrane integrity
To detect lipid peroxidation and membrane integrity, the malondialdehyde (MDA) content was determined using the thiobarbituric acid (TBA) reaction (Wang et al. Citation2010). The relative electrolyte leakage (REL) ratio was measured following previously published protocols (Gong et al. Citation1998).
Analysis of superoxide anion (
 ) and hydrogen peroxide (H2O2)
) and hydrogen peroxide (H2O2)
To detect the levels of ROS in tomato leaves, the rate of generation and H2O2 content were measured. The generation rate of
was measured following Yan et al. (Citation1996).
The H2O2 content was measured by spectrophotometry measurements at 415 nm after potassium iodide treatment, as previously described (Ibrahim and Jaafar Citation2012). Briefly, tissues were ground in 0.1% trichloroacetic acid, and the homogenate was centrifuged at 15,000 g for 15 min at 4°C, and the supernatant was used to measure H2O2 levels.
Analysis of antioxidant enzymes
Assay of antioxidant enzymes, including superoxide (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), glutathione S-transferase (GST), glycolate oxidase (GO) and glutathione peroxidase (GPX) were performed following published protocols (Yin et al. Citation2014; Suo et al. Citation2015; Yin et al. Citation2017; Zhang et al. Citation2019). The protein content was measured in all the enzymatic preparations using the Bradford method (Bradford Citation1976).
Measurements of chlorophyll a fluorescence transient (OJIP)
The polyphasic rise of fluorescence transients (OJIP fluorescence induction curve) were measured using the third fully expanded leaf from plants in different treatment groups using a Plant Effciency Analyser (PEA, Hansatech Instruments Ltd., King's Lynn, Norfolk PE32 1JL, UK) following protocols from Strasser et al. (Strasser and Srivastava Citation1995; Strasser and Strasser Citation1995). The leaves were adapted to darkness for 30 min before testing. The characteristic points O, J, I and P on the OJIP curve corresponded to time points 0, 2, 30 and 1000 ms, and the corresponding relative fluorescence intensities were expressed as FO, FJ, FI and Fm. The 0.15 and 0.3 ms time points on the OJIP curve were defined as L and K, respectively, and the corresponding relative fluorescence intensities were expressed as FL and FK, respectively. Standardizations of O-P, O-J and O-K on the OJIP curves of different treatments were carried out by defining the relative fluorescence intensity of O as 0, and the relative fluorescence intensities of P, J and K as 1. The formulas for the standardization were: VO–P = (Ft−Fo)/(FP−Fo), VO-J = (Ft−Fo)/(FJ−Fo), VO-K = (Ft−Fo)/(FK−Fo), where Ft is the relative fluorescence intensity at different time points. The relative variable fluorescence at the three characteristic points L, K and J on the standardized curve were expressed as VL, VK and VJ, respectively, and were calculated using the following formulas: VL = (FL−Fo)/(FK−Fo), VK = (FK−Fo)/(FJ−Fo), VJ = (FJ−Fo)/(FP −Fo). The differences between CK and VO-P , VO-J and VO-K curves of the plant leaves under different treatment conditions were determined and expressed as △VO-P, △VO-J and △VO-K, respectively (Srivastava et al. Citation1997; Strasser, Citation1997) (Strasser and Strasser Citation1995). The PSII maximum photochemical efficiency (Fv/Fm) was obtained through JIP-test analysis of OJIP curves following methods described in Strasser and Strasser Citation1995.
Protein extraction and western blotting
Total protein from tomato leaves that were subjected to different treatments were extracted using the Minute™ total protein extraction kit (Invent Biotechnologies, USA). Protein samples were boiled for 5 min in a water bath and separated by 10% SDS-PAGE gel, then transferred onto polyvinylidene difuoride (PVDF) membranes using the Trans-BlotTurbo™ (Bio-Rad, USA) transfer system. Subsequently, the PVDF membranes were blocked by 5% skimmed milk and incubated for 2 h with the primary antibody (1:1000) at 4°C overnight and secondary antibody (1:5000) for 1 h. The primary antibodies against Psb O and D1 antibodies obtained from Agrisera (Umeo, Sweden), and anti-β-actin and secondary antibodies were obtained from Kangwei (Beijing, China). The chemical coloration Azure c600 Western blot imaging system (Azure, USA) was used after the DAB Horseradish Peroxidase Color Development Kit to visualize the bands on the Western blots.
Statistical analysis
Data were analyzed with SPSS 17.0 (SPSS Inc., Chicago, USA) using ANOVA and LSD (p < 0.05). All the results are presented as means ± standard error (SE) and all experiments had more than three biological replicates.
Results
Effects of MT on chlorophyll content and carbon assimilation under NaCl stress
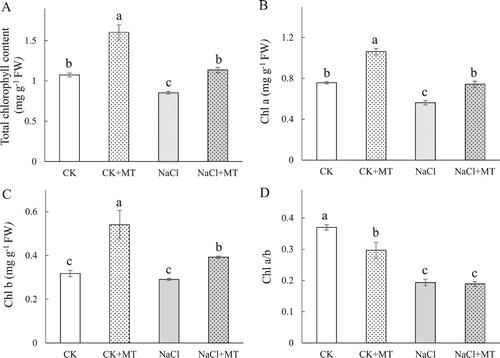
To test whether MT treatment reduced the effects of NaCl stress in tomato plants, chlorophyll content of the leaves was measured by evaluating changes in leaf color (). In NaCl treated plants, Chls, Chl a and Chl b contents in tomato leaves decreased by 20.68%, 25.82% and 8.44%, respectively. Compared to the NaCl treatment alone, plants that were treated with MT and NaCl had increased Chls, Chl a and Chl b levels by 33.06%, 32.04%, and 35.03%, respectively, ((A–C)), suggesting that the MT treatment mitigated the effects of NaCl. Seedlings that were treated with only MT had significantly higher Chls, Chl a and Chl b levels compared to the control ((A–C)). The ratio of chl a/b were similar across all treatments ((D)), which may be due to MT’s ability to mitigate changes in Chl a and Chl b at a similar level.
Figure 1. Effects of root-applied MT on the total chlorophyll content (A), Chl a (B), Chl b (C), and Chl a/b (D) at seven days NaCl treatment in tomato leaves. Means ± SE of three replicates are presented. Different letters above the columns indicate significant differences at p < 0.05 between different treatments.
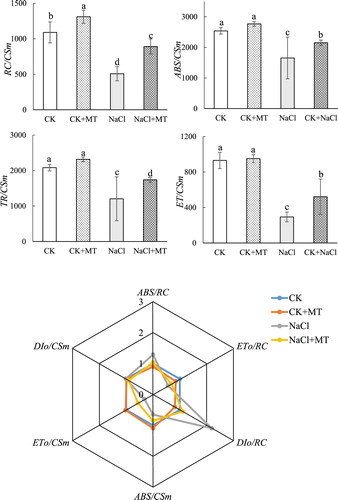
Seedlings that were treated with NaCl for seven days showed significant 40.65% reduction in net photosynthetic rate (Pn) compared to untreated plants, while Pn decreased by 20.33% in seedlings that were exposed to MT and NaCl compared to the untreated control. These results suggest that the adverse effects of NaCl stress on tomato photosynthesis were significantly reduced by pre-treatment with MT. Compared to untreated seedlings, stomatal conductance (Gs) and intercellular CO2 concentration (Ci) in NaCl treated plants decrease by 31.35% and 30.42%, respectively (), suggesting that stomatal limitation was an important factor that reduced photosynthetic rates in tomato plants undergoing NaCl stress. Nevertheless, the decrease in Ci was lower than Gs, suggesting that the decline of Pn was caused by non-stomatal limitation, suggesting that NaCl stress inhibited the light system, carbon assimilation and enzyme activation to some extent. In contrast, Gs and Ci were decreased by 13.43% and 17.12%, respectively, in plants that were treated with MT and NaCl compared to control seedlings (), and these measurements were higher than the NaCl treatment alone, indicating that MT can alleviate NaCl-induced reductions in the photosynthetic rate of leaves by overcoming stomatal factors ().
Table 1. Effects of melatonin on Pn, Gs, Ci, and CE in tomato under seven days’ salt stress.
Carboxylation efficiency (CE) is usually used to measure the amount of Rubisco in leaves, and NaCl stress often influences CE of vegetables more than other species (Cheng et al. Citation2016). When compared to the untreated controls, the CE of tomato seedlings decreased by 27.28% under NaCl stress and by 13.64% in seedlings that were pre-treated with MT and exposed to NaCl stress (), indicating that MT lessened the effect of Pn in tomato seedlings caused by NaCl stress by enhancing the activity of Rubisco.
Effects of MT on membrane integrity and key enzymes of the Ascorbate–Glutathione (AsA–GSH) cycle
To evaluate the effects of MT on membrane stability under NaCl stress, we measured the MDA content and REL in leaves. The MDA and REL contents of tomato leaves were significantly increased under NaCl stress, however, the application of exogenous MT significantly reduced the MDA and REL contents ((A)). Specifically, the MDA content increased from 10.32 nmol g−1 fresh weight (FW) to 28.73 nmol g−1 FW under NaCl stress, but the MDA content was 17.99 nmol g−1 FW in seedlings that were pretreated with MT and exposed to NaCl stress ((A)). The REL content increased from 41.48 nmol g−1 FW to 85.35 nmol g−1 FW under NaCl stress, but it was also decreased to 71.95 nmol g−1 FW in seedlings that were pretreated with MT and exposed to salt stress ((A)). The results indicated that pretreatment with 150 mΜ MT was sufficient to improve the salt tolerance of tomato seedlings, reduce the damage on the cell membrane caused by NaCl stress, and inhibit further oxidation of membrane lipids, thereby maintaining the integrity of the cell membrane under salt stress.
Figure 2. Effects of MT on antioxidant systems in tomato leaves. (A) generation rate and H2O2 content in leaves; (B) Malondialdehyde (MDA) content and relative electrolyte leakage (REL); (C) Superoxide dismutase (SOD) and peroxidase (POD) activities; (D) Catalase (CAT) and ascorbate peroxidase (APX) activities in leaves; (E) Monodehydroascorbate reductase (MDHAR) and dehydroascorbate reductase (DHAR) activities; (F) Glutathione peroxidase (GPX) and glutathione reductase (GR) activities. The values were determined after plants were treated with 150 mM NaCl for three days, and were presented as means ± standard deviation (n ≥ 3). The asterisks indicate significant differences (*P < 0.05; **P < 0.01).
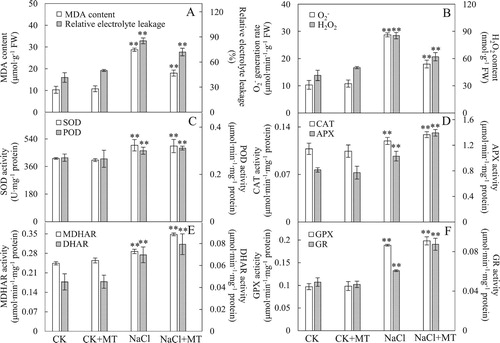
To monitor the effect of MT on ROS homeostasis in tomato leaves under NaCl stress, the generation rate, H2O2 content and the activity levels of enzymes involved in the ascorbate–glutathione cycle were analyzed. The
generation rate and H2O2 content were higher under NaCl treatment, while samples that were treated with both MT and NaCl showed reverted values ((B)), indicating that MT can effectively alleviate oxidative stress caused by NaCl stress.
Various antioxidant enzymes were involved in ROS scavenging ((C)). In particular, SOD activity increased by 20.91% under NaCl treatment compared to the untreated control, yet SOD activity levels were similar between the control samples treated with or without MT ((C)). In addition, among the enzymes involved in reducing H2O2 levels, POD, CAT and GPX activities were significantly increased by 10.95%, 10.74% and 94.07% ((C,D,F)), respectively, under NaCl treatment. Compared to the NaCl treated leaves, MT and NaCl treatment resulted in additional increases in POD, CAT, and GPX activities by 3.71, 7.77 and 5.12 %, respectively ((C,D,F)). Interestingly, the activity of enzymes involved in the AsA-GSH cycle showed significant increases under NaCl stress, and particularly under the NaCl and MT treatment conditions. The activities of APX, MDHAR, DHAR, and GR were further promoted by the application of exogenous MT, leading to an increase by 35.43%, 21.28%, 13.76% and 44.23%, respectively, compared with those treated with NaCl alone ((D,E,F)). These results indicated that exogenous applications of MT could promote the activity of key enzymes in the AsA–GSH cycle under salt stress.
Effects of MT on donor and acceptor sides of photosystem II (PS II) under NaCl stress
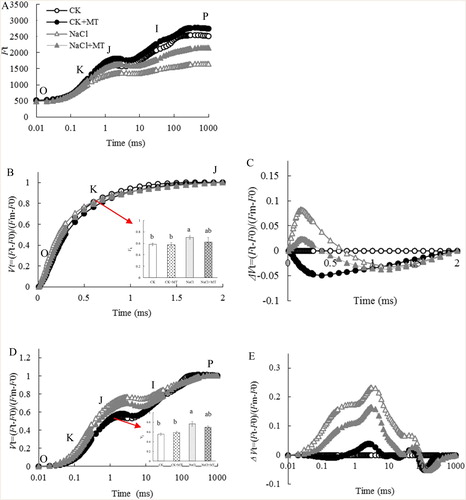
The fluorescence transient showed a polyphasic rise when leaves are illuminated by a high density of actinic light, which provides measurements for phase O, J, I, and P of PSII, such as electron transport levels in the donor and acceptor sides (Srivastava et al. Citation1997; Strasser and Strasser Citation1995). As shown in (A), the shapes of the OJIP curves of the tomato leaves were significantly different under the four treatments, and the ranges of the variations of the relative fluorescence intensity were also different at each characteristic point on the OJIP curve. OJIP curves were normalized to the (Fm−Fo) level ((B,D)), and ΔVt was obtained by subtracting the kinetics of plants that were untreated from kinetics of MT or NaCl treated plants ((C,E)). The shape of the OJIP transient changed over time, where the J, K and L points increased significantly under NaCl stress, and this increase was not as pronounced in the MT and NaCl treated samples when compared to the NaCl treated samples ((B,C)). This suggests that NaCl stress blocked electron transport from QA to QB, and MT treatment alleviated this effect. The K step (at 300 μs) of the chlorophyll a fluorescence transient (quantified as VK) is an indicator for OEC damage (Strasser, Citation1997; TóTh et al. Citation2005). We observed a 14.34% decrease in Vk in samples treated with NaCl alone, while NaCl and MT treatment led to a 11.86% increase in Vk compared with the NaCl only treatment ((D,E)), suggesting that NaCl stress led to the inhibition of the donor side of PSII activity and damaged OEC, while MT alleviated PSII inhibition and OEC damage. According to the Grouping Concept (Strasser and Strasser Citation1995) and JIP test (Strasser et al. Citation2004; Lin et al. Citation2009), a positive step in JIP indicates low grouping or low energy exchange between PSII units. Because the grouped conformation is more stable than the ungrouped one (Strasser et al. Citation2004; Lin et al. Citation2009), the decreased grouping indicates that PSII units may have become unstable or more fragile. This observation implies that NaCl stress damaged the PSII units, and exogenous application of MT reduced this NaCl-induced damage of PSII.
Figure 3. Effects of MT on the chlorophyll fluorescence (fluo) OJIP curves of tomato leaves treated with 150 mM NaCl. (a) OJIP transient; (b) normalized OJIP transient, the inserted column chart present the relative fluorescence intensity of step J (VJ); (c) ΔVt was obtained by subtracting the kinetics of untreated leaves from the kinetics of leaves treated with melatonin or salt between O and P; (d) normalized transient between O and J, the column chart presents the relative fluorescence intensity of step K (VK); (e) ΔVt was obtained by subtracting the kinetics of untreated leaves from the kinetics of leaves treated with melatonin or salt between O and J. O indicates the O step at about 20 ms; J indicates the J step at about 2 ms; I indicates the I step at about 30 ms; P indicates the P step, the maximum fluorescence. Each datum is the average of 6 independent measurements.
Effects of MT on thylakoid membrane proteins D1 and PsbO under NaCl stress
Western blots were performed to determine the effects of photoinhibition induced by NaCl stress on the degradation of D1 and PsbO proteins. Compared to the untreated controls, NaCl stress led to a decrease in D1 and PsbO levels by 58.67% and 35.72%, respectively. However, plants treated with MT and NaCl had 29.27% and 26.56% higher D1 and PsbO levels compared to NaCl stress ().
Discussion
Inhibitory effect of MT on chlorophyll degradation and photosynthesis reduction in tomatoes under NaCl stress
MT is a natural agent that has been widely used to promote growth and photosynthesis of plants under NaCl stress (Li et al. Citation2012; Wang et al. Citation2016). In this study, MT effectively lessened the decrease of Chls, Chl a and Chl b contents caused by NaCl stress () and promoted the increase of Pn and CE (), indicating that MT could alleviate chlorophyll degradation induced by NaCl stress (Kudoh and Sonoike Citation2002). In addition, Chls, Chl a and Chl b contents were much higher when plants were treated with MT than under normal conditions, suggesting that MT may facilitate the chloroplast gene expression and protein turnover to promote the accumulation of chlorophyll (Suo et al. Citation2015). The results are consistent with a recent study on tomato showing that MT increases ferredoxin, which promotes the synthesis of GSH and inhibits the degradation of Chl (Lin et al. Citation2013; Wei et al. Citation2015; Siddiqui et al. Citation2019). However, further research into the connection between the photosynthetic machinery and chlorophyll metabolic pathways is needed to further understand under NaCl stress.
Stomatal limitation and non-stomatal limitation factors can both decrease the photosynthetic rate in plants under stressful conditions (Zhu Citation2002). Stomatal limitation is usually caused by the partial closure of the stomata, which can reduce the CO2 concentration in mesophyll cells and inhibit photosynthesis. Non-stomatal factors restrict the diffusion of CO2 in carboxylation sites in mesophyll cells or reduce enzymatic activities during carbon assimilation (Sharkey et al. Citation2007). The lower levels of Gs and Ci that were observed under NaCl stress indicated that the decrease of Pn may be due to the effect of NaCl stress on root activity, leading to the partial closure of stomata, thus resulting in the decrease of Ci (). This finding suggested that stomatal limitation under NaCl stress was the main reason for the decrease of Pn that was observed in tomato seedling leaves. However, Gs and Ci increased with increasing Pn in MT and NaCl treated plants, indicating that application of MT led to maintaining of high potential PSII activity. The above results showed that MT regulated photosynthesis and enhanced the adaptability of tomato leaves in response to NaCl tolerance (Wang et al. Citation2016). This may be through maintaining higher Rubisco activity and solar utilization efficiency, and overcoming the stomatal limitation, thereby reducing the adverse effects caused by NaCl stress (Zhang et al. Citation2013).
MT promoted ROS scavenging by increasing the activities of enzymes in the AsA–GSH cycle
NaCl stress reduced the efficiency of photosynthesis, resulting in accumulation of excess light energy and obstruction of electron transfer. Excess light energy and electrons can influence the metabolic balance of ROS in plants, leading to an accumulation of ROS (Wilhelm and Selmar Citation2011). Excess ROS causes peroxidation of lipids and pigments on the cell membrane, resulting in increased cell membrane permeability and destruction of its functions (Zhang et al. Citation2012). The antioxidant capacity of MT has been widely reported, and it can significantly reduce the adverse effects of abiotic stress, such as cold-induced injury, heavy metals, UV radiation and NaCl injury (Hasan et al. Citation2015; Shi et al. Citation2015; Li et al. Citation2016b). This study showed that MT could improve the activity of SOD under NaCl stress ((C)), which was consistent with a previous study that showed that application of MT on apple leaves reduced the effects of NaCl stress (Li et al. Citation2012). In addition, under NaCl stress, MT promoted the activities of enzymes in the AsA–GSH cycle (APX, MDHAR, DHAR and GR), which can effectively increase ROS scavenging (Palma and Rio Citation2006). Previous results showed that MT promoted the activities of enzymes in the AsA–GSH cycle in cucumber chloroplasts exposed to cold stress (Zhao et al. Citation2016). Interestingly, the activities of these enzymes increased more than POD, CAT and GPX (). The results are consistent with a recent study on tomato under NaCl stress, which showed that exogenous application of MT induced the AsA–GSH cycle by activating antioxidant enzymes and non-antioxidant enzymes, and these changes led to higher tolerance to environmental stress (Siddiqui et al. Citation2019). Therefore, application of MT alleviated the oxidative damage caused by NaCl stress by promoting the activities of SOD and enzymes involved in the AsA–GSH cycle, thereby balancing ROS generation and scavenging and reducing the damage on PSII. This may be a special protective mechanism that MT plays a role in tomato under NaCl stress.
MT inhibited ROS production by promoting PSII repair
The information on the photochemistry of the donor and acceptor sides of PSII can be obtained using chlorophyll fluorescence technology (Strasser and Strasser Citation1995; Strasser, Citation1997; Lin et al. Citation2009). In this study, chlorophyll fluorescence was conducted to analyze the possible mechanism of MT in alleviating PSII from photosynthetic non-stomatal factors under NaCl stress. The increase of the relative variable fluorescence Vk at the K point (300 μs) was due to the inhibition of the water-splitting system and the partial suppression of the acceptor side before QA ((D,E)). The K step of the chlorophyll a fluorescence transient (quantified as VK) is an indicator of OEC injury in the photosynthetic apparatus (Strasser Citation1997; Zhang et al. Citation2017). The K phase was clearly visible in the standardized O-J curve ((D,E)), and VK also increased significantly under NaCl stress ((D,E)). The increase in VK is considered to be a sign of damage to the oxygen-evolving complex (OEC) on the electron donor side of PSII (Zhang et al. Citation2017). However, the increase in VK was reduced when exogenous MT was applied ((D,E)). In addition, O2 release capacity and the degradation of the PsbO protein (OEC) in plant leaves decreased significantly under NaCl stress ((D,E)). The decrease in the activity of OEC at the electron donor side of PSII would lead to incomplete cleavage of water, producing H2O2. The blockage of the electron transfer at the acceptor side of PSII would lead to leakage of the excess electrons, which can attack the free O2 in cells and generate superoxide anions. Reactive oxygen species such as H2O2 and superoxide anions can increase the peroxidation of cell membranes, leading to electrolyte extravasation, which can affect the normal functioning of cell membranes (Venkatesh et al. Citation2012).
The relative variable fluorescence at the J-step (VJ) represents the kinetic bottleneck of the electron transport chain, resulting in the momentary maximum accumulation of (Li et al. Citation2009; Strasser and Strasser Citation1995). VJ is an indicator of closure of PSII reaction centers or the redox state of
(Haldimann and Strasser Citation1999). The increase of VJ in the OJIP curve ((B,C)) indicated that NaCl stress suppressed the acceptor side of PSII ((B,C)), while the application of exogenous MT lessened the damage on the donor and acceptor sides of PSII ((B,C)). The degradation of D1 and PsbO in PSII were alleviated in plants that were pre-treated with MT under NaCl stress (). This confirmed that MT might promote the electron transport of the PSII acceptor side by protecting OEC function of the PSII donor side and prevent the degradation of D1 (Zhou et al. Citation2016).
Conclusion
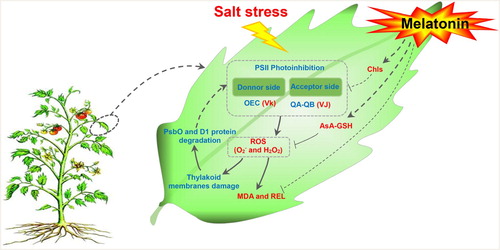
This study evaluated the response mechanism of PSII to NaCl stress in tomato leaves (). The donor and acceptor sides of the photosystems were damaged by NaCl stress, indicating that photoinhibition and photoinhibition-like damage had occurred. PsbO and D1 proteins in donor and acceptor sides of PSII were degraded and there was an over-accumulation of ROS. Application of exogenous MT increased NaCl tolerance in tomato by reducing chlorophyll, balancing the distribution of photosynthetic electrons, thereby suppressing ROS production, and by promoting the activities of enzymes involved in the ascorbate–glutathione cycle to enhance ROS scavenging. Taken together, these mechanisms suppressed damages caused by salt stress in plants that were treated with MT ().
Figure 5. Quantitative image analysis of core protein levels at seven days’ salt stress in tomato leaves. D1(A) and PsbO (B) protein levels were evaluated. Total proteins were analyzed using antibodies raised against D1 and PsbO. Immunoblotting of actin (45 kDa) served as loading controls. CK, control; CK+MT, 150 μM melatonin; NaCl, 150 mM NaCl stress; NaCl+MT, 150 μM melatonin with 150 mM NaCl.
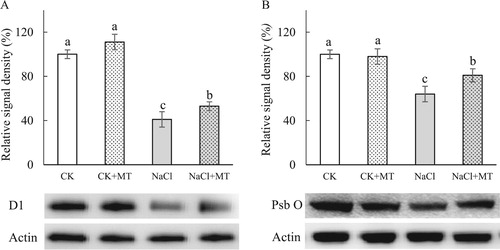
Figure 6. Schematic presentation of MT regulation systematic in tomato leaves under salt stress. Salt stress photoinhibition in the donor and acceptor sides of the photosystems II, and lead to ROS burst, which resulting in the damage to cell membrane and thylakoid membranes. To alleviate ROS toxicity, specific ROS scavenging pathways (e.g. APX, MDHAR, DHAR, and GR pathways) are induced. Exogenous MT increase the salt tolerance in tomato seedling leaves by reducing chlorophyll degradation to adjust photosynthetic electro flux and by enhancing the AsA-GSH cycle to increase the ROS scavenging capacity so as to suppress the damage caused by salt stress. Solid line with arrow and ‘T’ shape line represents stimulation and inhibition, respectively. The red and blue words indicate induced and reduced cellular processes by salt stress/MT, respectively. Dashed lines indicate indirect regulations. Abbreviations: AsA-GSH, ascorbate–glutathione cycle; Chls, chlorophyll; H2O2, hydrogen peroxide; MDA, malondialdehyde; , superoxide anion; REL, relative electrolyte leakage; ROS, reactive oxygen species; VJ, the relative variable fluorescence at the J step; Vk, the normalized relative variable fluorescence at the K step.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Zepeng Yin
Zepeng Yin from College of Horticulture, Shenyang Agricultural University, Shenyang 110866, China.
Jiazhi Lu
Jiazhi Lu from College of Horticulture, Shenyang Agricultural University, Shenyang 110866, China.
Sida Meng
Sida Meng from College of Horticulture, Shenyang Agricultural University, Shenyang 110866, China.
Yiling Liu
Yiling Liu from College of Horticulture, Shenyang Agricultural University, Shenyang 110866, China.
Islam Mostafa
Islam Mostafad from Department of Pharmacognosy, Faculty of Pharmacy, Zagazig University, Zagazig 44519, Egypt.
Mingfang Qi
Mingfang Qi from College of Horticulture, Shenyang Agricultural University, Shenyang 110866, China.
Tianlai Li
Tianlai Li from College of Horticulture, Shenyang Agricultural University, Shenyang 110866, China.
References
- Afreen F, Zobayed SM, Kozai T. 2006. Melatonin in Glycyrrhiza uralensis: response of plant roots to spectral quality of light and UV-B radiation. J Pineal Res. 41:108. doi: 10.1111/j.1600-079X.2006.00337.x
- Arnao M, Hernández-Ruiz J. 2009. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J Pineal Res. 46:58–63. doi: 10.1111/j.1600-079X.2008.00625.x
- Arnao M, Hernández-Ruiz J. 2015. Functions of melatonin in plants: a review. J Pineal Res. 59:133. doi: 10.1111/jpi.12253
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. doi: 10.1016/0003-2697(76)90527-3
- Cheng DD, Zhang ZS, Sun XB, Zhao M, Sun GY, Chow WS. 2016. Photoinhibition and photoinhibition-like damage to the photosynthetic apparatus in tobacco leaves induced by pseudomonas syringae pv. tabaci under light and dark conditions. BMC Plant Biol. 16:1–11. doi: 10.1186/s12870-015-0700-5
- Fan X, Zhang Z, Gao H, Yang C, Liu M, Li Y. 2014. Photoinhibition-like damage to the photosynthetic apparatus in plant leaves induced by submergence treatment in the dark. Plos One. 9:e89067. doi: 10.1371/journal.pone.0089067
- Foyer CH, Noctor G. 2009. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal. 11:861–905. doi: 10.1089/ars.2008.2177
- Galano A, Tan DX, Reiter RJ. 2011. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x
- Gong M, Li YJ, Chen SZ. 1998. Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J Plant Physiol. 153:488–496. doi: 10.1016/S0176-1617(98)80179-X
- Haldimann P, Strasser RJ. 1999. Effects of anaerobiosis as probed by the polyphasic chlorophyll a fluorescence rise kinetic in pea (Pisum sativum L). Photosynth Res. 62:67–83. doi: 10.1023/A:1006321126009
- Han Y, Wang S, Zhao N, Deng S, Zhao C, Li N. 2016. Exogenous abscisic acid alleviates cadmium toxicity by restricting Cd2+ influx in Populus euphratica cells. J Plant Growth Regul. 35:827–837. doi: 10.1007/s00344-016-9585-2
- Hasan MK, Ahammed GJ, Yin L, Shi K, Xia X, Zhou Y. 2015. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicuml. Front Plant Sci. 6:601. doi: 10.3389/fpls.2015.00601
- Ibrahim MH, Jaafar HZ. 2012. Primary, secondary metabolites, H2O2, malondialdehyde and photosynthetic responses of Orthosiphon stamineus Benth to different irradiance levels. Molecules. 17:1159–1176. doi: 10.3390/molecules17021159
- Kang K, Lee K, Park S, Kim Y S, Back K. 2010. Enhanced production of melatonin by ectopic overexpression of human serotonin N-acetyltransferase plays a role in cold resistance in transgenic rice seedlings. J Pineal Res. 49:176–182.
- Kolář J, Macháčková I, Eder J, Prinsen E, Dongen WV, Onckelen HV. 1997. Melatonin: occurrence and daily rhythm in Chenopodium rubrum. Phytochem. 44:1407–1413. doi: 10.1016/S0031-9422(96)00568-7
- Kudoh H, Sonoike K. 2002. Irreversible damage to photosystem I by chilling in the light: cause of the degradation of chlorophyll after returning to normal growth temperature. Planta. 215:541–548. doi: 10.1007/s00425-002-0790-9
- Lei XY, Zhu RY, Zhang GY, Dai YR. 2004. Attenuation of cold-induced apoptosis by exogenous melatonin in carrot suspension cells: the possible involvement of polyamines. J Pineal Res. 36:126–131. doi: 10.1046/j.1600-079X.2003.00106.x
- Li P, Cheng L, Gao H, et al. 2009. Heterogeneous behavior of PSII in soybean (glycine max) leaves with identical PSII photochemistry efficiency under different high temperature treatments. J Plant Physiol. 166:1607. doi: 10.1016/j.jplph.2009.04.013
- Li H, He J, Yang X, Li X, Luo D, Wei C. 2015. Glutathione-dependent induction of local and systemic defense against oxidative stress by exogenous melatonin in cucumber (Cucumis sativus L.). J Pineal Res. 60:206–216. doi: 10.1111/jpi.12304
- Li C, Liang B, Chang C, Wei Z, Zhou S, Ma F. 2016a. Exogenous melatonin improved potassium content in Malus under different stress conditions. J Pineal Res. 61:218–229. doi: 10.1111/jpi.12342
- Li X, Tan D, Jiang D, Liu F. 2016b. Melatonin enhances cold tolerance in drought-primed wild type and abscisic acid-deficient mutant barley. J Pineal Res. 61:328–339. doi: 10.1111/jpi.12350
- Li C, Wang P, Wei Z, Liang D, Liu C, Yin L. 2012. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J Pineal Res. 53:298–306. doi: 10.1111/j.1600-079X.2012.00999.x
- Lin ZH, Chen LS, Chen RB, Zhang FZ, Jiang HX, Ning T. 2009. CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport probed by the JIP-test, of tea leaves in response to phosphorus supply. BMC Plant Biol. 9:43. doi: 10.1186/1471-2229-9-43
- Lin YH, Pan KY, Hung CH, Huang HE, Chen CL, Feng TY, Huang LF. 2013. Overexpression of ferredoxin, PETF, enhances tolerance to heat stress in Chlamydomonas reinhardtii. Int J Mol Sci. 14:20913–20929. doi: 10.3390/ijms141020913
- Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A. 2015. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res. 59:403. doi: 10.1111/jpi.12267
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Ann Rev Plant Biology. 59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911
- Palma JM, Río LAD. 2006. Antioxidative enzymes from chloroplasts, mitochondria, and peroxisomes during leaf senescence of nodulated pea plants. J Exp Bot. 57:1747. doi: 10.1093/jxb/erj191
- Park S, Back K. 2012. Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. J Pineal Res. 53:385–389. doi: 10.1111/j.1600-079X.2012.01008.x
- Pelagio-Flores R, Muñozparra E, Ortizcastro R, López-Bucio J. 2012. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J Pineal Res. 53:279–288. doi: 10.1111/j.1600-079X.2012.00996.x
- Porra RJ. 2002. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res. 73:149–156. doi: 10.1023/A:1020470224740
- Posmyk MM, Kuran H, Marciniak K, Janas KM. 2008. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J Pineal Res. 45:24. doi: 10.1111/j.1600-079X.2007.00552.x
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. 2007. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 30:1035–1040. doi: 10.1111/j.1365-3040.2007.01710.x
- Shi H, Chan Z. 2014. The cysteine2/histidine2-type transcription factor ZINC FINGER OF Arabidopsis Thaliana 6-activated C-REPEAT-BINDING FACTOR pathway is essential for melatonin-mediated freezing stress resistance in Arabidopsis. J Pineal Res. 57:185–191. doi: 10.1111/jpi.12155
- Shi H, Wang X, Tan DX, Reiter RJ, Chan Z. 2015. Comparative physiological and proteomic analyses reveal the actions of melatonin in the reduction of oxidative stress in Bermuda grass (cynodon dactylon (l). pers. J Pineal Res. 59:120–131. doi: 10.1111/jpi.12246
- Siddiqui MH, Alamri S, Al-Khaishany MY. 2019. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int J Mol Sci. 20(2):353. doi: 10.3390/ijms20020353
- Srivastava A, Guissé B, Greppin H, Strasser RJ. 1997. Regulation of antenna structure and electron transport in photosystem II of Pisum sativum, under elevated temperature probed by the fast polyphasic chlorophyll a, fluorescence transient: OKJIP. BBA-Bioenergetics. 1320:95–106. doi: 10.1016/S0005-2728(97)00017-0
- Strasser BJ. 1997. Donor side capacity of Photosystem II probed by chlorophyll a fluorescence transients. Photosynth Res. 52(2):147–155. doi: 10.1023/A:1005896029778
- Strasser RJ, Srivastava A. 1995. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 61:32–42. doi: 10.1111/j.1751-1097.1995.tb09240.x
- Strasser BJ, Strasser RJ. 1995. Measuring fast fluorescence transients to address environmental questions: the JIP-test. In: P Mathis, editor. Photosynthesis: from light to biosphere. Dordrecht: Kluwer Academic Publishers; p. 977–980.
- Strasser RJ, Tsimilli-Micheal M, Srivastava A. 2004. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou, GC Govindjee, editor. Chlorophyll a fluorescence: a signature of photosynthesis. Dordrecht: Springer; p. 321–362.
- Suo J, Qi Z, Zhang Z, Chen S, Cao J, Liu G. 2015. Cytological and proteomic analyses of Osmunda cinnamomea germinating spores reveal characteristics of fern spore germination and rhizoid tip growth. Mol Cell Proteomics. 14(9):2510–2534. doi: 10.1074/mcp.M114.047225
- Takahashi S, Murata N. 2008. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 13:178–182. doi: 10.1016/j.tplants.2008.01.005
- Tal O, Haim A, Harel O, Gerchman Y. 2011. Melatonin as an antioxidant and its semi-lunar rhythm in green macroalga Ulva sp. J Exp Bot. 62:1903. doi: 10.1093/jxb/erq378
- Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. 2007. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x
- Tiryaki I, Keles H. 2012. Reversal of the inhibitory effect of light and high temperature on germination of Phacelia tanacetifolia seeds by melatonin. J Pineal Res. 52:332–339. doi: 10.1111/j.1600-079X.2011.00947.x
- TóTh SZ, Schansker G, Kissimon J, Ková CL, Garab G, Strasser RJ. 2005. Biophysical studies of photosystem ii-related recovery processes after a heat pulse in barley seedlings (Hordeum vulgare L.). J Plant Physiol. 162:181–194. doi: 10.1016/j.jplph.2004.06.010
- Venkatesh J, Upadhyaya CP, Yu JW, Hemavathi A, Kim DH, Strasser RJ. 2012. Chlorophyll a fluorescence transient analysis of transgenic potato overexpressing D-galacturonic acid reductase gene for salinity stress tolerance. Hortic Environ Biotechnol. 53:320–328. doi: 10.1007/s13580-012-0035-1
- Wang X, Chen S, Zhang H, Shi L, Cao F, Guo L. 2010. Desiccation tolerance mechanism in resurrection fern-ally Selaginella tamariscina revealed by physiological and proteomic analysis. J Proteome Res. 9:6561–6577. doi: 10.1021/pr100767k
- Wang LY, Liu JL, Wang WX, Sun Y. 2016. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica. 54:19–27. doi: 10.1007/s11099-015-0140-3
- Wang P, Sun X, Chang C, Feng F, Liang D, Cheng L. 2013a. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J Pineal Res. 55:424–434. doi: 10.1111/jpi.12069
- Wang P, Sun X, Li C, Wei Z, Liang D, Ma F. 2013b. Long term exogenous application of melatonin delays drought induced leaf senescence in apple. J Pineal Res. 54:292–302. doi: 10.1111/jpi.12017
- Wang P, Yin L, Liang D, Li C, Ma F, Yue Z. 2012. Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate-glutathione cycle. J Pineal Res. 53:11. doi: 10.1111/j.1600-079X.2011.00966.x
- Wang L, Zhao Y, Reiter RJ, He C, Liu G, Lei Q. 2014. Changes in melatonin levels in transgenic ‘Micro-Tom’ tomato overexpressing ovine AANAT and ovine HIOMT genes. J Pineal Res. 56:134–142. doi: 10.1111/jpi.12105
- Wei W, Li QT, Chu YN, Reiter RJ, Yu XM, Zhu DH, Zhang WK, Ma B, Lin Q, Zhang JS. 2015. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot. 66:695–707. doi: 10.1093/jxb/eru392
- Wilhelm C, Selmar D. 2011. Energy dissipation is an essential mechanism to sustain the viability of plants: The physiological limits of improved photosynthesis. J Plant Physiol. 168:79–87. doi: 10.1016/j.jplph.2010.07.012
- Yan B, Dai Q, Liu X, Huang S, Wang Z. 1996. Flooding-inducedmembrane damage, lipid oxidation and activated oxygengeneration in corn leaves. Plant Soil. 179:261–268. doi:10.1007/bf00009336.
- Yang Y, Chang D, Wang Y, Zhang FC. 2015. Effects of exogenous JA and MeJA on seed germination and seeding physiological characteristics of Gossypium hirsutum under salt stress. Seed. 34:8–18.
- Yin ZP, Li S, Ren J, Song XS. 2014. Role of spermidine and spermine in alleviation of drought-induced oxidative stress and photosynthetic inhibition in Chinese dwarf cherry (Cerasus humilis) seedlings. Plant Growth Regul. 74:209–218. doi: 10.1007/s10725-014-9912-1
- Yin Z, Ren J, Zhou L, Sun L, Wang J, Liu Y. 2017. Water deficit mechanisms in perennial shrubs Cerasus humilis leaves revealed by physiological and proteomic analyses. Proteome Sci. 15:9. doi: 10.1186/s12953-017-0117-1
- Zhan GM, Li RJ, Hu ZY, Liu J, Deng LB, Lu SY. 2014. Cosuppression of RBCS3B, in Arabidopsis, leads to severe photoinhibition caused by ROS accumulation. Plant Cell Rep. 33:1091. doi: 10.1007/s00299-014-1597-4
- Zhang Z, Li G, Gao H, Zhang L, Yang C, Liu P. 2012. Characterization of photosynthetic performance during senescence in stay-green and quick-leaf-senescence, Zea mays, L. inbred lines. Plos One. 7:e42936. doi: 10.1371/journal.pone.0042936
- Zhang HH, Li X, Zhang SB, Yin ZP, Zhu WX, Li JB, Meng L, Zhong HX, Wu YN, Xu N, Sun GY. 2018. Rootstock alleviates salt stress in grafted mulberry seedlings: physiological and PSII function responses. Front Plant Sci. 9:1806. doi: 10.3389/fpls.2018.01806
- Zhang D, Ren L, Chen GQ, Zhang J, Reed BM, Shen XH. 2015a. ROS-induced oxidative stress and apoptosis-like event directly affect the cell viability of cryopreserved embryogenic callus in Agapanthus praecox. Plant Cell Rep. 34:1499–1513. doi: 10.1007/s00299-015-1802-0
- Zhang N, Sun Q, Zhang H, Cao Y, Weeda S, Ren S. 2015b. Roles of melatonin in abiotic stress resistance in plants. J Exp Bot. 66:647. doi: 10.1093/jxb/eru336
- Zhang HH, Xu N, Li X, Jin WW, Tian Q, Gu SY. 2017. Overexpression of 2-Cys Prx increased salt tolerance of photosystem II in tobacco. Int J Agric Biol. 19:735–745. doi: 10.17957/IJAB/15.0348
- Zhang HH, Xu N, Teng ZY, Wang JR, Ma SL, Wu XY, Li X, Sun GY. 2019. 2-Cys Prx plays a critical role in scavenging H2O2 and protecting photosynthetic function in leaves of tobacco seedlings under drought stress. Journal of Plant Interaction. 14(1):119–128. doi: 10.1080/17429145.2018.1562111
- Zhang N, Zhao B, Zhang HJ, Weeda S, Yang C, Yang ZC. 2013. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L). J Pineal Res. 54:15–23. doi: 10.1111/j.1600-079X.2012.01015.x
- Zhao H, Ye L, Wang Y, Zhou X, Yang J, Wang J. 2016. Melatonin increases the chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. Front Plant Sci. 7:e85996.
- Zheng Q, Liu J, Liu R, Wu H, Jiang C, Wang C. 2015. Temporal and spatial distributions of sodium and polyamines regulated by brassinosteroids in enhancing tomato salt resistance. Plant & Soil. 400:147–164. doi: 10.1007/s11104-015-2712-1
- Zhou X, Zhao H, Cao K, Hu L, Du T, Baluška F, Zou Z. 2016. Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of d1 protein in tomato seedlings under salt stress. Front Plant Sci. 7. doi:10.3389/fpls.2016.01823.
- Zhu JK. 2002. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 53:247. doi: 10.1146/annurev.arplant.53.091401.143329