?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study was implemented to investigate the diurnal biochemical changes that occur in Ceratophyllum demersum in four growth habitats under in situ experiments. The results showed that compared to the other treatments, the open-flow treatments had the highest hydrogen peroxide (H2O2) concentration that occurred with the acceleration of antioxidant activity at midday, mainly due to the independent effect of irradiance and flow movement. The plant diurnal H2O2 and antioxidant activity rhythms had varying fluctuations, and peroxidase (POD) activity showed a rapid response to oxidative stress. The increase in POD activity followed a more similar pattern aligned with H2O2 synthesis, while catalase (CAT) and ascorbate peroxidase (APX) activities were dominant in the late hours of the day. The ability of this species to successfully grow in these habitats is related to the adaption of the diurnal cycles of antioxidant activity according to flow and irradiance changes.
Introduction
The growth and development of aquatic macrophytes depend on various environmental factors. Among these factors, water, temperature, flow and sediment nutrients play important roles (Dale Citation1986; Dawson and Szoszkiewicz Citation1999). Therefore, aquatic habitats are heterogeneous environments and are characterized by temporal and spatial fluctuations in chemical and physical parameters (Asaeda et al. Citation2013). In terms of zonation, aquatic plants can grow in stagnant, flowing or shaded habitats, which are classified into four categories: floating, floating leaves, submerged or emergent (Chambers and Kalff Citation1987).
Over the past few decades, aquatic habitats have been affected by anthropogenic activities, causing drastic changes in macrophyte species diversity (Giller Citation2005; Zhang et al. Citation2015). For example, the construction of dams and reservoirs changes flow dynamics and influences the abundance of macrophytes. Water level and flow dynamics influence macrophytes in different ways (Mjelde et al. Citation2013). Hydraulic forces generated by waves result in physical changes in macrophytes, e.g. growth retardation (Madsen et al. Citation2001; Ellawala et al. Citation2011), stem breakage and uprooting (Riis and Biggs Citation2003). Some submerged aquatic macrophytes, for instance, increase leaf nutrient uptake and oxygen absorption with moderate water flows (Stevens and Hurd Citation1997) by thinning the leaf blade (Madsen et al. Citation1993).
The same environmental factors exceed their threshold levels, and they become stressors for aquatic plant communities. Compared to other abiotic stressors, solar irradiance fluctuations are common in aquatic habitats and have been reviewed in different studies (López-Figueroa Citation1992; Conde-Álvarez et al. Citation2011; Yuan et al. Citation2016). In addition, photosynthesis alterations in aquatic plants due to physical restrictions and light availability are much more significant than those in terrestrial plants (Sand-Jensen Citation1997). In aquatic ecosystems, light energy is one of the key determinants that is crucial for photosynthesis because light provides energy for electron transport (Binzer et al. Citation2006), and thus, its limitation retards the growth of macrophytes (Barko et al. Citation1986). On the other hand, some aquatic plant species prefer shaded natural environments and behave as shade-adapted species (Barko and Smart Citation1986). These irradiance and flow changes may have positive or negative consequences for macrophytes, which may be related to the type of macrophyte species. Thus, based on these resource fluctuations, adaptive strategies may be developed for survival (Chen et al. Citation2016). Biochemical changes that take place at the cellular level might be an effective adaptive mechanism for abiotic stress that is controlled genetically and physiologically (Asaeda et al. Citation2013) and may be expressed as an antioxidant system. The activation of an antioxidant system occurs due to different abiotic stressors. Changing environmental factors disrupt plant cell homeostasis and cause an imbalance in internal plant cell metabolism (Aro et al. Citation1993), eventually causing oxidative stress. If received light energy exceeds the absorbance capacity of chlorophyll, then the generation of reactive oxygen species (ROS) is dramatically elevated (Nishiyama et al. Citation2001), which reduces photosynthesis potential (Powles Citation1984; Nishiyama et al. Citation2004). Often, this imbalance leads to the generation of ROS. Major ROS are identified as singlet oxygen, superoxide and hydrogen peroxide (H2O2) that accumulate within plant cells (Polesskaya et al. Citation2006). Water cycle-produced ROS are rapidly scavenged by antioxidative enzymes (Asada Citation2006).
Ceratophyllum demersum (Ceratophyllaceae) is a perennial submerged macrophyte that grows in streams, lakes, and shallow aquatic habitats. C. demersum originated in North America and is presently considered an invasive species in many areas of the world (DiTomaso et al. Citation2013). The invasion success of this noxious species depends on its rapid spreading and adaptation to a wide range of aquatic habitats (Mishra et al. Citation2009). Moreover, based on its growing area, this species expresses changing phenotypic plasticity. For example, the development of modified leaves that attach to deep water sediment (Sculthorpe Citation1967; Keskinkan et al. Citation2004) is a good adaptation for survival in deep water habitats. Most studies related to C. demersum have focused on sediment effects (Stiers et al. Citation2011), biotransformation (Stiers et al. Citation2011), and phytoremediation (Aravind and Prasad Citation2004; Mishra et al. Citation2008). However, little attention has been given to understanding the diurnal biochemical adaptation changes that occur in different C. demersum habitats.
In the present study, we experimentally simulated four potential habitats considering flow regime and solar irradiance, and we hypothesized that biochemical changes may vary diurnally with each simulated condition. Thus, our objectives were (1) to evaluate adaptation to light irradiance and water flow based on photosynthesis (electron transport rates (ETRs) and dark yield) and (2) to assess diurnal biochemical changes in C. demersum through enzyme activities (ascorbic peroxidase (APX), guaiacol peroxidase (POD), and catalase (CAT)) and ROS that corresponded to the flow and irradiance. The results provide an understanding of environmental conditions suitable for this plant, and this understanding may help effectively minimize the invasion potential of this noxious weed through alteration of the optimum growing conditions.
Methodology
Plant materials
Experimental plants were collected from the Toda River (35°48'13.9"N, 139°39'23.8"E), Japan, where this plant is considered a weed. After collection, plants were immediately transported to the indoor experimental facility located in the Department of Environmental Science, Saitama University, Japan (35°53'51.4"N, 139°60'77.8"E). Prior to the experiment, approximately 8 cm of C. demersum apical shoots were cultivated separately within three glass microcosms (60 cm × 30 cm × 36 cm) in the indoor laboratory for up to 4 weeks. Each microcosm was aerated to provide sufficient dissolved oxygen, and the growth chamber temperature was maintained at 23 ± 2°C with a light intensity of approximately 100 µmol m−2s−1 and a 16:8 light dark cycle. The nutrient medium was a 5% Hoagland solution to meet the nutrient requirements of the plant (Hoagland and Arnon Citation1950).
After one month, homogeneously developed plants were randomly selected, and propagules that were 10 cm long were cultivated within the PVC pots (6 cm in diameter and 7 cm high) for further acclimatization in an outdoor greenhouse. Plant fragment sizes were determined based on previous literature (Ellawala et al. Citation2011; Zaman and Asaeda Citation2013). The room temperature was maintained at 25 ± 2°C under a natural light: dark cycle (16:8) for 1 week before starting the experiment. A total of 300 mL of thoroughly washed commercial river sand (90% <1 mm particle size) was used for the planting medium, and the plant density was 6 fragments per pot.
Experimental setup
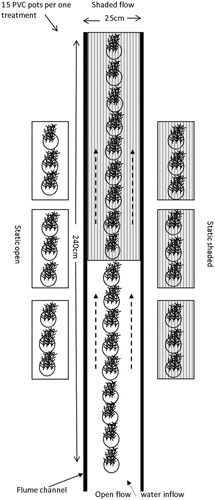
All experiments were conducted in an outdoor flume channel (240 cm × 25 cm × 22 cm) at Saitama University, Japan, in May 2018. After pretreatment for 7 days, each set of 15 PVC pots (6 cm in diameter and 7 cm high) was transferred into three glass microcosms (40 cm ×25 cm ×28 cm) that represented one treatment. To minimize adaptation shock to the plants, tanks were filled with tap water and aerated for 24 h. We incorporated four conditions, which were static open, static shaded, open flow, and shaded flow treatments, consisting of 3 replicates (). Black-colored commercially purchased polyethylene shade sheets (DIY, Doito, Japan) were cut into equal sizes and used for shade. The solar irradiance reduction efficiency of a particular shade cloth evaluated using a light intensity meter (Apogee, MQ-200, USA) was 72%. The outer surface of the glass tank was covered by 70% shade sheets, whereas uncovered tanks served as the static open environment and received 100% ambient photosynthetic active radiation (PAR). In contrast to the static treatments, the flume experiment setup was maintained, and water was circulated in the flume channel through regulated water pumps. Half of the flume channel was covered by a shade sheet and represented the shaded flow treatment. Just above the plant canopy, the water velocity was measured with an ultrasonic velocimeter (Tokyo Keisoku Co. Ltd, Japan). The mean flow velocity of water was approximately 25 cms−1, which was comparable to the mean flow velocities found in Japanese field conditions (Asaeda et al. Citation2017).
Figure 1. Top view of the schematic diagram showing open flow and shaded flow treatments with two static (open vs shaded) treatments.
Evaporation losses were compensated for by using preaerated tap water to maintain a constant water level (18 cm) among the four treatments. The water pH and temperature were measured using a portable water quality meter (Model No. U-53, Horiba, Japan). The observed water temperature remained constant over the experimental period at approximately 23.5°C ± 2°C, and the pH was within the range of 7.6 ± 0.3. The PAR in each treatment was recorded in three-hour intervals using a digital light intensity meter (Apogee, MQ-200, USA). After adjusting the treatment conditions, the experiment was continued for 5 days. On the last day of the experiment, three planting pots from each treatment were randomly selected for the biochemical assays every 3 h. Samples were collected between 6:00 am to 6:00 pm during the daylight regime for biochemical analysis.
Photosynthesis measurements
Photosynthesis was measured with a pulse amplitude modulation (PAM) fluorometer (Junior-PAM, Walz, Germany) at 12:00 pm on the day of harvest. Apical parts of the C. demersum shoots were connected to the junior PAM. Plants were adapted to the dark for 15 min before light curve measurements of 25, 45, 65, 90, 125, 190, 285, 420, 625, 820, and 1150 µmol m−2 s−1 were obtained. Each irradiation level was applied with a saturation flash (pulse length of 2.5 s), which was set by the PAM, for 1 min. Quantum yield was determined by the software WinControl-3. Thereafter, the relative ETRs of photosystem II were calculated using the model of (Walsby Citation1997). There were 4 replicates for each treatment.
Photosynthetic pigment analysis
The chlorophyll a, chlorophyll b and carotenoid contents were measured spectrophotometrically after extractions in N,N-dimethylformamide for 24 h. Green-colored supernatants were obtained, and the absorbance was read at wavelengths of 664, 647 and 480 nm using a UV spectrophotometer (Wellburn Citation1994). The pigment content was calculated using the following formulas:
(1)
(1)
(2)
(2)
(3)
(3)
Antioxidant enzyme analysis
Harvested C. demersum plants were thoroughly cleaned and blotted, and approximately 100 mg of fresh plant samples were extracted in chilled (50 mM, pH 6.0) potassium phosphate buffer with 2% (w/v) polyvinylpyrrolidone (PVP). Homogenate was centrifuged at 3000 g for 10 min at 4°C. The resultant supernatant was immediately stored at −80°C for further biochemical analysis.
H2O2 concentrations were estimated using a standard curve prepared from a known concentrations of H2O2 following the method of (Jana and Choudhuri Citation1982). The reaction mixture contained 750 µL of enzyme extract and 2.5 mL of 1% (v/v) TiSO4 in 20% (v/v) H2SO4. The intensity of color development was measured spectrophotometrically at 410 nm.
The APX activity (EC 1.11.1.11) was measured in a reaction mixture that contained 100 µL of enzyme extract, 200 µL of 0.5 mM ascorbic acid and 2.0 mL of 50 mM potassium phosphate buffer (pH 7.0). The reaction was started by the addition of 60 µL of 1 mM H2O2. The absorbance change was recorded at 290 nm every 10 s for a total of 3 min (Nakano and Asada Citation1981). The results are presented in µmol min−1 g−1 FW.
The CAT activity (EC 1.11.1.6) was determined following the method of (Aebi Citation1984). The reaction mixture contained 100 µL of 10 mM H2O2, 2.00 mL of 100 mM potassium phosphate buffer (pH 7.0), and 500 µL of enzyme extract. The decrease in absorbance at 240 nm was recorded for 10 s for 3 min. The CAT activity was evaluated using the extinction coefficient of 40 mM−1 cm−1.
For the measurement of the POD activity (EC 1.11.1.7), the reaction mixture contained 3.0 mL that had a pH 6, 50 mM potassium phosphate buffer, 50 µL of 0.2 mol guaiacol, 40 µL of 30 mM H2O2 and 100 µL enzyme (MacAdam et al. Citation1992). The POD activity was obtained using an extinction coefficient of 26.6 mM−1 cm−1. Each enzyme activity above is represented as µmol min−1 g−1 FW. The experiments were replicated three times (n = 3) for each growth condition.
Statistical analyses
Two-way analysis of variance (ANOVA) was performed for all corresponding variables when normality and homogeneity assumptions were met. In this study, we considered flow conditions (flow vs static) and irradiance level (open vs shaded) as fixed factors. When a significant effect was found, one-way ANOVA was carried out to test differences between treatments in each time interval (Gómez et al. Citation1998). The data were expressed before by their means and standard deviations followed by Tukey’s post hoc test (p < 0.05) to identify homogeneous subgroups of significantly different means. All statistical analyses were performed using SPSS version 16.0 (SPSS, Chicago, IL, USA).
Results
Photosynthesis responses
Dark yield responded significantly to the interactive effects of water flow and irradiance at midday (p < 0.05) (). The measured rETR in response to increasing irradiance up to 1200 µmol m−2 s−1 showed notable variation among the four treatments (). In comparison to the other plants, the plants subjected to the open flow treatment had the highest responsiveness to light in this experiment, while static shading resulted in the lowest responsiveness. The light curves of shaded plants in both the static and flow treatments exhibited a lower response for the given irradiance sequences compared to the plants in the unshaded treatments (). Based on the two-way ANOVA, at noon, the rETR max was influenced by irradiance, and there was no significant interaction between flow dynamics and irradiance (p < 0.05) ().
Figure 2. Rapid light curves of Ceratophyllum demersum under the four habitat conditions. The data presented are the means of four replicates, and the vertical bars denote the standard deviation.
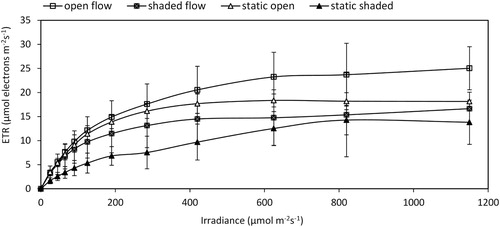
Table 1. Two-way ANOVA results showing the effects of irradiance and water flow on the physiological conditions of Ceratophyllum demersum at noon (12:00 pm). ANOVA results were significant at P < 0.05. For all response variables, DF = 1 for water flow, DF = 1 for irradiance, and DF = 1 for water flow × irradiance
Pigment concentration
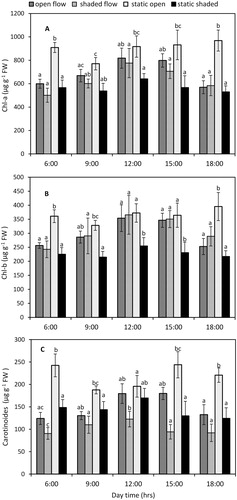
At 06:00 am, the Chl a concentration was significantly influenced by the interaction of water flow and irradiance level (p < 0.05) (A). Of the plants in the different treatments, the plants subjected to static open water had the highest Chl a concentration over the experimental period, whereas the plants subjected to shade exhibited the lowest pigment content (A). Between 06:00 am and 12:00 pm, plants subjected to open flow and shaded flow conditions had Chl a concentrations that followed a relatively linear trend that reached a peak at 12:00 pm. However, the opposite scenario was observed for Chl b during the middle of the day, as it was influenced only by the irradiance level (p < 0.05), but there were no interactive effects of flow and irradiance (). During the diurnal cycle at 06:00 pm, all treatments showed Chl a concentrations of approximately 500 µg g−1 FW except for that of the static open treatment. In the static open treatment, Chl b followed similar diurnal fluctuations to those of Chl a and continued to increase to approximately 350 µg g−1 FW between 03:00 pm and 06:00 pm (B). Compared to the steadier trend of the static open treatment, the other three treatments followed a more symmetrical pattern in which the concentration peaked at noon. The highest carotenoid levels were observed in the static open flow treatment, while the lowest carotenoid levels were found in the shaded flow treatment (C). At 06:00 am and 06:00 pm, water flow and irradiance interactively affected the carotenoid concentration, whereas the irradiance factor had a significant effect only at 12:00 pm (F = 7.973, p < 0.05) (). The carotenoids peaked at mid-day at 44.35% compared to those in the early morning in the open flow treatment (C). Thereafter, the concentration (179 µg g−1 FW) remained almost the same until 03:00 pm and then declined. Interestingly, carotenoids reached a maximum level after 03:00 pm in the static open flow treatment. Irradiance had a significant effect on carotenoid fluctuations (p < 0.05), but there was no significant interaction between irradiance and water flow ().
Figure 3. Pigment (Chl a, Chl b and carotenoid) concentrations in Ceratophyllum demersum plants grown under the four growth conditions. The data presented are the means of three replicates, and the different lower case letters at each time point indicate significant differences between treatments.
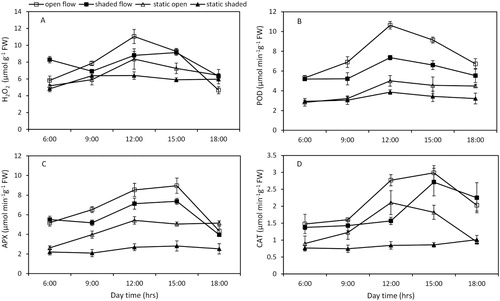
Reactive oxygen species
ROS were measured every three hours for C. demersum in the four treatments. Of the treatments, the open flow treatment showed the highest increase in H2O2 content throughout the experiment (A), followed by the shaded flow treatment. In addition, the static open and shaded treatments fluctuated and had lower amounts of ROS than the other flow treatments. Initially, the mean H2O2 concentration was approximately 5 µmol g−1 FW for each treatment at 06:00 am. Then, the plants grown under open conditions showed a two-fold increase in H2O2 at 12:00 pm, while the static open and shaded treatment values remained at 8.3 and 6.4 µmol g−1 FW, respectively. The H2O2 concentration was significantly higher at mid-day than over the 12-hour sampling period (p < 0.05). Moreover, at noon, the H2O2 content was significantly influenced by water flow (F = 19.762, p = 0.002) and irradiance (F = 13.248, p = 0.006), but there were no interactive effects. The H2O2 concentration reached its lowest level at approximately 06:00 pm in all treatments.
Antioxidant enzymatic activities
For all treatments, POD activity followed similar daily cycles as those of the H2O2 levels. POD activity increased in the first half of the day, and it declined in the latter part of the day (B). In comparison to the plants in static water, the plants exposed to continuous water movement had the highest POD activity. At mid-day, in all four treatments, the POD activity peaked and was significantly affected (16.54, p < 0.05) by flow (F = 303.45, p < 0.05) and irradiance (F = 71.77, p < 0.05). The mean POD activity of the open flow treatment was 10.62 ± 0.38 µmol min−1 g−1 FW at noon and was approximately two times greater than that of the static open and shaded treatments. However, POD activity declined by 30.79% in the shaded flow treatment compared to that in the open flow treatment at 12:00 pm.
The APX activity was delayed during the day and peaked at approximately 03:00 pm in all treatments. Compared to the static treatments, the flow treatments had significant effects on APX enzyme activity, which rapidly decreased at 06:00 pm (C). Moreover, the APX activity was relatively unchanged between 03:00 pm and 06:00 pm in the static shaded and static open treatments. However, the APX activity was reduced by 15.9% in the flow treatments compared to that in the static treatments at 06:00 pm.
The CAT activity increased along the solar irradiance gradient and peaked after mid-day at 03:00 pm in both the open and shaded flow treatments. The next highest level was observed in the static open treatment at 12:00 pm, whereas the CAT activity did not drastically fluctuate among the five sampling periods in the static shade treatment. The CAT activity was significantly influenced by irradiance in the early morning at 06:00 am and in the late evening at 06:00 pm in the four treatments, whereas significant interactive effects of irradiance and water flow were observed at noon (12.00 pm) (F = 70.54, p < 0.05) and 15:00 (F = 11.78, p < 0.05) (D).
Discussion
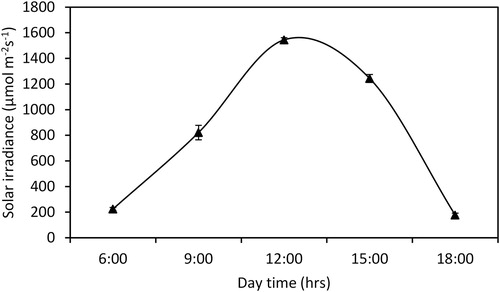
Light energy fluctuations in aquatic community production vary between 0 and 2000 µmol m−2s−1 (Binzer and Middelboe Citation2005). In our experiment, the maximum PAR was 1544 µmol m−2 s−1 at midday, and the lowest value was 224 µmol m−2 s−1 (). The data indicate that the received irradiance quantum yield was satisfactory in terms of the general photosynthesis performance of the C. demersum population. When light energy is sufficient, it promotes photoautotrophic organism growth in an aquatic environment (Wersal and Madsen Citation2013). On the other hand, excess light can cause photoinhibition (Kull Citation2002). For C. demersum, in comparison to the other plant treatments, the plants subjected to the static open water chlorophyll a concentration were upregulated at all five sampling time points. This finding may be related to the adaptation of absorbing excessive light with chlorophyll a pigments that follows the diminishing law of returns (Frost-Christensen and Sand-Jensen Citation1992). Madsen et al. (Citation1993) argued that an available high chlorophyll a concentration causes an increase in the photosynthesis rate of hydrophytes, and our results validated their findings. However, plants exposed to the flow with the natural irradiance chlorophyll a concentration declined. The reduction in the chlorophyll content in the plants grown in the flow treatments compared to that of the plants grown in the static treatments was related to the accumulation of ROS (Demidchik Citation2015). These results indicate that C. demersum exhibited an oxidative stress response when subjected to flow movement. The consequences of flow movement-related chlorophyll reduction were discussed in previous studies (Ellawala et al. Citation2011; Atapaththu and Asaeda Citation2015). Chlorophyll b and carotenoids exhibited the same pattern as chlorophyll a among treatments. Our research findings suggest that pigment fluctuation is an adaptive strategy for coping with available irradiance. Plant species follow various adaptations to alter photosynthesis capacity, and one of these adaptations is changing pigment content (Belshe et al. Citation2007).
Figure 5. Diurnal variation in irradiance over a 12-hour period. The data presented are the means of three replicates, and the vertical bars denote the standard deviation.
The ETR at light saturation is a good indicator of photosynthesis capacity based on fluorescence measurement (Genty et al. Citation1989). In addition to the chlorophyll pigment, we observed a higher elevated ETR in the flow-exposed plants than in the other plants. This observation may be related to the boundary layer hypothesis that a reduced thickness of the boundary layer in macrophyte leaves due to water flow and enhanced uptake of CO2 and dissolved nutrients (Wheeler Citation1980; Søndergaard et al. Citation1992) are important for photosynthesis. In addition, in comparison to that of whole-leaf plants, the morphological structure of C. demersum was identified as having a narrow-leaved, rigid shoot shape, which provides an advantage for resisting flow forces. Schutten and Davy (Citation2000) explained that macrophyte species with narrow leaves have a high level of resistance against hydraulic forces. In both the open static and open flow treatment propagules, the ETR results revealed that photosynthesis capacity was higher than that in the shaded treatment propagules. A low photosynthesis rate under low light is a typical observation in the plant community (Biswal et al. Citation2012). During photosynthesis, accelerated ROS production was also high in those two treatments. This phenomenon occurs because ROS are directly generated through natural photosynthesis with increased ETR, as demonstrated by earlier studies (Rijstenbil et al. Citation2000; Pospíšil Citation2009, Citation2016).
Often, a major adaptive strategy for scavenging ROS is the activation of antioxidant enzymes. Plant cell-metabolized ROS are identified as singlet oxygen, superoxide, hydroxyl radicles and H2O2 that are produced through light-driven processes (Pospíšil Citation2016). Among all ROS, H2O2 is considered a relatively stable nonradical ROS (Slesak et al. Citation2007). In addition, ROS react as intracellular messengers to produce cell antioxidants (Sauer et al. Citation2001). According to the results, we observed an excessive H2O2 concentration in the bare plants in the flow treatments in comparison to the plants in the static treatment at midday, indicating that C. demersum was in a state of oxidative stress. This result may be attributed to the mechanical shear stress forced on the plant leaf tissue by water movement (Pasternak et al. Citation2005; Asaeda et al. Citation2013), which is further aggravated by irradiance (Mittler Citation2002). However, excessive ROS generation signals an initiated scavenging system, and antioxidant concentrations increased drastically at mid-day. Our research findings are consistent with those of previous studies of (Atapaththu and Asaeda Citation2015) that explain water flow movement caused by elevated antioxidant activities in the submerged macrophyte species Elodea nuttallii.
In our study, the triggered antioxidant system was measured using three antioxidants: POD, APX and CAT. In the scavenging process, POD was considered to be a rapidly responding antioxidant (Knörzer et al. Citation1996). In addition, with increased solar irradiance in the morning at 0600, plants exposed to flow exhibited 1.84-fold higher POD activities than those at other times. These data indicate that POD activity in C. demersum plants tended to be upregulated with flow movement in contrast to those with static flow as a stress-responsive mechanism. Furthermore, the synthesis of POD activity markedly peaked at 12:00 pm in comparison to other sampling times under each of the four growth conditions. This finding suggests that under a natural day: night cycle, POD activity is dominant at noon to scavenge ROS. Intensification of POD activity is more closely associated with the early response mechanism to light stress and provides cellular protection against synthesized H2O2 (Zolfaghari et al. Citation2010). In our experiment, plants grown in the flow treatments increased their POD generation more dramatically, while plants grown under static conditions exhibited lower POD activity.
In addition to the POD activity, the APX activity plays a crucial role in the flexible antioxidant system. Asada (Citation1992) argued that APX is a key enzyme present in the ascorbate glutathione cycle and is responsible for H2O2 detoxification. Furthermore, elevated APX activity with abiotic stress has been observed in other aquatic plant species (Mishra et al. Citation2009; Chandani et al. Citation2017; Parveen et al. Citation2017). In our experiment, the results showed gradually elevated APX activity at approximately 03:00 pm in both flow treatments. At the end of the experimental period, the average activity declined by 51%. However, static-induced plant APX values did not sharply fluctuate and indicated a low level of oxidative stress. However, similar to the APX activity, a closely varied diurnal trend pattern was observed in the CAT activity that tended to increase in the late day time period. The delayed dominance of the CAT activity might have been related to the susceptibility of a particular enzyme to photoinactivation by excess sunlight (Shang and Feierabend Citation1999). These two antioxidants are responsible for transforming the H2O2 substrate into H2O and O2, and this activity takes place in two separate places: peroxisomes for CAT and chloroplasts for APX (Foyer et al. Citation1994). Our data emphasized that the three antioxidant enzymes considered in the habitat experiment responded at different times in the daily cycle for generated H2O2. CAT and APX fluctuated in the later part of the sampling period, while the POD cycle was triggered at 12:00 pm.
However, at the end of the day, continuous water flow movement caused balanced H2O2 generation, where the H2O2 concentration was close to both static treatment values. This finding demonstrated that perhaps an elevated response of antioxidant activities at noon resulted in a substantial recovery of ROS accumulation in the cell. The proper timing of plant responses to environmental changes is considered an adaptive advantage in plant physiology and metabolic activities (Michael et al. Citation2003; Dodd et al. Citation2005). Butow et al. (Citation1997) explained that antioxidant enzyme regulation takes place when the total daily irradiance is aligned with the diurnal rhythm. The activation of enzymatic patterns and key genetic stimuli is not fully understood and open for further exploration. This scenario suggests that future studies should evaluate the relative importance of biological enzymatic pattern activation to oxidative stress responses.
Conclusion
The diurnal physiological conditions of the C. demersum plants significantly varied among the four growth conditions. Their ability to successfully grow in habitats was related to modulating the diurnal cycles of antioxidant activities according to flow and irradiance changes. In particular, we observed POD as an early responding antioxidant against ROS, whereas CAT and POD scavenging occurred after the POD response. Interestingly, the irradiance effect significantly elevated oxidative stress at noon; however, with the activated antioxidant system, the effect was mediated a considerable amount in the late evening. Moreover, among the four treatments, the static open conditions can be considered the most favorable for the growth of this macrophyte. The lower level of H2O2 concentration coupled with the highest photosynthesis performance occurred in the plants under static open conditions. This result implies that a healthy cell environment for optimum plant growth may facilitate the invasive potential of the species. Additionally, in comparison to unshaded habitats, shaded habitats tend to decrease photosynthesis capacity. If these habitats exist, then those places may be vulnerable to invasion by these species. Therefore, ecologists should consider managing aquatic ecosystems to control this species. Further studies should focus on understanding the gene-level regulators that control these diurnal activities.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Viraj Prasanna Ranawakage
Viraj Prasanna Ranawakage is a PhD student at Department of Environmental Science, Saitama University Japan with research interest in fresh water macrophytes.
Takashi Asaeda
Takashi Asaeda is a professor emeritus at Saitama University Japan. His research interests have broadly covered ecology, hydrology, and fluid mechanics in the environment, including physical processes of watered area, structure of urban heat island and field observation and mathematical modeling of aquatic ecology and biology.
References
- Aebi H. 1984. [13] Catalase in vitro. In: Methods in enzymology. New York: Elsevier; p. 121–126.
- Aravind P, Prasad MNV. 2004. Zinc protects chloroplasts and associated photochemical functions in cadmium exposed Ceratophyllum demersum L., a freshwater macrophyte. Plant Sci. 166(5):1321–1327. doi: 10.1016/j.plantsci.2004.01.011
- Aro E-M, Virgin I, Andersson B. 1993. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1143(2):113–134. doi: 10.1016/0005-2728(93)90134-2
- Asada K. 1992. Ascorbate peroxidase–a hydrogen peroxide scavenging enzyme in plants. Physiol Plant. 85(2):235–241. doi: 10.1111/j.1399-3054.1992.tb04728.x
- Asada K. 2006. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 141:391–396. doi:10.1104/pp.106.082040.
- Asaeda T, Sanjaya K, Kaneko Y. 2017. Effects of mechanical stressors caused by mean flow and turbulence on aquatic plants with different morphologies. Ecohydrology. 10(5):e1873. doi: 10.1002/eco.1873
- Asaeda T, Zaman T, Rashid MH. 2013. Biochemical responses of aquatic macrophytes to two abiotic stresses. Peroxidases: Biochemical Characteristics, Functions and Potential Applications. 125–140.
- Asaeda T, Zaman T, Takahashi Y. 2013. Effect of trial impoundment on downstream macroinvertebrate community during construction of a large dam. Pol J Ecol. 61(4):785–796.
- Atapaththu KSS, Asaeda T. 2015. ‘Growth and stress responses of Nuttall’s waterweed Elodea nuttallii (Planch) St. John to water movements’. Hydrobiologia. 747(1):217–233. doi:10.1007/s10750-014-2141-9.
- Barko JW, Adams MS, Clesceri NL. 1986. Environmental factors and their consideration in the management of submersed aquatic vegetation: a review. J Aquat Plant Manag. 24(1):1–10.
- Barko JW, Smart RM. 1986. Sediment related mechanisms of growth limitation in submersed macrophytes. Ecology. 67(5):1328–1340. doi: 10.2307/1938689
- Belshe EF, Durako MJ, Blum JE. 2007. Photosynthetic rapid light curves (RLC) of Thalassia testudinum exhibit diurnal variation. J Exp Mar Biol Ecol. 342(2):253–268. doi: 10.1016/j.jembe.2006.10.056
- Binzer T, Middelboe AL. 2005. From thallus to communities: scale effectsand photosynthetic performance in macroalgae communities. Mar Ecol Prog Ser. 287:65–75. doi: 10.3354/meps287065
- Binzer T, Sand-Jensen K, Middelboe A-L. 2006. Community photosynthesis of aquatic macrophytes. Limnol Oceanogr. 51(6):2722–2733. doi: 10.4319/lo.2006.51.6.2722
- Biswal AK, et al. 2012. Light intensity-dependent modulation of chlorophyll b biosynthesis and photosynthesis by overexpression of chlorophyllide a oxygenase in tobacco. Plant Physiol. 159(1):433–449. doi: 10.1104/pp.112.195859
- Butow BJ, Wynne D, Tel Or E. 1997. Antioxidative protection of Peridinium Gatunense in Lake Kinneret: seasonal and daily variation. J Phycol. 33(5):780–786. doi: 10.1111/j.0022-3646.1997.00780.x
- Chambers PA, Kalff J. 1987. Light and nutrients in the control of aquatic plant community structure. I. In situ experiments. J Ecol. 75:611–619. doi: 10.2307/2260193
- Chandani H, Silva CD, Asaeda T. 2017. Effects of heat stress on growth, photosynthetic pigments, oxidative damage and competitive capacity of three submerged macrophytes. J Plant Interactions. 12(1):228–236. doi:10.1080/17429145.2017.1322153.
- Chen J, et al. 2016. Differential photosynthetic and morphological adaptations to low light affect depth distribution of two submersed macrophytes in lakes. Sci Rep. 6:34028. doi: 10.1038/srep34028
- Conde-Álvarez RM, et al. 2011. Photosynthetic performance of the aquatic macrophyte Althenia orientalis to solar radiation along its vertical stems. Oecologia. 166(4):853–862. doi: 10.1007/s00442-011-1941-0
- Dale HM. 1986. Temperature and light: The determining factors in maximum depth distribution of aquatic macrophytes in Ontario, Canada. Hydrobiologia. 133(1):73–77. doi:10.1007/BF00010804.
- Dawson FH, Szoszkiewicz K. 1999. Relationships of some ecological factors with the associations of vegetation in British rivers. In: Biology, Ecology and Management of aquatic plants. Springer; p. 117–122.
- Demidchik V. 2015. Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot. 109:212–228. doi: 10.1016/j.envexpbot.2014.06.021
- DiTomaso JM, et al. 2013. Weed control in natural areas in the western United States. Davis: Weed Research and Information Center, University of California.
- Dodd AN, et al. 2005. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 309(5734):630–633. doi: 10.1126/science.1115581
- Ellawala KC, Asaeda T, Kawamura K. 2011. The effect of flow turbulence on plant growth and several growth regulators in Egeria densa Planchon. Flora-Morphology, Distribution, Functional Ecology of Plants. 206(12):1085–1091. doi: 10.1016/j.flora.2011.07.014
- Foyer CH, Lelandais M, Kunert KJ. 1994. Photooxidative stress in plants. Physiol Plant. 92(4):696–717. doi: 10.1111/j.1399-3054.1994.tb03042.x
- Frost-Christensen H, Sand-Jensen K. 1992. The quantum efficiency of photosynthesis in macroalgae and submerged angiosperms. Oecologia. 91(3):377–384. doi: 10.1007/BF00317627
- Genty B, Briantais J-M, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta (BBA)-General Subjects. 990(1):87–92. doi: 10.1016/S0304-4165(89)80016-9
- Giller PS. 2005. ‘River restoration: seeking ecological standards. Editor’s introduction’. J Appl Ecol. 42(2):201–207. doi: 10.1111/j.1365-2664.2005.01020.x
- Gómez I, et al. 1998. Effects of solar radiation on photosynthesis, UV-absorbing compounds and enzyme activities of the green alga Dasycladus vermicularis from southern Spain. J Photochem Photobiol, B. 47(1):46–57. doi: 10.1016/S1011-1344(98)00199-7
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. Circular California Agricultural Experiment Station. 347:1–32. (2nd edit).
- Jana S, Choudhuri MA. 1982. Glycolate metabolism of three submersed aquatic angiosperms during ageing. Aquat Bot. 12:345–354. doi: 10.1016/0304-3770(82)90026-2
- Keskinkan O, et al. 2004. Heavy metal adsorption properties of a submerged aquatic plant (Ceratophyllum demersum). Bioresour Technol. 92(2):197–200. doi: 10.1016/j.biortech.2003.07.011
- Knörzer OC, Burner J, Boger P. 1996. Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol Plant. 97(2):388–396. doi: 10.1034/j.1399-3054.1996.970225.x
- Kull O. 2002. Acclimation of photosynthesis in canopies: models and limitations. Oecologia. 133(3):267–279. doi: 10.1007/s00442-002-1042-1
- López-Figueroa F. 1992. Diurnal variation in pigment content in Porphyra laciniata and Chondrus crispus and its relation to the diurnal changes of underwater light quality and quantity. Mar Ecol. 13(4):285–305. doi: 10.1111/j.1439-0485.1992.tb00356.x
- MacAdam JW, Nelson CJ, Sharp RE. 1992. Peroxidase activity in the leaf elongation zone of tall fescue: I. spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol. 99(3):872–878. doi: 10.1104/pp.99.3.872
- Madsen JD, et al. 2001. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia. 444(1–3):71–84. doi: 10.1023/A:1017520800568
- Madsen TV, Enevoldsen HO, Jørgensen TB. 1993. Effects of water velocity on photosynthesis and dark respiration in submerged stream macrophytes. Plant, Cell Environ. 16(3):317–322. doi: 10.1111/j.1365-3040.1993.tb00875.x
- Madsen TV, Sand-Jensen K, Beer S. 1993. Comparison of photosynthetic performance and carboxylation capacity in a range of aquatic macrophytes of different growth forms. Aquat Bot. 44(4):373–384. doi: 10.1016/0304-3770(93)90078-B
- Michael TP, et al. 2003. Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science. 302(5647):1049–1053. doi: 10.1126/science.1082971
- Mishra S, et al. 2008. Thiol metabolism and antioxidant systems complement each other during arsenate detoxification in Ceratophyllum demersum L. Aquat Toxicol. 86(2):205–215. doi: 10.1016/j.aquatox.2007.11.001
- Mishra S, et al. 2009. Thiol metabolism play significant role during cadmium detoxification by Ceratophyllum demersum L. Bioresour Technol. 100(7):2155–2161. doi: 10.1016/j.biortech.2008.10.041
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7(9):405–410. doi: 10.1016/S1360-1385(02)02312-9
- Mjelde M, Hellsten S, Ecke F. 2013. A water level drawdown index for aquatic macrophytes in Nordic lakes. Hydrobiologia. 704(1):141–151. doi: 10.1007/s10750-012-1323-6
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22(5):867–880.
- Nishiyama Y, et al. 2001. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 20(20):5587–5594. doi: 10.1093/emboj/20.20.5587
- Nishiyama Y, et al. 2004. Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry. 43(35):11321–11330. doi: 10.1021/bi036178q
- Parveen M, Asaeda T, Rashid MH. 2017. Biochemical adaptations of four submerged macrophytes under combined exposure to hypoxia and hydrogen sulphide. PLoS ONE. 12(8):1–12. doi:10.1371/journal.pone.0182691.
- Pasternak T, et al. 2005. Morphogenic effects of abiotic stress: Reorientation of growth in Arabidopsis thaliana seedlings. Environ Exp Bot. 53(3):299–314. doi:10.1016/j.envexpbot.2004.04.009.
- Polesskaya OG, Kashirina EI, Alekhina ND. 2006. Effect of salt stress on antioxidant system of plants as related to nitrogen nutrition. Russ J Plant Physiol. 53(2):186–192. doi: 10.1134/S1021443706020063
- Pospíšil P. 2009. Production of reactive oxygen species by photosystem II. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1787(10):1151–1160. doi: 10.1016/j.bbabio.2009.05.005
- Pospíšil P. 2016. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front Plant Sci. 7:1950. doi: 10.3389/fpls.2016.01950
- Powles SB. 1984. Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol. 35(1):15–44. doi: 10.1146/annurev.pp.35.060184.000311
- Riis T, Biggs BJF. 2003. Hydrologic and hydraulic control of macrophyte establishment and performance in streams. Limnol Oceanogr. 48(4):1488–1497. doi: 10.4319/lo.2003.48.4.1488
- Rijstenbil JW, Coelho SM, Eijsackers M. 2000. A method for the assessment of light-induced oxidative stress in embryos of fucoid algae via confocal laserscan microscopy. Mar Biol. 137(5–6):763–774. doi: 10.1007/s002270000443
- Sand-Jensen K. 1997. Broad-scale comparison of photosynthesis in terrestrial and aquatic plant communities. Oikos. 80:203–208. doi: 10.2307/3546536
- Sauer H, Wartenberg M, Hescheler J. 2001. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 11(4):173–186. doi: 10.1159/000047804
- Schutten J, Davy AJ. 2000. Predicting the hydraulic forces on submerged macrophytes from current velocity, biomass and morphology. Oecologia. 123(4):445–452. doi: 10.1007/s004420000348
- Sculthorpe CD. 1967. Biology of aquatic vascular plants. London: St. Martin’s Press.
- Shang W, Feierabend J. 1999. Dependence of catalase photoinactivation in rye leaves on light intensity and quality and characterization of a chloroplast-mediated inactivation in red light. Photosynth Res. 59(2–3):201–213. doi: 10.1023/A:1006139316546
- Slesak I, et al. 2007. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochimica Polonica-English Edition-. 54(1):39–50. doi: 10.18388/abp.2007_3267
- Søndergaard M, Kristensen P, Jeppesen E. 1992. Phosphorus release from resuspended sediment in the shallow and wind-exposed Lake Arresø, Denmark. Hydrobiologia. 228(1):91–99. doi: 10.1007/BF00006480
- Stevens CL, Hurd CL. 1997. Boundary-layers around bladed aquatic macrophytes. Hydrobiologia. 346(1–3):119–128. doi: 10.1023/A:1002914015683
- Stiers I, Njambuya J, Triest L. 2011. Competitive abilities of invasive Lagarosiphon major and native Ceratophyllum demersum in monocultures and mixed cultures in relation to experimental sediment dredging. Aquat Bot. doi:10.1016/j.aquabot.2011.05.011.
- Walsby AE. 1997. Numerical integration of phytoplankton photosynthesis through time and depth in a water column. New Phytol. 136(2):189–209. doi: 10.1046/j.1469-8137.1997.00736.x
- Wellburn AR. 1994. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 144(3):307–313. doi: 10.1016/S0176-1617(11)81192-2
- Wersal RM, Madsen JD. 2013. Influences of light intensity variations on growth characteristics of Myriophyllum aquaticum. J Freshw Ecol. 28(2):147–164. doi: 10.1080/02705060.2012.722067
- Wheeler WN. 1980. Effect of boundary layer transport on the fixation of carbon by the giant kelp Macrocystis pyrifera. Mar Biol. 56(2):103–110. doi: 10.1007/BF00397128
- Yuan G, et al. 2016. Growth and C/N metabolism of three submersed macrophytes in response to water depths. Environ Exp Bot. 122:94–99. doi: 10.1016/j.envexpbot.2015.09.009
- Zaman T, Asaeda T. 2013. Effects of NH4–N concentrations and gradient redox level on growth and allied biochemical parameters of Elodea nuttallii (Planch.). Flora-Morphology, Distribution, Functional Ecology of Plants. 208(3):211–219. doi: 10.1016/j.flora.2013.02.009
- Zhang K, et al. 2015. The fate of Amazonian ecosystems over the coming century arising from changes in climate, atmospheric CO 2, and land use. Glb Chg Bio. 21(7):2569–2587. doi: 10.1111/gcb.12903
- Zolfaghari R, Hosseini SM, Korori SAA. 2010. Relationship between peroxidase and catalase with metabolism and environmental factors in Beech (Fagus orientalis Lipsky) in three different elevations. Int J Env Sci. 1(2):243–252.