 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Salinity of cultivable land is a growing global concern that has been affecting the yields of major crops worldwide such as pigeon pea. In the current study, transgenic pigeon pea plants were developed using an in-planta Agrobacterium-mediated genetic transformation method wherein OsRuvB, a rice DNA helicase gene, was incorporated to induce salt tolerance in pigeon pea plants. Transformation efficiency of 35.7% was achieved with stable insertion of OsRuvB in transgenic lines. When subjected to salinity stress induced by 75 mM NaCl increase in chlorophyll content, relative water content, peroxidase and catalase activity in transgenic lines was observed over the wild type plants. Membrane injury index and lipid peroxidation were significantly reduced in transgenic lines. Proline and Total Soluble Sugar content were enhanced in both transgenic plants and wild type strains. It was inferred that transgenic lines were tolerant to salinity stress and tolerance may be imparted through an alternative unknown pathway.
GRAPHICAL ABSTRACT
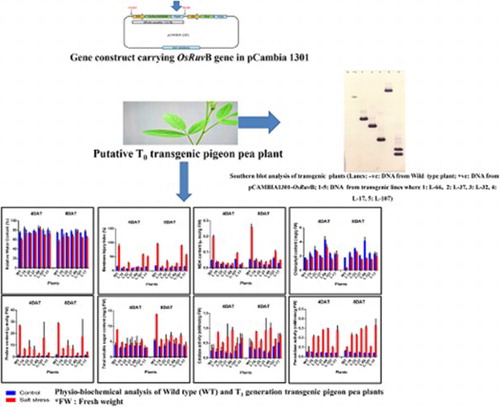
Introduction
A major setback in agricultural productivity has been observed on a global scale, an attribute aggravated due to the changing climatic conditions. The effect of the changes in climates may be observed as increasing salinity of cultivable land, drought, submergence and extreme temperatures. All these factors, shortly termed as abiotic stress by agriculturalists, have severe detrimental effect on crop productivity (Boyer Citation1982; Mahajan and Tuteja Citation2005; Rao et al. Citation2013). Of these salinity has been identified as one of the major environmental constraints restricting productivity of crops worldwide. Excessive irrigation has resulted in increasing salinity of croplands at a rate wherein 50% of the cultivable land may be lost to salinity on a global scale 2050 (Wang et al. Citation2003). Salinity affects crop production by inducing other stresses inclusive of water stress, ionic stress, oxidative stress and nutritional imbalance (Tsugane et al. Citation1999; Hernandez et al. Citation2001; Isayenkov Citation2012). Salinity initially induces osmotic stress causing drought like symptoms followed by ionic imbalance. This in turn leads to salinity induced ionic toxicity or stress in plants. Major processes such as photosynthesis, protein and lipid metabolisms, etc are affected due to salinity stress which in turn results in low crop yields (Rasool et al. Citation2013). Major effects of salinity on plants include reduced CO2 assimilation: mainly due to stomatal closure, membrane damage, reduced activity of key enzymes of necessary cellular mechanisms such as CO2 fixation, ATP synthesis and increase in ROS due to the enhanced metabolite flux through photo-respiratory pathway.
Plants respond to salinity stress at cellular as well as whole-organism level. The effects are significant at physiological as well as the biochemical level and can be observed as reduced leaf size, disturbed plant water relations and decreased water-use efficiency. To withstand the salinity stress, plants adapt several mechanisms like accumulation of compatible osmolyte molecules such as proline and glycine betaine, enhanced uptake of water through water channels in membrane, improved root architecture, modified leaves as well as stems and stomatal closure to reduce transpirational loss of water.
With the decreasing agricultural lands and indifferent production practices in the current times remediation of salt-affected soil appears to be an economically challenging task. It thus calls for a probable solution in improving the plant genotype to make it resilient/tolerant to salinity. Attempts to impart tolerance in plants to salinity stress so as to meet the rising food demand for the growing population have been made. Molecular breeding strategies have been adopted for crop improvement programs in several crops, including legumes such as soybean and common bean and attempts have also been made for improvement in field pea (Leonforte et al. Citation2013). However, genetic engineering studies have demonstrated fruitful results wherein development of transgenic plants showed successful adaptation to laboratory induced salinity stress. In plants, DNA helicases function as molecular motor proteins in various cellular mechanisms and are essential for almost all DNA metabolic activities including pre-mRNA splicing (Tuteja and Tuteja Citation2004a).Studies have shown that NTP-dependent transcription activators play a major role in abiotic stress response (Tuteja and Tuteja Citation2004b). RuvB DNA helicases have demonstrated the ability to impart tolerance in plants for salinity stress. However, the expression of the same has been reported in only three plants- one in Arabidopsis (Holt et al. Citation2002) and two in rice (Wang et al. Citation2011; Saifi et al. Citation2018a). The pathway through which RuvB DNA helicases induces stress tolerance in other crop plants is yet to be elucidated. It was thus suggested to apply transgenic approach and incorporate genes encoding RuvB DNA helicases in study plant, pigeon pea, and investigate the potential for abiotic stress tolerance.
Pigeon pea [Cajanus cajan (L.) Millsp.] is a diploid legume crop (2n = 2x = 22) which belongs to the family Fabaceae. Pigeon pea is the second most important legume crop after chickpea in India and is normally cultivated during the kharif season. Pigeon pea shows a salt sensitive growth response. Excess salt causes physiological drought in pigeon pea and continuous and high exposure is lethal to the same. Genetically engineering the pigeon pea plant to induce salt tolerance is one such solution. While genetic transformation of pigeon pea is fastidious, it is recalcitrant to tissue culture regeneration. In the present study, tissue culture-independent plant transformation strategy was employed to demonstrate the functionality of RuvBDNA helicase in transgenic pigeon pea and the potential applicability for improving the yields of the same under salinity stress.
Methods
Plant material and transformation
Seeds of pigeon pea (cv. Manak) used for genetic transformation in this study were obtained from Pulses Section, Department of Genetics and Plant Breeding, CCS HAU, Hisar. The Agrobacterium tumefaciens strain LBA4404 harboring pCAMBIA 1301-OsRuvB gene was used for transformation. Transgenic plants were developed using a rapid and efficient Agrobacterium-mediated transformation protocol (Patent Application no. 201811012099).
Screening of putative transformants
Genomic DNA was isolated from young leaves of putative transformed and wild type pigeon pea plants using CTAB DNA isolation protocol (Saghai-Maroof et al. Citation1984) and subjected to PCR amplification in C 1000 Touch™ Thermal Cycler (Bio-Rad, USA) using OsRuvB gene-specific primers (OsRuvB-F 5′-ACGGATCCCTCGAGATGAGGATCGAGGAGGTGCAGTCGG-3′ and OsRuvB-R 5′-ACGGATCCGAATTCTTAGGTGATGTATCTTTCC-3) for detection of the transgene. The PCR amplification steps comprised of initial denaturation at 94°C for 5 min followed by 35 cycles with 1 min at 94°C, 1 min at 50°C and 1 min 30 s at 72°C. The final extension was carried out for 10 min at 72°C.
Detection of transgene copy number
Stable integration and copy number of the transgene in the T0 transgenic pigeon pea lines was confirmed by Southern hybridization using Total Blot+ nylon membrane, Biotin Decalabel DNA Labelling Kit and Biotin Chromogenic Detection Kit (Fermentas, USA). The genomic DNA isolated from wild type pigeon pea plants was used as a negative control.
The genomic DNA isolated from transgenic and wild type plants was digested, electrophoresed on 1.0% agarose gel and transferred onto Total BLOT+ nylon membrane. PCR amplified fragment of OsRuvB gene was biotin-labeled using Biotin Decalabel DNA Labelling Kit (Thermo Scientific) and used as a probe to hybridize to the target DNA by incubating overnight at 42°C with moderate shaking in the hybridization oven. Hybridization of Biotin-labeled probe to the transgene was detected with alkaline phosphatase-conjugated streptavidin using Biotin Chromogenic Detection Kit (Fermentas, USA). Plasmid DNA was used as positive control.
Efficacy of transgene for salt tolerance:
Wild type and six T1 transgenic lines were subjected to 75 mM NaCl stress 15 days after germination, to assess the efficacy of OsRuvB gene for salt stress tolerance. The plants were grown in the transgenic greenhouse facility of the Department. The plants were watered with 75 mM NaCl solution up to saturation point of the soil to avoid any leaching of water. Following physio-biochemical parameters were studied at 0, 4 and 8 days after salt stress treatment.
Relative water content (RWC)
Relative Water Content was studied using the method given by Smart and Bingham (Citation1974). Leaf discs, (diameter – 1.5 cm) were cut, weighed and put in de-ionized water for 4 h for hydration to full turgidity, blotted dried and weighed to obtain fully turgid weight. Then the samples were oven-dried at 80°C for 24 h and weighed to get the dry weight. RWC was calculated using the following formula:
Electrolyte leakage or membrane injury (MI)
Membrane Injury was analyzed according to the protocol of Sullivan (Citation1972) (22) wherein leaf samples (100 mg) were taken in 10 ml de-ionized water and incubated for 24 h at 4°C. The conductance of the liquid was recorded at 25°C with a conductivity meter (ECa = before boiling). Following this, the samples were boiled at 100°C in a water bath for 10 min, allowed to cool and the electrical conductivity (ECb = after boiling) was measured again. MI was calculated by the following formula:
Malondialdehyde (MDA) content
Malondialdehyde content was measured by modified Heath and Packer (Citation1968) method. Here, Fresh leaf tissue (300 mg) was homogenized in 5 ml of 0.1% Trichloroacetic acid (TCA) (w/v) solution and centrifuged at 8000 rpm for 15 min. One ml of the supernatant was taken in a test tube and precipitated by addition of 4 ml 20% TCA containing 0.5% thiobarbituric acid (TBA) and placed in a water bath shaker at 95°C for 30 min followed by quickly cooling in an ice-bath. After centrifugation at 8000 rpm for 10 min the absorbance of the reaction mixture was read at 532 nm and the value for non- specific absorption at 600 nm was subtracted. The amount of MDA was calculated using its extinction coefficient of 155 mM−1 cm−1.
Chlorophyll content
Sawhney and Singh's (Citation2002) dimethyl sulfoxide (DMSO) method was followed to assess the chlorophyll content in the WT and transgenic pigeon pea plants. Here, leaf discs (30 mg) were washed, blotted dried and dipped overnight in 3 ml of DMSO to extract chlorophyll. Chlorophyll content in DMSO was estimated using a spectrophotometer after the incubation period wherein absorbance was read at 663 and 64 nm, respectively and its content was calculated using the formula:
where V is volume of DMSO, A is absorbance at specific wavelengths and W is the weight of tissue taken.
Proline content
The proline content in the leaves was estimated as per the method given by Bates et al. (Citation1973) (5). Leaf samples (300 mg) were homogenized in 3 ml of 3% sulfosalicylic acid and centrifuged at 12,000 g for 10 min. To 2 ml of the supernatant in a test tube, 2 ml glacial acetic acid and 2 ml acid ninhydrin were added and homogenized. The tubes were kept in a water bath for 1 h at 100°C and cooled in ice bath. The reaction mixture was extracted by addition of 4 ml toluene. The tubes were then left at room temperature for 30 min for separation of the two phases. The optical density of the chromophore-containing toluene (upper phase) was measured at 520 nm using toluene as blank. The proline concentration was determined from a standard curve developed using L-Proline.
Total soluble sugar (TSS) content
Yemm and Willis (Citation1954) (33) protocol was followed to determine the TSS content . Fresh leaf samples weighing 300 mg were homogenized in 80% ethanol using acid-washed sand and refluxed thrice with 80% ethanol. The supernatant from different extractions was pooled and the final volume was made to 5 ml with 80% ethanol. The extract so obtained was used for estimation of TSS content. An aliquot (0.2 ml) from the extract was evaporated dry in a boiling water bath and the residue was dissolved in 1 ml of distilled water. The resultant solution was mixed with 4.0 ml of anthrone reagent and heated in a water bath for 10 min. After cooling, absorbance was recorded at 620 nm. The TSS content was estimated from the standard curve prepared from gradient concentrations of D-glucose.
Catalase (EC 1.11.1.6) activity
Catalase activity was estimated according to the procedure described by Aebi (Citation1984) (1). All the steps of extraction were carried out at 0–4°C. Leaf tissue (1 g) was washed with distilled water; blot dried and macerated using a chilled pestle and mortar in 3.0 ml of cold extraction buffer (0.1 M potassium phosphate buffer pH 7.0 containing 0.1 mM EDTA, 1% (w/v) PVP, 0.5% triton X-100 and 20% glycerol with a pH 7.8). The resultant homogenate was centrifuged at 10,000 rpm for 15 min at 4°C. The supernatant was carefully decanted and used as the cell free extract. The reaction mixture in a final volume of 3 ml, contained 0.1 M phosphate buffer (pH 7.0), 10 mM H2O2 and 100 µl of cell free extract. Reaction was initiated with the addition of H2O2 and enzyme activity was determined by following the degradation of H2O2 at 240 nm for 2 min. Enzyme activity was calculated using the value 39.4 mM−1 cm−1 – extinction coefficient value for H2O2. One unit of enzyme activity corresponded to one mmol of H2O2 consumed per minute during the reaction.
Peroxidase (EC 1.11. 1.7) activity
The procedure of Siegel (Citation1993) was followed for estimating peroxidase activity (20). The extraction protocol for the enzyme was same as that for catalase estimation. Three ml of reaction mixture contained 0.1 M potassium phosphate buffer (pH 7.0), 0.1 mM guaiacol, 0.1 mM H2O2 and 100 µl cell free extract. Reaction was started with the addition of H2O2 and increase in absorbance at 470 nm was recorded for 2 min. The activity was calculated using the extinction coefficient value of 26.6 mM−1 cm−1 for guaiacol. One unit of enzyme activity was equivalent to µmol of H2O2 oxidized per minute during the reaction.
Inheritance pattern of OsRuvB gene in T1 generation
The inheritance pattern of OsRuvB gene in T1 generation was assessed by the presence of transgene detected through direct PCR amplification with gene-specific primers. Segregation data was analyzed by Chi-square test (p ≤ 0.05, χ2 = 3:841).
Statistical analysis
Analysis in all the physio-biochemical parameters was carried out in triplicates (three plants for each parameter). A two-way ANOVA was performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla California USA, www.graphpad.com) to calculate the least significant difference (LSD) between mean values within treatments at 0.05 significance levels.
Equipments and settings
The gel images were captured using GenoSens capture software under a BenchTop Gel-Documentation system with trans UV operating at 302nm and exposure settings set to auto exposure.
Results
Development and selection of transgenic pigeon pea plants carrying OsRuvB
An in-planta method of Agrobacterium-mediated genetic transformation was used to develop the transgenic pigeon pea plants (Patent Application No. 201811012099).The genomic DNA isolated from putative T0 transgenic plants was subjected to PCR amplification for confirming presence of OsRuvB gene using gene-specific primers. Amplification of 1.4 kb fragment confirmed the presence of OsRuvB gene in transgenic plants while the same was not observed in the genomic DNA isolated from wild type plants. Out of 70 putative T0 plants screened, 25 plants read positive for the presence of the gene of interest, thereby, presenting a transformation efficiency of 35.70% (). Six PCR positive T0 plants with better vigor and yield were advanced to T1 generation (). PCR analysis of T1 plants demonstrated the stability of transgene in these plants. Southern analysis with OsRuvB as a probe demonstrated that the T-DNA was integrated either as single copy (transgenic events L-10, L-17, L-32, L-37, L-66) or double copy (transgenic event L-107) (). The inheritance pattern of OsRuvB gene in T1 generation was assessed by PCR analysis. Segregation data was analyzed by Chi square test. The results indicate that the transgenes segregated according to the Mendelian ratio (p ≤ 0.05, χ2 = 3.841). The T1 plants of the line L-10, L-17, L-32, L-37 and L-66, segregated in a normal Mendelian ratio with a 3:1 ratio for the transgene, suggesting a single copy of the integrated transgene while the ratio for line L-107 was 15:1 suggesting two copies of the integrated transgene (). Stress-response experiments were carried out with the 6 selected transgenic lines in T1 generation.
Figure 1. 1.5% Agarose gel showing PCR amplification of OsRuvB gene using gene specific primers (Lanes: M: 1 kb DNA ladder (Fermentas); −ve: negative control; 1–70: T0 plant samples; +ve: positive control). Full length gels are displayed in supplementary figure 1. Different gels separated by white spacing in between.
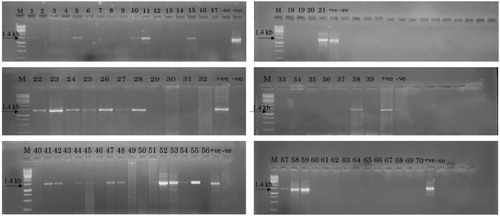
Figure 2. Southern blot analysis of transgenic plants (Lanes: –ve: DNA from Wild type plant; +ve: DNA from pCAMBIA1301-OsRuvB; 1–6: DNA from transgenic lines where 1: L-66, 2: L-37, 3: L-32, 4: L-17, 5: L-107, 6: L-10). Full length blots are displayed in supplementary figure 2. Different blots separated by white spacing in between.
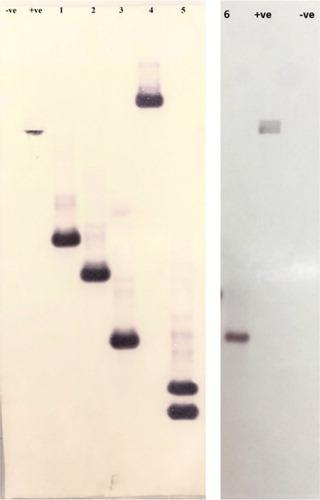
Table 1. Seed yield (g/plant) in T0 transgenic and wild type pigeon pea plants.
Table 2. Segregation analyses of the transgene in T1 progeny of transgenic pigeon pea based on PCR analysis.
Evaluation of salt tolerance in T1 transgenic plants
The wild type plants and the 6 selected transgenic pigeon pea lines from T1 generation were subjected to 75 mM NaCl stress after 15 days of germination and observations on physio-biochemical parameters were recorded. After 4days of inducing salinity stress the WT demonstrated poor growth and wrinkling and browning of leaves while pigeon pea plants carrying OsRuvB gene showed a healthy growth with an increase in plant height and greener leaves (). A steady decline in Relative water content (RWC) with increased duration of 75 mM salt stress was recorded in wild type (WT) as well as transgenic lines. The WT and the lines (L-10, L-17, L-32, L-37, L-66, L-107) showed 19.89%, 13.42%, 1.37%, 14.16%, 3.43%, 11.93% and 9.48% decline in RWC (at 75 mM NaCl stress), respectively, on 4th day of treatment. The decline in RWC on 8th day of treatment for WT and transgenic lines was 25.59%, 20.49%, 6.49%, 4.59%, 8.05%, 14.35% and 17.89%, respectively. Thus, it was inferred that under stress conditions the RWC was significantly more in transgenic pigeon pea lines than in WT (a). The membrane injury (MI) as evident from the histogram (b) increased in both the wild type and transgenic pigeon pea plants under salinity stress. However, the extent of injury was quite less in transgenic pigeon pea lines as compared to the wild type plants after 4 and 8 days of treatment. The wild type plants under stress had an increase of 4.65-fold in MI whereas in the transgenic lines the maximum MI was recorded for line L-107 with a 4.28-fold increase after four days of treatment. Similar results were observed in MI after 8 days of treatment (b). The MDA content as consistent with the membrane injury was also found to increase in both WT and transgenic pigeon pea lines. The MDA content as evident from the histogram (c) was higher in WT plants as compared to the transgenic lines. The increase in MDA in WT plants was 3.25 and 4.20-fold on 4th and 8th day of treatment respectively while in transgenic lines (L-10, L-17, L-32, L-37, L-66, L-107) it was between 0.16 and 1.35-fold on 8th day of treatment.
Figure 4. Effect of salt stress (75 mM NaCl) on (a) Relative Water content (%), (b) Membrane Injury Index (%), (c) MDA content (µmol/g FW), (d) Chlorophyll content (mg/g FW), (e) Proline content (µmol/g FW), (f) Total Soluble Sugar content (mg/g FW), (g) Catalase activity (mM/min/g FW), (h) Peroxidase activity (mM/min/g FW) in wild type and T1 transgenic pigeon pea lines. (Detailed analysis given in supplementary table 1–8).
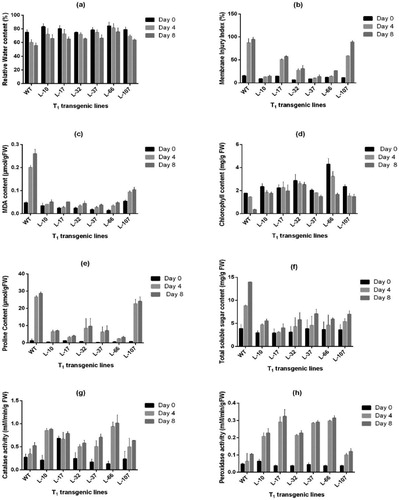
A significant reduction in total chlorophyll content in both WT and transgenic lines was observed at 4 and 8 days after salt stress treatment (d). At 75 mM NaCl stress, however, the transgenic lines showed 0.25 to 1.24-fold and 3.2 to 6.2-fold higher chlorophyll content than the WT on 4th and 8th days of treatment respectively. Comparisons of the proline content under controlled growth conditions and salinity stress were made simultaneously after 4th and 8th day of induced salinity stress. It was noted that the proline content was relatively low in the WT and transgenic lines under the control conditions on both 4 and 8 days after treatment. However, a drastic increase was observed under salt stress conditions (e). Both transgenic lines and WT under salt stress recorded significantly higher levels of proline on the 4th and 8th day after treatment as compared to the control conditions. However, the proline content in transgenic lines was 14–88% (2.46 to 22.70 µmol/g FW) lesser compared to the WT (26.62 µmol/g FW) on the 4th day of treatment. A similar observation was made for both after 8 days of treatment. Likewise, the TSS content was evaluated in both WT and transgenic lines under controlled and salinity stress growth conditions on the 4th and 8th day of treatment. Results were similar to proline content wherein relatively low TSS content was observed in the WT and transgenic lines under the control conditions while in the treated plants the content was higher (f). As with proline content, the TSS content was relatively higher in WT plants (8.82 mg/g FW and 13.95 mg/g FW) under salt stress conditions as compared to the transgenic lines (3.06 to 5.38 mg/g FW and 4.01–5.53 mg/g FW) after four and eight days of treatment respectively.
A significant increase in the catalase and peroxidase activities was observed in both WT and transgenic plants grown under 75mM salt stress over-controlled conditions. The catalase activity in WT under stress had an increase of 0.25-fold and in transgenic lines it varied from 0.68 to 4.6-fold after four days of treatment. Increased activities were observed after 8 days of treatment with a 0.48-fold rise in WT and 1.08 to 6.23-fold in transgenic lines in comparison to the control conditions (g). Likewise, there was a significant increase in peroxidase activity in both WT and transgenic lines under 75 mM salt stress condition over control (h). WT under stress had an increase of 0.20-fold and 0.66-fold after the 4th and 8th days, respectively, of treatment respectively while the activity in transgenic lines varied from 1.50 to 6.25-fold and 1.80 to 6.75-fold after four and eight days of treatment respectively. It was noted that the increase in peroxidase activities was more profound in WT over transgenic plants between the 4th and 8th days of salt stress treatment.
Discussion
Salinity stress has been identified as a major concern affecting the agricultural yields on a global scale with the arid and semi-arid regions being affected more (Vaidyanathan et al. Citation2003). Imbalance in water-plant relations, ionic toxicity, salinity induced oxidative stress and nutritional imbalance are the major concerns in this regards as the stresses individually and together damage crop production both qualitatively and quantitatively (Beebe Citation2012; Yang et al. Citation2013). Since remediation of saline soils to cultivable quality is not economically feasible, solutions to impart tolerance to salt stress in major crop plants are being researched exceptionally in an attempt to improve crop productivity and meet the current food demands of the growing population. Development of transgenic plants that can tolerate salt stress and salt induced afore stresses have been and continue to be the ideal strategy to date.
Salinity stress manifests itself in different forms as a result of which salinity induced crop losses are more. Higher salt concentrations result in drought-like conditions initially as a result of which the plant-water relationship is imbalanced (Rasool et al. Citation2013). Salt induced osmotic stress leads to ionic toxicity and oxidative stress at the cellular level. With the induction of salt stress one may observe stomatal closure and inhibition of cell growth, both prominent in the shoot (Munns and Passioura Citation1984; Munns and Termaat Citation1986). Water deficit affects photosynthesis which in turn leads to carbon deficit and high photochemical competence (Chaves Citation1991). This in turn leads to the synthesis of reactive oxygen species and initiation of oxidative stress. Oxidative stress result in damage to cellular components, degradation of chlorophyll and peroxidation of the membrane lipids; all of which result in impaired plant metabolism and improper growth of the crop plant and thereby poor yield (Rasool et al. Citation2013; Isayenkov and Maathuis Citation2019).
Plants to in attempt to tolerate salinity stress trigger multiple responses at the wholeplant, physiological, biochemical, cellular and molecular levels. Indepth investigations have been carried out to study these mechanisms and induce the same in crop plants to tolerate salinity stress leading to better yields. By far, genetic transformation studies have demonstrated successful induction of tolerance to salt stress and a better understanding of the physiological processes, a milestone that was not achieved through biochemical approaches (Birch Citation1997; Araújo et al. Citation2015). Transgenics is being exploited to induce tolerance to salinity stress in major crop plants and several reports of successful transformations have been reported.
The current study was an attempt at imparting tolerance to salinity stress in pigeon pea plants through the application of transgenics. Here transgenic pigeon pea lines carrying OsRuvB gene for salt tolerance were developed using an efficient Agrobacterium-mediated transformation protocol (Patent Application no.201811012099). RuvB is a conserved gene family of DNA helicases present in both single and multicellular organisms. The gene family has been identified to impart stress tolerance due to abiotic factors in Oryza sativa (rice) (Saifi et al. Citation2018b). The resultant was a stable transgene integration in the transgenic pigeon pea lines with a transformation efficiency of 35.7% which is quite high for a recalcitrant legume like pigeon pea. The percentage of confirmed transgenic plants was found to be higher as compared to the previous studies on pigeon pea that reported transformation efficiency at 19.3% (Surekha et al. Citation2014), 15 (Surekha et al. Citation2005) and 30% (Lawrence and Koundal Citation2001) respectively. Agrobacterium tumefaciens mediated transgenic studies for inducing salinity tolerance in chickpea using OsRuvB demonstrated a transformation efficacy of 17% (Preeti Citation2018). It could thus be stated that the in-planta Agrobacterium-mediated transformation protocol in the current study can be exploited for further transformation associated studies owing to its better efficacy.
Six selected PCR positive T0 plants harboring OsRuvB gene were subjected to Southern blot analysis and a single copy insertion was observed in five out of six lines under study whereas one line L-107 was found to carry two-copies of the transgene. The analysis also confirmed stable transgene integration in the pigeon pea plants. Segregation analysis of the transgene in T1 plants of the line L-10, L-17, L-32, L-37 and L-66 showed segregation in a monogenic Mendelian ratio of 3:1, further confirming a single-copy insertion of the transgene while, the line L-107 which had two copies of the transgene, segregated in 15:1 ratio (). Higher transformation efficacy here could be indicative of a potential development of transgenic pigeon pea plants for commercial application so as to sustain the food needs of the current population.
Higher salt concentrations impair the water-plant relations and in lieu of the disturbed osmotic potential the relative water uptake is reduced in plants. This result in drought-like symptoms and reduced stomatal conductance, so as to conserve water, in the plants (Rasool et al. Citation2013; Isayenkov and Maathuis Citation2019). Relative water content is a physiological index used to study the water retention capacity and it acts as an appropriate parameter to measure water status and osmotic adjustments of plants under abiotic stresses (Ravikumar et al. Citation2014). The RWC declined in both WT and transgenic lines under salt stress but the reduction was more prominent in WT plants implying thereby that the transgenic lines could effectively retain more water in their tissues than WT under increasing durations of salt stress. The findings were observed to be in par with related transformation studies that reported better water retention in transgenic crops inclusive of maize (Lu et al. Citation2013), tomato (Rai et al. Citation2013) and tobacco(Liu et al. Citation2008) for drought stress and tobacco (Yadav et al. Citation2012) and pigeon pea (Surekha et al. Citation2014) for salinity stress. Better water retention indicates better adjustment of the plant under osmotic stress and thereby better growth and productivity of the transgenic pigeon pea plant. The results also imply that the OsRuvB was effective in inducing tolerance to the transgenic plants.
Reduced stomatal conductance due to salinity stress results in reduced carbon assimilation which in turn results in decreased concentrations of biomolecules such as carbohydrates(Chaves et al. Citation2009; Rasool et al. Citation2013). Apart from carbon assimilation, carbohydrates are known to play a vital role in osmoprotection and osmotic adjustments and also in scavenging radical species under salt stress (Nemati et al. Citation2011; Rasool et al. Citation2013). Carbon deprivation also leads to increased photochemical activities that lead to the synthesis of reactive oxygen species (ROS). ROS in turn damages cell components, degrades chlorophyll and brings about membrane lipid peroxidation. These parameters are indicative of salinity and stress (Rasool et al. Citation2013; Isayenkov and Maathuis Citation2019) and thus were considered in the current study.
Current transformation study has demonstrated higher concentrations of total soluble sugars in both wild type and transgenic plants. Higher proline concentrations were also observed. Proline is an osmoprotectant that has a key role in maintaining the osmotic balance in crop plants, protecting the cell organelles, enzymes and enhancing the osmolarity of the plant cells under stress conditions (Surekha et al. Citation2014). While higher concentrations of these biomolecules are indicative of tolerance capacity of both plants to stress, they are also indicative of better stomatal conductance and improved carbon assimilation. Higher concentrations of proline and total soluble sugars also imply the adjustments to the osmotic potential of the pigeon pea plants to tolerate salt stress as well as to enhance water uptake under the same. However, the increase in proline and total soluble sugars was significantly more in wild type plants as compared to the transgenic plants under stress (e,f) suggesting that other osmolytes like glycine, betaine or polyamines etc., that were not considered in the present study may be playing a vital role in osmo-protection (Ahmad et al. Citation2010). It could also be suggested that the transgene OsRuvB may be having an alternate pathway to induce tolerance to salt stress which has not been studied yet.
Oxidative stress inhibits the synthesis of chlorophyll as well as initiates its degradation. Reduced chlorophyll means reduced photosynthesis which in turn indicates low yields. The transgenic lines also maintained a higher chlorophyll content under salt stress conditions and studies have demonstrated that higher chlorophyll content positively influences the photosynthetic rate (Augustine et al. Citation2015). This indicates that the transformation has imparted an effective management of the oxidative stress and that the photochemical activity of photosystem II has not been reduced. It can thus be concluded that OsRuvB gene transformation can improve the production of the pigeon pea crops under salinity stress.
Salt stress is also linked with the inability to manage oxidative stress either by reduction in the production of ROS or by effectively scavenging them. ROS act as signaling components in plants controlling various cellular processes. However, a relatively high concentration of the ROS results in impairment of cellular components and thereby cell damage. Plants through the production of antioxidant enzymes keep the ROS concentration in check. Under salinity stress management of the ROS becomes challenging and the inability to scavenge could result in poor yields. A significantly higher catalase and peroxidase activity in transgenic lines of pigeon pea demonstrated their ability to scavenge ROS. Improved catalase and peroxidase activity have been cited in Arabidopsis (Zhao et al. Citation2017), peanut (Singh et al. Citation2014) and Sorghum bicolor (Anjaneyulu et al. Citation2014; Surender Reddy et al. Citation2015) under salt stress wherein the enhanced productivity resulted in effective management and scavenging of ROS. Production of antioxidant enzymes under salt stress allows effective management of the ROS and thereby improves the longevity of the cells and its components.
Additional proof for reduced oxidative damage was demonstrated by less membrane injury index and MDA content in the transgenic plants compared to wild type pigeon pea plants. MDA is an indicator of membrane lipid peroxidation and thus is considered an effective marker of oxidative stress-induced cellular damage. Lower the MDA content better the management of oxidative stress and lower the membrane injury index. It can thus be inferred that under salt stress conditions the transgene OsRuvB is playing a key role in maintaining a lower ROS content, thereby, preventing membrane damage in plants. Reduced membrane damage and MDA content in related transformation studies on pigeon pea have been reported (Surekha et al. Citation2014). The results were observed to be in par with related studies on transgenic plants inclusive of tobacco (Yadav et al. Citation2012), apple (Li et al. Citation2013), alfalfa (Tang et al. Citation2013; Li et al. Citation2014), tobacco (Vaid et al. Citation2015) and cotton (Zhang et al. Citation2009) under salinity stress. The findings are indicative of effective ROS management and successful induction of tolerance in the transgenic plants towards salinity stress.
Transformation studies for salt stress have also reported improved physio-biochemical traits in the transgenic lines over WT in the absence of salinity stress i.e. control growth conditions. The transgenic lines here include peanut (Bhatnagar-Mathur et al. Citation2014), soybean (de Paiva Rolla et al. Citation2014), tomato (Rai et al. Citation2013) and other plant species (Kasuga et al. Citation1999). Likewise, enhanced physio-biochemical traits were observed in transgenic pigeon pea plants in the current transformation studies. This could be due to the constitutive expression of OsRuvB under the regulatory control of CaMV35S promoter. From the observation it can be concluded that the transgene OsRuvB induced tolerance in the transgenic pigeon pea lines to salt stress.
Conclusion
From the results it can thus be concluded that OsRuvB transformation in pigeon pea plants can better impart tolerance in the plants under osmotic stress. Also, that the transformation can mediate improved plant growth, better regulation of plant processes and improved yields under salt stress. Based on the higher transformation efficacy achieved in the current study it can be stated that OsRuvB integrated transgenic pigeon peas provides a higher scope of studying plant physiology under stress and otherwise. Also, that these transgenic plants demonstrate better potential for commercial application in terms of sustainable agriculture compared to previous transformation studies with pigeon pea. Nevertheless, the pathway through which OsRuvB rendered improved physio-biochemical outcomes under salt stress needs to be further investigated. Also, the role of other osmolytes in imparting tolerance to the pigeon pea plants needs to be elucidated.
Acknowledgements
The authors would like to thank the Director of Research, CCS Haryana Agricultural University, Hisar for providing the necessary Financial support to carry out this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ORCID
Rakshita Singh http://orcid.org/0000-0002-4606-6932
Additional information
Notes on contributors
Rakshita Singh
Dr Rakshita Singh PhD from Department of Department of Molecular biology, Biotechnology and Bioinformatics, CCS HAU, Hisar, Haryana, India. Experience in plant transformation and generation of transgenics in different crops and studying physiology of plants under stress.
Sudhir Sharma
Sudhir Sharma currently pursuing PhD from UMass, Amherst, USA. He has experience in working on salinity tolerance in wheat and is currently working on genome editing in rice.
Pushpa Kharb
Dr Pushpa Kharb presently, Prof. & Head, Department of Molecular biology, Biotechnology and Bioinformatics, CCS HAU, Hisar, Haryana, India. She served as Director (Technical), Centre for Plant Biotechnology for three years. She completed her MSc and PhD in Genetics from CCSHAU, Hisar. She was a Rockefeller Foundation Post-doctoral Fellow for two years at Texas A & M University, USA. She has been granted three patents so far out of seven patents filed. She is a recipient of ICAR sponsored Best Teacher award. She is a member of several academic societies and has published more than 50 research papers in national and international journals.
Shabnam Saifi
Dr Shabnam Saifi pursued PhD from International Centre for Genetic Engineering and Biotechnology, New Delhi, India and worked on stress tolerance in rice and characterization of the OsRuvB gene.
Narendra Tuteja
Dr Narendra Tuteja (b.1955), PhD, D.Sc., FNA, FNASc., FASc, FNESA, FNAAS, FTWAS, Group Leader & Senior Scientist, International Centre for Genetic Engineering & Biotechnology (ICGEB), New Delhi. Sp: Plant molecular biology/biotechnology/stress tolerance/plant-microbes interactions. He has made significant contributions in crop improvement under adverse conditions. Dr Tuteja has reported the first DNA helicase from plant and human systems and discovered novel roles of helicases, G-proteins, Ca2+-binding proteins & LecRLK in abiotic stress tolerance in plants; developed salinity/drought tolerant rice, groundnut and sugarcane. Many genes for high salinity stress tolerance isolated from plant and fungus P.indica. Engaged in developing marker/reporter-free transgenics. His results indicate the potential for improving crop production at suboptimal conditions. Total publications: 255; Book edited: 9; Citations: 6928; h-index: 40; i10-index: 107.
References
- Aebi H. 1984. [13] Catalase in vitro. Methods Enzymol. 105:121–126. doi: 10.1016/S0076-6879(84)05016-3
- Ahmad P, Jaleel CA, Sharma S. 2010. Antioxidant defense system, lipid peroxidation, proline-metabolizing enzymes, and biochemical activities in two Morus alba genotypes subjected to NaCl stress. Russian J Plant Physiol. 57:509–517. doi: 10.1134/S1021443710040084
- Anjaneyulu E, Reddy PS, Sunita MS, Kishor PBK, Meriga B. 2014. Salt tolerance and activity of antioxidative enzymes of transgenic finger millet overexpressing a vacuolar H+-pyrophosphatase gene (SbVPPase) from Sorghumbicolor. J Plant Physiol. 171:789–798. doi: 10.1016/j.jplph.2014.02.001
- Araújo SS, Beebe SE, Crespi MD, González B, Gruber EM, Lejeune-Henaut V, Link I, Monteros W, Prats MJ, Rao E, et al. 2015. Abiotic stress responses in legumes: strategies used to cope with environmental challenges. Crit Rev Plant Sci. 34(1–3):237–280. doi:10.1080/07352689.2014.898450.
- Augustine SM, Cherian AV, Syamaladevi DP, Subramonian N. 2015. Erianthus arundinaceus HSP70 (EaHSP70) acts as a key regulator in the formation of anisotropic interdigitation in sugarcane (Saccharum spp. hybrid) in response to drought stress. Plant Cell Physiol. 56:2368–2380. doi: 10.1093/pcp/pcv142
- Bates LS, Waldren RP, Teare ID. 1973. Rapid determination of free proline for water-stress studies. Plant Soil. 39:205–207. doi: 10.1007/BF00018060
- Beebe S. 2012. 5 common bean breeding in the tropics. Plant Breed Rev. 36(36):357–426.
- Bhatnagar-Mathur P, Rao JS, Vadez V, Dumbala SR, Rathore A, Yamaguchi-Shinozaki K, Sharma KK. 2014. Transgenic peanut overexpressing the DREB1A transcription factor has higher yields under drought stress. Mol Breed. 33:327–340. doi: 10.1007/s11032-013-9952-7
- Birch RG. 1997. Plant transformation: problems and strategies for practical application. Annu Rev Plant Physiol Plant Mol Biol. 48:297–326. doi: 10.1146/annurev.arplant.48.1.297
- Boyer JS. 1982. Plant productivity and environment. Science. 218:443–448. doi: 10.1126/science.218.4571.443
- Chaves MM. 1991. Effects of water deficits on carbon assimilation. J Exp Bot. 42:1–16. doi: 10.1093/jxb/42.1.1
- Chaves MM, Flexas J, Pinheiro C. 2009. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 103:551–560. doi: 10.1093/aob/mcn125
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 125(1):189–198. doi: 10.1016/0003-9861(68)90654-1
- Hernandez JA, Ferrer MA, Jimenez A, Barcelo AR, Sevilla F. 2001. Antioxidant systems and O2−/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 127:817–831. doi:10.1104/pp.010188.
- Holt BF, Boyes DC, Ellerström M, Siefers N, Wiig A, Kauffman S, Grant MR, Dangl JL. 2002. An evolutionarily conserved mediator of plant disease resistance gene function is required for normal Arabidopsis development. Devel Cell. 2:807–817. doi: 10.1016/S1534-5807(02)00174-0
- Isayenkov SV. 2012. Physiological and molecular aspects of salt stress in plants. Cytol Genet. 46:302–318. doi:10.3103/S0095452712050040.
- Isayenkov SV, Maathuis FJM. 2019. Plant salinity stress: many unanswered questions remain. Front Plant Sci. 10:80. doi:10.3389/fpls.2019.00080.
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. 1999. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nature Biotechnol. 17:287–291. doi: 10.1038/7036
- Lawrence PK, Koundal KR. 2001. Agrobacterium tumefaciens-mediated transformation of pigeon pea (Cajanus cajan L. Millsp.) and molecular analysis of regenerated plants. Curr Sci. 80:1428–1432.
- Leonforte A, Sudheesh S, Cogan NO, Salisbury PA, Nicolas ME, Materne M, Kaur S. 2013. SNP marker discovery, linkage map construction and identification of QTLs for enhanced salinity tolerance in field pea (Pisum sativum L.). BMC Plant Bio. 13(1):161. doi: 10.1186/1471-2229-13-161
- Li C, Wei Z, Liang D, Zhou S, Li Y, Liu C, Ma F. 2013. Enhanced salt resistance in apple plants overexpressing a Malus vacuolar Na+/H+ antiporter gene is associated with differences in stomatal behavior and photosynthesis. Plant Physiol Biochem. 70:164–173. doi: 10.1016/j.plaphy.2013.05.005
- Li H, Wang Z, Ke Q, Ji CY, Jeong JC, Lee HS, Lim YP, Xu B, Deng XP, Kwak SS. 2014. Overexpression of codA gene confers enhanced tolerance to abiotic stresses in alfalfa. Plant Physiol Biochem. 85:31–40. doi: 10.1016/j.plaphy.2014.10.010
- Liu X, Hua X, Guo J, Qi D, Wang L, Liu Z, Jin Z, Chen S, Liu G. 2008. Enhanced tolerance to drought stress in transgenic tobacco plants overexpressing VTE1 for increased tocopherol production from Arabidopsis thaliana. Biotech Lett. 30:1275–1280. doi: 10.1007/s10529-008-9672-y
- Lu Y, Li Y, Zhang J, Xiao Y, Yue Y, Duan L, Zhang M, Li Z. 2013. Overexpression of Arabidopsis molybdenum cofactor sulfurase gene confers drought tolerance in maize (Zea mays L.). PLoS ONE. 8.
- Mahajan S, Tuteja N. 2005. Cold, salinity and drought stresses: an overview. Arch Biochem Biophys. 444:139–158. doi: 10.1016/j.abb.2005.10.018
- Munns R, Passioura JB. 1984. Hydraulic resistance of plants. Effects of NaCl in barley and lupin. Aust J Plant Physiol. 11:351–359. doi:10.1071/PP9840351.
- Munns R, Termaat A. 1986. Whole-plant responses to salinity. Aust J Plant Physiol. 13:143–160. doi:10.1071/PP9860143.
- Nemati I, Moradi F, Gholizadeh S, Esmaeili MA, Bihamta MR. 2011. The effect of salinity stress on ions and soluble sugars distribution in leaves, leaf sheaths and roots of rice (Oryza sativa L.) seedlings. Plant Soil Environ. 57(1):26–33. doi: 10.17221/71/2010-PSE
- de Paiva Rolla AA, de Fátima Corrêa Carvalho J, Fuganti-Pagliarini R, Engels C, do Rio A, Marin SRR, de Oliveira MCN, Beneventi MA, Marcelino-Guimarães FC, Farias JRB, et al. 2014. Phenotyping soybean plants transformed with rd29A:AtDREB1A for drought tolerance in the greenhouse and field. Transgenic Res. 23:75–87. doi: 10.1007/s11248-013-9723-6
- Preeti. 2018. Development and characterization of transgenic chickpea (Cicer arietinum L.) plants with OsRuvB gene for salt stress tolerance [thesis]. https://krishikosh.egranth.ac.in/handle/1/5810089618.
- Rai AC, Singh M, Shah K. 2013. Engineering drought tolerant tomato plants over-expressing BcZAT12 gene encoding a C2H2 zinc finger transcription factor. Phytochemistry. 85:44–50. doi: 10.1016/j.phytochem.2012.09.007
- Rao IM, Beebe SE, Polania J, Ricaurte J, Cajiao C, Garćıa R, Rivera M. 2013. Can tepary bean be a model for improvement of drought resistance in common bean. Afr Crop Sci J. 21:265–281.
- Rasool S, Hameed A, Azooz MM, Rehman M-U, Siddiqi TO, Ahmad P. 2013. Salt stress: causes, types and responses of plants, chapter 1: soil and water management for sustained agriculture in alluvial plains and flood plains exposed to salinity: a case of Neretva river valley (pp. 1–24).
- Ravikumar G, Manimaran P, Voleti SR, Subrahmanyam D, Sundaram RM, Bansal KC, Viraktamath BC, Balachandran SM. 2014. Stress-inducible expression of AtDREB1A transcription factor greatly improves drought stress tolerance in transgenic indica rice. Transgenic Res. 23:421–439. doi: 10.1007/s11248-013-9776-6
- Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW. 1984. Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Nat Acad Sci. 81:8014–8018. doi: 10.1073/pnas.81.24.8014
- Saifi SK, Passricha N, Tuteja R, Tuteja N. 2018a. Stress-induced Oryza sativa RuvBL1a is DNA-independent ATPase and unwinds DNA duplex in 3′ to 5′ direction. Protoplasma. 255:669–684. doi: 10.1007/s00709-017-1178-9
- Saifi S, Passricha N, Tuteja N, Swain D. 2018b. Prediction of cis-regulatory elements for a detailed insight of RuvB family genes from Oryza sativa. ORYZA - Int J Rice. 54. doi:10.5958/2249-5266.2017.00019.4.
- Sawhney V, Singh DP. 2002. Effect of chemical desiccation at the post-anthesis stage on some physiological and biochemical changes in the flag leaf of contrasting wheat genotypes. Field Crops Res. 77(1):1–6. doi: 10.1016/S0378-4290(01)00192-7
- Siegel BZ. 1993. Plant peroxidases-an organismic perspective. Plant Growth Regul. 12:303–312. doi: 10.1007/BF00027212
- Singh N, Mishra A, Jha B. 2014. Over-expression of the peroxisomal ascorbate peroxidase (SbpAPX) gene cloned from halophyte Salicornia brachiata confers salt and drought stress tolerance in transgenic tobacco. Marine Biotechnol. 16:321–332. doi: 10.1007/s10126-013-9548-6
- Smart RE, Bingham GE. 1974. Rapid estimates of relative water content. Plant Physiol. 53(2):258–260. doi: 10.1104/pp.53.2.258
- Sullivan CY. 1972. Mechanisms of heat and drought resistance in grain sorghum and methods of measurement. Sorghum in Seventies. New Delhi: Oxford & IBH Pub. Co.
- Surekha C, Beena MR, Arundhati A, Singh PK, Tuli R, Dutta-Gupta A, Kirti PB. 2005. Agrobacterium-mediated genetic transformation of pigeon pea (Cajanus cajan (L.) Millsp.) using embryonal segments and development of transgenic plants for resistance against Spodoptera. Plant Sci. 169:1074–1080. doi: 10.1016/j.plantsci.2005.07.011
- Surekha C, Kumari KN, Aruna LV, Suneetha G, Arundhati A, Kavi Kishor PB. 2014. Expression of the Vigna aconitifolia P5CSF129A gene in transgenic pigeonpea enhances proline accumulation and salt tolerance. Plant Cell Tissue Organ Cul. 116:27–36. doi: 10.1007/s11240-013-0378-z
- Surender Reddy P, Jogeswar G, Rasineni GK, Maheswari M, Reddy AR, Varshney RK, Kavi Kishor PB. 2015. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol Biochem. 94:104–113. doi: 10.1016/j.plaphy.2015.05.014
- Tang L, Cai H, Ji W, Luo X, Wang Z, Wu J, Wang X, Cui L, Wang Y, Zhu Y, Bai X. 2013. Overexpression of GsZFP1 enhances salt and drought tolerance in transgenic alfalfa (Medicago sativa L.). Plant Physiol Biochem. 71:22–30. doi: 10.1016/j.plaphy.2013.06.024
- Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H. 1999. A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell. 11:1195–1206. doi:10.2307/3870742. doi: 10.1105/tpc.11.7.1195
- Tuteja N, Tuteja R. 2004a. Prokaryotic and eukaryotic DNA helicases: essential molecular motor proteins for cellular machinery. Eur J Biochem. 271:1835–1848. doi: 10.1111/j.1432-1033.2004.04093.x
- Tuteja N, Tuteja R. 2004b. Unraveling DNA helicases Motif, structure, mechanism and function. Eur J Biochem. 271:1849–1863. doi: 10.1111/j.1432-1033.2004.04094.x
- Vaid N, Pandey P, Srivastava VK, Tuteja N. 2015. Pea lectin receptor-like kinase functions in salinity adaptation without yield penalty, by alleviating osmotic and ionic stresses and upregulating stress-responsive genes. Plant Mol Bio. 88:193–206. doi: 10.1007/s11103-015-0319-9
- Vaidyanathan H, Sivakumar P, Chakrabarty R, Thomas G. 2003. Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.): differential response in high yielding and sensitive varieties. Plant Sci. 165:1411–1418. doi: 10.1016/j.plantsci.2003.08.005
- Wang C-W, Chen W-C, Lin L-J, Lee C-T, Tseng T-H, Leu W-M. 2011. OIP30, a RuvB-like DNA helicase 2, is a potential substrate for the pollen-predominant OsCPK25/26 in rice. Plant Cell Physiol. 52:1641–1656. doi: 10.1093/pcp/pcr094
- Wang W, Vinocur B, Altman A. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 218:1–14. doi: 10.1007/s00425-003-1105-5
- Yadav NS, Shukla PS, Jha A, Agarwal PK, Jha B. 2012. The SbSOS1 gene from the extreme halophyte Salicornia brachiata enhances Na+ loading in xylem and confers salt tolerance in transgenic tobacco. BMC Plant Bio. 12:188. doi:10.1186/1471-2229-12-188.
- Yang ZB, Rao IM, Horst WJ. 2013. Interaction of aluminium and drought stress on root growth and crop yield on acid soils. Plant Soil. 372(1–2):3–25. doi: 10.1007/s11104-012-1580-1
- Yemm EW, Willis AJ. 1954. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 57:508–514.
- Zhang H, Dong H, Li W, Sun Y, Chen S, Kong X. 2009. Increased glycine betaine synthesis and salinity tolerance in AhCMO transgenic cotton lines. Mol Breed. 23:289–298. doi: 10.1007/s11032-008-9233-z
- Zhao X, Wei P, Liu Z, Yu B, Shi H. 2017. Soybean Na+/H+ antiporter GmsSOS1 enhances antioxidant enzyme activity and reduces Na+ accumulation in Arabidopsis and yeast cells under salt stress. Acta Physiol Plant. 39:19. doi: 10.1007/s11738-016-2323-3

