ABSTRACT
In this study, two bread wheat (Triticum aestivum L.) cultivars, Pishgam (drought-tolerant) and Shahryar (drought-sensitive), were grown in the greenhouse under control and moisture stress conditions. Based on phenological and morpho-physiological results, Pishgam was confirmed as a moisture stress tolerant cultivar. In the fallowing, at the start of heading time, its treated and untreated flag leaves were sampled for two-dimensional electrophoresis (2-DE) based on proteomics approach. Among approximately 263 protein spots appearing in two-dimensional gels, 23 and 10 protein spots were up- and down-regulated, respectively. Among these differentially expressed proteins, 11 proteins with more differences were identified by MALDI TOF/TOF MS which allocated to six functional protein groups involved in photosynthesis or respiration, carbohydrate metabolism, energy metabolism, chaperon, lipid metabolism and unknown function. We report this for the first time that chloroplastic acyl carrier protein synthase I and chloroplastic 20 kDa chaperonin proteins were significantly changed in wheat in response to moisture stress.
Introduction
Abiotic stresses can change physiological and biochemical traits (Jamshidi Goharrizi, Baghizadeh, et al. Citation2020; Jamshidi Goharrizi, Moosavi, et al. Citation2020), genes expression template (Jamshidi Goharrizi et al. Citation2018) as well as proteome pattern of plants (Nazari et al. Citation2018). One crucial issue in plant production is access to water, which affects the plant growth cycle. Low moisture leads to water stress, which is most often observed in areas with low rainfall and low irrigation (Wang et al. Citation2005). Wheat (Triticum aestivum L.), as one of the most important crops in the world, accounts for about 20% of the calories consumed by humans (Brenchley et al. Citation2012); meanwhile, due to the phenomenon of global warming and limitation of available water resources, its performance is decreasing annually. In recent years, numerous studies have focused on interactions between bread wheat and drought stress, which their results have demonstrated that recognizing genetic diversity in various traits can be effective in tolerance to stress (Hameed et al. Citation2011). Moisture stress leads to adverse effects on the quantity and quality of wheat yield, in addition, it creates complex responses at the cellular and physiological levels in the plant (Nazari et al. Citation2019). Obtaining and identifying high yielding plants with identifying and improving molecular mechanisms under water stress conditions could be the best strategy to deal with drought stress (Kamal et al. Citation2010). Proteins are essential biomolecules in organisms that affect all of cell functions, and their expression measurements can provide a broad overview of molecular events and specific physiological conditions (Ngara and Ndimba Citation2014). Proteomics is known as an effective technique for identifying potential proteins which are presented in tissues, cells or subcellular compartments, in different conditions (Ghatak et al. Citation2017). Proteomics is a powerful technique to directly assess of proteins influenced by a particular environmental stimuli, to identification of biochemical pathways and the complex response of plants to environmental stress. So far, several proteomics studies have been performed on crop species of wheat under drought stress (Caruso et al. Citation2009; Bazargani et al. Citation2011; Ge et al. Citation2012; Budak et al. Citation2013; Kamal et al. Citation2013; Cheng et al. Citation2015, Citation2016; Fotovat et al. Citation2017; Li et al. Citation2018).
In the present study, we first assessed the effect of imposing moisture stress in two bread wheat cultivars; Pishgam (drought-tolerant) and Shahryar (drought-sensitive). After determining the tolerant cultivar to moisture stress, we used a proteomics approach to identify responsive protein classes involved in tolerance to moisture stress in tolerant bread wheat (Pishgam cultivar). This study provides more information about the flag leaf protein profiles of tolerant bread wheat in response to moisture stress and identification of molecular differences and tolerance mechanisms.
Materials and methods
Plant materials and moisture stress treatment
The materials used in the greenhouse pot experiment included two bread wheat cultivars (Triticum aestivum L.): drought-sensitive Shahryar and drought-tolerant Pishgam developed in Seed and Plant Improvement Institute (S.P.I.I), Karaj, Iran. Pishgam cultivar was breeded and introduced as a tolerant cultivar for arid and semi-arid regions of Iran by S.P.I.I. ().
Table 1. Some characteristics of the plant materials used in the present research.
Before the start of the proteomics experiment, tolerance to moisture stress in Pishgam was re-approved using our phenological and morpho-physiological data analysis. So that the preliminary experiments was conducted in a research-greenhouse at Bu-Ali Sina University (located in Hamedan province, west of Iran) in 2014–2015 growing season. The seeds were surface sterilized by washing with 70% ethanol, followed by immersion in 5% sodium hypochlorite for 30 min and were finally washed three times with distilled water. Then, wheat seeds were germinated and vernalized in the dark condition for 21 days at 4°C before transplanted into pots. Next, five germinated seedlings of similar growth were transferred to black plastic pots, with 40 cm of diameter and 80 cm of height, containing 15 kg soil comprised of 50% agronomy-field soil (silty-loam), 25% sand and 25% manure. Plants were grown under two controlled and imposed moisture stress conditions with 10–12 h photoperiod and 25 ± 3°C temperature in three replications. In the following, soil moisture was maintained at 95% and 45% soil pod capacity (S.P.C) in controlled and imposed moisture stress conditions, respectively. At the first-three weeks of normal growth, the irrigation of plants was applied daily with tap water while adding the necessary volume to bring soil to field capacity (determined by weighing pots). After the first-three weeks, the applying moisture stress treatment (45% S.P.C.) was started when the seedling had approximately 4–6 leaves and followed until the harvest time. At heading time, the expanded flag leaves of treated and untreated plants were harvested, quickly wrapped in aluminum foil pouch, immediately frozen in liquid nitrogen and stored at −80 °C for protein extraction.
Measurement of the morpho-physiological traits
In both non-stress and stress conditions, 31 traits related to phenology, morpho-physiology, root-characters, and grain yield were measured at heading and harvest times on five plants in each pot. These traits were included days to heading (DTH), days to anthesis (DTA), days to maturity (DTM), grain filling period (GFP), chlorophyll content (SPAD), plant height (PH), peduncle length (PEL), tiller number per plant (TN), leaves number per plant (LN), fertile spike number per plant (FSNPP), spikelet number per spike (SNPS), seed number per main spike (SNPMS), seed number per plant (SNPP), main spike weight (MSW), seed weight per main spike (SWPMS), peduncle weight (PEW), main stem weight (MSTW), 1000-grain weight (TGW), economical yield per plant (EYPP), biological yield per plant (BYPP), plant harvest index (PHI), leaf area index (LAI), relative water content (RWC), water use (WU), excised leaf water retention (ELWR), water use efficiency (WUE), main root length (MRL), root volume (RV), root dry weight (RDW), root area (RA), root to shoot dry weight ratio (RDW/SDW).
At heading time, leaf physiological traits including relative water content (RWC), excised leaf water retention (ELWR) and chlorophyll content (SPAD index) were measured on the second leaves per plant. So that, RWC and ELWR were measured according to Mguis et al. (Citation2013), and SPAD index was recorded using a chlorophyll meter (SPAD-502; Konica Minolta Sensing, Inc. Osaka, Japan). The total water use (WU) was calculated as the amount of water use during the plant growth.
At physiological maturity, all of plants were cut off from the soil surface, the different parts of the plant were separated, and the characters related to root and grain yield were measured. Water use efficiency (WUE) determined by the ratio between the economical yield per plant (EYPP) and the total water use (WU).
Data analysis for phenological and morpho-physiological traits
Before analyzing data, the normal distribution of the total data and homogeneity of variance were verified using the Kolmogorov–Smirnov test. A t-test was used to test the statistical differences between the means of wheat materials in non-stress and stress conditions using SPSS software.
Protein extraction
The flag leaves of tolerant cultivar (Pishgam) were selected for this experiment based on statistical analysis from previous stage. Leaf samples were ground in liquid nitrogen and acetone/trichloroacetic acid (TCA) precipitated according to Damerval et al. (Citation1986) with some modifications. The protein concentrations were measured according to the Bradford assay using BSA as standard.
Two-dimensional electrophoresis (2-DE)
2-DE was carried out according to Görg et al. (Citation1988). At the first dimension separation, 320 µL of rehydration solution containing 120 µg of protein was taken up into an immobilized pH gradient (IPG) strip (17 cm, pH 4–7 linear, BioRad) during rehydration over night, then proteins were separated by subjecting the IPG gel strips to electrophoresis for 100 kV-h in a PROTEAN IEF system. At the second dimension separation, the IPG strips were equilibrated in DTT-containing equilibration solution at room temperature for 30 min, sealed at the top of a 12.5% SDS-PAGE gel. Proteins were separated based on molecular weight by using a PROTEAN II Xi Cell two electrophoresis unit (BioRad, USA). Protein spots in analytical gel were visualized by silver staining according to Blum et al.’s protocol (Citation1987).
Image analysis
Six silver-stained 2-D gels provided from three replication of each non-treated and treated plants, were scanned using a GS800 calibrated densitometer (Bio-Rad). The scanned images were processed and statistically evaluated with Melanie 7 software (Genebio, Geneva, Switzerland). The spot volumes were normalized as a percentage of the total volume, quantified, and subjected to t-test. Finally, protein spots with significant differences (p ≤ .01) were considered as regulated proteins for further analysis.
In-gel digestion and protein identification
Protein spots were carefully excised from the preparative CBB stained gels and subjected to in-gel trypsin digestion according to previous study from our group (Nazari et al. Citation2018). Afterward, peptide mixtures were analyzed using MALDI-TOF/TOF MS. MALDI matrix, a–cyano–4–hydroxycinnamic acid (CHCA), was prepared as 5 mg/mL in 6 mM ammonium phosphate monobasic, 50% acetonitrile, 0.1% trifluoroacetic acid and mixed with the peptide sample at 1:1 ratio (v/v). Mass spectrometry data were obtained using an AB Sciex 5800 TOF/TOF System, MALDI TOF TOF (Framingham, MA, USA). Data acquisition and data processing were respectively done using a TOF TOF Series Explorer and Data Explorer (both from AB Sciex). Reflectron positive mode was calibrated at 50 and 10 ppm mass tolerance as external and internal standard, respectively. Each mass spectrum was obtained as a sum of 500 shots. In the final step, data from mass spectrometry were analyzed by MASCOT software (http://www.matrixscience.com) and NCBI non-redundant protein (NCBInr) database to identify proteins. The parameters such as enzyme, trypsin; variable modifications, oxidation (M); Peptide tolerance, 200 ppm; MS/MS tolerance, 0.8 Da; carbamidomethylation of cysteine as fixed modification were used (Rezaee et al. Citation2018).
Results
Phenological and morpho-physiological responses of wheat to moisture stress
Overall, imposed moisture stress significantly (p < .05) decreased the whole phenological and morpho-physiological traits, except excised leaf water retention (ELWR) and water use efficiency (WUE) that increased in both wheat cultivars (). Some of the traits related to root-characters including root dry weight (RDW) and root to shoot dry weight ratio (RDW/SDW) only increased in Pishgam cultivar (). As expected, mean values of the most measured traits in Pishgam (drought-tolerant cultivar) was significantly (p < .05) higher than Shahryar (drought-sensitive) under different moisture conditions (). High mean values of measured traits in Pishgam, especially economical yield per plant (EYPP), yield-related trait and water use efficiency (WUE) indicate that its productivity and moisture stress-tolerance was significantly greater than Shahryar cultivar under different moisture conditions. As tolerance to moisture stress in Pishgam has previously been reported by the S.P.I.I, according to our results, tolerance to moisture stress was again confirmed in this cultivar. Therefore, its flag leaves were used in the proteomic experiment to evaluate the changes on flag leaf proteome in response to imposed moisture stress.
Table 2. Mean comparison of two wheat cultivars subjected to non-stress and moisture stress conditions.
2-DE analysis of moisture stress-responsive proteins
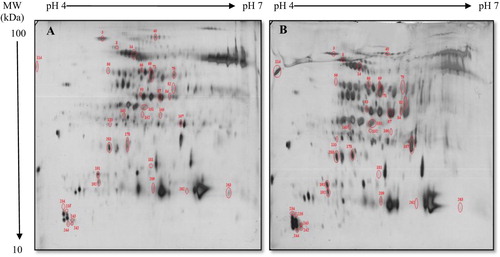
2-DE analysis of the flag leaves proteins of Pishgam (tolerant cultivar) was used to monitor changes in response to imposed moisture stress in three replications. shows the reference proteome maps obtained from different samples of Pishgam cultivar in control and moisture stress conditions ((A,B)). The broad distribution of the protein spots was uniformly displayed in the pI range from 4.0–7.0 and the molecular masses from 10 to 100 kDa. The changes in protein spot volume were quantified by software analysis (see ‘Materials and methods’). Approximately 263 protein spots were reproducibly detected and matched on silver stained gels. Of these identified spots, only 33 spots were differentially expressed (p < .05) between control and moisture stress-treated plants; 23 spots were upregulated and 10 spots were downregulated by the moisture stress ().
Database search and functional classification of moisture stress-responsive proteins
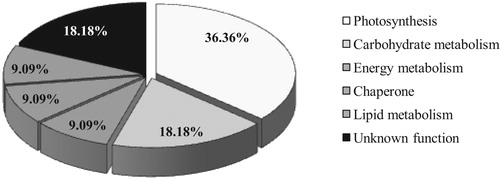
11 spots of 33 spots with more differences (at least two-fold) were selected for identification. These spots were excised from the gels, in-gel digested by trypsin, and analyzed by MALDI-TOF/TOF mass spectrometry. According to Mass data and analyzing them using Mascot program and NCBI non-redundant protein database, ten unique proteins were classified into six functional categories: photosynthesis/respiration (36.36%), carbohydrate metabolism (18.18%), energy metabolism (9.09%), chaperon (9.09%), lipid metabolism (9.09%) and unknown function (18.18%), as listed and shown in , Supplementary Table 1 and .
Table 3. Identified moisture responsive proteins using MALDI TOF-TOF in Pishgam cultivar.
Discussion
Comparison of the effects of imposed moisture stress on different cultivars
In this study, under different moisture stress conditions, the phenological and morpho-physiological characteristics of wheat cultivars changed significantly (p < .05). Pishgam, moisture stress-tolerant cultivar, showed higher mean values of all phenological traits except grain filling period (GFP) compared with Shahryar. It seems longer growth period of Pishgam, helps to make optimum use of the environment. It also holds the promise of increasing overall Pishgam productivity by extending potential growing season. As shown in , mean values of the most morpho-physiological traits of Pishgam cultivar was significantly (p < .05) higher than Shahryar. Pishgam cultivar had higher value of grain yield and characteristics related to grain yield. It also used less water during its growth and showed higher water use efficiency (WUE) than Shahryar cultivar under different moisture conditions. So that, these characteristics can increase adaptation and tolerance of Pishgam cultivar to moisture stress. The related to root traits, including main root length (MRL), root volume (RV), root dry weight (RDW), root area (RA) and root to shoot dry weight ratio (RDW/SDW), showed higher mean values in Pishgam cultivar under moisture stress condition. This increase in the related to root traits can reveal this cultivar maintains its root absorption efficiency under moisture stress conditions. In general, agronomic, morphological and phenological traits are very important with suitable potential for detecting suitable wheat genetic resources (Pagnotta et al. Citation2005; Ahmadi et al. Citation2012). The results showed that the studied traits were suitable for indirect selection to improve grain yield and identifying valuable germplasm that contains useful genes for tolerance to moisture stress.
Photosynthesis-related proteins
Photosynthesis occurs primarily in the leaves, and some photosynthesis/respiration proteins change by applying moisture stress. In this group, four responsive proteins to moisture stress were identified, including ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) large subunit (spot 114), chloroplastic ribulose bisphosphate carboxylase small chain (spot, 263), and two isoforms of ribulose bisphosphate carboxylase/oxygenase activase A (Rubisco activase A, spots 69 and 75). In this study, rubisco large subunit was strongly upregulated (10.39-fold) and found at the highest levels of protein expression or accumulation by imposed moisture stress. Also, Rubisco small chain (spot 263) was downregulated (3.5-fold) and each of the two rubisco activase isoforms (spots 69 and 75) were upregulated (3.26-fold and 3.17-fold, respectively) by imposed moisture stress. Rubisco is the key enzyme of Calvin cycle (Parry et al. Citation2003), the only enzyme which can catalyze carboxylation or oxygenation reaction depending upon the molecular concentration of CO2 or O2, and constitutes more than 50% of soluble leaf protein which indicates that this enzyme is important in the plants (Sudhakar et al. Citation2016). Subunits of rubisco enzyme are susceptible to fragmentation under moisture stress conditions. This phenomenon, possibly leading to isoforms of slightly different molecular weight and isoelectric point, an increase in the number of rubisco large subunit (Salekdeh et al. Citation2002; Ge et al. Citation2012; Budak et al. Citation2013). Some researchers reported upregulation of rubisco large subunits in wheat in response to drought stress (Caruso et al. Citation2009; Bazargani et al. Citation2011; Budak et al. Citation2013; Kamal et al. Citation2013; Cheng et al. Citation2015), whereas others found downregulation of rubisco small subunits in wheat (Caruso et al. Citation2009; Ge et al. Citation2012) in response to drought stress. Because of rubisco is involved in photosynthetic carbon assimilation and could improve crop yield in C3 plants (Raines Citation2011), increasing the expression of this enzyme can lead to plant tolerance in moisture stress.
Rubisco activase A identified in 2 spots (spots 69 and 75), probably the causes of this phenomenon are the existence of protein isoforms, post-translational changes and translation from alternative spliced mRNA (Caruso et al. Citation2008; Maleki et al. Citation2014; Liu et al. Citation2015). The amount of active rubisco in a leaf is an important factor regulating the rate of photosynthetic carbon fixation (Servaites et al. Citation1984). Rubisco activases can activate the rubisco enzyme by carbamylation, remove tight binding inhibitors from rubisco, thus play a key role in regulating photosynthesis in plants (Keown et al. Citation2013). It seems upregulation of rubisco activase could lead to more carbon assimilation, more products of photosynthesis, and tolerance to moisture stress in plant. Upregulation of rubisco activase was observed in previous study from our group (Nazari et al. Citation2018) on tolerant Aegilops wheat in response to moisture stress, also in other studies on tolerant plants in response to various stresses (Budak et al. Citation2013; De Abreu et al. Citation2014; Maleki et al. Citation2014; Boustani et al. Citation2017; Yan et al. Citation2017). On the other hand, downregulation of rubisco activase was reported in sensitive plants in response to various stresses (Sobhanian et al. Citation2010; Beritognolo et al. Citation2011; Cheng et al. Citation2015).
Carbohydrate metabolism
Two different proteins were identified in this group including chloroplastic-like fructose-bisphosphate aldolase (spot 93) and phosphoglycerate mutase (spot 181). As shown in and Supplementary Table 1, chloroplastic fructose-bisphosphate aldolase was upregulated (3.92-fold) and phosphoglycerate mutase was downregulated (2.13-fold) in response to moisture stress. Fructose-1,6-bisphosphate aldolase (FBA or FBPA) is a key metabolic enzyme in carbon fixation and sucrose metabolism, catalyzes a reversible reaction that splits the aldol fructose 1,6-bisphosphate (FBP), into dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP) during the pathways of glycolysis or gluconeogenesis (Berg et al. Citation2010). In this study, the increased expression of FBA enzyme could indicate the maintenance of carbohydrate metabolism and signal transduction in tolerant cultivar (Pishgam) during the period of moisture stress and can be identified as moisture stress-responsive marker protein in chloroplast. In several research studies, upregulation of FBA enzyme was reported from crop plants responses to drought and salt stresses (Kamal et al. Citation2012; Zadražnik et al. Citation2013; Faghani et al. Citation2015; Nouri et al. Citation2015).
Phosphoglycerate mutase (PGM), the other protein in this group, is a key enzymatic activity in glycolysis and catalyses the reversible interconversion of 3-phosphoglycerate to 2-phosphoglycerate (Zhao and Assmann Citation2011). This enzyme was often affected by various stresses, observed with upregulated (Caruso et al. Citation2008; Vítámvás et al. Citation2015; Nazari et al. Citation2018) and downregulated (Caruso et al. Citation2009) expression, in wheat and barley plants. Various observations may be due to long-term exposure of the plant to environmental stresses, and the plant adapts to changes without the needing to excess energy.
Energy metabolism
Soluble inorganic pyrophosphatase (spot 152) was only identified in the group of proteins related to energy metabolism, which was downregulated (2.82-fold) by imposing moisture stress. Soluble inorganic pyrophosphatase participates in the assimilation of mineral nutrients, especially in sulfur metabolism (Schmidt and Jäger Citation1992), however, there is little knowledge about the details of this protein function in response to various stresses. It has been reported a decreased abundance of this protein in wheat by drought stress (Bazargani et al. Citation2011), and only detected in drought-stressed plants (Riccardi et al. Citation1998).
Chaperone
In the group of chaperone proteins, the identified protein was chloroplastic 20 kDa chaperonin (spot 133) which was downregulated (2.83-fold) by imposing moisture stress. Chaperones, a group of functional accompanying proteins, are involved in protein folding, assembly, degradation, and protection of nascent proteins during their transport into specific organelles, in both optimal and adverse conditions (Wang et al. Citation2004; Cheng et al. Citation2015). Chaperonin 20 kDa (CPN20) is a well-known chloroplast-localized co-chaperonin and help chaperonin CPN60s in protein folding in an ATP-dependent reaction (Horwich et al. Citation2007). Several studies have shown that CPN20 mediates an antioxidant enzyme (FeSOD) activation in Arabidopsis chloroplasts (Kuo et al. Citation2013), and negatively regulates abscisic acid signaling in Arabidopsis (Zhang et al. Citation2013). It seems the reduction of CPN20 was first observed in wheat in response to moisture stress. Also, low-abundance of this protein was reported in maize leaves under drought stress (Zhao et al. Citation2016).
Lipid metabolism
Chloroplastic 3-oxoacyl [acyl-carrier-protein] synthase I (spot 235) was only identified in this group, which was strongly upregulated (5.52-fold) in response to imposed moisture stress. Acyl carrier protein (ACP) is one of the most abundant proteins in the cell which plays an important role in the pathway of fatty acids biosynthesis in the most of organisms. In this pathway, ACP is converted to its active form by acyl carrier protein synthases (AspS) (White et al. Citation2005). Fatty acids are essential components of cellular membranes, suberin, and cutin waxes which can be structural barriers to the environment. Also, they lead to resistance to environmental stresses through the remodeling of membrane fluidity (Upchurch Citation2008). In this study, high increased abundance of AspS was observed in wheat in response to moisture stress for the first time which can be shown this protein is probably able to regulate the fluidity of the membrane to maintain the function of the essential proteins in tolerant wheat under moisture stress conditions. In the study of Kamal et al. (Citation2012) on a wheat cultivar, this protein showed an increase in abundance in response to salinity stress.
Unknown/hypothetical proteins
In this group, two differentially expressed unknown/hypothetical proteins were detected, including an unnamed protein product (spot 167) with high upregulation (5.45-fold), and hypothetical protein GLYMA-13G084400GLYMA (spot 242) with an upregulation (3-fold). An unknown protein could be defined as a protein whose function has not yet been characterized, and a hypothetical protein could be defined as a protein that is supposed to exist in an organism however its existence has not been shown experimentally (Park et al. Citation2012). These unknown or hypothetical proteins may contribute to moisture-stress tolerance in wheat cultivar.
Conclusion
In this study, we performed a phenological and morpho-physiological analysis with two wheat cultivars subjected to non-stress and moisture stress conditions. After evaluating and determining the moisture stress-tolerant cultivar (Pishgam), its protein changes in flag leaves were identified by 2-DE and MALDI-TOF-TOF MS. Differential expression of 33 moisture stress-responsive proteins revealing the significant effect of moisture stress on the flag leaf proteome of tolerant wheat and the use of that from various signaling pathways and molecular processes in response to moisture stress. Imposed moisture stress significantly increased the abundance of some proteins involved in photosynthesis (rubisco large subunit and rubisco activase A isoform), carbohydrate metabolism (FBA), lipid metabolism (AspSI) and two unknown or hypothetical proteins. In this study, strongly upregulation of AspSI protein in wheat was observed for the first time, in response to moisture stress. Since the highest frequency and level of expression was observed in the upregulated proteins, they can candidate for major roles in tolerance to moisture stress. The abundance of some proteins was significantly reduced by moisture stress which involved in photosynthesis (rubisco small subunit), carbohydrate metabolism (phosphoglycerate mutase), energy metabolism (soluble inorganic pyrophosphatase) and CPN20 chaperonin protein. Furthermore, significant reduction in expression of CPN20 protein in wheat was first observed in this study. The differences in proteome levels may provide an insight into the high tolerance of bread wheat to abiotic stresses. According to the obtained results, proteomics, as a complementary tool, could be useful for identifying candidate genes or proteins to moisture stress-tolerance in bread wheat.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Maryam Nazari
Maryam Nazari is Ph.D. of Plant Breeding from Bu-Ali Sina University, Hamedan, Iran. Her M.Sc. degree is in Plant Breeding from Kerman University, Kerman, Iran. For about 8 years, she has studied response of different plants under abiotic stresses with various techniques such as proteomics, gene expression, statistical analysis, HPLC, UPLC, biochemical traits, and….. She has several papers on tolerance of plants to abiotic stress.
Sayyed Saeed Moosavi
Sayyed Saeed Moosavi is Ph.D. academic staff of Department of Agronomy and Plant Breeding, Faculty of Agriculture, Bu-Ali Sina University, Hamedan, Iran. His Ph.D. and M.Sc. Degree are in Plant Breeding from Tehran University. His research focuses on breeding for tolerance to abiotic stress. He has several papers on this research area. He has studied response of different plants under abiotic stresses with various techniques such as proteomics, gene expression, statistical analysis, HPLC, UPLC, biochemical traits, and…..
Mahmood Maleki
Mahmood Maleki is Ph.D. academic staff of Department of Biotechnology, Institute of Science and High Technology and Environmental Science, Graduate University of Advanced Technology, Kerman, Iran. His Ph.D. and M.Sc. Degree are in Agricultural Biotechnology from Tehran University. His research focuses on breeding for tolerance to abiotic stress. He has several papers on this research area. He has studied response of different plants under abiotic stresses with various techniques such as proteomics, gene expression, statistical analysis, HPLC, UPLC, biochemical traits, and…..
Kiarash Jamshidi Goharrizi
Kiarash Jamshidi Goharrizi currently hold a Ph.D. in Plant Breeding from Azad University of Yazd, Iran (3 September 2019) and an M.Sc. degree in Agricultural Engineering in the study field of Biotechnology and Molecular Genetics in Horticultural Products from Azad University of Jiroft, Iran (1 September 2015). He has studied response of different plants under abiotic stresses with various techniques such as proteomics, gene expression, statistical analysis, HPLC, UPLC, biochemical traits, breeding programs (pistachio rootstocks and wheat) and gene registration. He has 26 papers, 13 of which were published by the best journals in the world.
References
- Ahmadi M, Farshadfar E, Veisi S. 2012. Evaluation of genetic diversity in land races of bread wheat under irrigated and rain fed conditions. Int J Agric Crop Sci. 4:1627–1636.
- Bazargani MM, Sarhadi E, Bushehri AAS, Matros A, Mock HP, Naghavi MR, Ehdaie B. 2011. A proteomics view on the role of drought-induced senescence and oxidative stress defense in enhanced stem reserves remobilization in wheat. J Proteomics. 74:1959–1973. doi: 10.1016/j.jprot.2011.05.015
- Berg IA, Kockelkorn D, Ramos-Vera WH, Say RF, Zarzycki J, Hügler M, Fuchs G. 2010. Autotrophic carbon fixation in archaea. Nat Rev Microbiol. 8:447. doi: 10.1038/nrmicro2365
- Beritognolo I, Harfouche A, Brilli F, Prosperini G, Gaudet M, Brosché M, Salani F, Kuzminsky E, Auvinen P, Paulin L. 2011. Comparative study of transcriptional and physiological responses to salinity stress in two contrasting Populus alba L. genotypes. Tree Physiol. 31:1335–1355. doi: 10.1093/treephys/tpr083
- Blum H, Beier H, Gross HJ. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 8:93–99. doi: 10.1002/elps.1150080203
- Boustani A, Fatehi F, Azizinezhad R. 2017. The proteome response of Hordeum marinum to long-term salinity stress. Cereal Res Commun. 45:401–410. doi: 10.1556/0806.45.2017.020
- Brenchley R, Spannagl M, Pfeifer M, Barker GL, D’Amore R, Allen AM, Kay S. 2012. Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature. 491:705. doi: 10.1038/nature11650
- Budak H, Akpinar BA, Unver T, Turktas M. 2013. Proteome changes in wild and modern wheat leaves upon drought stress by two-dimensional electrophoresis and nanoLC-ESI–MS/MS. Plant Mol Biol. 83:89–103. doi: 10.1007/s11103-013-0024-5
- Caruso G, Cavaliere C, Foglia P, Gubbiotti R, Samperi R, Laganà A. 2009. Analysis of drought responsive proteins in wheat (Triticum durum) by 2D-PAGE and MALDI-TOF mass spectrometry. Plant Sci. 177:570–576. doi: 10.1016/j.plantsci.2009.08.007
- Caruso G, Cavaliere C, Guarino C, Gubbiotti R, Foglia P, Laganà A. 2008. Identification of changes in Triticum durum L. leaf proteome in response to salt stress by two-dimensional electrophoresis and MALDI-TOF mass spectrometry. Anal Bioanal Chem. 391:381–390. doi: 10.1007/s00216-008-2008-x
- Cheng L, Wang Y, He Q, Li H, Zhang X, Zhang F. 2016. Comparative proteomics illustrates the complexity of drought resistance mechanisms in two wheat (Triticum aestivum L.) cultivars under dehydration and rehydration. BMC Plant Biol. 16:188. doi: 10.1186/s12870-016-0871-8
- Cheng Z, Dong K, Ge P, Bian Y, Dong L, Deng X, Li X, Yan Y. 2015. Identification of leaf proteins differentially accumulated between wheat cultivars distinct in their levels of drought tolerance. PLoS One. 10:e0125302. doi: 10.1371/journal.pone.0125302
- Damerval C, De Vienne D, Zivy M, Thiellement H. 1986. Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis. 7:52–54. doi: 10.1002/elps.1150070108
- De Abreu CEB, dos Santos Araújo G, de Oliveira Monteiro-Moreira AC, Costa JH, de Brito Leite H, Moreno FBMB, Prisco JT, Gomes-Filho E. 2014. Proteomic analysis of salt stress and recovery in leaves of Vigna unguiculata cultivars differing in salt tolerance. Plant Cell Rep. 33:1289–1306. doi: 10.1007/s00299-014-1616-5
- Faghani E, Gharechahi J, Komatsu S, Mirzaei M, Khavarinejad RA, Najafi F, Farsad LK, Salekdeh GH. 2015. Comparative physiology and proteomic analysis of two wheat genotypes contrasting in drought tolerance. J Proteomics. 114:1–15. doi: 10.1016/j.jprot.2014.10.018
- Fotovat R, Alikhani M, Valizadeh M, Mirzaei M, Salekdeh GH. 2017. A proteomics approach to discover drought tolerance proteins in wheat Pollen grain at Meiosis stage. Protein Pept Lett. 24:26–36. doi: 10.2174/0929866523666161130143446
- Ge P, Ma CY, Wang SL, Gao LY, Li XH, Guo GF, Ma WJ, Yan YM. 2012. Comparative proteomic analysis of grain development in two spring wheat varieties under drought stress. Anal Bioanal Chem. 402:1297–1313. doi: 10.1007/s00216-011-5532-z
- Ghatak A, Chaturvedi P, Weckwerth W. 2017. Cereal crop proteomics: systemic analysis of crop drought stress responses towards marker-assisted selection breeding. Front Plant Sci. 8:757. doi: 10.3389/fpls.2017.00757
- Görg A, Postel W, Domscheit A, Günther S. 1988. Two-dimensional electrophoresis with immobilized pH gradients of leaf proteins from barley (Hordeum vulgare): method, reproducibility and genetic aspects. Electrophoresis. 9:681–692. doi: 10.1002/elps.1150091103
- Hameed A, Bibi N, Akhter J, Iqbal N. 2011. Differential changes in antioxidants, proteases, and lipid peroxidation in flag leaves of wheat genotypes under different levels of water deficit conditions. Plant Physiol Biochem. 49:178–185. doi: 10.1016/j.plaphy.2010.11.009
- Horwich AL, Fenton WA, Chapman E, Farr GW. 2007. Two families of chaperonin: physiology and mechanism. Annu Rev Cell Dev Biol. 23:115–145. doi: 10.1146/annurev.cellbio.23.090506.123555
- Jamshidi Goharrizi K, Baghizadeh A, Kalantar M, Fatehi F. 2020. Combined effects of salinity and drought on physiological and biochemical characteristics of pistachio rootstocks. Sci Hortic. 261:108970. doi: 10.1016/j.scienta.2019.108970
- Jamshidi Goharrizi K, Moosavi SS, Amirmahani F, Salehi F, Nazari M. 2020. Assessment of changes in growth traits, oxidative stress parameters, and enzymatic and non-enzymatic antioxidant defense mechanisms in Lepidium draba plant under osmotic stress induced by polyethylene glycol. Protoplasma. 257:459–473. doi: 10.1007/s00709-019-01457-0
- Jamshidi Goharrizi K, Wilde HD, Amirmahani F, Moemeni MM, Zaboli M, Nazari M, Moosavi SS, Jamalvandi M. 2018. Selection and validation of reference genes for normalization of qRT-PCR gene expression in wheat (Triticum durum L.) under drought and salt stresses. J Genet. 97:1433–1444. doi: 10.1007/s12041-018-1042-5
- Kamal A, Cho K, Choi JS, Jin Y, Park CS, Lee J, Woo S. 2013. Patterns of protein expression in water-stressed wheat chloroplasts. Biol Plant. 57:305–312. doi: 10.1007/s10535-012-0290-0
- Kamal AHM, Cho K, Kim DE, Uozumi N, Chung KY, Lee SY, Woo SH. 2012. Changes in physiology and protein abundance in salt-stressed wheat chloroplasts. Mol Biol Rep. 39:9059–9074. doi: 10.1007/s11033-012-1777-7
- Kamal AHM, Kim KH, Shin KH, Choi JS, Baik BK, Tsujimoto H, Woo SH. 2010. Abiotic stress responsive proteins of wheat grain determined using proteomics technique. Aust J Crop Sci. 4:196.
- Keown JR, Griffin MD, Mertens HD, Pearce FG. 2013. Small oligomers of ribulose-bisphosphate carboxylase/oxygenase (rubisco) activase are required for biological activity. J Biol Chem. 288(28):20607–20615. doi: 10.1074/jbc.M113.466383
- Kuo WY, Huang CH, Liu AC, Cheng CP, Li SH, Chang WC, Jinn TL. 2013. CHAPERONIN 20 mediates iron superoxide dismutase (FeSOD) activity independent of its co-chaperonin role in Arabidopsis chloroplasts. New Phytol. 197:99–110. doi: 10.1111/j.1469-8137.2012.04369.x
- Li N, Zhang S, Liang Y, Qi Y, Chen J, Zhu W, Zhang L. 2018. Label-free quantitative proteomic analysis of drought stress-responsive late embryogenesis abundant proteins in the seedling leaves of two wheat (Triticum aestivum L.) genotypes. J Proteomics. 172:122–142. doi: 10.1016/j.jprot.2017.09.016
- Liu H, Sultan MARF, Li Liu X, Zhang J, Yu F, Zhao H. 2015. Physiological and comparative proteomic analysis reveals different drought responses in roots and leaves of drought-tolerant wild wheat (Triticum boeoticum). PLoS One. 10:e0121852. doi: 10.1371/journal.pone.0121852
- Maleki M, Naghavi M, Alizadeh H, Poostini K, Mishani CA. 2014. Comparison of protein changes in the leaves of two bread wheat cultivars with different sensitivity under salt stress. Ann Res Rev Biol. 4:1784–1797. doi: 10.9734/ARRB/2014/7795
- Mguis K, Albouchi A, Abassi M, Khadhri A, Ykoubi-Tej M, Mahjoub A, Brahim NB, Ouerghi Z. 2013. Responses of leaf growth and gas exchanges to salt stress during reproductive stage in wild wheat relative Aegilops geniculata Roth. and wheat (Triticum durum Desf.). Acta Physiol Plant. 35:1453–1461. doi: 10.1007/s11738-012-1185-6
- Nazari M, Jamshidi Goharrizi K, Moosavi SS, Maleki M. 2019. Expression changes in the TaNAC2 and TaNAC69-1 transcription factors in drought stress tolerant and susceptible accessions of Triticum boeoticum. Plant Genet. Resour. 17:471–479. doi: 10.1017/S1479262119000303
- Nazari M, Moosavi SS, Maleki M. 2018. Morpho-physiological and proteomic responses of Aegilops tauschii to imposed moisture stress. Plant Physiol Biochem. 132:445–452. doi: 10.1016/j.plaphy.2018.09.031
- Ngara R, Ndimba BK. 2014. Understanding the complex nature of salinity and drought-stress response in cereals using proteomics technologies. Proteomics. 14:611–621. doi: 10.1002/pmic.201300351
- Nouri MZ, Moumeni A, Komatsu S. 2015. Abiotic stresses: insight into gene regulation and protein expression in photosynthetic pathways of plants. Int J Mol Sci. 16:20392–20416. doi: 10.3390/ijms160920392
- Pagnotta MA, Mondini L, Atallah MF. 2005. Morphological and molecular characterization of Italian emmer wheat accessions. Euphytica. 146:29–37. doi: 10.1007/s10681-005-8607-0
- Park SJ, Son WS, Lee BJ. 2012. Structural analysis of hypothetical proteins from helicobacter pylori: an approach to estimate functions of unknown or hypothetical proteins. Int J Mol Sci. 13:7109–7137. doi: 10.3390/ijms13067109
- Parry MAJ, Andralojc PJ, Mitchell RA, Madgwick PJ, Keys AJ. 2003. Manipulation of rubisco: the amount, activity, function and regulation. J Exp Bot. 54:1321–1333. doi: 10.1093/jxb/erg141
- Raines CA. 2011. Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiol. 155:36–42. doi: 10.1104/pp.110.168559
- Rezaee F, Lahouti M, Maleki M, Ganjeali A. 2018. Comparative proteomics analysis of whitetop (Lepidium draba L.) seedlings in response to exogenous glucose. Int J Biol Macromol. 120:2458–2465. doi: 10.1016/j.ijbiomac.2018.09.016
- Riccardi F, Gazeau P, de Vienne D, Zivy M. 1998. Protein changes in response to progressive water deficit in maize: quantitative variation and polypeptide identification. Plant Physiol. 117:1253–1263. doi: 10.1104/pp.117.4.1253
- Salekdeh GH, Siopongco J, Wade LJ, Ghareyazie B, Bennett J. 2002. Proteomic analysis of rice leaves during drought stress and recovery. Proteom Int Ed. 2:1131–1145. doi: 10.1002/1615-9861(200209)2:9<1131::AID-PROT1131>3.0.CO;2-1
- Schmidt A, Jäger K. 1992. Open questions about sulfur metabolism in plants. Annu Rev Plant Biol. 43:325–349. doi: 10.1146/annurev.pp.43.060192.001545
- Servaites JC, Torisky RS, Chao SF. 1984. Diurnal changes in ribulose-1,5-bisphosphate carboxylase activity and activation state in leaves of field grown soybeans. Plant Sci Lett. 35:115–121. doi: 10.1016/0304-4211(84)90184-6
- Sobhanian H, Razavizadeh R, Nanjo Y, Ehsanpour AA, Jazii FR, Motamed N, Komatsu S. 2010. Proteome analysis of soybean leaves, hypocotyls and roots under salt stress. Proteome Sci. 8:19. doi: 10.1186/1477-5956-8-19
- Sudhakar P, Latha P, Reddy PV. 2016. Phenotyping crop plants for physiological and biochemical traits. Amsterdam: Elsevier/Academic Press.
- Upchurch RG. 2008. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett. 30:967–977. doi: 10.1007/s10529-008-9639-z
- Vítámvás P, Urban MO, Škodáček Z, Kosová K, Pitelková I, Vítámvás J, Renaut J, Prášil IT. 2015. Quantitative analysis of proteome extracted from barley crowns grown under different drought conditions. Front Plant Sci. 6:479. doi: 10.3389/fpls.2015.00479
- Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA. 2005. Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J Plant Physiol. 162:465–472. doi: 10.1016/j.jplph.2004.09.009
- Wang W, Vinocur B, Shoseyov O, Altman A. 2004. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 9:244–252. doi: 10.1016/j.tplants.2004.03.006
- White SW, Zheng J, Zhang YM, Rock CO. 2005. The structural biology of type II fatty acid biosynthesis. Annu Rev Biochem. 74:791–831. doi: 10.1146/annurev.biochem.74.082803.133524
- Yan L, Fan G, Deng M, Zhao Z, Dong Y, Li Y. 2017. Comparative proteomic analysis of autotetraploid and diploid Paulownia tomentosa reveals proteins associated with superior photosynthetic characteristics and stress adaptability in autotetraploid Paulownia. Physiol Mol Biol Plants. 23:605–617. doi: 10.1007/s12298-017-0447-6
- Zadražnik T, Hollung K, Egge-Jacobsen W, Meglič V, Šuštar-Vozlič J. 2013. Differential proteomic analysis of drought stress response in leaves of common bean (Phaseolus vulgaris L.). J Proteomics. 78:254–272. doi: 10.1016/j.jprot.2012.09.021
- Zhang XF, Jiang T, Wu Z, Du SY, Yu YT, Jiang SC, Zhang DP. 2013. Cochaperonin CPN20 negatively regulates abscisic acid signaling in Arabidopsis. Plant Mol Biol. 83:205–218. doi: 10.1007/s11103-013-0082-8
- Zhao F, Zhang D, Zhao Y, Wang W, Yang H, Tai F, Hu X. 2016. The difference of physiological and proteomic changes in maize leaves adaptation to drought, heat, and combined both stresses. Front Plant Sci. 7:1471.
- Zhao Z, Assmann SM. 2011. The glycolytic enzyme, phosphoglycerate mutase, has critical roles in stomatal movement, vegetative growth, and pollen production in Arabidopsis thaliana. J Exp Bot. 62:5179–5189. doi: 10.1093/jxb/err223