ABSTRACT
Plants must cope with the stress conditions to survive. Plant growth promoting rhizobacteria can improve plant growth either directly or indirectly under stress conditions. However, the possible mechanisms remain unclear. Here we report that Bacillus megaterium strain A12 (BMA12) maintains hormonal and redox homeostasis and restores the photosynthetic efficacy of tomato plants through multiple mechanisms to survive under salinity stress conditions. Tomato plants were co-cultivated with BMA12 under saline conditions. The application of BMA12 significantly increased plant growth and photosynthetic capacity. BMA12 decreased production of ROS and ethylene but increased expression levels of selected genes responsible for repairing of damaged photosynthetic apparatus and maintenance of redox homeostasis. Furthermore, BMA12 significantly altered metabolic profile to restore perturbations of tomato plant physiology impaired with salinity stress. This study proves that BMA12 can be used in the conventional agriculture system in the salinity effected fields.
1. Introduction
According to an estimate, nearly 7% of the land is affected by salinity (Ruizlozano et al. Citation2012; Abdel-Ghani et al. Citation2015). Abiotic stresses inhibit plant growth and cause considerable yield reduction (Baniasadi et al. Citation2015; Khan et al. Citation2018). Salinity stress may cause >30% losses in crop plants in saline effected fields (Ashraf and Pjc Citation2004). The area of salinity affected agricultural land is increasing because of natural processes and conventional agricultural practices (Berger et al. Citation2012). High levels of salinity adversely affects plant growth and development (Hashem et al. Citation2016). Salinity causes osmotic and ionic stresses to limit plant growth (Flowers and Colmer Citation2008). Additionally, high salinity manifests an oxidative stress inside plant body, resulting in malfunctioning of photosynthesis and ion homeostasis to retard plant growth (Porcel and Ruiz-Lozano Citation2012; Alqarawi, Hashem, et al. Citation2014; Rahneshan et al. Citation2018).
Abiotic stresses affect photosynthesis in plants (Ozakca Citation2013). The changes in photosynthesis process are associated with distresses in carbon and nitrogen assimilation pathways (Tejera et al. Citation2004). These stress cause reduction of the electron transport chain leading to photo-oxidation (Grbić and Bleecker Citation1995; Balota et al. Citation2004). Furthermore, abiotic stresses can damage the PSII protein system of the photosynthetic machinery, that is an unavoidable process (Gururani, Mohanta, et al. Citation2015; Gururani, Venkatesh, et al. Citation2015). Plants have developed the process to recover the damaged PSII through a specific repairing system (Melis Citation1999; Murata et al. Citation2007). Reactive oxygen species (ROS) are key deleterious products hindering plant metabolism under stress conditions. It has been proved that ROS hinder the PSII repairing mechanism by obstructing the formation of the D1 protein of PSII system encoded by PsbA gene (Nishiyama et al. Citation2011; Gururani, Venkatesh and Tran Citation2015; Yu et al. Citation2015). Secondly, the increased accumulation of ROS causes oxidative damage and affects the integrity of the cell (Ahmad et al. Citation2010). ROS accumulation in leaves causes the oxidation of certain molecules and ultimately programed cell death (Pang and Wang Citation2008).
Plants cope with a suite of biotic and abiotic stress factors in natural habitats (Nguyen et al. Citation2016). Plants employ self-defense mechanisms to prevent oxidative damages under salinity stress. The hyper activation of antioxidant systems and increased production of compatible osmolytes are included among several defensive strategies used by plants against abiotic stress conditions (Vardharajula et al. Citation2011; Alqarawi, Allah, et al. Citation2014). The enzymatic and non-enzymatic components work together to neutralize toxic ROS in antioxidant systems of plants. Osmolytes like free proline, sugars and amino acids help to sustain the water level to regulate cellular metabolism and functionality (Hossain et al. Citation2015). Secondly, the plants respond to abiotic stress through changes in some major metabolic pathways such as photosynthesis, TCA cycle and (Lewis et al. Citation2001; Rai Citation2002; Lotfi et al. Citation2010). Different metabolites may participate in plant stress tolerance. However, the knowledge regarding salinity tolerance-related metabolomics is limited. Metabolomic analyses can help to determine the specific responses of plant physiological systems to cope environmental stresses (Oliver et al. Citation2011). A variety of small signaling molecules modulate molecular responses of plants against abiotic stresses through complicated networks (Pieterse et al. Citation2012; Vleesschauwer et al. Citation2014). Phytohormones play a key role in controlling certain molecular mechanisms inside plant body and hence optimize plant responses against abiotic stresses (Nguyen et al. Citation2016). Absisc acid (ABA) is involved in many developmental processes like growth inhibition, stomatal conductance and primary root growth (Liang et al. Citation2014). This hormone is an important controlling factor of plant responses to different environmental stresses as salinity (Sharp and Lenoble Citation2002; Agata and Iwona Citation2013).
Previous studies have suggested that beneficial soil microbes including plant growth promoting rhizobacteria (PGPR) rescue plant growth and yield under stress conditions (Berg Citation2009; Cho et al. Citation2015; Abd_Allah et al. Citation2018). Some important roles of PGPR include bio-fertilization, restriction of pathogen growth and induction of abiotic stresses tolerance in plants (Adesemoye et al. Citation2008; Bhattacharyya and Jha Citation2012; Jing et al. Citation2018). PGPR can promote plant growth by both directly and indirectly mechanisms. The direct mechanisms included root growth promotion, rhizoremediation and stress mitigation (Vaishnav et al. Citation2016; Jha and Subramanian Citation2018). In addition to that, PGPR modulate physiological processes through perturbation of metabolism of plants (Ilangumaran and Smith Citation2017). It is important to know how PGPR repair osmotic homeostasis and photosynthesis process after onset of salinity stress in plants and what inducible mechanisms make plants to survive salinity stress in the presence of these beneficial microbes. In our previous research, B. megaterium strain A12 (BMA12) stimulated the growth of tomato plants under salinized conditions (Aslam et al. Citation2018). The objectives of this study were to understand the responses, adaptation and tolerance of salinity stress in tomato plants at physiological, molecular and biochemical levels under influence of BMA12. In this research work, the possible adaptations are also described made by tomato plants to alter osmotic homeostasis and improve photosynthesis under stress conditions mediated by this beneficial bacterium.
2. Materials and methods
2.1. Strains and culture condition
The pure B. megaterium strain A12 (BMA12) culture was procured from the conservatory of the Plant Biotechnology laboratory, Institute of Agricultural Sciences, University of the Punjab, Lahore, Pakistan, and grown in a nutrient agar medium overnight on a rotating shaker (200 rpm) at 30°C.
2.2. Plant materials and bacterial inoculation
Tomato seeds (L. esculantum cv. RioGrande) were purchased from commercial seed market. The seeds were surface sterilized using standard sodium hypochlorite method. Following the sterilization, the seeds were germinated in plastic pots of 6-inch diameter filled with sterilized commercial potting mix. After germination, one healthy seedling was left in each pot. As in our previous study, the plant’s growth was the most retarded at 200 mM NaCl (Aslam et al. Citation2018). Therefore this concentration was used for experiments. Plants were treated after 10 days of emergence. Treatment details are as follow: Control = 100 mL of distilled sterilized water to serve as non-treated control. T1 = 100 mL of 200 mM aqueous NaCl solution to act as salinity control. T2 = 100 mL of aqueous BMA12 formulation (1 × 107 colony forming units mL−1) to serve as bacterial control, T3 = 100 mL of 200 mM NaCl and 100 mL of aqueous formulation of BMA12 (1 × 107 colony forming units mL−1). The pots were irrigated with distilled sterilized water when needed. Each experiment was repeated twice with five replicate plants of each treatment. After ten days of treatment applications, the plants were analyzed for growth attributes and rest of the analyses.
2.3. Leaf pigment analysis
Young leaves of tomato plants were excised from plants after ten days of treatment applications. The leaf material was powdered in liquid nitrogen and extracted in 30 mL of solution (1:1 (v/v) acetone and ethanol) overnight at 30°C. Chlorophyll a, b and carotenoid contents in the leaves were quantified by the spectrophotometric method as described by (Kaźmierczak Citation1998).
2.4. Photosynthetic rate measurements
The fully expanded leaves of tomato plants from each treatment were selected for photosynthetic parameters measurements using LI-6400 system (Li-Cor Inc., Lincoln, NE, USA). The samples were illuminated with the saturated photosynthetic photon flux density (PPFD) with the help of a light-emitting diode (LED) light source for half hour prior to measurements for full induction of the photosynthesis process. Afterwards, the net photosynthetic rate (Pn), transpiration rate (Tr), and stomatal conductance (Gs) were measured simultaneously. All parameters for measurement were adopted as described by (Chen et al. Citation2010). Intrinsic water use efficiency (iWUE) was calculated from the ratio of Pn and Tr.
2.5. Analysis of changes in soluble sugars, free amino acids, soluble protein contents and some related metabolites
Tomato leaf material was grinded as fine powdered in liquid nitrogen and (0.1 g) was extracted with 80% (v/v) ethanol at 80°C. This extract was used to determine total soluble sugars, sucrose, and free amino acid. Total soluble sugar contents were measured adopting the anthrone reagent method (Turakainen and Hartikainen Hseppanen Citation2004). Five milliliters of anthrone sulfuric acid solution (75% v:v) was added to 0.1 mL of supernatant previously prepared. This mixture was warmed up to 90°C for 20 min and cooled in water bath in cold water. OD was taken at 620 nm. Free amino acid contents were determined using ninhydrin reagent (Moore and Stein Citation1954). One millilitter of acetate buffer (pH = 5.4), 1 ml chromogenic agent and 1 mL of extraction material were mixed thoroughly and heated in boiling water bath for twenty minutes. The solution was cooled, 3 mL ethanol (60%, v/v) was further added and OD was taken at 510 nm. Lastly, soluble protein contents were quantified using method of (Kruger Citation1988).
2.6. Quantifications of enzymatic and non-enzymatic antioxidants
For quantification of enzymatic antioxidants, plants protein was extracted in protein extraction buffer (50 mM Tris–HCl buffer (pH7.0) containing 3 mM MgCl2, 1 mM EDTA) as described by (Kang et al. Citation2014). Here ascorbate (APX) activity was determined by measuring the oxidation of ascorbic acid substrate at 290 nm as suggested by Nakano and Asada (Citation1981) and expressed as mol ASA min−1. Superoxide dismutase (SOD) activity was measured by monitoring the photoreduction of nitroblue tetrazolium (NBT) at 560 nm, as advised by Beyer and Fridovich (Citation1987). One unit of SOD was defined as the amount of enzyme that caused a 50% decrease of the SOD-inhibited NBT reduction. Catalase (CAT) activity was determined as advised by Azevedo et al. (Citation1998). For that purpose, the initial rate of decrease in ascorbate concentration caused by the consumption of H2O2 was measured at 240 nm and expressed as mm H2O2 min−1. Peroxidase (POD) activity was based on the determination of guaiacol oxidation at 470 nm caused by H2O2 as suggested by Putter (Citation1974) and expressed as µmol oxidized guaicol. Polyphenoloxidase (PPO) activity was quantified by measuring the rate of increase in absorbance at 410 nm in the presence of 0.1 M catechol substrate as suggested by Halpin and Lee (Citation1987).
To measure changes in quantities of non-enzymatic antioxidants, plant leaf samples were homogenized in 5% trichloro acetic acid and clear supernatant was collected by centrifugation at 12000rpm for 15 min at 4°C. Ascorbate (ASC) and dehydro-ascorbate (DHA) were quantified using methodology of (Arrigoni et al. Citation1992). Reduced glutathione (GSH) and oxidized glutathione (GSSH) contents were estimated by method of (Chevone and Hess Citation1992).
2.7. H2O2 quantification
Hydrogen peroxide was extracted by snap freezing 100 mg of plant material in liquid nitrogen (Veljovicjovanovic et al. Citation2002) Plant material was taken from the top, middle and bottom part of tomato plants and homogenized in 1.5 mL of 1 M HClO4. Phenolic compounds were removed by using insoluble polyvinylpyrrolidone. This mixture was centrifuged at 12000 × g for 15 min at 4°C. H2O2 contents were quantified as described by Cheeseman (Citation2006). Briefly, 60 μL of this material was mixed with 600 μL of eFOX reagents (250 Μm ferrous ammonium sulfate, 100 μM sorbitol, 100 μM xylenol orange, and 1% ethanol in 25 mM H2SO4). OD was taken at 550 and 800 nm and the difference was recorded. H2O2 quantity was estimated using a standard H2O2 curve.
2.8. Analysis of metabolomic perturbations and phytohormones quantification by UPLC-ESI MS/MS
This analysis was performed to observe perturbations in important physiological processes and assessment of changes in quantities of some important liquid phytohormones. For that purpose, UPLC-ESI MS/MS analysis was performed to simultaneously quantify phytohormones and some other plant metabolites as described by (Yu et al. Citation2015).
To observe metabolic perturbations, leaf material was grinded to fine powder in liquid nitrogen. This powdered material was mixed in pure methanol following sonication for 5 min. The solution was passed through cellulose filters (0.2 µm pore size). Whereas, optimized sample preparation strategy was used for phytohormones quantification as described by (Yu et al. Citation2015). Afterwards 0.2 μL of prepared samples were injected into ‘UPLC/ESI-Qtof/ MS’ instrument separately. The chromatographic separation was performed on a Waters ACQUITY UPLC I-class system (Waters Corporation, Dublin, Ireland) fitted with Waters ACQUITY UPLC BEH C18 column. The composition of mobile phases and details of flow rate parameters can be seen in (Molina-Calle et al. Citation2017). The chromatography system was coupled with electrospray ionization (ESI) to a Waters Xevo Qtof-MS, operating in full scan mode. The parameters of ESI source and mass spectrometer were exactly followed as described by (Vieira et al. Citation2017). Cinnamic acid was used as an internal standard for phytohormones quantifications, as suggested by (Yu et al. Citation2015).
MzMine version 2.30 (mzmine.github.io) was used for both qualitative and quantitative analysis of UPLC-ESI MS/MS data regarding phytohormones and other metabolites. The alignment was carried out as a function of retention time, using a tolerance window of 0.2 min and 10 ppm mass accuracy (Molina-Calle et al. Citation2017). Metabolites were identified by matching mass spectra with mass spectral libraries (NIST and Wiley) and using online database MassBank (http://www.massbank.jp/). Metabolites were considered identified with a spectral match factor higher than 800.
2.9. Analysis of changes in ethylene production
Ethylene quantification was performed by gas chromatograph as described by (Yasin et al. Citation2018). Briefly, freshly removed leaf pieces were placed in 1 mL of water in falcon tube. The tube was immediately covered with a gas-proof septum and placed in dark at 30°C for 4 h. Afterwards, 1 mL gas was withdrawn using Hamilton gastight syringe and injected inside a gas chromatograph for ethylene quantification.
2.10. RNA extraction and qRT-PCR analysis
qRT-PCR analysis was performed to analyze changes in transcriptome levels of some selected genes involved in photosynthesis system, maintenance of redox homeostasis and stress related processes. Total RNA was isolated from leaves using the TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Quantitative RT–PCR (qRT-PCR) was performed using the SYBR green based qRT-PCR Kit (TaKaRa, Dalian, China). Details of primers are given in the supplementary (). The details of the qRT-PCR process can be seen in (Hu et al. Citation2016). The reactions were carried out in triplicate of each treatment. Actin gene was used as internal standard.
Table 1. Changes in growth parameters of tomato plants under influence of salinity and BMA12.
2.11. Statistical analysis
All the experiments were repeated twice with five technical replicates. One-way ANOVA was performed using the DSAASTAT software (Onofri, Italy). The significant differences between different treatments were determined through Duncan’s new multiple range test (DNMRT) test.
3. Results
3.1. Resistance to salt stress
Inoculating tomato plants with halotolerant BMA12 allowed for an evaluation of the ameliorative effect on plants against the salinity stress. The growth indices attained by the plants in either conditions are shown in . Plants cultivated with B. megaterium A12 grew to a significantly greater extent in comparison to the plants that were raised without bacterium (). Fresh and dry weights accumulation was significantly greater in bacterially treated plants under either condition (). Plant growth was inhibited in the absence of bacteria in salinized growth media (). It was seen that the plants cultivated with BMA12 and salinity stress accumulated fresh and dry weights to nearly similar as non-inoculated control plants raised in the absence of salinity stress ().
3.2. Photosynthetic pigment analysis
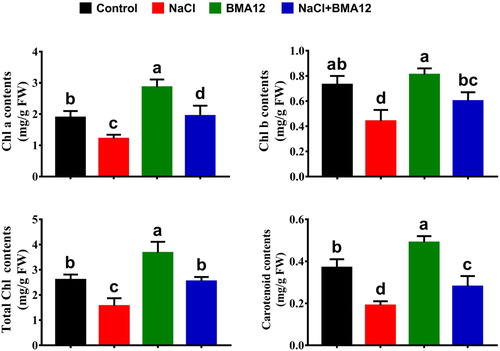
The chlorophyll (Chl a, Chl b) and carotenoid contents in leaves of tomato plants were increased with BMA12 treatment (). The leaf Chl a and Chl b contents were higher by 38.1 and 21.5% respectively in tomato plants inoculated with BMA12 under salinized conditions (). Changes in the carotenoid contents in salinized tomato plants showed a same trend and increased by 32.1% with BMA12 symbiosis compared to the control plants (). This beneficial bacterium also increased chlorophyll and carotenoids contents of tomato plants under non-salinized conditions compared to the control plants ().
Figure 1. Effect of Bacillus megaterium strain A12 (BMA12) and salinity stress on chlorophyll and carotenoids contents of tomato plants. Results provided here are mean values of two independent experiments. Vertical bard represents standard error. Small letters represent level of significance among different treatments as governed by ANOVA and DNMRT at = p ≥ 0.05.
3.3. Leaf gas exchange and photochemistry
The photosynthetic rate, transpiration rate, stomatal conductance and water use efficacy in tomato plants were greatly affected by BMA12, under both salinized and normal growth media (). Compared to the plants under salinity stress alone, these parameters were increased from 19% to 43% in tomato plants inoculated with BMA12 (). Salinity stress significantly affected the photosynthetic rate in tomato plants. Bacterial inoculated tomato plants showed significantly higher photosynthetic rate even after being salinized (). This bacterium demonstrated high recovery potential for photosynthetic rate as compared to transpiration rate and stomatal conductance and led to higher water use efficiency (photosynthetic rate /stomatal conductance) compared to the salinized control plants ().
Table 2. Change in the photosynthetic parameters of tomato plants under influence of salinity and BMA12.
When the effects on transpiration rate were evaluated, the salinity stress reduced the transpiration rate in tomato plants (). Inoculation of BMA12 increased the transpiration rate (not less than 21.7%) in salinized tomato plants in either combination (). The non-salinized tomato plants receiving BMA12 were less sensitive to this condition (). More pronounced alleviation in transpiration rate (43.4%) was seen where salinized tomato plants were co cultivated with BMA12 (). Similarly, the stomatal conductance increased up to 19.6% under salinity stress and 15.2% in non-salinized plants in the presence of BMA12 as compared to their respective control plants grown without BMA12 (). The symbiosis of BMA12 increased water use efficacy by 23.7% in tomato plants grown under salinized condition as compared to the plants raised under salinity stress alone ().
3.4. Changes in redox homeostasis
The treatment of BMA12 modulated the redox status under salinity stress. BMA12 treated tomato plants were differed in the pool of the antioxidant enzyme system during salinity stress (). These differences were more evident in SOD, APX and PPO activities. The activities were increased up to 2.5, 1.3 and 2.8 folds in leaves of tomato plants co-cultivated with BMA12 than with control plants under salinized conditions (). Salinity stress alone also changed the concentrations of these enzymes but the effects were more evident in the presence of BMA12 ().
Table 3. Changes in activities of antioxidant enzymes in leaves of tomato plants under influence of salinity and BMA12.
Similarly, the levels of non-enzymatic antioxidants were significantly changed in BMA12 treated tomato plants under stress conditions in a similar manner to that antioxidant enzymes (). The comparison of quantities of these non-enzymatic antioxidants showed varying trend. The total quantities of DHA (15.8%), GSH (49.5%) and GSSG (37.9%) were higher in the BMA12 treated plants under salinity stress conditions compared to the plants grown without BMA12 under salinity stress conditions (). However, the reduced ascorbate contents were higher in salinized tomato plants in the absence of BMA12 (). The composition of these of non-enzymatic antioxidants was slightly varied with salinity alone, whereas major increases were induced by BMA12 ().
Table 4. Changes in reduced and oxidized ascorbate and glutathione contents in the leaves of tomato plants under influence of salinity and BMA12.
3.5. Phytohormones profile
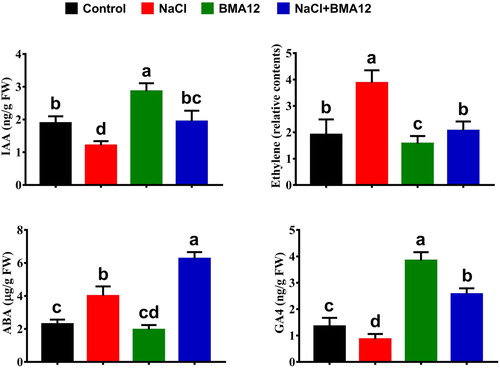
The concentrations of ABA, GA, SA and ethylene were significantly changed in tomato plants after salt and BMA12 treatment compared with the control condition (). The well-known stress response hormones ethylene and ABA increased significantly (2.1 and 1.7 folds) in tomato plants under salinity stress. The ethylene quantity was decreased (1.8 folds) after BMA12 treatment in comparison to the salinity control plants (). In contrast, the quantities of IAA (1.2 folds), ABA (1.6 folds), salicylic acid (1.2 folds) and GA4 (2.3 folds) were increased in tomato plants receiving BMA12 as compare to the plants grown under salinity stress alone ()
Figure 2. Effect of Bacillus megaterium strain A12 (BMA12) and salinity stress on phytohormones profile of tomato plants. Results provided here are mean values of two independent experiments. Vertical bard represents standard error. Small letters represent level of significance among different treatments as governed by ANOVA and DNMRT at = p ≥ 0.05.
3.6. Changes in expression levels of photosynthesis and stress related genes
The transcriptome levels of selected genes of the photosynthesis process (PsbA, PBGD, Chlase), redox regulation (Trxf, Trxm2, Trx m1/2) and stress related (SOS1, APX1, LERBOH1) genes in tomato plants were analyzed after 07 days of treatments application. Regarding photosynthesis related genes, the salinity stress significantly decreased expression levels of PsbA and PBGD genes as compared to the control plants (). When tomato plants treated with NaCl along with BMA12, the expression of both genes was up-regulated to a level significantly higher than the salinity control plants (). In the same way, NaCl increased expression level of Chlase gene (). However, BMA12 was unable to have significant effects on expression levels of this gene ().
Figure 3. Effect of Bacillus megaterium strain A12 (BMA12) and salinity stress on expression of some photosynthesis and redox homeostasis related genes of tomato plants. Gene expression was analyzed one week after treatments applications. Results provided here are mean values of two independent experiments. Vertical bard represents standard error. * represents level of significance among different treatments as governed by ANOVA. (* = p ≥ 0.05, ** = p ≥ 0.01).
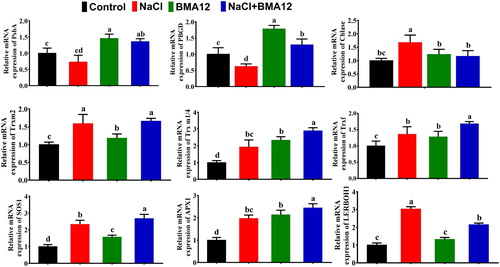
In the same way, symbiosis of BMA12 showed altered expression of redox regulation and stress related genes in tomato plants (). Interestingly, the exposure of salinity stress also increased Trxf, Trxm2 and Trm x1/2 genes expression levels but at varying extant. The presence of BMA12 in combination to salinity stress showed more pronounced expression of these genes (). When salinized tomato plants received BMA12, the expression of Trxf gene was scientifically increased as compared to the non-treated control plants (). Even in the absence of salinity, B. megaterium positively increased expression levels of Trxf gene (). Regarding Trxm2 gene, salinity exposure significantly increased its expression levels (). However, BMA12 increased expression levels of Trxm2 in combination to salinity stress but with non-significant differences as compared to the salinized control plants ().
BMA12 symbiosis positively influenced stress related genes (SOS1, APX1) of tomato plants both under salinized and non-salinized conditions. Here salinity stress alone was also effective enough to up-regulate expression levels of these genes at significant places (). Contrastingly, BMA12 decreased expression levels of LERBOH1 gene. It showed maximum expression levels under salinity stress alone (). Taken together, BMA12 mostly increased expression levels of selected genes governing photosynthesis process, stress management and redox regulation in tomato plants under stress conditions.
3.7. Change in soluble sugars, soluble proteins, free amino acid contents and related metabolites in tomato plants
Results showed pronounced effects of salinity and BMA12 applications on soluble sugars contents (SSC), free amino acids contents (FAAC), soluble protein contents (SPC) and some related metabolites (). Seven days after treatment, concentrations of SSC (39.0%), FAAC (18.7%), and SPC (37.8%) significantly increased in tomato plants exposed to BMA12 under salinity stress conditions as compared to salinized control plants (). The same increase was seen for proline that is considered an important osmolyte in plants. Importantly, under the exposure of salinity stress, SSC, FAAC and SPC were 24.6%, 44.8%, and 26.6% lower in comparison to the non-treated control plants respectively (). In the same way, onset of salinity stress decreased ß-carotene contents (39.3%) in tomato plants as compared to the non-treated control plants (). Whereas, BMA12 increased ß-carotene contents up to 21.6 and 07.4% in tomato plants under salinized and normal conditions as compared to the non-treated control plants respectively ().
Table 5. Changes in soluble sugar contents, soluble protein contents, free amino acid contents and some stress related metabolites in tomato plants under influence of salinity and BMA12.
3.8. Changes in photosynthesis related metabolism
UPLC-ESI MS/MS analysis detected changes in concentrations of several metabolites. We compared the metabolite’s peak area with internal standard to make comparative analysis among different treatments. We observed that the concentrations of several metabolites belonging to glycolysis process were different in the tomato plants with varying treatments (). We focused on perturbations in photosynthesis related metabolism in tomato plants under salinity and BMA12 (). Sugars such as mannose, xylose, fructose and glucose were more abundant in tomato plants under the influence of BMA12 under all conditions (). Salinity stress decreased the quantities of most of the sugars inside tomato plants. However, symbiosis of BMA12 significantly increased sugar production under all conditions ().
Figure 4. Changes in metabolomics of tomato plants induced by Effect of Bacillus megaterium strain A12 (BMA12) and salinity stress. Metabolites were quantified by UPLC/ESI MS/MS after one week after treatments applications. Metabolites were extracted from leaf samples of tomato plants. Results provided here are mean values of two independent experiments.
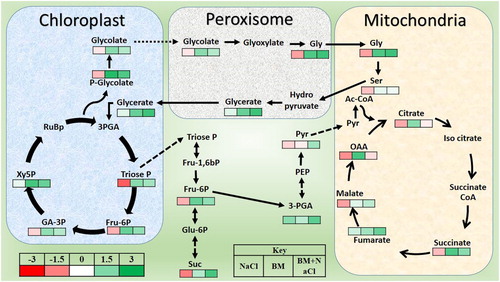
Similarly, the concentrations of most of the tricarboxylic acid (TCA) compounds were increased in tomato plants co-cultivated with BMA12 in comparison to the respective control plants (). However, some deviations were seen from this trend. For example, changes in citrate and oxaloacetate were opposite from the above-mentioned scenario. Apart of sugars and organic acids, some amino acids (glycine, threonine) were also increased by BMA12 under salinity and normal conditions ().
4. Discussions
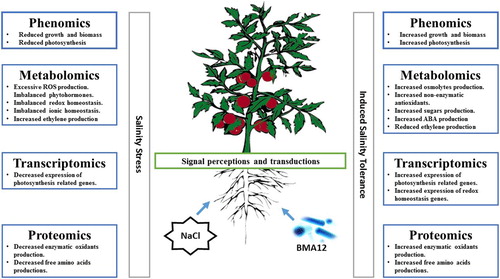
Abiotic stress not only effect the normal growth of plant and crop productivity but also the extent of recovery after the damage has taken place. High salinity is a major factor that significantly limits crop productivity. Plants exposure to salt stress conditions cause various morphological, physiological and biochemical changes (Yin et al. Citation2017). The establishment of plant-BMA12 interaction, showed multiple positive effects in tomato plants under salinized conditions. BMA12 was previously shown to induce salinity tolerance in tomato plants, as well as increasing plant growth under both stress and normal conditions (Aslam et al. Citation2018). In this study we elucidated the possible mechanisms behind stress tolerance of tomato plants mediated by BMA12 using molecular, physiological and biochemical techniques. The relevant role of BMA12 is proved in the acclimation of tomato plants grown under salinized conditions by different mechanisms including the maintenance of redox homeostasis and the restoration of photosynthetic capabilities (). The details of these ameliorative mechanisms are discussed below.
Figure 5. Possible mechanisms of induced salinity tolerance in tomato plants mediated by Bacillus megaterium strain A12 (BMA12).
4.1. Symbiosis of BMA12 can rescue damaged photosynthetic machinery of tomato plants under salinity stress by multiple mechanisms
Abiotic stress severely affect the photosynthesis processes leading to photoinhibition (Gururani et al. Citation2015). It is sensible to measure photosynthetic parameters to study the response of plants during stress conditions (Gururani et al. Citation2015). Abiotic stress can affect photosynthesis of plants by stomatal and non-stomatal limitation factors (Zhou et al. Citation2016). Salinity stress inhibited net photosynthetic rate. In the same way, transpiration rate, stomatal conductance and water use efficacy were adversely affected in tomato plants under salinized conditions. However, in the symbiosis of BMA12, the photosynthetic parameters were increased significantly. This may have been caused by the regulation of stomatal and non-stomatal limitation factors by BMA12.
Some physiological indices like chlorophyll contents are closely linked with the plant photosynthetic process (Foyer and Shigeru Citation2011) and considered to assess the plant’s tolerance to stress conditions (Orellana et al. Citation2010). The total chlorophyll contents decreased up to 40% in the leaves of tomato plants under salt stress as compared to the non-treated control plants. Whereas, this parameter decreased only up to 07% in the leaves of tomato plants co-cultivated with BMA12 in the same regards, showing that the total chlorophyll degradation rate in BMA12 treated plants was slower than the plants cultivated under salt stress alone. As chlorophyll contents imitate the extent of damage of photosynthetic machinery, these results showed that symbiosis of BMA12 can minimize the adverse effects of salt stress on photosynthetic machinery of tomato plants.
It has been proved that excessive salt accumulation lowers the transcription and translation of PsbA gene responsible for the biosynthesis of the D1 protein of PSII (Allakhverdiev et al. Citation2002). This protein plays a key role in repairing of damaged photosystem after stress induced photoinhibition (Krishna et al. Citation2013). Secondly, the stress mediated increased accumulation of ROS in plants impair the biosynthesis of D1 protein after induced photoinhibition (Nishiyama, et al. Citation2011; Yoshitaka and Norio Citation2014). RT–PCR analysis showed the higher expression levels of PsbA gene that encoded D1 protein, in tomato plants under influence of BMA12. Here possible mechanism behind increased transcriptome levels of D1 protein encoding gene can be the reduced accumulation of ROS in tomato plants co-cultivated with BMA12.
Chlorophyll biosynthesis involves porphobilinogen deaminase enzyme encoded by the PBGD gene (Roberts et al. Citation2012). This enzyme plays key role in the formation of tetrapyrrole molecules. Whereas, chlorophyllase (Chlase) encoded by Chlase, catalyzes the breakdown of chlorophyll thorough de-esterification process (Harpazsaad et al. Citation2007). We observed decreased expression of PBGD gene but increased expression of Chlase gene after onset of salinity in tomato plants. These results are consistent with chlorophyll quantifications as its quantity decreased significantly under saline conditions. Hence, the decrease of chlorophyll contents under salinity stress could be due to decline of PBGD activity or increased Chlase activity. Here symbiosis of BMA12 reversed the scenario by increasing expression levels of PBGD that may have led to the increased biosynthesis of chlorophyll in tomato plants.
4.2. BMA12 mediated decrease in ROS can help in restoration of photosynthetic activity
ROS can cause oxidation of different molecules and disturbance of normal cellular processes leading to cell death (Andrzej et al. Citation2010). Redox signals are the key regulators of plant photosynthesis, metabolism, growth and development (Foyer and Allen Citation2003). Secondly, redox regulation is of crucial importance for the biosynthesis of the photosynthetic apparatus and its efficacy (Kieselbach Citation2013). Therefore, the aerobic organisms have developed an enzymatic and non-enzymatic antioxidant systems against ROS. Salinity-induced alterations in the ion homeostasis can contribute to the malfunctioning of photosynthetic apparatus of tomato plants. Inactivation of enzymatic antioxidants like SOD and APX are considered as limitations of photosynthetic efficiency under different stress conditions in plants (Ishikawa and Shigeoka Citation2008) and thus potential targets for improvement (Foyer and Shigeru Citation2011).
It was seen that co-cultivation of BMA12 mostly increased the quantities of non-enzymatic antioxidants and activities of antioxidant system related enzymes in tomato plants. The increased activities of antioxidant system related enzymes may contribute to the recovery of the redox state under stress conditions. In the same way, increases in non-enzymatic antioxidants (DHA, GSH and GSSG) pools of tomato plants induced by BMA12 can help plants to better adopt stress conditions (Orellana et al. Citation2010). The redox state of cell is also involved in the regulation of photosynthetic electron flow (Andrzej et al. Citation2010) and activity of some of the photosynthesis related enzymes (Rochaix Citation2011). As evident from results, the treatment of BMA12 can assist tomato plants to retain the redox balance and it might be a contributing factor to restore the functionality of photosynthetic system.
The benefits of BMA12 can be further seen regarding its effect of expression of some redox regulation (Trxf, Trxm2) genes. Compared with the non-treated control plants, BMA12 significantly increased expression levels of both genes. Trxf gene displayed reduced expression under salinity. Presence of BMA12 in the rhizosphere of tomato plants significantly increased its expression levels. Regarding Trxm2 gene, salinity stress alone positively influenced its expression levels. The possible reason can be the oxidative signaling during salinity stress conditions that can act as an elicitor for this gene (Fernández-Trijueque et al. Citation2012). Here BMA12 resulted in further increase of expression levels of Trxm2 gene but with non-significant differences as compared to the salinized control plats.
Likewise, different regulation of SOS1, APX1 and LERBOH1 was observed in tomato plants in response to salinity stress and BMA12 symbiosis. The SOS pathway is of critical importance in regulating Na+/K+ homeostasis and salinity stress tolerance (Munns Citation2002). APX1 gene is involved in scavenging of ROS. BMA12 induced increased expression levels of both of these genes in tomato plants. This suggests that this beneficial bacterium enhanced the sensitivity of tomato plants towards salinity stress. The application of salinity alone also led to a significant upregulation of LERBOH1 gene involved in ROS biosynthesis which is consistent with the increased H2O2 quantities observed under salinity stress. This fact could also serve to explain the lowering of H2O2 contents in tomato plants receiving BMA12 that significantly decreased expression levels of this gene.
4.3. BMA12 induced changes in plant hormones can modulate photosynthesis and salinity stress response
The symbiosis of BMA12 was responsive to the phytohormones production in tomato plants grown under salinity conditions suggesting that BMA12 played a key role in hormonal signal transduction. We found that BMA12 showed maximum increase in ABA production in tomato plants under salinity stress that may have acted in ABA dependent signaling pathways in response to the abiotic stress conditions. In addition, salinity stress alone also increased ABA production in tomato plants but at lower extant. Ethylene affects plant stress tolerance and regulates senescence (Bleecker and Kende Citation2000). Increased ethylene level can induce senescence in plants (Grbić and Bleecker Citation1995, Morgan and Drew Citation1997). Ethylene increase in plants under abiotic stresses including salinity (Balota et al. Citation2004; Hays et al. Citation2007). Decrease in the levels of ethylene production has been correlated with stress tolerance in plants (Hays et al. Citation2007). As demonstrated by the results, ethylene levels were decreased significantly in tomato plants co-cultivated with BMA12. These findings also indicate that this balance between ethylene and abscisic acid might have regulated the response of tomato plants to mitigate salinity stress.
Similarly, cytokinins have been shown to slower the degradation of photosynthetic protein and increased expression of photosystem related genes under stress conditions (Hare et al. Citation1997; Rivero et al. Citation2010). The exogenous applications of cytokinins have been shown to increase abiotic stress tolerance in bent grass (Zhao et al. Citation2008; Merewitz et al. Citation2010). We observed increased levels of cytokinins in tomato plants induced by BMA12. These results indicate that BMA12 mediated changes in phytohormones production may play an important role in adaptation to salinity stress conditions. As phytohormones effect the expression of photosynthesis related genes and play the role in PSII damage repair mechanism (Bartoli et al. Citation2013; Anne et al. Citation2014), hence, it is quite imperative to consider these changes in the levels of hormones interlinked with improved photosynthesis efficacy of tomato plants under influence of BMA12.
4.4. BMA12 positively influences osmolites production and restores metabolomic perturbations in tomato plants
Findings of this studies have demonstrated that besides improving photosynthesis efficacy, BMA12 also played a positive role by restoring the perturbations in metabolomics of tomato plants induced by salinity stress. Under salinity stress plants accumulate compatible solutes that are known for their osmo-protection activity (Chellichaabouni et al. Citation2010). This is one of the common responses of plants to change in the external osmotic potential (Hasegawa et al. Citation2000). Proline act as a biochemical marker of salt stress level in plants (Shamshiri and Fattahi Citation2014). This acts as free radical scavenger, stabilize cytosolic pH for subcellular structures and balance cell redox process (Verbruggen and Hermans Citation2008). In this study, salinity induced an increase in the proline content in the leaves of tomato plants. The increase was significantly remarkable in bacterized tomato plants under salinized conditions. Likewise, symbiosis of BMA12 increased sugar production in tomato plants. Sugars help in storage and transportation of carbon inside plants body fixed through photosynthesis (Xu et al. Citation2013). Sugars also act as signaling molecules to regulate other physiological process in plants (Koch Citation2004). Some disaccharides accumulate under stress conditions and help to maintain the membrane integrity and cell hydration levels (Dracup et al. Citation1986; Koch Citation2004). Here increased production of some sugars was found in tomato plants under influence of BMA12. This further highlights the active photosynthetic supply of carbohydrates and increased carbon reserves that can help to rescue growth of tomato plants under salinity stress. Apart of sugars, some amino acids were clearly increased in tomato plants co-cultivated with BMA12. Opposite to symbiosis of BMA12, a predominate decrease in the amino acid concentrations was seen in tomato plants under salinity stress.
Globally, changes in the production of sugars and amino acids discriminated differential effects of salinity and BMA12. Their accumulation was reduced under salinity stress alone, whereas symbiosis of BMA12 increased biosynthesis of these photosynthesis intermediates. These metabolites are recognized as important players in the growth and development of plants and abiotic stress tolerance.
5. Conclusion
Our results showed that BMA12 symbiosis in tomato plants restored redox homeostatsis and restored photosynthesis system, consequently improving the growth of tomato plants against salinity stress. This study suggests that BMA12 could act as a source to ameliorate salinity stress and, possibly, offers a source that can be used in conventional agriculture system to make plants survive under salinity stress.
Supplemental Material
Download MS Word (14.1 KB)Acknowledgements
WA, GL, HA, AA carried out the experiments. WA, GL, AA, TA, NAY, TW and WL performed data analysis. WA, JG, TW drafted the manuscript. WA, TA designed the experiments. SA, TA, supervised the whole work.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Waheed Akram http://orcid.org/0000-0003-3811-6677
Sajid Rashid Ahmad http://orcid.org/0000-0003-0122-472X
Additional information
Funding
Notes on contributors
Waheed Akram
Dr Waheed Akram is working as a postdoctoral fellow at Vegetable Research Institute, Guangdong Academy of Agricultural Sciences, China. He is working in the field of plant stress physiology.
Hina Aslam
Miss. Hina Aslam is a PhD scholar in the College of Earth and Environmental Sciences, University of the Punjab, Pakistan.
Sajid Rashid Ahmad
Prof. Dr Sajid Rashid Ahmad is serving a director at College of Earth and Environmental Sciences, University of the Punjab, Pakistan.
Tehmina Anjum
Prof. Dr Tehmina Anjum is leading a research group working on changes in plant biochemistry under stress conditions in the Institute of Agricultural Sciences, University of the Punjab, Lahore Pakistan.
Nasim Ahmad Yasin
Dr Nasim Ahmad Yasin is leading researchers in the field of plant stress physiology.
Waheed Ullah Khan
Dr Waheed Ullah Khan is leading researchers in the field of plant stress physiology.
Aqeel Ahmad
Dr Aqeel Ahmad is also working as a postdoctoral fellow at Vegetable Research Institute, Guangdong Academy of Agricultural Sciences, China. His research work deals with the plant metabolomics and proteomics.
Juxian Guo
Miss. Juxian Guo is working as research associates at Vegetable Research Institute, Guangdong Academy of Agricultural Sciences, China.
Tingquan Wu
Prof. Dr Tingquan Wu is leading a research work in the field of plant pathology at Vegetable Research Institute, Guangdong Academy of Agricultural Sciences, China.
Wenlong Luo
Dr Wenlong Luo is working as research associates at Vegetable Research Institute, Guangdong Academy of Agricultural Sciences, China.
Guihua Li
Dr Guihua Li is an expert in the field of plant genomics. She is a group leader at Vegetable Research Institute, Guangdong Academy of Agricultural Sciences, China.
References
- Abd_Allah EF, Alqarawi AA, Hashem A, Radhakrishnan R, Al-Huqail AA, Al-Otibi FON, Malik JA, Alharbi RI, Egamberdieva D. 2018. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J Plant Interact. 13:37–44.
- Abdel-Ghani AH, Neumann K, Wabila C, Sharma R, Dhanagond S, Owais SJ, Börner A, Graner A, Kilian B. 2015. Diversity of germination and seedling traits in a spring barley (Hordeum vulgare L.) collection under drought simulated conditions. Genet Resour Crop Evol. 62:275–292.
- Adesemoye AO, Torbert HA, Kloepper JW. 2008. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can J Microbiol. 54:876–886.
- Agata DG, Iwona S. 2013. Open or close the gate – stomata action under the control of phytohormones in drought stress conditions. Front Plant Sci. 4:138–138.
- Ahmad P, Jaleel CA, Sharma S. 2010. Antioxidant defense system, lipid peroxidation, proline-metabolizing enzymes, and biochemical activities in two Morus alba genotypes subjected to NaCl stress. Russ J Plant Physiol. 57:509–517.
- Allakhverdiev SI, Yoshitaka N, Sachio M, Hiroshi Y, Noritoshi I, Yu K, Norio M. 2002. Salt stress inhibits the repair of photodamaged photosystem II by suppressing the transcription and translation of psbA genes in synechocystis. Plant Physiol. 130:1443–1453.
- Alqarawi AA, Allah EFA, Hashem A. 2014. Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. J Plant Interact. 9:802–810.
- Alqarawi AA, Hashem A, Abd Allah EF, Alshahrani TS, Huqail AA. 2014. Effect of salinity on moisture content, pigment system, and lipid composition in Ephedra alata Decne. Acta Biol Hung. 65:61–71.
- Andrzej K, Elzbieta KN, Ireneusz S, Zbigniew M. 2010. The key role of the redox status in regulation of metabolism in photosynthesizing organisms. Acta Biochim Pol. 57:143–151.
- Anne C, Silvia N, Marion K, Hamada A, Han A, Bernhard G, Michael R, Thomas S. 2014. A novel protective function for cytokinin in the light stress response is mediated by the Arabidopsis histidine kinase2 and Arabidopsis histidine kinase3 receptors. Plant Physiol. 164:1470–1483.
- Arrigoni O, De Gara L, Tommasi F, Liso R. 1992. Changes in the ascorbate system during seed development of Vicia faba L. Plant Physiol. 99:235–238.
- Ashraf M, Harris PJC. 2004. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 166:3–16.
- Aslam H, Ahmad SR, Anjum T, Akram W. 2018. Native halotolerant plant growth promoting bacterial strains can ameliorate salinity stress on tomato plants under field conditions. Int J Agric Biol. 20:315–322.
- Azevedo RA, Alas RM, Smith RJ, Lea PJ. 1998. Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiol Plant. 104:280–292.
- Balota M, Cristescu S, Payne WA, te Lintel Hekkert S, Laarhoven LJJ, Harren FJM. 2004. Ethylene production of two wheat cultivars exposed to desiccation, heat, and Paraquat-induced oxidation. Crop Sci. 44:812–818.
- Baniasadi F, Saffari VR, Moud AAM. 2015. Effect of putrescine and salinity on morphological and biochemical traits and pigment content of marigold plant (Calendula officinalis L.). J Sci Technol Greenhouse Cult. 6(21):125–133.
- Bartoli CG, Casalongué CA, Simontacchi M, Marquez-Garcia B, Foyer CH. 2013. Interactions between hormone and redox signalling pathways in the control of growth and cross tolerance to stress. Environ Exp Bot. 94:73–88.
- Berg G. 2009. Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol. 84:11–18.
- Berger B, de Regt B, Tester M. 2012. Trait dissection of salinity tolerance with plant phenomics. Methods Mol Biol. 913:399–413.
- Beyer WF, Fridovich I. 1987. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 161:559–566.
- Bhattacharyya PN, Jha DK. 2012. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 28:1327–1350.
- Bleecker AB, Kende H. 2000. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 16:1–18.
- Cheeseman J. M. 2006. Hydrogen peroxide concentrations in leaves under natural conditions. J Exp Bot. 57(10):2435–2444. http://dx.doi.org/10.1093/jxb/erl004.
- Chellichaabouni A, Ben Mosbah A, Maalej M, Gargouri K, Gargouribouzid R, Drira N. 2010. In vitro salinity tolerance of two pistachio rootstocks: Pistacia vera L. and P. atlantica Desf. Environ Exp Bot. 69:302–312.
- Chen LS, Qi YP, Jiang HX, Yang LT, Yang GH. 2010. Photosynthesis and photoprotective systems of plants in response to aluminum toxicity. Afr J Biotechnol. 9:9237–9247.
- Chevone BI, Hess JL. 1992. Seasonal Variation in the antioxidant system of eastern white pine needles: evidence for thermal dependence. Plant Physiol. 98:501–508.
- Cho ST, Chang HH, Egamberdieva D, Kamilova F, Lugtenberg B, Kuo CH. 2015. Genome analysis of Pseudomonas fluorescens PCL1751: a rhizobacterium that controls root diseases and alleviates salt stress for its plant host. Plos One. 10:e0140231.
- Dracup M, Gibbs J, Greenway H. 1986. Melibiose, A suitable, non-permeating osmoticum for suspension-cultured tobacco cells. J Exp Bot. 37:1079–1089.
- Fernández-Trijueque J, Barajas-López JDD, Chueca A, Cazalis R, Sahrawy M, Serrato AJ. 2012. Plastid thioredoxins f and m are related to the developing and salinity response of post-germinating seeds of Pisum sativum. Plant Sci. 188–189:82–88.
- Flowers TJ, Colmer TD. 2008. Salinity tolerance in halophytes *. New Phytol. 179:945–963.
- Foyer CH, Allen JF. 2003. Lessons from redox signaling in plants. Antioxid Redox Signaling. 5:3–5.
- Foyer CH, Shigeru S. 2011. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 155:93–100.
- Grbić V, Bleecker AB. 1995. Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J. 8:595–602.
- Gururani MA, Mohanta TK, Bae H. 2015. Current understanding of the interplay between phytohormones and photosynthesis under environmental stress. Int J Mol Sci. 16:19055–19085.
- Gururani MA, Venkatesh J, Tran LS. 2015. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol Plant. 8:1304–1320. Epub 2015/05/23.
- Halpin BE, Lee CY. 1987. Effect of blanching on enzyme activity and quality changes in green peas. J Food Sci. 52:1002–1005.
- Hare PD, Cress WA, van Staden J. 1997. The involvement of cytokinins in plant responses to environmental stress. Plant Growth Regul. 23:79–103.
- Harpazsaad S, Azoulay T, Arazi T, Benyaakov E, Mett A, Shiboleth YM, Hörtensteiner S, Gidoni D, Galon A, Goldschmidt EE. 2007. Chlorophyllase is a rate-Limiting enzyme in chlorophyll catabolism and is posttranslationally regulated. Plant Cell. 19:1007–1022.
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. 2000. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 51:463–499.
- Hashem A, Abd_Allah EF, Alqarawi AA, Al-Huqail AA, Wirth S, Egamberdieva D. 2016. The interaction between Arbuscular Mycorrhizal fungi and Endophytic bacteria enhances plant growth of Acacia gerrardiiunder salt stress. Front Microbiol. 7:1089.
- Hays DB, Do JH, Mason RE, Morgan G, Finlayson SA. 2007. Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Sci. 172:1113–1123.
- Hossain MA, Burritt DJ, Fujita M. 2015. Proline and glycine betaine modulate cadmium-induced oxidative stress tolerance in plants. Hoboken: John Wiley & Sons, Ltd.
- Hu L, Xiang L, Li S, Zou Z, Hu XH. 2016. Beneficial role of spermidine in chlorophyll metabolism and D1 protein content in tomato seedlings under salinity-alkalinity stress. Physiol Plant. 156:468–477.
- Ilangumaran G, Smith DL. 2017. Plant growth promoting rhizobacteria in Amelioration of salinity stress: a systems biology perspective. Front Plant Sci. 8:1768.
- Ishikawa T, Shigeoka S. 2008. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate Peroxidase in Photosynthesizing organisms. Biosci Biotech Biochem. 72:1143–1154.
- Jha Y, Subramanian RB. 2018. From interaction to gene induction: an eco-friendly mechanism of PGPR-mediated stress management in the plant.
- Jing LI, Cao Y, Ding J, Sun Y, Zheng Y, Guanmo HU, Shen Z, Rong LI, Shen Q. 2018. Development of bio-nursery substrates containing PGPR flora and evaluation of their seedlings growth promoting effect. Journal of Nanjing Agricultural University.
- Kang SM, Khan AL, Waqas M, You YH, Kim JH, Kim JG, Hamayun M, Lee IJ. 2014. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact. 9:673–682.
- Kaźmierczak A. 1998. Studies on morphology and metabolism of prothalli during GA 3 -induced formation of antheridia in Anemia phyllitidis. Acta Physiol Plant. 20:277–283.
- Khan N, Bano A, Rahman MA, Rathinasabapathi B, Babar MA. 2018. UPLC-HRMS based untargeted metabolic profiling reveals changes in Chickpea (Cicer arietinum) Metabolome following long-term drought stress. Plant Cell Environ. 42(1):115–132.
- Kieselbach T. 2013. Oxidative folding in chloroplasts. Antioxid Redox Signal. 19:72–82.
- Koch K. 2004. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol. 7:235–246.
- Krishna N, Anjana J, Roshan Sharma P, Rupak T, Shin PY, Eva-Mari A, Gil NH, Lee C-H. 2013. Towards a critical understanding of the photosystem II repair mechanism and its regulation during stress conditions. FEBS Lett. 587:3372–3381.
- Kruger NJ. 1988. The Bradford method for protein quantitation. Methods Mol Biol. 32:9–15.
- Lewis BD, Hirsch RE, Sussman MR, Spalding EP. 2001. Functions of AKT1 and AKT2 Potassium channels determined by studies of single and double mutants of Arabidopsis. Plant Physiol. 127:1012–1019.
- Liang C, Wang Y, Zhu Y, Tang J, Hu B, Liu L, Ou S, Wu H, Sun X, Chu J. 2014. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc Natl Acad Sci U S A. 111:10013–10018.
- Lotfi N, Vahdati K, Amiri R, Kholdebarin B, Mcneil DL. 2010. Drought-induced accumulation of sugars and proline in radicle and plumule of tolerant walnut varieties during germination phase. Acta Hortic. 861:289–296.
- Melis A. 1999. Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci. 4:130–135.
- Merewitz EB, Gianfagna T, Huang B. 2010. Effects of SAG12-ipt and HSP18.2-ipt expression on Cytokinin production, root growth, and leaf senescence in creeping bentgrass exposed to drought stress. J Am Soc Hortic Sci. 135:230–239.
- Molina-Calle M, de Medina VS, Priego-Capote F, de Castro MDL. 2017. Establishing compositional differences between fresh and black garlic by a metabolomics approach based on LC–QTOF MS/MS analysis. J Food Compos Anal. 62:155–163. http://dx.doi.org/10.1016/j.jfca.2017.05.004.
- Moore S, Stein WH. 1954. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 211:907–913.
- Morgan PW, Drew MC. 1997. Ethylene and plant responses to stress. Physiol Plant. 100:620–630.
- Munns R. 2002. Comparative physiology of salt and water stress. Plant Cell Environ. 25:239–250.
- Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. 2007. Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta. 1767:414–421.
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in Spinach chloroplasts. Plant Cell Physiol. 22:867–880.
- Nguyen D, Rieu I, Mariani C, Dam NM. 2016. How plants handle multiple stresses: hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol Biol. 91:727–740.
- Nishiyama Y, Allakhverdiev SI, Murata N. 2011. Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol Plant. 142:35–46.
- Oliver MJ, Guo L, Alexander DC, Ryals JA, Wone BWM, Cushman JC. 2011. A Sister Group contrast using Untargeted Global Metabolomic analysis Delineates the biochemical regulation Underlying Desiccation tolerance in Sporobolus stapfianus. Plant Cell. 23:1231–1248.
- Orellana S, Yañez M, Espinoza A, Verdugo I, González E, Ruiz-Lara S, Casaretto JA. 2010. The transcription factor SlAREB1 confers drought, salt stress tolerance and regulates biotic and abiotic stress-related genes in tomato. Plant Cell Environ. 33:2191–2208.
- Ozakca DU. 2013. Effect of abiotic stress on photosystem I-related gene transcription in photosynthetic organisms. Am J Respir Crit Care Med. 186:804–804.
- Pang CH, Wang BS. 2008. Oxidative stress and salt tolerance in plants. Berlin Heidelberg: Springer.
- Pieterse CM, Van d, Zamioudis C, Leonreyes A, Van Wees SC. 2012. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 28:489–521.
- Porcel R, Ruiz-Lozano JM. 2012. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron Sustain Dev. 32:181–200.
- Putter J. 1974. Front matter – methods of enzymatic analysis. 2nd ed., Vol. 2. Methods of Enzymatic Analysis.
- Rahneshan Z, Nasibi F, Moghadam AA. 2018. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J Plant Interact. 13:73–82.
- Rai VK. 2002. Role of amino acids in plant responses to stresses. Biol Plantarum. 45:481–487.
- Rivero RM, Jacinta G, Allen VD, Harkamal W, Eduardo B. 2010. Enhanced cytokinin synthesis in tobacco plants expressing PSARK::IPT prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiol. 51:1929–1941.
- Roberts A, Gill R, Hussey RJ, Mikolajek H, Erskine PT, Cooper JB, Wood SP, Chrystal EJ, Shoolingin-Jordan PM. 2012. Crystallization and preliminary X-ray characterization of the tetrapyrrole-biosynthetic enzyme porphobilinogen deaminase from Arabidopsis thaliana. Acta Crystallogr. 68:1491–1493.
- Rochaix JD. 2011. Regulation of photosynthetic electron transport. Biochim Biophys Acta. 1807:375–383.
- Ruizlozano JM, Porcel R, Azcón C, Aroca R. 2012. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. J Exp Bot. 63:4033–4044.
- Shamshiri MH, Fattahi M. 2014. Evaluation of two biochemical markers for salt stress in three Pistachio rootstocks inoculated with Arbuscular Mycorrhiza (Glomus mosseae). J Stress Physiol Biochem. 10:335–346.
- Sharp RE, Lenoble ME. 2002. ABA, ethylene and the control of shoot and root growth under water stress. J Exp Bot. 53:33–37.
- Tejera NA, Campos R, Sanjuan J, Lluch C. 2004. Nitrogenase and antioxidant enzyme activities in Phaseolus vulgaris nodules formed by Rhizobium tropici isogenic strains with varying tolerance to salt stress. J Plant Physiol. 161:329–338.
- Turakainen M, Hartikainen Hseppanen MM. 2004. Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J Agric Food Chem. 52:5378–5382.
- Vaishnav A, Varma A, Tuteja N, Choudhary DK. 2016. PGPR-mediated Amelioration of crops under salt stress. Singapore: Springer.
- Vardharajula S, Ali SZ, Grover M, Reddy G, Bandi V. 2011. Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Interact. 6:1–14.
- Veljovicjovanovic S, Noctor G, Christine H. 2002. Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiol Biochem. 40:501–507.
- Verbruggen N, Hermans C. 2008. Proline accumulation in plants: a review. Amino Acids. 35:753–759.
- Vieira GS, Marques ASF, Machado MTC, Silva VM, Hubinger MD. 2017. Determination of anthocyanins and non-anthocyanin polyphenols by ultra performance liquid chromatography/electrospray ionization mass spectrometry (UPLC/ESI-MS) in jussara (Euterpe edulis) extracts. J Food Sci Technol. 54:2135–2144.
- Vleesschauwer DD, Xu J, Höfte M. 2014. Making sense of hormone-mediated defense networking: from rice to Arabidopsis. Front Plant Sci. 5:611.
- Xu Y, Du H, Huang B. 2013. Identification of metabolites associated with superior heat tolerance in thermal Bentgrass through metabolic profiling. Crop Sci. 53:1626–1635.
- Yasin NA, Akram W, Khan WU, Ahmad SR, Ahmad A, Ali A. 2018. Halotolerant plant-growth promoting rhizobacteria modulate gene expression and osmolyte production to improve salinity tolerance and growth in Capsicum annum L. Environ Sci Pollut Res. 25(23):1–15.
- Yin W, Hu Z, Hu J, Zhu Z, Yu X, Cui B, Chen G. 2017. Tomato (Solanum lycopersicum) MADS-box transcription factor SlMBP8 regulates drought, salt tolerance and stress-related genes. Plant Growth Regul. 83:55–68.
- Yoshitaka N, Norio M. 2014. Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Appl Microbiol Biotechnol. 98:8777–8796.
- Yu X, Song X, Jing Q, Zang Y, Li Y, Yong L, Liu C. 2015. An ultrahigh-performance liquid chromatography method with electrospray ionization tandem mass spectrometry for simultaneous quantification of five phytohormones in medicinal plant Glycyrrhiza uralensis under abscisic acid stress. J Nat Med. 69:278–286.
- Zhao WY, Xu S, Li JL, Cui LJ, Chen YN, Wang JZ. 2008. Effects of foliar application of nitrogen on the photosynthetic performance and growth of two fescue cultivars under heat stress. Biol Plantarum. 52:113–116.
- Zhou X, Zhao H, Cao K, Hu L, Du T, Baluška F, Zou Z. 2016. Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front Plant Sci. 30(7):1823.
