ABSTRACT
Flooding is an important limiting factor for plant growth and development. Recent studies have shown that endophytic fungi symbiotic with extreme environmental plants can help hosts resisting various stresses. Previously, a styrene antioxidant NFA (Z-N-(4-hydroxystyryl) formamide) in riparian endophytic Aspergillus fumigatus was reported to effectively improve drought resistance in rice. To evaluate NFA’s ability to resist flooding of plants, we tested the antioxidant activity in yeasts, and investigated the flooding tolerance of Arabidopsis seedlings through fully submergence. The results showed that NFA could effectively alleviated oxidative damage by H2O2 in yeast and improved the flooding resistance of Arabidopsis. The tendency of oxidative parameters responded to flooding can be reversed by NFA, such as malondialdehyde, superoxide dismutase, ethanol dehydrogenase and NADPH oxidase. Expression analyses of NADPH oxidases and ROP protein families indicated that Atrboh D, Atrboh F and AtROP 11 may be involved in NFA-mediated flooding resistance.
1. Introduction
Flooding seriously affects agroforestry production worldwide. In flooded plants, oxygen and light supply is limited, and the cells are forced to turn to anaerobic respiration, leading to the accumulation of reactive oxygen species (ROS) (Adkins et al. Citation2010; Miao et al. Citation2017). Mild flooding quickly induces plants to produce appropriate ROS, which triggers oxidative burst and promotes the expression of stress-resistant genes and protective enzymes (Jorge et al. Citation2016; Singh et al. Citation2016; Foyer et al. Citation2017). While severe flooding results in ROS accumulation exceeding scavenging activity of protective enzymes such as superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD), which would give rise to lipid peroxidation and damage membrane system, together with production of toxic malondialdehyde (MDA) to crosslink macromolecules (Pucciariello and Perata Citation2017).
In Arabidopsis, flooding or hypoxia treatment would up-regulate Alcohol dehydrogenase (ADH), through which ROS radicals would be partially removed by oxidation NAD(P)H into NAD(P)+ (Cao et al. Citation2017). Therefore, ADH activity of flooding-tolerant species increased significantly when subjected to flooding (Adkins et al. Citation2010; An et al. Citation2016).
As a key enzyme producing ROS, NADPH oxidase (known as Rboh in Arabidopsis) is closely related to the flooding resistance of plants (Kwak et al. Citation2003; Weyemi and Dupuy Citation2012). Studies have shown that both AtrbohD and AtrbohF mutants are very sensitive to hypoxia stress (Kwak et al. Citation2003; Liu et al. Citation2017). Additionally, NADPH oxidase in plants is activated by ROP, a small GTP binding protein (Fehér and Lajkó Citation2015; Ma et al. Citation2017), which is involved in ROS accumulation and response to abiotic stresses (Steffens and Sauter Citation2010).
Endophytic fungi played an important role in plants’ response to stress by promoting ecological adaptability of the hosts (Weyens et al. Citation2009; Khiralla et al. Citation2016). For example, endophytes from coastal mangroves are capable of enhancing the tolerance to saline-alkali (Mei and Flinn Citation2010). It is reported that the root fungal endophytes enhance heavy metal stress tolerance of the trees naturally distributed in several mines (Yamaji et al. Citation2016). To help plants to deal with various adversities, endophytes may produce numerous secondary metabolites such as alkaloids, terpenoids or phenols to enhance the activity of antioxidant enzymes, or elicit a certain pathway (McLellan et al. Citation2007; Mishra et al. Citation2016; Bilal et al. Citation2018).
In the previous study, we obtained lots of endophytic fungi with high antioxidant activity from Myricaria laxiflora (Qin et al. Citation2019), a plant living in the riparian zone of the Yangtze River and surviving from 6 months of flooding a year (Tian et al. Citation2015). Endophytic Aspergillus fumigatus SG-17 was reported to effectively alleviate drought stress in rice by styrene antioxidant of NFA (Qin et al. Citation2019). Herein, we evaluated the role of NFA in enhancing Arabidopsis against flooding, which was helpful to understand the flooding tolerance mediated by the antioxidants, and laid a foundation for improving anti-flood capacity of plants.
2. Materials and methods
2.1. Strains, medium and plant resources
The fungus of A. fumigatus SG-17 (CCTCCM 2015286, China Typical Culture Preservation Center) was isolated from the root of M. laxiflora and preserved in paraffin at 4°C (Qin et al. Citation2019). Yeast and the mutants were purchased from EUROSCARF library. Arabidopsis seeds of Col-0 were provided by Biotechnology Center of Three Gorges University. SG-17 was cultured in potato dextrose agar (PDA) medium, comprising 200 g potato, 20 g glucose and 15–20 g agar, made up with distilled water to 1000 mL. The pH was adjusted to 6.0 and the medium was sterilized at 121°C for 20 min. Yeast was cultured in YPD (Yeast Peptone Dextrose) medium, which consisted of 1% yeast extract, 2% tryptone, 2% glucose, and the solid medium was added to 2% agar.
2.2. Preparation of NFA
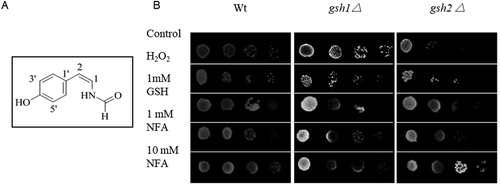
The fungus SG-17 was inoculated into shaking flask (200 mL/500 mL), and then grown for 14 d at 28°C. The fermented liquid was harvested by suction and extracted 3 times with an equal volume of ethyl acetate. HHitech ultra-pure water was applied. After thin layer chromatography and semi-preparative high performance liquid chromatography separation, the compound of NFA ((A)) was collected to test the activity (Qin et al. Citation2019).
2.3. Antioxidant activity of NFA in yeast
Wild type BY4741, gsh1Δ and gsh2Δ mutant strain (supplementary table 1) were pre-cultured in YPD medium at 30 °C until the final OD600 reached 0.05, then transferred to the fresh YPD. After the logarithmic stage growth, the cells were collected and washed with sterile water for three times, then diluted OD600 value to 2.0, 0.2 and 0.02, respectively. The suspension of 5 μL was placed onto YPD medium containing 2 mM H2O2, and then cultured at 30°C for 3 d before photograph. In the comparative group, 1–10 mM of NFA was used to evaluate the antioxidant activity, while 1 mM of GSH was used as the positive control (Koskimäki et al. Citation2016).
2.4. Flooding stress of Arabidopsis seedlings and the physiological response
The vernalized Col-0 seeds were evenly spotted into sterilized soil (nutrient soil: vermiculite = 1:1), then sealed with fresh-keeping film and placed in plant culture room. The plants grew under 20–22°C, 16 h/8 h alternating day and night. The light intensity was 120–150 µmol m−2 s−1. After 3–5 days, removed the film and kept the seedlings growing for 4 weeks until flowering stage. In our preliminary investigation, Arabidopsis plants during this period were more sensitive to flooding. The flooding condition was: Six similar seedlings per pot were fully submerged for 4 days, then pour out and grew normal for another 2 weeks before recording. The volume of the pot was 855 cm3 with a height of 9 cm. In the treatment group, NFA at 1, 10 and 100 µg/mL dissolved in DMSO were sprayed to the seedlings 4 h before flooding. Each treatment was repeated three times.
Four days after the plants submerged, MDA and SOD contents were assayed by kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). ADH and NADPH oxidase contents were determined by One-step sandwich ELISA, using kits from Jiangsu Baolai Biotechnology Co., Ltd. (Jiangsu, China). All the procedures were followed the manufacturer’s instructions.
2.5. Expression analysis of Atrbohs and AtROPs
The seedlings were treated with 1 µg/mL of NFA for 1, 2, 4 and 6 h, and then flooded for 4 days. After harvested 3–4 leaves for RNA extraction by EASYspin kit from Aidlab Biology Co., Ltd (Beijing, China), Real time RT–PCR was performed 30–35 cycles by the kit of GenStar Co., Ltd (Beijing, China) according to the instructions, and actin was used as an internal standard. All the primer sequences were listed in the supplementary table 2.
2.6. Data processing
The data analysis and drawing software used in this experiment was Graphpad prism 5 (GraphPad Software Inc., San Diego, CA, USA). One way ANOVA and LSD were used to analyze the significance. All data were expressed as means ± standard deviation.
3. Results
3.1. Antioxidant activity of NFA in yeast
In the previous study, we reported the antioxidant activity of NFA, a styrene derived from endophytic A. fumigatus. In order to confirm the protective effect of NFA on oxidative damage in vivo, a gradient dilution method was used to evaluate Saccharomyces cerevisiae cells and GSH biosynthesis singular gene deletions mutants (gsh1Δ and gsh2Δ) ((B)). The results showed that NFA at concentrations of 1–10 mM obviously shielded yeast against H2O2 stress. Compared with gsh1Δ, the mutant of gsh2Δ was more sensitive to oxidative stress. Anyhow, both of them showed reduced sensitivity to H2O2 when treated with NFA, indicating a clear antioxidant activity of NFA in vivo.
3.2. NFA effectively assisted Arabidopsis to resist flooding stress
Previously, NFA present in riparian fungus was reported to improve the drought resistance of rice by regulating the oxidation pathway (Qin et al. Citation2019). Herein, the role of NFA in plant flooding was assessed by fully submerged Arabidopsis seedlings. After 4 days’ flooding and 2 weeks’ recovery, the growth vigor and plant height were significantly lower than those of normal seedlings ((A)). Whereas NFA can partially rescue the phenotype ((B–D)), particularly the flooded plants approached almost normal at concentration of 100 μg/mL ((D)), indicated that NFA can effectively improve the flooding tolerance of Arabidopsis.
Figure 2. Effects of NFA on Arabidopsis seedlings under flooding. A: Flooded for 4d, B: NFA (1 μg/mL) and flooded, C: NFA (10 μg/mL) and flooded, D: NFA (100 μg/mL) and flooded, E: No flooded, F: NFA (100 μg/mL) no flooded. G–J: Contents of MDA, SOD, ADH and NADPH, ‘**’means significant differences in P < 0.01.
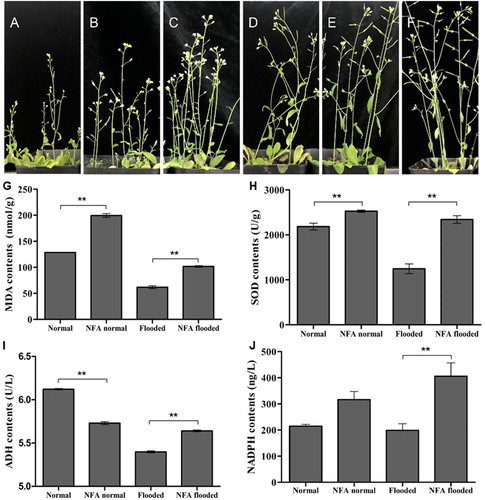
When plants suffered from adversity, the contents of MDA, SOD and ADH would change significantly. Therefore, these parameters are important indicators for evaluating stress degree or resistance ability (Adkins et al. Citation2010; Miao et al. Citation2017). Although instantaneous flooding resulted in increased levels of MDA, SOD and ADH, the contents would decrease significantly with the prolonged inundation (supplementary figure 1; Miao et al. Citation2017). After 4 days of flooding, the contents of MDA, SOD and ADH decreased by 51.83%, 42.92% and 11.76%, respectively ((G–I), p < 0.01). However, 1 μg/mL of NFA significantly increased MDA and SOD contents under normal condition ((G–H)), and reached the maximum value after treatment for 4 h (supplementary figure 2). Surprisingly, the positive effect of NFA in Arabidopsis was more obvious after flooding. In terms of SOD, it only increased by 15.79% before flooding, whereas reached 88.07% after flooding, which was close to the normal level ((H)). At the same time, before flooding NFA reduced ADH amounts, but after 4 days’ fully flooding, ADH contents significantly increased compared with non NFA treatment (p < 0.01, (I)), retarding the decline of ADH caused by flooding. These results indicated that after flooding, NFA was more conducive to maintaining ROS homeostasis, confirming that NFA alleviated the flooding stress level in plants through oxidative regulation.
3.3. Expression analysis of Atrbohs family responded to NFA
NADPH oxidase is a key enzyme producing ROS, which can be activated by NFA to improve drought resistance in rice (Qin et al. Citation2019). Similarly, whether or not the Arabidopsis plants were flooded, NFA would lead to an increase of NADPH oxidase content ((J)). Moreover, like SOD and ADH, the NADPH oxidase enhancement by NFA was more obvious after flooding than before, suggesting the role of NFA in plants against flooding.
Since there were 10 genes, Atrboh A-J, encoding NADPH oxidase in Arabidopsis genome, to further clarify which member was responsible for flooding response, qRT-PCR was used to analyze the family before and after flooding. These members responded differently to flooding stress in Arabidopsis. Among the 10 genes, only AtrbohD and AtrbohF decreased significantly after 4 days of flooding (p < 0.01, (A–B), supplementary figure 3), indicated that these two genes may be the main NADPH oxidases of Arabidopsis under flooding stress, consistent with Atrboh D and Atrboh F involving in hypoxia tolerance reported in the literature (Liu et al. Citation2017).
Figure 3. The relative level of Atrboh D and Atrboh F. A and B: The expression with flooding time. C and D: The expression of Atrboh D (C) and Atrboh F (D) after NFA treatment without flooding stress. E and F: The expression comparison of Atrboh D (E) and Atrboh F (F) after NFA treatment under normal or flooding conditions.
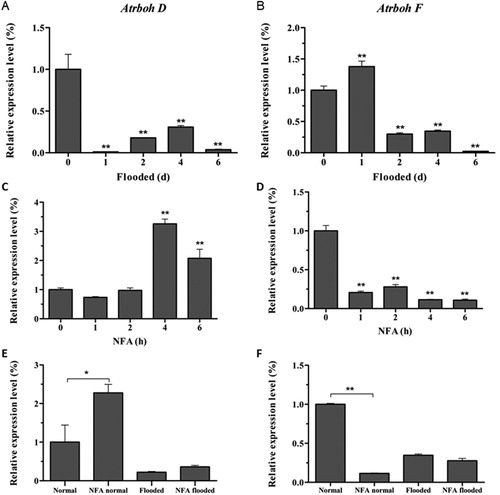
NFA may improve the flooding resistance of Arabidopsis by regulation Atrboh D and Atrboh F. Under normal condition, NFA at 1 μg/mL significantly increased the expression of AtrbohD in Arabidopsis (p < 0.01), and maximized when treated for 4 h ((C)). However, AtrbohF can be inhibited by NFA, and the down-regulation rate reached 88.6% at 4 h ((D)). Accordingly, after flooding stress AtrbohD was slightly up-regulated by NFA ((E)). However, flooding decreased the expression of AtrbohF by 65.4% in the absence of NFA, and only 19.9% after NFA treatment ((F)), suggesting AtrbohF was partially restored by NFA to participate in flooding stress.
3.4. Expression of AtROPs family affected by NFA
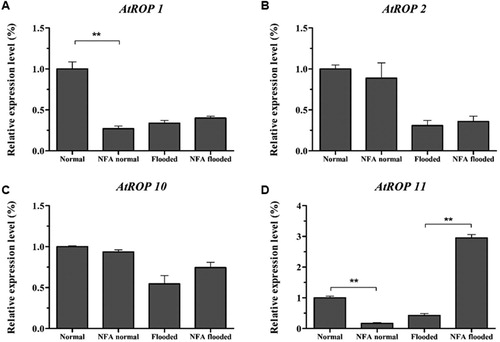
In higher plants, most NADPH oxidases were activated by ROP proteins (Ma et al. Citation2017). To determine whether NFA affects the expression of ROP in Arabidopsis, qRT-PCR was used to analyze AtROPs members related to oxidative metabolism under flooding stress. The results showed that AtROP1, AtROP2 and AtROP11 were significantly decreased in continuous flooding (supplementary figure 4, p < .01), just like the drop in expression of Atrboh D and Atrboh F. Furthermore, in non-flooding condition, NFA decreased the level of AtROP1, AtROP10 and AtROP11 (, supplementary figure 5). However, this regulatory effect of NFA can be reversed by flooding, which up-regulated the above 3 genes, especially AtROP11 increased by 6-fold (), indicating that AtROP11 should play an important role in NFA-mediated flooding resistance.
4. Discussion
In recent years, there have been more and more reports on endophytes assisting host plants to improve habitat adaptability, but few about flooding resistance. Based on views of chemical ecology, here we reported the endophytic A. fumigatus SG-17 from riparian M. laxiflora produced a styrene antioxidant NFA, which alleviated oxidative damage by H2O2 in yeast and improved the ability of Arabidopsis to resist flooding stress. These findings indicate that the endophytic fungi from plants adapted to waterlogging stress have a positive effect on plant resistance to flooding.
Nearly half a century ago, NFA was isolated as a selective antibiotic to tuberculosis and an inhibitor against platelet aggregation (Anzai et al. Citation1962; Umehara et al. Citation1984). So far, there are few reports on the antioxidant activity and improving plants to resist adversities. As a matter of fact, most NFA was obtained as a secondary metabolite from marine or aquatic fungi, such as Penicillium chrysogenum from the Yellow River Delta (Qu et al. Citation2012) or from the marine endophytic fungi (An et al. Citation2013), leading to a hypothesis that the substance may be related to water stress. In our results, NFA could slightly promote the growth of Arabidopsis ((E–F)), however, physiological parameters and gene expression showed that NFA had a more significant effect on the growth of Arabidopsis under flooding, suggesting that NFA might be more likely to function as a stress-resistant substance than as a biomass-promoting substance.
In Arabidopsis plants after flooding, NFA significantly affected the contents of NADPH oxidase to resist flooding. However, in terms of the detailed role of NFA, we found an apparent paradox: on the one hand NFA can reduce the content of ROS as an antioxidant; on the other hand it can improve the activity of NADPH oxidase in vivo to increase the content of ROS. The reason may be due to the dual regulation of NFA: induction ROS generation to launch stress response, and elimination excess ROS to improve the plant’s ability to resist flooding.
Physiological parameters such as MDA and SOD were closely related to plant varieties and stress resistance. As a product of oxidative damage, MDA generally went up in the early stress stage, and went down with the continuance, whereas the tendency in SOD was exactly the opposite (Guo et al. Citation2019). In our experiment, NFA could effectively reverse the trend of both MDA and SOD under flooding ((G–H)), which fully reflected the role of NFA in plants against flooding stress.
Atrboh D and Atrboh F, two members of NADPH oxidase, may be involved in NFA-mediated flooding resistance of Arabidopsis, whereas the function modes may be distinct. With regard to Atrboh D, NFA inhibited the down-regulation by flooding ((A,C,E)). However, the function way of Atrboh F may be more complex, by virtue that both flooding and NFA treatment led to its reduction ((B,D,F)), resulting in the fallacy that NFA promoted flooding stress. A possible reason was that the different treatment time was set in the experiment, 4 days in flooding versus 4 h in NFA treatment. Actually Atrboh F increased significantly at 1 day of flooding ((B)), and this trend was also inhibited by NFA, suggested that AtrbohF may play a role in the early stage of flooding, while AtrbohD may function in the late stage. Either AtrbohD or AtrbohF, NFA can counteract the alteration of expression level caused by flooding stress, thus improving the tolerance to flooding.
ROP proteins play a key role in plant response to flooding stress as a positive regulator of NADPH oxidase. In our study, AtROP11 was significantly activated by NFA under flooded for 4 days, implied the function in response to flooding stress in plants, however, the function needs to be further studied in the future.
Unfortunately, here we failed to probe whether NFA can improve the flooding resistance in the endophytic fungi SG-17’s natural host, M. laxiflora, due to the difficulty in obtaining aseptic seedlings without endophytic fungi. To further reveal the molecular mechanism of NFA improving plants flooding tolerance, future research would be focused on: (1) The effect of NFA on the flood resistance of the natural host, (2) How NFA regulates NADPH oxidase, (3) The role of NADPH oxidases and the instantaneous quantitative of ROS in various conditions, (4) Using NADPH oxidase inhibitors and mutants to analyze pathways related flooding.
Supplemental Material
Download MS Word (767 KB)Acknowledgements
XYH performed most of the experiments and wrote the manuscript under the supervision of LSP. GY analyzed the data. LCX provided technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Yanhong Xue
Yanhong Xue holds an MSc in Ecology from the Three Gorges University. Now she is a technician in Hubei laboratory of Natural Products Research and Development. Her current research interests focus on chemical ecology.
Yuan Gao
Yuan Gao is a graduate student majored in Microbiology in the Three Gorges University.
Chengxiong Liu
Chengxiong Liu holds a PhD in Biochemistry and Molecular Biology from the Wuhan University and is Lecturer at Biological and Pharmaceutical College, Three Gores University. He is interested in separation and structure identification of natural products.
Shiping Liu
Shiping Liu holds a PhD in Biochemistry and Molecular Biology and is a professor in Biological and Pharmaceutical College, Three Gores University. His current research interests include chemical biology and the interaction of plants and microbes, together with biological activity evaluation of natural products.
References
- Adkins SW, Shiraishi T, Mccomb JA. 2010. Submergence tolerance of rice–A new glasshouse method for the experimental submergence of plants. Physiol Plant. 80(4):642–646.
- An CY, Li XM, Li CS, Gao SS, Shang Z, Wang BG. 2013. Triazoles and other N-containing metabolites from the marine-derived endophytic fungus Penicillium chrysogenum EN-118. Helv Chim Acta. 96:682–687.
- An Y, Qi L, Wang L. 2016. ALA pretreatment improves waterlogging tolerance of fig plants. PLoS One. 11(1):e0147202.
- Anzai K, Okuma K, Nagatsu J, Suzuki S. 1962. Chemical structure of tuberin. J Antibiot. 15:110–111.
- Bilal S, Khan AL, Shahzad R, Kim YH, Imran M, Khan MJ, Al-Harrasi A, Kim TH, Lee IJ. 2018. Mechanisms of Cr (VI) resistance by endophytic Sphingomonas sp. LK11 and its Cr (VI) phytotoxic mitigating effects in soybean (Glycine max L.). Ecotox Environ Safe. 164:648–658.
- Cao Y, Ma C, Chen G, Zhang J, Xing B. 2017. Physiological and biochemical responses of Salix integra Thunb under copper stress as affected by soil flooding. Environ Pollut. 225:644–653.
- Fehér A, Lajkó DB. 2015. Signals fly when kinases meet Rho-of-plants (ROP) small G-proteins. Plant Sci. 237:93–107.
- Foyer CH, Ruban AV, Noctor G. 2017. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem J. 474(6):877–883.
- Guo Y, Ping W, Chen J, Zhu L, Zhao Y, Guo J, Huang Y. 2019. Meta-analysis of the effects of overexpression of WRKY transcription factors on plant responses to drought stress. BMC Genet. 20(1):63.
- Jorge TF, Rodrigues JA, Caldana C, Schmidt R, van Dongen JT, Thomas-Oates J, António C. 2016. Mass spectrometry-based plant metabolomics: metabolite responses to abiotic stress. Mass Spectrom Rev. 35(5):620–649.
- Khiralla A, Mohamed IE, Tzanova T, Schohn H, Slezack-Deschaumes S, Hehn A, André P, Carre G, Spina R, Lobstein A, et al. 2016. Endophytic fungi associated with Sudanese medicinal plants show cytotoxic and antibiotic potential. FEMS Microbiol Lett. 363(11):89.
- Koskimäki JJ, Kajula M, Hokkanen J, Ihantola EL, Kim JH, Hautajärvi H, Hankala E, Suokas M, Pohjanen J, Podolich O, et al. 2016. Methyl-esterified 3-hydroxybutyrate oligomers protect bacteria from hydroxyl radicals. Nat Chem Biol. 12(5):332–338.
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. 2003. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22(11):2623–2633.
- Liu B, Sun L, Ma L, Hao FS. 2017. Both AtrbohD and AtrbohF are essential for mediating responses to oxygen deficiency in Arabidopsis. Plant Cell Rep. 36(6):947–957.
- Ma QH, Zhu HH, Han JQ. 2017. Wheat ROP proteins modulate defense response through lignin metabolism. Plant Sci. 262:32–38.
- McLellan CA, Turbyville TJ, Wijeratne EM, Kerschen A, Vierling E, Queitsch C, Whitesell L, Gunatilaka AA. 2007. A rhizosphere fungus enhances Arabidopsis thermotolerance through production of an HSP90 inhibitor. Plant Physiol. 145(1):174–182.
- Mei C, Flinn BS. 2010. The use of beneficial microbial endophytes for plant biomass and stress tolerance improvement. Recent Pat Biotechnol. 4(1):81–95.
- Miao LF, Yang F, Han CY, Pu YJ, Ding Y, Zhang LJ. 2017. Sex-specific responses to winter flooding, spring waterlogging and post-flooding recovery in Populus deltoids. Sci Rep. 7(1):2534.
- Mishra VK, Singh G, Passari AK, Yadav MK, Gupta VK, Singh BP. 2016. Distribution and antimicrobial potential of endophytic fungi associated with ethnomedicinal plant Melastoma malabathricum L. J Environ Biol. 37(2):229–237.
- Pucciariello C, Perata P. 2017. New insights into reactive oxygen species and nitric oxide signalling under low oxygen in plants. Plant Cell Environ. 40(4):473–482.
- Qin WGG, Liu CX, Jiang W, Xue Y, Wang G, Liu S. 2019. A coumarin analogue NFA from endophytic Aspergillus fumigatus improves drought resistance in rice as an antioxidant. BMC Microbiol. 19:50.
- Qu P, Liu PP, Fu P, Wang Y, Zhu W. 2012. Secondary metabolites of halotolerant fungus Penicillium chrysogenum HK14-01 from the Yellow River Delta area. Acta Microbiol Sinica. 52:1103–1112.
- Singh R, Singh S, Parihar P, Mishra RK, Tripathi DK, Singh VP, Chauhan DK, Prasad SM. 2016. Reactive oxygen species (ROS): beneficial companions of plants’ developmental processes. Front Plant Sci. 27(7):1299.
- Steffens B, Sauter M. 2010. G proteins as regulators in ethylene-mediated hypoxia signaling. Plant Signal Behav. 5(4):375–378.
- Tian W, Bi YH, Zeng W, Jiang W, Xue YH, Wang GX, Liu SP. 2015. Diversity of endophytic fungi of Myricaria laxiflora grown under pre- and post-flooding conditions. Gen Mol Res. 14:10849–10862.
- Umehara K, Yoshida K, Okamoto M, Iwami M, Tanaka H, Kohsaka M, Imanaka H. 1984. Studies on WF-5239, a new potent platelet aggregation inhibitor. J Antibiot. 37:469–474.
- Weyemi U, Dupuy C. 2012. The emerging role of ROS-generating NADPH oxidase NOX4 in DNA-damage responses. Mutat Res-Gen Tox En. 751(2):77–81.
- Weyens N, van der Lelie D, Taghavi S, Vangronsveld J. 2009. Phytoremediation: plant-endophyte partnerships take the challenge. Curr Opin Biotechnol. 20(2):248–254.
- Yamaji K, Watanabe Y, Masuya H, Shigeto A, Yui H, Haruma T. 2016. Root fungal endophytes enhance heavy-metal stress tolerance of Clethra barbinervis growing naturally at mining sites via growth enhancement, promotion of nutrient uptake and decrease of heavy-metal concentration. PLoS One. 11(12):e0169089.