ABSTRACT
Two stink bug-resistant soybean cultivars, Dowling and IAC 100, and one sucepible, Silvânia were selected to evaluate their influence on Euschistus heros development. Evaluation of the biology of E. heros was conducted on nymph development, adult longevity, female fecundity and egg fertility in seeds of each cultivar. The results showed that E. heros nymphs reared on cv Dowling did not complete their biological cycle, but it was completed on cvs Silvânia and IAC 100. The total amount of six isoflavonoids identified and one flavonol was higher in cv Silvânia compared to the levels quantified in cv Dowling. The results showed that cvs Dowling and IAC 100 have constitutive resistance affecting E. heros development and that the major falvonoids quantified in these cultivars are not involved in soybean antibiotic effect observed in this study.
Introduction
Plants have developed a number of direct defences that can act negatively on herbivores. They present morphological barriers to avoid herbivory and/or oviposition, such as spines and trichomes, or chemical barriers, the secondary metabolites such as alkaloids, terpenoids, steroids and isoflavonoids (Arimura et al. Citation2005; Chen Citation2008; Mitchell et al. Citation2016). The plant chemical defences play an important role not only in the initial choice of the herbivore, but also in determining the amount of food that the insect will consume (Srivastava et al. Citation1999; War et al. Citation2012).
Flavonoids are phenolic compounds derived from the shikimic acid pathway via the phenylpropanoid route that play an important role in the mechanism of antibiosis against insects (Treutter Citation2006; Chen Citation2008; O’Neil et al. Citation2010). However, insect co-evolving with toxic plants learn to deal with them using diferent approaches, detoxifying and expelling them in frass or sequestering them in their body cuticle as a defence against predators or into their wings to attract mates (Treutter Citation2006; Fürstenberg-Hägg et al. Citation2013). In addition, there are specific phytochemicals that in small amounts can act as phagostimulants in insect-plant interactions, and the same compounds in higher amounts are toxic (Simmonds Citation2001, Citation2003; Chacón-Fuentes et al. Citation2015).
Studies have reported that resistance of soybean to stink bugs and caterpillars is related to the concentration of flavonoids in seeds and leaves (Hoffmann-Campo et al. Citation2001; Salvador et al. Citation2010; Bansal et al. Citation2013). The compounds daidzin and genistin, rutin and genistein, were reported to be involved in soybean seed resistance against Nezara viridula L. (Hemiptera: Pentatomidae) and Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae), respectivelly (Piubelli, Hoffmann-Campo, Arruda, Franchini, et al. Citation2003; Piubelli et al. Citation2005). The induction of two isoflavonoids, daidzein and daidzin, after Spodoptera litura F. (Lepidoptera: Noctuidae) herbivory has also been reported (Murakami et al. Citation2014). Recently, Graça et al. (Citation2016) described that cis-jasmone, a plant phytohormone, increased the levels of flavonoids on three different soybean cultivars, including cvs IAC 100 and Dowling. The resistance of IAC 100 to stink bugs (Carrão-Panizzi and Kitamura Citation1995) and lepidopterans (Piubelli et al. Citation2005; McPherson and Buss Citation2007) has been proposed to be an antibiotic effect. For cv Dowling, an antixenotic and antibiotic effect against aphids, which stop feeding and die after 48 h, was suggested (Li et al. Citation2004, Citation2008). In contrast, the cv Silvânia has high susceptibility to stink bugs herbivory in field conditions, but no studies were conducted to determine what makes it susceptible when compared to other cultivars (Laumann et al. Citation2008).
The Neotropical brown stink bug, Euschistus heros F. (Hemiptera: Pentatomidae), together with other stink bugs of the family Pentatomidae, is considered a major pest of soybean, causing serious economic losses to Brazilian farmers (Borges et al. Citation2011; Borges and Blassioli-Moraes Citation2017). Despite its economic importance and the information about sources of resistance in soybean to stink bugs, there is little knowledge about the mechanism of defence involved and about the effect of resistant cultivars on the development of this stink bug species, which is an important pest on grain crops in South America.
Among the three categories of resistance, antibiosis is the mechanism with advantageous characteristics for investigation of the development of new cultivars compared to antixenotic and tolerance. Antixenosis can only be detected if there is a choice by herbivores between at least two cultivars and tolerance cannot be manifested when the population of herbivores reaches high levels. Moreover, antibiosis directly affects the development and survival of the herbivore, that can be characterized by mortality in immature stages, extended development and oviposition periods, weight reduction and decrease in female fecundity and fertility (Vendramin and Guzzo Citation2009; Mitchell et al. Citation2016). The mechanism of antibiosis in plant resistance to herbivores has been widely observed in different crops and has been an important characteristic for integrated pest management.
Antibiosis combined to the reduction of preference for one host may affect the dynamics of populations and community structure of herbivores and natural enemies (Agrawal Citation2005). In this study, the hypothesis tested was that the level of direct defence in resistant soybean cultivars (Dowling and IAC 100) is different from that of susceptible cultivar (Silvânia) and that antibiosis and/or antixenosis are the mechanisms of resistance involved. Thus, the aims of this study were to evaluate: (i) the effect of these cvs on E. heros nymphal development, female fecundity, egg fertility and adult survival and (ii) the isoflavonoid levels in soybean seeds, correlating these levels with the biological parameters of E. heros measured in this investigation.
Material and methods
Plant and insect rearing
Euschistus heros individuals were obtained from a laboratory colony started from adults collected in soybean fields near Embrapa Genetic Resources and Biotechnology, Brasília, Brazil (15°47′S, 47°55′W). Stink bugs were reared in 8 L plastic containers on a diet of soybean seeds (cv Conquista), sunflower seeds (Helianthus annuus), raw peanuts (Arachis hypogaea), fresh green beans (Phaseolus vulgaris) and water. The food supply was renewed twice a week. To provide an oviposition substrate and shelter for the bugs, a 15 cm2 piece of nylon mesh screen was placed inside the cages. They were kept in a controlled-environment room at L14:D10 photoperiod, 26 ± 0.3°C and 70 ± 10% r.h.
Soybean (cultivars Dowling, IAC 100 and Silvânia) seeds were obtained from the Embrapa Cerrados Research Center (Brasília, DF, Brazil). For biological development experiments and chemical analysis of isoflavonoids, soybean seeds were planted in a small 2.5 × 3.0 m plot. The plot consisted of three rows of plants spaced 0.4 m between rows and with a density of eight plants per linear meter. Two herbicide applications were made (Dual Gold, 1.5 l/ha), soil was limed (lime and gypsum) and fertilizer was applied with NPK (4:14:20), superphosphate and micronutrients. Planting was performed with topdressing lines using NPK (2:10:10). The pods in stage R6, completely full, but not ripe, were harvested manually on the day that they were used. Approximately 1 h elapsed from harvest to being used in the experiments where the seeds were offered to the insects and used for isoflavonoid extractions.
Biology of Euschistus heros in different soybean cultivars
Egg masses of stink bugs were obtained from a colony maintained at Semiochemicals laboratory of Embrapa Genetic Resources and Biotechnology. The pods of the three cultivars (Dowling, IAC 100 and Silvânia) were used as a food source for stink bugs.
When nymphs reached the second instar, they were transferred to plastic pots (9 cm diameter × 15 cm high) containing three pods of one cultivar and a cotton swab moistened with water. The harvested pods were used to facilitate the insect observation, and to avoid bias due degradation of chemicals or contamination by fungi and other microorganisms the pods were replaced every two days. To replace the pods, the pots were open and three new pods were offered to the nymphs. The nymphs move to the new pods without any external interference, and when all nymphs changed to the new pods, the olds ones were removed from the pots. In all replicates the movement of the nymphs from the olds soybean pods to the new ones took around 2–3 h. Six pots were used per cultivar, each with 40 nymphs, and the experiment was repetead three times. The duration of each nymphal instar was recorded when at least 50% of individuals had moved to the next stage.
After their imaginal moult and cuticular hardening, the adults were sexed based on external morphology of the genitalia (Borges et al. Citation2006) and weighed using an analytical balance (Shimadzu AUW220D, Japan). One male and one female were placed inside a plastic cage containing a 5 cm2 piece of nylon mesh screen for the oviposition substrate and fed on the pods of the tested cultivars. Eggs were collected daily, glued on pieces of cardboard (1 cm2) with gum arabic and kept in Petri dishes until hatching. The pods were replaced for new ones every two days.
The insects and eggs were kept in a controlled-environment room at L14:D10 photoperiod, 26 ± 0.3°C and 70 ± 10% r.h. and were observed daily to determine the influence of the soybean seed cultivar on the biology development of adults and nymphs. For this proporse, the following parameters were measured: (i) the survival of nymphs and adults, (ii) adult longevity, (iii) adult weight, (iv) the pre-oviposition and (v) oviposition period, (vi) number of eggs per female (fecundity), (vii) the viability of eggs (fertility) and (viii) number of nymphs/female.
Extraction of flavonoids from soybean seeds
To evaluate the influence of herbivory and the cultivars on the isoflavonoid levels in soybean seeds, pods at the R6 reproductive stage were subjected to herbivory damage by E. heros females or remained undamaged. A soybean twig with pods was placed in a cage with five E. heros virgin females starved for 24 h (herbivory treatment) or without insects (control). At 0–24, 0–48 and 0–72 h after herbivory damage, the pods were harvested and three seeds removed for extraction. The cultivars that had lower (Silvânia) and higher (Dowling) negative effects on the stink bug development were used for this experiment and six replicates were conducted of each treatment/cultivar.
Soybean seeds from both treatments had their isoflavonoid quantities evaluated using a protocol modified from Kim et al. (Citation2005). Soybean seeds were macerated using liquid nitrogen in a porcelain mortar and pestle to obtain a fine flour. An aliquot of 2 g was extracted with 10 mL of methanol and 2 mL of H2O with 0.1 M HCl for 2 h with stirring. Then, the extract was filtered with a vacuum (80 g/m2 weight, 205 μm thick, 14 μm average pores, Qualy J Lab Pro) to remove bigger solid residues. Next, the extract was filtrered using a syringe filter with a hydrophilic PTFE membrane (25 mm diameter × 0.45 μm pores Millex Millipore) before analysis with high-performance liquid chromatography (HPLC) and high-performance liquid chromatography coupled to mass spectrometry (LC-MS).
Flavonoids analysis
An aliquot of 10 μL of each sample was injected and analyzed by HPLC (Flexar, Perkin Elmer) equipped with a quaternary pump and photodiode array detector. The HPLC analyses were conducted using a reverse phase C18 analytical column (4.6 mm diameter × 150 mm length and 3 μm film). Analyses were conducted using a gradient composed of two solvents: (A) acetonitrile and (B) water with 2% acetic acid. The initial gradient consisted of 90% solvent B and 10% A for 10 min, changed to 65% solvent B and 35% A for 55 min, and returned to the initial conditions for 10 min. The solvent flow rate was 0.65 ml/min, the oven temperature was maintained at 24°C and the total run time was 75 min. Six replicates for each sample were conducted. The isoflavonoids were detected at 260 nm wavelength. The quantification of flavonoids was obtained through calibration curves using synthetic standards. The calibration curves were constructed through injection of the standards at different concentrations of 1.5, 3, 15, 30, 60 and 90 µg/mL (daidzin, daidzein, genistin, genistein, glycitin, glycitein and rutin) using the same conditions for soybean sample analysis (Figure S1). All standards were prepared using methanol.
To confirm the identity of the flavonoids identified using LC-UV the same samples were analyzed by liquid chromatography (Agilent Infinity 1290 Infinity Series II) couple to a mass spectrometry detector with a triple quadrupole analyzer and equipped with a Jet Stream electrospray ionization source (Agilent 6470 Series Triple Quadrupole LC/MS), in positive ionization mode. Soybean samples and standard solutions were injected using a C18 reverse phase column (Agilent Zorbax Eclipse Plus C18 2.1 mm × 100 mm, 1.8 µm particle size), using as eluent: (A) water with 0.1% acetic acid and (B) acetonitrile at a 0.3 mL/min flow rate. The injection volume was 1 µL. The initial gradient consisted of 90% solvent A and 10% B for 0.5 min, changed to 50% solvent A for 6 min, then changed to 90% A and 10% B for 8 min, and returned to the initial conditions for 9 min. The mass spectrometer was operated in positive multiple reaction monitoring (MRM) mode using two specific transitions for each flavonoids under the following conditions: nitrogen sheath gas flow of 10 L/min at temperature of 300°C, flow rate of the nitrogen drying gas of 10 L/min at temperature of 300°C, nebulizer at 20 psi, capillary at 4.0 kV and nozzle voltage equal to 500 V. The identification of isoflavonoids was confirmed by the mass spectra of the fragment ions compared to those of the authentic standards in their respective retention times.
Chemicals
Standards of isoflavones daidzein (95%), genistein (98%), glycitein (98%), genistin (98%), daidzin (98%), glycitin (98%) and the flavonol rutin (98%) were purchased from Cayman Chemical Company (MI, USA). Acetonitrile and methanol HPLC grade, 2-isopropanol and acetic acid (99%) were purchased from Sigma Aldrich (St Louis, USA).
Statistical analysis
Data from biology development were tested for normality (Lilliefors) and homogeneity of variance (Bartlett), after which they were transformed into log(x + 1) or square root(x + 1) to meet the assumptions of ANOVA. Unifactorial analysis of variance (ANOVA) and comparison of means by Tukey’s test (significance level 5%) were used to compare the eitgh parameters measured, i.e. (i) the survival of nymphs and adults (ii) adult longevity, (iii) adult weight, (iv) the pre-oviposition and (v) oviposition period, (vi) the number of eggs per female (fecundity), (vii) the viability of eggs (fertility) and (viii) number of nymphs/female.
Data from chemical analysis were tested for normality (Lilliefors) and homogeneity of variance (Bartlett), after which they were transformed into log(x + 1) or square root(x + 1) to meet the assumptions of ANOVA. The mean total amount of isoflavonoids and the amount of each individual compound extracted from the pods of cultivars Dowling and Silvânia were subjected to multifactorial ANOVA 2 × 2 × 3 (2 cultivars (Dowling and Silvania) × 2 treatments (undamaged and herbivory damage) × 3 time points (0–24, 0–48 and 0–72 h)), with a completely randomized design. The means values obtained were analyzed by Tukey’s test at 5% significance. Analyses of biology study and total amount of flavonoids were performed using SAS 9.0 (SAS Institute Citation2001).
For the evaluation of survivorship, the Kaplan-Meier method was used that estimates the median survivorship time (MST) which corresponds to the time when 50% of the initial population is alive and compares the survivorship curves by the Log-Rank test.
A principal component analysis (PCA) was applied to the flavonoids data to evaluate their influence in separating treatments and cultivars. The PCA was performed using a correlation matrix and analysis was carried out using Paleontological Statistic Software (PAST version 2.17).
Results
Biology of Euschistus heros on different soybean cultivars
The development of E. heros nymphs was affected by soybean cultivars (). The duration of each instar nymph (2nd instar: F = 14.65, P < 0.001; 3rd instar: F = 18:36, P < 0.001; 4th instar: F = 5.30, P = 0.006; and 5th instar: F = 478.72, P < 0.001), the complete nymphal stage (F = 478.72, P < 0.001) and the total period from egg to adult (F = 16.14, P < 0.001) were significantly different among cultivars. The duration of nymphal stages was significantly higher when the stink bugs were fed on cvs Dowling and IAC100 than on cv Silvânia (). The estimated duration of the nymphal stage did not surpass 19 days with cv Dowling owing to the mortality of all the nymphs before reaching the fifth instar ( and ). There was a significant difference between the weight of females (F = 19.78, P < 0.001) and males (F = 6.13, P = 0.013) reared on cvs IAC 100 and Silvânia. The weight of females fed on cv IAC 100 was lower (47.3 ± 0.9 mg) than that for females fed on cv Silvânia (52.9 ± 0.7 mg). The same result was observed for males (IAC 100: 45.2 ± 0.8 mg and Silvânia: 49.5 ± 0.8 mg).
Figure 1. Survival curves of Euschistus heros (nymphs + adults) over a 32 days supply with pods of soybean cultivars Dowling, IAC 100 and Silvânia (Log-Rank test, P < 0.01).
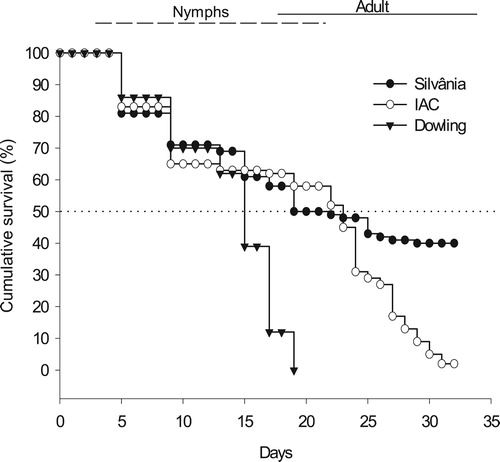
Table 1. Time in days (mean ± SEM) for the development of nymphal stages (2nd to 5th instar) and total immature development (egg to adult), of Euschistus heros fed on pods of soybean cultivars Dowling, IAC100 and Silvânia (27 ± 1°C, 65 ± 10% RH and photophase of 14 h).
The use of cvs IAC 100 and Silvânia as food for the nymphs of E. heros did not affect the duration of the pre-oviposition period (F = 2.78, P = 0.112), egg incubation (F = 2.84, P = 0.106) and even egg viability (F = 1.55, P = 0.221) (). However, there were significant differences in the oviposition period (F = 26.40, P < 0.001), number of eggs per female (F = 12.06, P < 0.001) and nymphs per female (F = 8.72, P = 0.006). All these features were lower for females fed on cv IAC 100 than those reared with pods of cv Silvânia. In addition, the number of females that laid eggs was different between cvs IAC 100 and Silvânia (F = 15.54, P < 0.001); a higher number of females reared on cv Silvânia, 50% of individuals, laid eggs compared with only 10.6% females that were reared on IAC 100 pods ().
Table 2. Biological characteristics (mean ± SEM) of Euschistus heros female from nymphs fed on the soybean pods, IAC 100 and Silvânia (27 ± 1°C, 65 ± 10% RH and photophase of 14 h).
The median survival time (MST) estimated values from Kaplan-Meier survival analysis were 15, 19 and 23 days for cvs Dowling, IAC 100 and Silvânia, respectively (). From overlapping confidence intervals for this parameter, the cv Dowling differed from other cvs and was less favorable to the stink bug (). However, considering the entire period of the experiment (32 days), all three soybean cvs differed in relation to survival curves of E. heros (Log-Rank test, χ2 = 255.66, P < 0.001) ( and ).
Table 3. Survival time (mean, median and interval (minimal and maximal)), in days, of Euschistus heros over 32 days old supply with pods of soybean cultivars Dowling, IAC 100 and Silvânia. P values were associated with the comparison of the survival curves by Log-Rank test.
Comparing the curves, it was observed that E. heros survived significantly longer on cv Silvânia than on cvs Dowling and IAC 100 (Log-Rank test, P < 0.01). Clear differences between the survival curves were observed after the 15th day of the experiment, with a rapid decline in the survival of insects fed on pods of cv Dowling that culminated in the mortality of all individuals on the 19th day. In this period, the cvs IAC 100 and Silvânia had statistically similar survival curves (Log-Rank test, P = 0.06). The distinction between these two cvs occurred only after the 23rd day, when insects showed a higher survival on pods of cv Silvânia ().
Flavonoids in seeds of soybean cultivars
The presence of six isoflavonoids, three aglycones (genistein, daizein and glycitein) and three glycosidic forms (daidzin, genistin and glycitin), was identified in soybean seeds of the two cvs evaluated in this study. For the fisrt time, the flavonol rutin was identified in soybean seeds. The identification of the isoflavonoids by HPLC with a diode array detector was further confirmed by LC-MS/MS analysis using a multiple reaction monitoring (MRM) approach, monitoring three specific transitions for each isoflavonoid () and confirmed comparing the mass spectra with authentic standards and the retention time of each peak was also confimed with samples spiked the standards (Figure S2).
Table 4. Product ions obtained from [M-H+] ions in MS3 spectra showing the retention time, protonated molecular ion [M-H+] (Q1), and characteristics fragment ions (Q3).
The total amount of flavonoids in soybean seeds was evaluated during the time after the start of herbivore feeding (0–24, 0–48 and 0–72 h). This time was evaluated because it was the time that the insects were allowed to feed on soybean pods. The statistical analysis of the mean total amount of flavonoids showed a significant interaction between cultivar, treatment and time (df = 2, 60; F = 7.67; P = 0.001). The statistical analysis showed that cv Silvânia presented higher level of flavonoids than cv Dowling at 0–48 and 0–72 h time-points ( and Table S1). For cv Dowling no difference was observed in the total amount of flavonoids over the time evaluated (0–24, 0–48 and 0–72 h) comparing undamaged soybean seeds with seeds exposed to herbivore ( and Table S1). Whereas cv Silvania presented enhanced production of flavonoids in seeds exposed to herbivore than undamaged soybean seeds ( and Table S1).
Figure 2. Mean total amount of isoflavonoids (μg/g) (mean ± SEM) extracted from undamaged soybean pods (control) and pods with Euschistus heros herbivory damaged. Treatments: Dow: cultivar Dowling and Sil: cultivar Silvânia. Means followed by the same letter within each cultivar (lower case) and between cultivars (capital letter) did not differ significantly by Tukey’s test (P > 0.05).
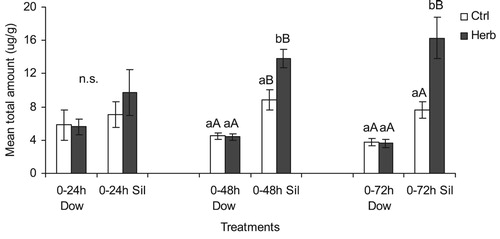
When evaluating individual compounds, ANOVA analysis showed a significant interaction between cultivar, treatment and time to daidzin (df = 2, 60; F = 10.04; P < 0.0001), glycitin (df = 2,60; F = 3.23; P = 0.046) (0–48 and 0–72 h), daidzein (df = 2,60; F = 3.37; P = 0.04), genistein (df = 2,60; F = 3.21; P = 0.047) (0–72 h) and rutin (df = 2, 60; F = 3.31; P = 0.043). cv Silvania presented higher levels of daidzin (0–48 and 0–72) and glycitin (0–72 h) in soybean seeds exposed to E. heros herbivory than undamaged seeds (Figure S3 and Table S2). Undamaged seeds of cv Dowling presented higher level of rutin than soybean seeds exposed to E. heros herbivory at 0–48 h time point (Figure S3 and Table S2). The statiscal analysis did not indicate significant interaction between cultivars, treatment and time to glycitein (df = 2, 60; F = 0.42, P = 0.658) and genistin (df = 2,60; F = 1.26; P = 0.292) (Figure S3 and Table S2).
The PCA analysis separated the soybean cultivars evaluated, Dowling and Silvânia, by the amount of flavonoids extracted from the seeds at the three time points (0–24, 0–48 and 0–72 h). The first two components of the PCA explained 61.58% of the data variability at 0–24 h, 63.3% at 0–48 h and 84.65% at 0–72 h. The main compounds responsible for separation of the cultivars at 0–24 h were glycitin, genistin, daidzin and glycitein which are related to the cv Silvânia, considered the first component ( and ). At 0–48 and 0–72 h, the compounds daidzin, genistin, glycitin, daidzein and rutin were related to cv Silvânia (). PCA analysis separated the treatments Herb and Ctrl of cv Silvânia at 0–48 and 0–72 h time points ().
Figure 3. Principal Component Analysis (PCA) ordination for components 1 and 2 of isoflavonoids compounds emitted by undamaged and herbivore-damage soybean plants from cultivars Dowling and Silvânia.
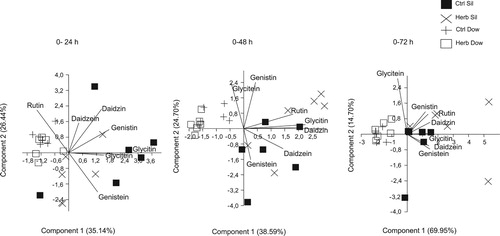
Table 5. Coefficients of correlation with the first two components of the Pricipal Component Analysis (PCA) (Loadings).
Discussion
The results showed that the cvs Dowling and IAC 100 have constitutive resistance against Euschistus heros, and in the former cv, nymphs had 100% mortality. In cv IAC 100 nymphs can develop but their performance was negatively affected compared with cv Silvânia (susceptible). The antibiosis observed may be associated with phenolic compounds which in general, play an important role in plant defence against herbivores and pathogens (Dixon and Steele Citation1999). Some of them, such as isoflavonoids, depending on the concentration, may be attractants, deterrents, repellents, or toxic to insects (Hoffmann-Campo et al. Citation2001; Simmonds Citation2001; Chacón-Fuentes et al. Citation2015). Recently, it was shown that soybean seeds of cv Dowling treated with cis-jasmone had their direct defence activated, influencing the weight of E. heros adults, and the authors related this result to the higher production of flavonoids (Graça et al. Citation2016). The results obtained here, similar to the results reported by Graca et al (Citation2016), showed that E. heros adults and nymphs feed with Dowling seeds (0–48 h) had their development negatively affected. Although this significant effect on stink bug development was observed, it was not possible to identified if phytochemicals are involved in the E. heros nymphs death. The chemical analysis of Dowling seeds, over the time points evaluated did not show significant influence of E. heros herbivory on the isoflavonoids and flavonol levels (daidzein, glycitein, and genistein, with their respective glycosidic forms, and rutin). In addition, the total amount of flavonoids was higher in cv Silvânia than in cv Dowling, indicating that these flavonoids could not be directly related to the resistance observed in Dowling seeds.
The direct defence of cv Dowling against E. heros may be related to other phytochemicals, such as the malonyl derivatives (Graça et al. Citation2016), or amino acids found in soybeans that could be responsible for resistance of the plants, as observed by Chiozza et al. (Citation2010), who showed that the amino acids in soybean leaves affected the development of the aphid Aphis glycines Matsumura (Hemiptera: Aphididae). In addition, the higher flavonoids level in cv Silvânia compared to cv Dowling did not affect the development of E. heros, and this breakdown plant-resistance can be related with the insect gut microbiota, that can degraded or deactivate the soybean chemical defence, as observed to N. viridula (Medina et al. Citation2018). Isoflavonoids (genistin, genistein, daidzein) also did not affect development or preference of other insect species, as observed for Lymantria dispar L. (Lepidoptera: Erebidae) (Karowe and Radi Citation2011) and Chilesia rudis (Lepidoptera: Arctiidae) Butler (Chacón-Fuentes et al. Citation2015).
The previously reported resistance of cv IAC 100 and susceptibility of cv Silvânia (Piubelli, Hoffmann-Campo, Arruda, Lara Citation2003; Laumann et al. Citation2008) towards stink bugs was confirmed in this work. Those works related this resistance to the higher isoflavonoid levels compared to susceptible cultivars in laboratory experiments (Piubelli, Hoffmann-Campo, Arruda, Lara Citation2003) and with higher stink bug population levels on cv Silvânia in field experiments compared with resistant cvs (Laumann et al. Citation2008). In addition, for the first time, it was shown that soybean cv Dowling has a natural resistance against E. heros. This cultivar appears to be particularly resistant to sucking insects, because it was previously described to be resistant towards A. glycines (Hill et al. Citation2004; Hesler et al. Citation2012). The results suggest that cvs IAC 100 and Dowling are less adequate for the development of stink bugs, because according to Soo Hoo and Fraenkel (Citation1966), insect development on a host is adequate when it has a shorter development time, heavier larva and/or pupa and low mortality rates. In addition, the resistance to E. heros involves the category of antibiosis, since changes in the life cycle of the insect, such as an increase in the development time and reduced adult emergence, depending on the genotype of the plant where the insect was maintained, are considered indicative of antibiosis (Crompton and Ode Citation2010; Mitchell et al. Citation2016).
The results of the present study showed that seeds of cvs IAC 100 and Dowling have constitutive defence, and previous studies showed that they have indirect defence activated when subjected to feeding injury by E. heros, releasing herbivory-induced plant volatiles that attract the main natural enemy of this pest, the egg parasitoid Telenomus podisi (Hymenoptera: Platygastridae) (Michereff et al. Citation2011, Citation2015). Therefore, these soybean cultivars present an optimal defence, investing in indirect defence in the vegetative stage and direct defence in the reproductive and seed stages. One prediction of the optimal defence theory is that chemical defence will be allocated within a plant as a function of tissue value, where value is correlated with the cost of having that tissue removed. Therefore, the plants can invest in traits to defend the parts that have the highest value for their development and that are more susceptible to pest attack (Heil Citation2008; McCall and Fordyce Citation2010; Schuman et al. Citation2016).
Further studies should investigate which compounds or morphological characteristics are involved in the resistance of cv Dowling, how the interaction between constitutive and induced defences of soybean plants occurs in the vegetative and reproductive stages, and whether there is a relationship between the categories of resistance in different phenological stages of the plants. In the search for new cultivars for use in integrated pest management, plants attractive to natural enemies and with moderate levels of resistance to herbivores could be considered, which might keep the pests at low infestation levels, thereby retaining the natural enemies in the field. These cultivars, in combination with other control methods, could reduce the use of insecticides thus contributing to the ecological sustainability of agricultural systems. It is also necessary to investigate the influence that harvested pods have in the flavonoids production, since it is known that harvesting parts of the plant can change its physiology due the mechanical damage. In this biology study, the insects fed on harvested pods during 0–48 h, all three cultivars evaluated were submitted to the same mechanical damage, so all of them were at the same conditions when were offer to the insects.
Supplemental Material
Download Zip (240.1 KB)Acknowledgements
We thank Priscila Souza and Warley Dias for helping with laboratory rearing of the insects.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Maria Carolina Blassioli-Moraes http://orcid.org/0000-0001-7569-9985
Additional information
Funding
Notes on contributors
Mirian F. F. Michereff
Dr Mirian F.F. Michereff is an associative Biologist Researcher at Embrapa Genetic Resources and Biotechnology.
Miguel Borges
Dr Miguel Borges is a Biologist Researcher at Embrapa Genetic Resources and Biotechnology.
Raúl A. Laumann
Dr Raul A. Laumann is a Biologist Researcher at Embrapa Genetic Resources and Biotechnology.
Daniela Daniel
Dr Daniela Daniel is an associative Chemist Researcher at University of São Paulo-Chemistry Institute of Chemistry and Scientist of Application at Agilent Corporation.
Claudimir Lucio do Lago
Dr Claudimir Lucio do Lado is a Professor and chemist Scientist at University of São Paulo – Institute of Chemistry.
Maria Carolina Blassioli-Moraes
Dr Maria Carolina Blassioli Moraes is a Chemist Researcher at Embrapa Genetic Resources and Biotechnology.
References
- Agrawal AA. 2005. Future directions in the study of induced plant responses to herbivory. Entomol Exp Appl. 115:97–105. doi: 10.1111/j.1570-7458.2005.00294.x
- Arimura GI, Kost C, Boland W. 2005. Herbivore-induced, indirect plant defences. Biochim Biophy Acta. 1734:91–111. doi: 10.1016/j.bbalip.2005.03.001
- Bansal R, Jun TH, Mian MAR, Michel AP. 2013. Developing host-plant resistance for hemipteran soybean pests: lessons from soybean aphid and stink bugs. In: El-Shemy HA, editor. Soybean – pest resistance. Rijeka, Croatia: INTECH Open Access Publisher; p. 19–46.
- Borges M, Blassioli-Moraes MC. 2017. The semiochemistry of pentatomidae in stink bugs biorational control based on communication processes. Boca Raton, FL: CRC Press; p. 95–124.
- Borges M, Laumann RA, Silva CCA, Moraes MCB, Santos HM, Ribeiro DT. 2006. Metodologias de criação e manejo de colônias de percevejos da soja (Hemiptera: Pentatomidae) para estudos de comportamento e ecologia química. Brasília: Embrapa – Cenargen. 18p. Documentos, n. 182.
- Borges M, Moraes MCB, Peixoto MF, Pires CSS, Sujii ER, Laumann RA. 2011. Monitoring the Neotropical brown stink bug Euschistus heros (F.) (Hemiptera: Pentatomidae) with pheromone-baited traps in soybean fields. J Appl Entomol. 135:68–80. doi: 10.1111/j.1439-0418.2010.01507.x
- Carrão-Panizzi MC, Kitamura K. 1995. Isoflavone content in Brazilian soybean cultivars. Breed Sci. 45:295–300.
- Chácon-Fuentes M, Parra L, Rodriguez-Saona C, Seguel I, Ceballos R, Quiroz A. 2015. Domestication in murtilla (Ugni molinae) reduced defensise flavonol levels but increased resistance against a native herbivorous insect. Environ Entomol. 44:627–637. doi: 10.1093/ee/nvv040
- Chen MS. 2008. Inducible direct plant defense against insect herbivores: A review. Insect Sci. 15:101–114. doi: 10.1111/j.1744-7917.2008.00190.x
- Chiozza MV, O’Neal ME, MacIntosh GC. 2010. Constitutive and induced differential accumulation amino acid in leaves of susceptible and resistant soybean plants in response to the soybean aphid (Hemiptera: Aphididae). Environ Entomol. 39:856–864. doi: 10.1603/EN09338
- Crompton DS, Ode PJ. 2010. Feeding behavior analysis of the soybean aphid (Hemiptera: Aphididae) on resistant soybean ‘dowling’. J Econ Entomol. 103:648–653. doi: 10.1603/EC09370
- Dixon RA, Steelf CL. 1999. Flavonoids and isoflavonoids – a gold mine for metabolic engineering. Trends Plant Sci. 4:394–400. doi: 10.1016/S1360-1385(99)01471-5
- Fürstenberg-Hägg J, Zagrobelny M, Bak S. 2013. Plant defense against insect herbivores. Int J Mol Sci. 14:10242–10297. doi: 10.3390/ijms140510242
- Graça JP, Ueda TE, Janegitz T, Vieira SS, Salvador MC, Oliveira MCN, Zingaretti SM, Powers SJ, Pickett JA, Birkett MA, Hoffmann-Campo CB. 2016. The natural plant stress elicitor cis-jasmone causes cultivar-dependent reduction in growth of the stink bug Euschistus heros and associated changes in flavonoid concentrations in soybean, Glycine max. Phytochemistry. 131:84–91. doi: 10.1016/j.phytochem.2016.08.013
- Heil M. 2008. Indirect defence via tritrophic interactions. New Phytol. 178:41–61. doi: 10.1111/j.1469-8137.2007.02330.x
- Hesler LS, Prischmann DA, Dashiell KE. 2012. Field and laboratory evaluations of soybean lines against soybean aphid (Hemiptera: Aphididae). J Econ Entomol. 105:608–615. doi: 10.1603/EC11339
- Hill CB, Li Y, Hartman GL. 2004. Resistance to the soybean aphid in soybean germplasm. Crop Sci. 44:98–106. doi: 10.2135/cropsci2004.9800
- Hoffmann-Campo CB, Harborne JB, McCaffery AR. 2001. Pre-ingestive and post-ingestive effects of soyabean extracts and rutin on Trichoplusia ni growth. Entomol Exp Appl. 98:181–194. doi: 10.1046/j.1570-7458.2001.00773.x
- Karowe DN, Radi JK. 2011. Are the phytoestrogens ganistein and daidzein anti-herbivore defenses? A test using the gypsy moth (Lymantria dispar). J Chem Ecol. 37:830–837. doi: 10.1007/s10886-011-9986-4
- Kim JJ, Kim SH, Hahn SJ, Chung IM. 2005. Changing soybean isoflavone composition and concentrations under two different storage conditions over three years. Food Res Int. 38:435–444. doi: 10.1016/j.foodres.2004.11.001
- Laumann RA, Farias Neto AL, Blassioli-Moraes MC, Silva AP, Vieira CR, Moraes SVP, Hoffman-Campo CB, Borges M. 2008. Dinâmica populacional de percevejos (Hemiptera: Pentatomidae) em diferentes genótipos de soja. In: IX Simpósio Nacional Cerrado; II Simpósio Internacional Savanas Tropicais, Brasília, DF. Desafios e estratégias para o equilíbrio entre sociedade, agronegócio e recursos naturais: anais. Planaltina, DF: Embrapa Cerrados, http://www.cpac.embrapa.br/download/747/t.
- Li Y, Hill CB, Hartman GL. 2004. Effect of three resistant soybean genotypes on the fecundity, mortality and maturation of soybean aphid (Homoptera: Aphididae). J Econ Entomol. 97:1106–1111. doi: 10.1093/jee/97.3.1106
- Li Y, Zou J, Li M, Bilgin DD, Vodkin LO, Hartman GL, Clough SJ. 2008. Soybean defense responses to the soybean aphid. New Phytol. 179:185–195. doi: 10.1111/j.1469-8137.2008.02443.x
- McCall AC, Fordyce JA. 2010. Can optimal defence theory be used to predict the distribution of plant chemical defences? J Ecol. 98:985–992. doi: 10.1111/j.1365-2745.2010.01693.x
- McPherson RM, Buss GR. 2007. Evaluation lepidopteran defoliation resistance in soybean breeding lines containing the stink bug (Hemipetra: Pentatomidae) resistance IAC100 cultivar in their pedigrees. J Econ Entomol. 100:962–968. doi: 10.1093/jee/100.3.962
- Medina V, Sardoy PM, Soria M, Vay CA, Gutkind GO, Zavala JA. 2018. Characterized non-transient microbiota from stink bug (Nezara viridula) midgut deactivates soybean chemical defenses. Plos One. 13:e0200161.. doi: 10.1371/journal.pone.0200161
- Michereff MFF, Laumann RA, Borges M, Michereff Filho M, Diniz IR, Farias Neto A, Moraes MCB. 2011. Volatiles mediating plant-herbivory-natural enemy interaction in resistant and susceptible soybean cultivars. J Chem Ecol. 37:273–285. doi: 10.1007/s10886-011-9917-4
- Michereff MFF, Michereff-Filho M, Blassioli-Moraes MC, Laumann RA, Diniz IR, Borges M. 2015. Effect of resistant and susceptible soybean cultivars on the atrraction of egg parasitoids under field conditions. J Appl Entomol. 139:207–216. doi: 10.1111/jen.12148
- Mitchell C, Brennan RM, Graham J, Karley AJ. 2016. Plant defense against herbivorous pests: exploting resistance and tolerance traits for sustainable crop protection. Front Plant Sci. 7:1–8. doi: 10.3389/fpls.2016.01132
- Murakami S, Nakata R, Aboshi T, Yoshinaga N, Teraishi M, Okumoto Y, Ishihara A, Morisaka H, Huffaker A, Schmelz EA, Mori N. 2014. Insect-induced daiddzein, formonetin and their conjugates in soybean leaves. Metabolites. 4:532–546. doi: 10.3390/metabo4030532
- O’Neil BF, Zangeri AR, Dermody O, Bilgin DD, Casteel CL, Zavala JA, DeLucia EH, Berenbaum MR. 2010. Impact of elevated levels of atmospheric CO2 and herbivory on flavonoids of soybean (Glycine max Linnaeus). J Chem Ecol. 36:35–45. doi: 10.1007/s10886-009-9727-0
- Piubelli GC, Hoffmann-Campo CB, Arruda IC, Franchini JC, Lara FM. 2003. Flavonoid increase in soybean as a response to Nezara viridula injury and its effects on insect-feeding preference. J Chem Ecol. 29:1223–1233. doi: 10.1023/A:1023889825129
- Piubelli GC, Hoffmann-Campo CB, Arruda IC, Lara FM. 2003. Nymphal development, lipid content, growth and weight gain of Nezara viridula (L.) (Heteroptera: Pentatomidae) fed on soybean genotypes. Neotrop Entomol. 32:127–132. doi: 10.1590/S1519-566X2003000100019
- Piubelli GC, Hoffmann-Campo CB, Moscardi F, Miyakubo SH, Oliveira MCN. 2005. Are chemical compounds important for soybean resistance to Anticarsia gemmatalis? J Chem Ecol. 31:1509–1525. doi: 10.1007/s10886-005-5794-z
- Salvador MC, Boiça AL, de Oliveira MCN, Graça JP, Silva DM, Hoffmann Campo CB. 2010. Do different casein concentrations increase the adverse effect of rutin on the biology of Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae)? Neotrop Entomol. 39:774–783. doi: 10.1590/S1519-566X2010000500017
- SAS Institute. 2001. SAS User’s Guide: Statistics, version 9.0. 6th ed. Cary, NC: SAS Institute.
- Schuman M, Allmann A, Baldwin IT. 2016. The layers of plant responses to insect herbivores. Ann Rev Entomol. 61:373–394. doi: 10.1146/annurev-ento-010715-023851
- Simmonds MSJ. 2001. Importance of flavonoids in insect-plant interactions: feeding and oviposition. Phytochemistry. 56:245–252. doi: 10.1016/S0031-9422(00)00453-2
- Simmonds MSJ. 2003. Flavonoid-insect interactions: recent advances in our knowledge. Phytochemistry. 64:21–30. doi: 10.1016/S0031-9422(03)00293-0
- Soo Hoo CF, Fraenkel G. 1966. The selection of food plants in a polyphagous inect, Prodenia eridania (Cramer). J Insect Physiol. 12:693–709. doi: 10.1016/0022-1910(66)90115-6
- Srivastava R, Shukls YN, Kumar S. 1999. Recent advances in the chemistry of insect antifeedants. J Med Arom Plant Sci. 21:59–76.
- Treutter D. 2006. Significance of flavonoids in plant resistance: a review. Environ Chem Lett. 4:147–157. doi: 10.1007/s10311-006-0068-8
- Vendramin JD, Guzzo EC. 2009. Resistência de plantas e a bioecologia e nutrição dos insetos. In: Panizzi AR, Parra JR, editors. Bioecologia e nutrição de insetos: base para o manejo integrado de pragas. Brasília-DF: Embrapa Informação Tecnológica; p. 1055–1105.
- War AR, Paulraj MG, Ahmad T, Buhroo A, Hussain B, Ignacimuthu S, Sharma HC. 2012. Mechanisms of plant defense against inscet herbivores. Plant Sign Behav. 7:1306–1320. doi: 10.4161/psb.21663
