ABSTRACT
Most flowering plants are visited by various pollinator insects. To understand floral specialization for pollinators, the relative importance of different flower visitors to the focal plant species should be revealed. In the present study, we observed the insects that visited the orchid Platanthera hologlottis throughout the day and night using interval timer photography to reveal the relative importance of diurnal and nocturnal flower visitors. We observed visitation by both diurnal (e.g. the butterfly Ochlodes ochraceus) and nocturnal (e.g. the moth Thysanoplusia intermixta) insects and examined their relative contribution to fruit production and pollinarium removal experimentally. Results showed that the fitness was higher in flowers visited by nocturnal insects than in those visited by diurnal insects. These results suggest that the floral traits of P. hologlottis may be specialized for nocturnal flower visitors rather than diurnal flower visitors.
Introduction
Most flowering plants depend on animals, mostly insects, for their pollination (Ollerton et al. Citation2011). The relationship between plants and pollinator insects (i.e. pollination mutualism) strongly influences their reproductive success. To improve reproductive success, floral traits can become ecologically, evolutionary, and phenotypically specialized for their pollinators (Boberg and Ågren Citation2009; Newman et al. Citation2015; Armbruster Citation2017). For example, the evolution of long nectar spurs in Aquilegia was driven by a pollinator shift associated with changes in tongue length of pollinators (Whittall and Hodges Citation2007). Additionally, Sletvold et al. (Citation2012) reported that diurnal pollinators mediated stronger selection on traits influencing floral display than nocturnal pollinators and that this selection varied between populations of Gymnadenia conopsea. These studies also showed that different pollinator species exerted different selection pressures on floral traits. Therefore, to understand floral specialization, it is important to reveal the pollinator species that exert selection pressure on the plant species under investigation.
In some situations, flowers are visited by different pollinator insects, and the specific species of pollinators change not only among populations (Nagano et al. Citation2014; Kuriya et al. Citation2015; Hattori et al. Citation2016) but also within a population (Wolff et al. Citation2003; Cordeiro et al. Citation2016; Funamoto and Ohashi Citation2017). For example, Isertia laevis (Rubiaceae) is pollinated mainly by hummingbirds (Trochilidae) during the day and by hawk moths at night. Furthermore, the reproductive success per visit of I. laevis pollinated by hawk moths was significantly higher than that of those pollinated by hummingbirds (Wolff et al. Citation2003). Funamoto and Ohashi (Citation2017) reported an adaptation to nocturnal moths in Adenophora triphylla var. japonica (Campanulaceae) that have flowers that appear to fit with a bee-pollination syndrome but are visited by diurnal and nocturnal insects. Although several studies have provided support for pollination syndromes (Danieli-Silva et al. Citation2012; Murúa and Espíndola Citation2015; Strelin et al. Citation2016), the pollination syndrome does not predict the pollinators of most plant species (Ollerton et al. Citation2009). Furthermore, secondary pollinators are common and play important roles in plant reproduction (Rosas-Guerrero et al. Citation2014). Therefore, to understand floral specialization for pollinators, it is necessary to observe pollinator species throughout the day and night to identify the relative importance of pollinator species to a focal plant.
It is sometimes difficult to directly observe nocturnal pollinators because their flower visitation frequency is very low (Suetsugu and Fukushima Citation2014). To overcome this problem, there has been a recent increase in studies using interval photography to observe nocturnal pollinators (e.g. Suetsugu and Tanaka Citation2013; Suetsugu et al. Citation2015). In the present study, we observed flower visitors to the orchid Platanthera hologlottis (Orchidaceae) throughout the day and night using interval photography. P. hologlottis is a perennial herb broadly distributed in Japan (Hayashi Citation2009). It has upright white flowers with spurs that open from June to July (). They have a small ridge on the midrib in front of a ca. 15-mm-long spur that partially obstructs the center of the flower (Inoue Citation1983; ). Furthermore, P. hologlottis is called ‘JYAKOU CHIDORI (JYAKOU means musk in Japanese)’ in Japan because their flowers are fragrant. Although it has been previously reported that P. hologlottis flowers were visited by more diurnal insects than nocturnal insects, the flower morphology of P. hologlottis is similar to that of P. flava and P. ussuriensis that are pollinated by small moths (Inoue Citation1983). These floral characters match a moth-pollination syndrome (Fægri and van der Piji Citation1971; Fenster et al. Citation2004). Thus, flower morphology may imply that P. hologlottis flowers are ecologically specialized for moth pollination. However, it has been previously reported that pollen vectors of P. hologlottis were by butterflies (Ochlodes venata, Colias erate, Pieris melete) mainly (Inoue Citation1983). It is therefore unclear whether the effective pollinators of P. hologlottis are diurnal flower visitors. In the present study, we hypothesized that nocturnal visitors contribute more to pollen removal and fruit production than diurnal visitors.
Figure 1. (a) Platanthera hologlottis in the wild, (b) an enlarged photograph of P. hologlottis flower, (c) a visiting species of Ochlodes ohraceus in the day, (d) a visiting species of Plussiinae in the night.

In such species, using interval photography to observe diurnal and nocturnal flower visitors is useful for identifying the flower visitors (Suetsugu and Tanaka Citation2013; Suetsugu et al. Citation2015). Furthermore, the experimental approach to reveal the relative contribution of diurnal and nocturnal flower visitors to fruit production and pollinarium removal is useful for gaining knowledge to identify the effective pollinators because the pollination effectiveness of flower visitors can be estimated by measuring paternal (pollen removal) and maternal (producing seeds) perspectives (Schupp et al. Citation2017). Thus, in the present study, we determined the relative importance of diurnal and nocturnal pollinators of P. hologlottis.
Material & methods
Study site
We studied a population of P. hologlottis in Kaida Highland, central Japan (1155 m a.s.l., 35°56'50.5"N 137°38'21.4"E). In Kaida Highland, the mean monthly temperature is 7.4 °C, and the annual amount of precipitation is 2080mm. July is the rainiest month (342 mm). Our study site contained vegetation of the montane zone (<1600 m). This vegetation comprises a mosaic forest of old-growth coniferous and old-growth deciduous broad-leaved wood (Nakashizuka et al. Citation1993). Additionally, we also found planted coniferous forests.
The place where we studied P. hologlottis was a wetland and was populated by many plant species, including Platy codon grandiflous (Campanulaceae) and Myosotis scorpioides (Boraginaceae). Here, we found about 50 individuals of P. hologlottis within 20 m × 20 m area. In summer 2015 and 2016, we studied the pollinator assemblage associated with the flowers of P. hologlottis. In summer 2016, we measured the effect of pollination during the day and night on the fitness of P. hologlottis.
Pollinator assemblage
To investigate the visitors to P. hologlottis flowers, we used three digital cameras (Optio WG-40, 30, Pentax Japan) that were set-up using the interval-programming function to automatically take pictures at 30-second or 20-second intervals. The diurnal observations began at 7:00 am and ended at 5:00 pm and the nocturnal observations began at 6:00 pm and ended at 3:00 am because sunrise was about 7:00 am and sunset at 6:00 pm in observation place. We obtained 6973 photos from 19 P. hologlottis plants (n = 13 in 2015, n = 6 in 2016) during the day and 6525 photos from 20 P. hologlottis plants (n = 13 in 2015, n = 7 in 2016) during the night in 2 observation days in each year. For observations, we focused on the same individuals of P. hologlottis.
Effect of diurnal/nocturnal pollinators on plant fitness
To compare the relative contribution to plant fitness between diurnal and nocturnal flower visitors, we estimated their contribution to male fitness by determining the rate of pollinarium removal per individual (number of removed pollinariu/number of flowers), and their contribution to female fitness by determining the rate of fruit set per individual (number of fruits/number of flowers). To manipulate flower visitation from pollinators to P. hologlottis, we set three treatments using fine nylon mesh bags (0.75 mm): Control (D + N): open-pollinated control that pollinators could visit P. hologlottis flowers freely (male fitness, n = 9; female fitness, n = 10), Treatment 1 (D): pollinators could visit P. hologlottis flowers only during the daytime (6:00 am to 5:00 pm) (male fitness, n = 8; female fitness, n = 10), and Treatment 2 (N): pollinators could visit P. hologlottis flowers only during the night (6:00 pm to 5:00 am) (male fitness, n = 10; female fitness, n = 11). These experiments were conducted over 3 days. 1 month after the experiments, we observed all individuals and checked whether flowers formed a fruit or not. To measure the effect of pollinator removal of the bags, we set the bags at 9 individuals, 162 flowers (T0). By this manipulation, every flower did not produce any fruits or seeds. Thus, the bags can prevent any visitations from pollinators of P. hologlottis completely.
Data analysis
For the comparison of the rate of fruit set per individual and the pollinarium removal rate per individual between the control and treatment groups, the Kruskal–Wallis with Wilcoxon post-hoc test was used. All statistical analyses were conducted in JMP v. 14.0.0.
Results
In the observation site, P. hologlottis flowers opened from June to August. We directly observed that many lepidopteran species (Lycaenidae butterflies, Pieridae butterflies, Sphinigidae moths etc.) visited the flowers in the daytime. At night, the flowers were visited by mainly Plusiinae species.
During the day, we observed visitations by 36 insect individuals, including 24 butterflies (including Ochlodes ochraceus (1/24) and unknown species in Pieridae (9/24), Lycaenidae (7/24), and Hesperiidae (7/24)), and 12 moths (Macroglossum bombylans (1/12), Thysanoplusia intermixta (8/12), unknown species in Plusiinae (1/12) and other moths (2/12)) (). However, at night, we observed visitations 44 times by only moths (including unknown species in Plusiinae (13/44) (C), Thysanoplusia intermixta (7/44) and other moths (24/44)) visited the flowers (). When the butterfly species visited the flowers, they held the flower. Therefore, they posed only their mouth part into the flower and sucked the nectar while on the flower. Conversely, when the moth species visited the flowers, they could not hold the flower. Therefore, they posed their head into the flower and sucked the nectar while flying (c & d).
Table 1 The number of visitations to Platanthera hologlottis by different insect taxa during both day and night.
In the experiment, the rate of fruit set per individual significantly differed among treatments (Kruskal–Wallis test, χ2 = 18.04, P < 0.001; ). Although the rate of fruit set per individual did not differ between the control (D + N) and N (Wilcoxon test, Z = 1.44, P = 0.15), the rate of fruit set per individual that was pollinated by only nocturnal pollinators (N) was significantly higher than the rate per individual that was pollinated by only diurnal pollinators (D) (Wilcoxon test, Z = 3.84, P < 0.001; ). In terms of female fitness, the contribution of nocturnal pollinators was at least twice that of diurnal pollinators.
Figure 2. Effect of pollinators on plant fitness during both the day (D) and night (N), those with different letters are significantly different at P < 0.05 (Wilcoxon post hoc tests).
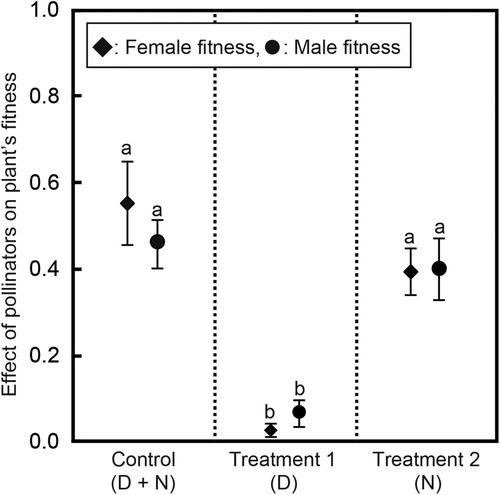
In the experiment, the pollinarium removal rate per individual differed significantly among treatments (Kruskal–Wallis test, χ2 = 13.50, P = 0.001; ). Although the pollinarium removal rate per individual did not differ between the control (D + N) and N (Wilcoxon test, Z = 0.57, P = 0.74), the pollinarium removal rate per individual that was pollinated by only nocturnal pollinators (N) was significantly higher than the rate per individual that was pollinated by only diurnal pollinators (D) (Wilcoxon test, Z = 2.92, P = 0.003; ). In terms of male fitness, the contribution of nocturnal pollinators was at least 5-fold higher than that of diurnal pollinators.
Discussion and conclusion
In the present study, we revealed the insect visitors to flowers of P. hologlottis using interval photography. P. hologlottis flowers were pollinated by different lepidopteran species. Visitation by Thysanoplusia intermixta, which was considered to be a principle pollinator of P. hologlottis by Inoue (Citation1983), was observed at all times during the day and night. However, our results indicated that T. intermixta does not pollinate P. hologlottis because only nocturnal flower visitors can influence the fitness of P. hologlottis. This result was caused by the lesser mechanical fit between the spur length of P. hologlottis and the proboscis length of T. intermixta. In our study site, the spur length of P. hologlottis was longer than the proboscis length of T. intermixta (Tamada et al., unpublished data). This implies that the pollinaria of P. hologlottis may not attach to the body parts of T. intermixta when T. intermixta visited the flower of P. hologlottis. Unfortunately, because we could not identify all flower visitors to species level, a principle pollinator species of P. hologlottis was not revealed in our study. However, our findings indicate that nocturnal pollinators, mostly moths other than T. intermixta, are relatively more important for P. hologlottis. This is no wonder because it has been previously reported that T. intermixa was not effective pollinator in also other Platanthera species (P. japonica) even though T. intermixta was higher than other flower visitors (Suetsugu and Tanaka Citation2013).
The misleadingly reported finding that T. intermixta is the main pollinator of P. hologlottis resulted from the previous study only focusing on the frequency of flower-visiting (quantity of pollination) and not on the effectiveness of pollination (combination of male and female fitness) (Inoue Citation1983). The effectiveness of pollinators should be evaluated from the various aspects because the effectiveness of pollinators can be affected by males, females, or both combined (Young Citation2002; Wolff et al. Citation2003; Schupp et al. Citation2017). In particular, the assumption that focusing on selection by a single ‘most effective pollinator’ (the most common functional group of visitors) (Stebbins Citation1970) sometimes leads to erroneous conclusions regarding the mechanisms of floral adaptation (Ollerton et al. Citation2009). Young (Citation2002) compared the effectiveness (seed production) of diunal and nocturnal pollinators by similar experimental evaluation of our study and showed that flowers exposed only to nocturnal-visiting insects (mostly sphingid and noctuid moths) produced significantly more seeds than flowers exposed only to the diurnal-visiting insects (bees, wasps, and flies) in Silene latifolia although diurnal-visiting insects were more abundant than nocturnal-visiting insects. In our study, too, the highly frequent visiting moth (i.e. T. intermixta) did not contribute to produce fruits of P. hologlottis in comparisons with the other nocturnal-visiting insects. Therefore, focusing only on the most common pollinator does not accurately predict the agents of floral adaptation (i.e. pollination syndrome).
Our results showed that moths other than T. intermixta are more important pollinators than butterflies for P. hologlottis. This difference may result from different flower visiting behaviors between butterflies and the moths. When the butterflies visited the flowers, the pollinarium may not attach to their head because they did not pose their head into the flowers. On the other hand, when the moths visited the flowers, the pollinarium may not have got attached to the base of their head because they posed their head into the flowers. These results imply that the floral traits of P. hologlottis (e.g. upright white flowers and sweet fragrance) may be ecologically specialized for moths (i.e. moth-pollination syndrome) because the pollination syndrome suggests that moths favor upright white flowers with fragrance (Fulton and Hodges Citation1999; Hodges et al. Citation2003; Fenster et al. Citation2004). Some orchid-moth-pollinated species exhibit the same floral traits (van der Niet et al. Citation2011). Furthermore, fragrance traits were recognized as a kind of moth-pollinated syndrome traits. Most hawk-moth-pollinated plants show convergent evolution of emitting a sweet-smelling scent (Thompson Citation1994). For example, hawk-moth-pollinated tobacco plants were reported to emit greater amounts of specific fragrances during the night (Raguso et al. Citation2003). P. hologlottis may have other traits of the pollination syndrome specialized for moths other than T. intermixta. Further research is required to focus not only on some floral traits (e.g. fragrance, morphology, anthesis period) but also on pollinator traits (e.g. behavior, the compatibility of flower and pollinator morphology), and especially reveal whether fragrance of P. hologlottis flowers is favored by moths.
Acknowledgements
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Dr. Keiichiro Shikata for moth identifications and Dr. Kenji Suetsugu for observation using digital cameras.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ORCID
Mitsuru Hattori http://orcid.org/0000-0003-3176-5893
Additional information
Funding
Notes on contributors
Mitsuru Hattori
Mitsuru Hattori is a associate professor at Nagasaki University Japan. His research interests have focused on evolutionary ecology of biological interactions.
Yoko Tamada
Yoko Tamada is a M.S. student at Department of Science, Shinshu University.
Takao Itino
Takao Itino is a professor at Shinshu University Japan.
References
- Armbruster WS. 2017. The specialization continuum in pollination systems: diversity of concepts and implications for ecology, evolution and conservation. Funct Ecol. 31:88–100. doi:10.1111/1365-2435.12783.
- Boberg E, Ågren J. 2009. Despite their apparent integration, spur length but not perianth size affects reproductive success in the moth–pollinated orchid Platanthera bifolia. Funct Ecol. 23:1022–1028. doi:10.1111/j.1365-2435.2009.01595.x.
- Cordeiro GD, Pinheiro M, Dötterl S, Alves-dos-Santos I. 2016. Pollination of Campomanesia phaea (Myrtaceae) by night-active bees: a new nocturnal pollination system mediated by floral scent. Plant Biol. 19:132–139. doi:10.1111/plb.12520.
- Danieli-Silva A, de Souza JMT, Donatti AJ, Campos RP, Vicente-Silva J, Varassin GI. 2012. Do pollination syndromes cause modularity and predict interactions in a pollination network in tropical high-altitude grass land? Oikos. 121:35–43. doi:10.1111/j.1600-0706.2011.19089.x.
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndromes and floral specialization. Annu Rev Ecol Evol Syst. 35:375–403. doi:10.1146/annurev.ecolsys.34.011802.132347.
- Fægri KP, van der Piji L. 1971. The principal of pollination ecology. Oxford: Pergamann press.
- Fulton M, Hodges SA. 1999. Floral isolation between Aquilegia formosa and Aquilegia pubescens. Proc Roy Soc B-Biol Sic. 266:2247–2252. doi:10.1098/rspb.1999.0915.
- Funamoto D, Ohashi K. 2017. Hidden floral adaptation to nocturnal moths in an apparently bee-pollinated flower, Adenophora tryphylla var. japonica (Campanulaceae). Plant Biol. 19:767–774. doi:10.1111/plb.12579.
- Hattori M, Nagano Y, Shinohara Y, Itino T. 2016. Pattern of flower size variation along an altitudinal gradient differs between Impatiens textori and Impatiens noli-tangere. J Plant Int. 11:152–157. doi:10.1080/17429145.2016.1226437.
- Hayashi Y. 2009. Nihon-no Yasou. Tokyo: Yama to Keikoku Sha.
- Hodges SA, Fulton M, Yang JY, Whittall JB. 2003. Verne grant and evolutionary studies of Aquilegia. New Phytol. 161:113–120. doi:10.1046/j.1469-8137.2003.00950.x.
- Inoue K. 1983. Systematics of genus Platanthera (Orchidaceae) in Japan and adjacent regions with special reference to pollination. J Fac Sci U Tokyo. 3:285–374.
- Kuriya S, Hattori M, Nagano Y, Itino N. 2015. Altitudinal flower size variation correlates with local pollinator size in a bumblebee-pollinated herb, Prunella vulgaris L. (Lamiaceae). J Evol Biol. 28:1761–1769. doi:10.1111/jeb.12693.
- Murúa M, Espíndola A. 2015. Pollination syndromes in a specialized plant-pollinator interaction: does floral morphology predict pollinators in Calceolaria? Plant Biol. 17:551–557. doi:10.1111/plb.12225.
- Nagano Y, Abe K, Kitazawa T, Hattori M, Hirao SA, Itino T. 2014. Changes in pollinator fauna affect altitudinal variation of floral size in a bumblebee-pollinated herb. Ecol Evol. 4:3395–3407. doi:10.1002/ece3.1191.
- Nakashizuka T, Iida S, Suzuki W, Tanimoto T. 1993. Seed dispersal and vegetation development on a debris avalanche on the Ontake volcano, central Japan. J Veg Sci. 4:537–542. doi:10.2307/3236081.
- Newman E, Manning J, Anderson B. 2015. Local adaptation: mechanical fit between floral ecotypes of Nerine humilis (Amaryllidaceae) and pollinator communities. Evolution. 69:2262–2275. doi:10.1111/evo.12736.
- Ollerton J, Alarcón R, Waser NM, Price MV, Watts S, Cranmer L, Hingston A, Peter CI, Rotenberry J. 2009. A global test of the pollination syndrome hypothesis. Ann Bot. 103:1471–1480. doi:10.1093/aob/mcp031.
- Ollerton J, Winfree R, Tarrant S. 2011. How many flowering plants are pollinated by animals? Oikos. 120:321–326. doi:10.1111/j.1600-0706.2010.18644.x.
- Raguso RA, Levin RA, Foose SE, Holmberg MW, McDade LA. 2003. Fragrance chemistry, nocturnal rhythms and pollination “syndromes” in Nicotiana. Phytochemistry. 63:265–284. doi:10.1016/S0031-9422(03)00113-4.
- Rosas-Guerrero V, Aguilar R, Martén-Rodríguez S, Ashworth L, Lopezaraiza-Mikel M, Bastida JM, Quesada M. 2014. A quantitative review of pollination syndromes: do floral traits predict effective pollinators? Ecol Lett. 17:388–400. doi:10.1111/ele.12224.
- Schupp EW, Jordano P, Gómez JM. 2017. A general framework for effectiveness concepts in mutualisms. Ecol Lett. 20:577–590. doi:10.1111/ele.12764.
- Sletvold N, Trunschke J, Wimmergren C, Ågren J. 2012. Separating selection by diurnal and nocturnal pollinators on floral display and spur length in Gymnadenia conopsea. Ecology. 93:1880–1891. doi:10.1890/11-2044.1.
- Stebbins GL. 1970. Adaptive radiation of reproductive characteristics in angiosperms: pollination mechanisms. Ann Rev Ecol Syst. 1:307–326. doi:10.1146/annurev.es.01.110170.001515.
- Strelin MM, Benitez-Vieyra S, Fornoni J, Klingenberg CP, Cocucci AA. 2016. Exploring the ontogenetic scaling hypothesis during the diversification of pollination syndromes in Caophora (Loasaceae, subfam. Loassoideae). Ann Bot. 117:937–947. doi:10.109./aob/mcw035 doi: 10.1093/aob/mcw035
- Suetsugu K, Fukushima S. 2014. Pollination biology of the endangered orchid Cypripedium japonica in a fragmented forest of Japan. Plant Spec Biol. 29:293–299. doi:10.1111/1442-1984.12016.
- Suetsugu K, Tanaka K. 2013. Pollination of Sedirea japonica (Orchidaceae) by Bombus diversus diversus (Hymenoptera: Apidae). Eur J Entomol. 110:545–548. doi:10.14411/eje.2013.074.
- Suetsugu K, Tanaka K, Okuyama Y, Yukawa T. 2015. Potential pollinator of Vanda falcata (Orchidaceae): Theretra (Lepidoptera: Sphingidae) hawkmoths are visitors of long spurred orchid. Eur J Entomol. 112:393–397. doi:10.14411/eje.2015.031.
- Thompson JN. 1994. The Coevolutionary Process. Chicago: University of Chicago Press.
- van der Niet T, Liltved WR, Johnson SD. 2011. More than meets the eye: a morphological and phylogenetic comparison of long-spurred, white-flowered Satyrium species (Orchidaceae) in South Africa. Bot J Linn Soc. 166:417–430. doi:10.1111/j.1095-8339.2011.01143.x.
- Whittall JB, Hodges SA. 2007. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature. 447:706–709. doi:10.1038/nature05857.
- Wolff D, Braun M, Liede S. 2003. Nocturnal versus diurnal pollination success in Isertia laevis (Rubiaceae): A sphingophilous plant visited by hummingbirds. Plant Biol. 5:71–78. doi:10.1055/s-2003-37977.
- Young HJ. 2002. Diurnal and nocturnal pollination of Silene alba (Caryophyllaceae). Amer J Bot. 89:433–440. doi:10.3732/ajb.89.3.433.
