ABSTRACT
In this study, the impacts of exogenous melatonin on freezing stress mitigation in pistachio (Pistacia vera L.) seedlings (30-days old) were investigated. Our results showed that in pistachio seedlings under freezing stress antioxidant enzyme activity, malondialdehyde (MDA) hydrogen peroxide (H2O2), electrolyte leakage, chlorophyll degradation, proline and soluble sugar and γ-aminobutyric acid (GABA) content increased. Phenolic component, phenylalanine ammonia lyase (PAL) activity and the expression of fatty acid desaturase (FAD2) gene increased whilst ascorbate and glutathione concentration significantly decreased in comparison with control. In this research, application of melatonin significantly reduced the antioxidant enzyme activity, MDA, H2O2, chlorophyll degradation, GABA concentration, soluble sugar, and proline contents under stress condition. In addition electrolyte leakage, PAL activity and transcripts of FAD2 gene markedly decreased when the leaves of pistachio seedlings sprayed with melatonin. The results of this study showed that MEL could alleviate the oxidative damages induced by freezing stress and to maintain the structure and mobility of cell membrane in pistachio seedlings under stress conditions. Considering that MEL is an inexpensive and safe bio-stimulator, it seems that MEL is a potential candidate that could be applied used to increase the resistant pistachio trees against variety of biotic and abiotic stresses in the field conditions.
Introduction
Freezing temperature is an environmental condition that causes adverse effects on the growth of plants and productivity of crops (Akula and Ravishankar Citation2011). Cold stress has various effects on plant physiology, biochemistry and molecular biology (Chinnusamy et al. Citation2010). There is an agreement that a plasma membrane is the first place of freezing damage due to its fundamental function in cellular action through a freeze–thaw cycle (Griffith and Antikainen Citation1996). In addition, it is well established that cold stress causes the alterations of lipid composition and an increase in the fatty acid saturation index (Lado et al. Citation2016). Secondary responses to cold have been associated with oxidative stress and consequently, leading to increased production of reactive oxygen species such as singlet oxygen, hydrogen peroxide, superoxide anions, hydroxyl and peroxyl radicals in plant cell (Hossain et al. Citation2012). Thus, plant tolerance to cold stress is also closely correlated to increased capacity of scavenging and detoxifying the ROS. In addition, plants attempt to neutralize the detrimental influences of cold stress by enhancing the synthesis of osmolytes such as proline, glycine betaine, soluble protein, and soluble sugars, which can vary depending upon a variety of plant and might be interchangeable (Erdal Citation2012; Shao et al. Citation2008). It has been reported that, some of these osmolytes such as free sugars, proline, and γ-aminobutyric acid accumulate at low temperatures, share cooperative solute-like characteristics and have been proved to play a role in preserving proteins and membranes from freezing detriment (Kaplan et al. Citation2007).
Melatonin (N-acetyl-5-methoxytryptamine) (MEL) is an endogenously produced molecule with excellent properties as natural antioxidant (Arnao and Hernández-Ruiz Citation2015).
MEL is a useful component for human health and plant preservation. MEL modulates circadian rhythms (Bano-Otalora et al. Citation2020) and inflammatory processes at the intestinal level (Mannino, Caradonna et al. Citation2019). Additionally, MEL is a strong agent against cancer, cardiovascular, fibrosis, neurodegenerative diseases, sepsis and osteoporosis (Li et al. Citation2019). Further studies by Wei et al. (Citation2020) indicated that melatonin was an efficient and tolerable drug in the treatment sleep onset insomnia in human. Also, MEL has been reported to cause a decrease in the proliferation of tumor cells and, increase survival (Pourhanifeh et al. Citation2019). Bizzarri et al. (Citation2013) concluded that MEL might act as a pro-oxidant in cancer cells. In previous study, it has been reported that melatonin can act as a direct antioxidant through the stabilization of biological membranes where it plays a relevant role in membrane fluidity and in counteracting lipid peroxidation under oxidative stress (García et al. Citation2014). Additionally, melatonin also indirectly increases the activities of reactive oxygen species scavenging enzymes (such as SOD, CAT, APX and POX) and maintaining the content of another important antioxidant such as glutathione and ascorbic acid at higher levels in comparison with control plants (Arnao and Hernández-Ruiz Citation2014; Wang et al. Citation2012). It has been showed that MEL is the first guard against the oxidative stress and could preservation of other antioxidants such as (β-carotenoids, vitamin E, vitamin C and glutathione), (Tan et al. Citation2012). Several reports suggest that treatment with melatonin enhances plant resistance to different types of stresses such as alkaline (Cen et al. Citation2020; Liu et al. Citation2015), cold (Kolodziejczyk et al. Citation2016; Turk et al. Citation2014), drought, heavy metal (Posmyk et al. Citation2008), UV irradiation (Zhang, Jia et al. Citation2012) and biotic stress (Lee et al. Citation2014; Moustafa-Farag et al. Citation2020) Jannatizadeh et al. (Citation2019) also reported that chilling injury in tomato fruits was alleviated by exogenous MEL application during cold storage.
Pistachio (Pistacia vera L.) a member of the Anacardiaceae family has been mainly cultivated in Iran, USA, Syria, Iraq, Turkey and some areas of Europe. Pistachio consumption is useful for human health and reduces risk of heart disease. In addition pistachio has economic importance in the lives of some people (Mannino, Gentile et al. Citation2019). Pistachio plant often experience serious cold injury in early spring which reduces yield and product quality. The effects of cold stress are not well studied in pistachio plant. Therefore, the information on how to protect pistachio plants from cold stress is very limited. It has been reported that spring cold in the 2004 and 2005, harmed in average 60% of pistachio trees in Iran (Afshari et al. Citation2010). The knowledge of the physiology of stress tolerance is undoubtedly helpful to counteract this adverse effect. In some researches relationship between melatonin and GABA as two signaling molecules were reported. For example, announced that the melatonin treatment delayed postharvest spoilage in strawberry fruits by enhancing GABA shunt activity (Aghdam and Fard Citation2017). Turi et al. (Citation2014) also identified and quantified melatonin and GABA in Artemisia tridentate under aseptic conditions. Nevertheless, there is no information in the literature about the relationship between melatonin and GABA in freezing-induced stress in plants. So in this research, the influence of MEL on some physiological and biochemical parameters, antioxidant enzyme activities, GABA content and the expression of fatty acid desaturase enzyme in pistachio seedlings during freezing stress were investigated.
Materials and methods
Plant materials and treatments
Ahmadaghaei pistachio cultivar is one of the most significant commercial pistachio cultivars which were considered in Kerman province. The surface of the pistachio seeds (Pistacia vera ‘Ahmadaghaii’) were sterilized with a solution of 7% (v/v) sodium hypochlorite for 5 min and rinsed with running water as well as distilled water. The sterilized seeds were imbibed in distilled water for 24 h and then germinated on moisture filter paper in the dark at 28°C. After emergence, the seedlings planted in pots of sand. Plants were grown at the constant thermal regime of 25/20°C (day/night) in a growth chamber using a 16/8-h light/dark cycle under a relative humidity of 45–50% for 30 days. Thirty-day-old seedlings were selected for foliar application of MEL (0.1, 0.25 and 0.5 µM). Deionized water was used for control with 2-day intervals extended for eight days. 0.01% v/v Tween-20 was utilized for surfactant in all treatments.
After pretreatment of seedlings with MEL, the seedlings were separated into two groups: one group was located in an incubator, in which the temperature was set 25°C. The temperature of the incubator changed to −4°C after 30 min and seedlings were stored at −4°C for 2 h. The other group was kept under optimal conditions. Duration and severity of cold stress were optimized in a preliminary experiment according to the severity of injury to pistachio seedlings. The stressed seedlings were transferred to the normal condition for 12 h to evaluate of biochemical and molecular changes in pistachio seedlings caused by freezing.
Malondialdehyde and other aldehydes concentration analysis
Malondialdehydes is a critical biomarker of lipid peroxidation. MDA concentration measured as illustrated by Heath and Packer (Citation1968). The Extinction coefficient (ε) of 1.55 × 105 M−1 cm−1 was used for the determination of MDA concentration and an extinction coefficient of 0.457 × 105 M−1 cm−1 was utilized for calculation of other aldehydes concentration (Meir et al. Citation1992).
Quantification of electrolyte leakage
Electrolyte leakage was measured according to the method of Yu et al. (Citation2006) with some modification.
Briefly, 10 leaf discs (1 cm2) were collected from pistachio seedlings leaves and washed three times with deionized water. The washed leaves were transferred into test tubes containing 15 mL distilled water and incubated at room temperature for 3 h. The initial electrical conductivity (EC1) of the medium was measured using a conductivity meter. The solution was autoclaved at 121°C for 7 min. The final conductivity (EC2) was measured after cooling the solution at room temperature. Electrolyte leakage was calculated as EC1/EC2 and expressed as a percentage.
Determination of hydrogen peroxide
The exact concentration of hydrogen peroxide was determined according to the methods by Alexieva et al. (Citation2001). Pistachio leaves were immersed in liquid nitrogen and homogenized with 0.1% (w/v) trichloroacetic acid (TCA) (1:4, w/v). Subsequently, the leaves were centrifuged at 4°C and 10,000 × g for 15 min, and the supernatants were separately collected. The reaction mixture contained 0.25 mL supernatant, 0.25 mL 100 mM potassium phosphate buffer (pH 7) and 1 mL 1 M KI. The reaction was developed for 1 h in darkness and absorbance was determined at 390 nm. The content of hydrogen peroxide was measured through a standard curve prepared with the known concentration of H2O2.
Enzyme extraction and activity determination
To determine the activities of antioxidant enzyme, leaves (0.5 g) were homogenized in 50 mM potassium phosphate buffer (pH 7.0) consisting of 1 mM ethylenediamine-tetraacetic acid (EDTA), 1% (w/v) polyvinyl-pyrrolidone (PVP), and 1 mM phenylmethylsulfonyl fluoride (PMSF). All procedures were accomplished in 4°C. The homogenate was centrifuged at 10,000 × g for 20 min and the supernatant was used for assay the activity of enzymes. Protein content was estimated using the Bradford method (Citation1976) with bovine serum albumin (BSA) as a standard.
Catalase (CAT) activity (EC 1.11.1.6)
Catalase activity was determined by monitoring the decomposition of H2O2 (extinction coefficient of 40 M−1 cm−1) at 240 nm following a method by Dhindsa et al. (Citation1981).
Guaiacol peroxidase (GPX) (EC1.11.1.7)
Oxidation of guaiacol to tetraguaiacol calculated by the monitoring of changes in absorbance at 470 nm and using an extinction coefficient of 25.5 mM−1 cm−1 in order to detect the activity of peroxidase (Plewa et al. Citation1991).
Ascorbate peroxidase (APX) (EC 1.11.1.11)
Ascorbate peroxidase was established spectrophotometrically consistent with the oxidation of ascorbate. The reaction solution contained of 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.1 mM H2O2, and 150 µL enzyme extract. H2O2-dependent oxidation of ascorbate was followed by evaluating the decrease in absorbance within 1 min at 290 nm (extinction coefficient of 2.8 mM−1 cm−1) (Nakano and Asada Citation1981).
PAL activity (EC 4.3.1.5)
PAL activity was determined based on the rate of cinnamic acid production. 1 ml of 10 mM L-phenylalanine, 0.4 ml of deionized water and 0.1 ml of enzyme extract were incubated at 37°C for 1 h. The reaction was terminated by the addition of 0.5 ml of 6 M HCl and the cinnamic acid concentration was measured spectrophotometrically by the absorbance at 260 nm. One unit of PAL activity is equal to 1 μM of cinnamic acid produced per min (D´cunha et al. Citation1996).
Carbohydrates and proline concentration analysis
The proline content in fresh leaves was ascertained by using a method by Bates (Citation1973). Total soluble sugar also was assayed using anthrone reagent and glucose as standard (Roe Citation1955).
Chlorophyll and carotenoids content determination
Chlorophyll concentration was measured in 80% acetone extracts as described by Lichtenthaler (Citation1987). Before measuring the absorbance with a UV/visible spectrophotometer at 663.2, 646.8, and 470 nm; the extracts were centrifuged at 3000 × g. The concentration of chlorophyll was evaluated by the equations presented by Lichtenthaler (Citation1987) given below:
Chla = (12.25 A663.2–2.79 A646.8) × volume of supernatant × dilution factor/sample mass (g)
Chl b = (21.21 A646.8–5.1 A663.2) × volume of supernatant × dilution factor/sample mass (g)
Car = [(1000 A470–1.8 Chla - 85.02 Chlb)/198] × volume of supernatant × dilution factor/sample mass (g)
Total phenolic content analysis
Total phenolic content was determined using the Folin-Ciocalteau method by Singleton et al. (Citation1999). Gallic acid was used for constructing the standard curve. Results were expressed as mg gallic acid (GA) per gram of the leaf fresh weight.
Glutathion (GSH) determination
The reduced glutathione (GSH) content was determined by a method by Ellman (Citation1959) with some modification. 0.2 g of frozen material was ground with 2 ml of 15% metaphosphoric acid and centrifuged at 10,000 × g for 30 min. Assay mixture was prepared by adding 2.6 ml sodium phosphate buffer (pH = 7.7), 200 µl of 6 mM 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB) and 200 µl of a sample extract. The mixture was stabilized at 25°C for 30 min. The absorbance was monitored at 412 nm.
Ascorbate and dehydroascorbate determination
The Ascorbate (ASC) and dehydroascorbate content were determined according to Kampfenkel et al. Citation1995.
This assay is based on the reduction of the ferric ion to ferrous ion by ASC. The ferrous ion then forms complexes with bipyridyl that absorbs at 525 nm. Total ascorbate (ASC + DHA) is determined through a reduction of DHA to ASC by dithiothreitol. DHA concentration was calculated by subtracting ascorbate from total ascorbate pool.
Determination of total antioxidant capacity using DPPH method
The DPPH scavenging activity was assayed using the method of Singh et al. (Citation2002). Frozen leaf samples (0.05 g) were extracted with 80% methanol using a pre-chilled mortar and a pestle on an ice bath. The homogenate was filtered through one layer of muslin cloth and centrifuged for 20 min at 5000 × g at 4°C. Two mL of 100 µM DPPH methanolic solution was mixed with 20 µL of the extracted sample and allowed to stand for 30 min at 29°C in complete darkness. Radical scavenging capacity was expressed as the inhibition percentage and was calculated by the following equation:
Radical scavenging activity (%) = (control optical density – sample optical density/control optical density) × 100
Ascorbic acid (20, 30, 50 µM) was chosen as a standard antioxidant and antioxidant activity of the extract was expressed in µM of ascorbic acid equivalents per µL of extract.
Analysis of GABA by HPLC
GABA was analyzed using HPLC on the ground of the method described by Akçay et al. (Citation2012) with minor changes. Leaves (0.4 g) were cryopreserved in liquid nitrogen, homogenized with 1 mL water: chloroform: methanol (3:5:12, v/v/v) solution. Following 3 min centrifugation, the supernatant was collected, 300 µL chloroform and 500 µL water was added. The resulting solution was vortexed and centrifuged for 3 min. The top phase was carefully withdrawn and dried. The dried samples were again dissolved in the mixture of 100 µL water, 150 µL Borax buffer (pH 8.0), and 250 µL of derivatization reagent 2-hydroxynaphthaldehyde (HN) (0.3%, w/v, in methanol). The solution was maintained in a water bath at 80°C for 20 min and cooled down to room temperature. The ultimate volume was set to 1 mL with methanol, and injection into the HPLC column followed by filtering the resulting mixture (Reprosil-pur C18-AQ; 200 × 4.6 mm, 5 µm). Sample injection volume was 20 µL, flow rate 0.5 mL min−1; mobile phase was consisted of methanol: water (62:38, v/v), and detection (UV-detector) was performed at 330 nm. Typical retention time was 10 min for GABA, and the whole elution time was 15 min. The amount of GABA was evaluated as a ratio of the surface area under the substrate HPLC peak compared to those obtained with the untreated control.
Quantitative real-time PCR analysis
Total RNA was extracted from pistachio seedlings leaves using the CinnaPure RNA purification kit. Quantity and quality of each RNA sample were routinely determined from absorption at 260 and 280 nm. RNA integrity was detected using 2% (w/v) agarose gel electrophoresis. RNA samples were treated with DNase І (Pishgam, RNase Free) to remove genomic DNA. FAD2 gene amplification was performed using primers designed from the plant of zucchini (Palma et al. Citation2015). The reference housekeeping gene (NADH dehydrogenase) designed from the plant of pistachio (Zamani Bahramabadi et al. Citation2011). Specific primer pairs were designed for omega-6 fatty acid desaturase (forward primers 5′-TTCTGTCCTCCGCTCATTCT-3′, reverse primers 5′-GACCCATACGCCAGTAAGGA-3′), and NADH dehydrogenase (forward primers 5′-GGAGACTCAAATGGTGGATA-3′, reverse primers 5′-ACCTGCTAGTGGA GGAAGAC-3′).
cDNA was synthesized from total RNA according to the manufacturer's protocols (Thermo Scientific). Real-time PCR reactions were performed using SYBR Green real-time PCR master mix (Ampliqon, Denmark) and a Bioer fluorescent quantitative PCR detection system at 95°C for 15 min, followed by 40 cycles at 95 for 15 s, 58°C for 30 s and 72°C for 30 s. The melting curve was analyzed after the reaction was completed. Efficiency of primers was analysed by dilution method. Relative quantifications were then calculated using the correction from reference gene and transferred to a liner relationship using 2−Δ ΔCT (Livak and Schmittgen Citation2001).
Statistical analysis
Experiments were established in a completely randomized pattern. Each treatment included tree replicates of 10 seedlings, and the entire procedures of the trial were repeated two times with similar results. Data were subjected to analysis of variance. Further statistical analyses were completed with the MSTATC software. LSD tests discovered considerable variations between means. P values lower than 0.05 were regarded statistically significant.
Results
Lipid peroxidation and electrolyte leakage
MDA and other aldehydes content and electrolyte leakage increased in those pistachios which were under freezing stress in comparison with control seedlings. Pretreatment of seedlings with exogenous MEL reduced the peroxidation of lipids and membrane leakage (50–60%) in plants which were under stress condition. In this research the 0.25 and 0.5 µM MEL was more effective than 0.1 µM ().
Table 1 Effects of exogenous application of melatonin on malondialdehyde (MDA), other aldehydes, membrane leakage and hydrogen peroxide (H2O2) content of pistachio seedlings exposed to freezing stress.
H2O2 production rate
The H2O2 contents increased in pistachio seedling during freezing stress, and was significantly alleviated by MEL application (). Hydrogen peroxide contents decreased about 50% in stressed-plants which were pretreatment with MEL.
Antioxidative enzymes activity
All of the antioxidant enzyme activity (GPX, CAT, and APX) increased in freezing stressed seedlings compared to the non-treated group. Melatonin pretreatment decreased these enzymes activity in seedlings during freezing stress (APX, GPX and CAT decreased about 50%, 75% and 50–75%, respectively), as compared to non-pretreated plants ().
Table 2. Effects of exogenous application of melatonin on activities of guaicol peroxidase (GPX), catalase (CAT) and ascorbate peroxidase (APX) in pistachio seedlings exposed to freezing stress.
Glutathione (GSH), ascorbate and dehydroascorbate content
The glutathione content significantly decreased in freezing stressed seedlings as compared with the control. MEL pretreatment markedly suppressed the reduction of glutathione content when plants were subjected to freezing stress (). Data showed that in plants which were pretreated with MEL, GSH content increased 3–4 more than in non-pretreated plants.
Table 3. Effects of exogenous application of melatonin on concentrations of glutathione, ascorbate, dehydroascorbate, ratio of ascorbate to dehydroascorbate and radical scavenging capacity in pistachio seedlings exposed to freezing stress.
Similarly, low ASC content induced by freezing stress was markedly increased by MEL pretreatment (about 5 folds). Also the ratio of ASC/DHA was calculated under freezing stress and this ratio significantly decreased comparison to control. Whereas, MEL treatment increased ASC/DHA ratio markedly in the freezing stressed plants ().
Total antioxidant capacity
In normal condition, MEL pretreatment with different concentrations had positive effects on radical scavenging activity. It also was shown that freezing stress increased antioxidant capacity and melatonin pretreatment resulted in further increases in antioxidant capacity as compared to freezing-stressed ones alone ().
Chlorophyll and carotenoids contents
Chlorophyll content was significantly decreased in pistachio seedlings which were exposed to freezing stress. The application of MEL improved chlorophyll content in seedlings which were under stress condition. Freezing stress and MEL pretreatment had no significant effects on carotenoids content ().
Table 4. Effects of exogenous application of melatonin on chlorophyll a, chlorophyll b, total chlorophyll and carotenoids content of pistachio seedlings exposed to freezing stress.
Soluble carbohydrates and proline
Soluble sugar and proline content increased in freezing stressed seedlings. Pretreatment of seedlings with MEL decreased the content of soluble sugar and proline (about 50-70%) in plants which were under stress conditions ().
Table 5. Effects of exogenous application of melatonin on concentrations of proline, soluble sugars, phenolic component, GABA (γ-aminobutyric acid) and activity of phenylalanine ammonia lyase (PAL) in pistachio seedlings exposed to freezing stress.
Total phenolic content and PAL activity
PAL activity and phenolic compounds increased in freezing stressed seedlings. Melatonin pretreated plants showed significantly lower levels of phenolic compounds in comparison with non-treated plants (70% decline in phenolic compounds and 50% decrease of PAL activity). There was a positive correlation between phenolic contents and PAL activity ().
GABA concentration
The GABA content increased in freezing stressed seedlings as compared with the control plants. MEL pretreatment decreased the GABA content in freezing stressed plants.
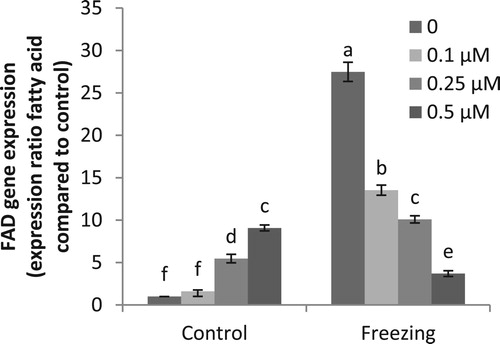
Effect of freezing stress and melatonin pretreatment on expression of FAD2gene
Freezing stress increased the expression of FAD gene in comparison with the control samples. Pretreatment with MEL significantly decreased the transcripts of this gene under stress condition. The most remarkable decreases were observed in the seedlings treated with 0.5 μM melatonin ().
Discussion
Subjecting plants to low temperature is believed to initiate biochemical and physiological modifications in plants, leading to loss of vigor. Low temperature causes some alterations in cell structure, cell membranes and cell wall compound. The systems of Cell membrane are the initial sites of cold injuries (Kratsch and Wise Citation2000).
Lipid peroxidation assessed by MDA content is a widely used indicator of membrane injury owing to the reaction of free radicals with phospholipids and fatty acids, which induces peroxidation of lipids, and increases the amount of MDA (Wan et al. Citation2015). Electrolyte leakage is also a good indicator of membrane permeability or the loss of membrane integrity under stress condition (Li et al. Citation2012). The results of this research showed that the concentration of MDA, other aldehydes, and membrane leakage in stressed plants pretreatment with melatonin were remarkably decreased compared with those seedlings which were just under stress. This is accompanied with depletion in superoxide anion and H2O2 content in melatonin pretreated plants. It seems that melatonin could attenuate oxidative stress caused by freezing stress in pistachio seedlings. It has been reported that in the cell membranes, melatonin can be placed between the polar heads of polyunsaturated fatty acids and therefore, can reduce the lipid peroxidation and maintain the optimal fluidity of the membrane (Ceraulo et al. Citation1999; Janas and Posmyk Citation2013). Positive impact of MEL on the membrane integrity, lipid peroxidation products and electrolyte leakage in water-stressed cucumber seedlings has been previously demonstrated (Zhang et al. Citation2013). Our results showed that melatonin treatment reduced the level of MDA, anion superoxide and H2O2 look to be also aline with other studies under UV-B, heat and cold stress (Turk et al. Citation2014; Xu et al. Citation2010).
Cold stress leads to changes in the certain antioxidant activity of an enzyme. These changes are illustrated as a mechanism, which resists the adverse impact of reactive oxygen species (Lee and Lee Citation2000). Overproduction of ROS damages the lipids, plant proteins, carbohydrates, and DNA, which eventually causes cell death (Wang, Miao et al. Citation2013). A great deal of preceding research concentrated on the function of melatonin in the decreasing of stress and scavenging of ROS directly or indirectly through raising the levels of antioxidants and the activities of related enzymes to scavenge ROS (Arnao and Hernández-Ruiz Citation2015; Li et al. Citation2012). These actions provide a way to assist in enhancing abiotic stress resistance in diverse plants (Zhang et al. Citation2014). Among the defense systems CAT, GPX, and APX are essential enzymes in breaking down H2O2 to H2O in plant cells (Mittler et al. Citation2011). In the present experiment, freezing stress provoked a significant increase in CAT, APX, and GPX activity in plants, whereas the exogenous application of MEL decreased the activity of these antioxidant enzymes in plants which were under stress. Considering the reduction of MDA, H2O2 and superoxide anion as the stress index, and weak activities of CAT, APX, as well as GPX in MEL- pretreated plants, it seems that melatonin did not induce the antioxidant activity and probably scavenged the ROS directly and alleviated oxidative damages caused by freezing stress. The direct interacts of melatonin with hydroxyl radical, hydrogen peroxide, singlet oxygen and superoxide anion was reported previously (Kostopoulou et al. Citation2015; Tan et al. Citation2013). In the present work, DPPH antioxidant capacity was higher in melatonin treated plants compared to control plants which is recommended the directly role of melatonin as excellent free radical scavengers and broad-spectrum antioxidant.
Under stress, non-enzymatic antioxidant systems such as ascorbate (ASC), reduced glutathione (GSH) and carotenoids are directly or indirectly responsible for ROS removing from cells (Bałabusta et al. Citation2016). The changes in the ratio of ASC to DHA are pivotal for the cell to sense oxidative stress and respond accordingly (Potters et al. Citation2002). In this study, under freezing stress, the ratio of ASC to DHA was decreased significantly when compared with controls. However, exogenously applied melatonin significantly increased the ratio of ASC/DHA under freezing or normal conditions. So, melatonin may leads to proper function of plant against oxidative stress with the increasing of ASC/DHA ratio. Also, glutathione is one of the major non-enzymatic tissue antioxidants (Franco and Cidlowski Citation2009) which has been demonstrated that decreased under cold stress (Foyer et al. Citation1997). Our data exhibit that the GSH content was decreased under freezing stress when compared to controls and increased in the presence of exogenous melatonin. The role of melatonin on alleviation of oxidative damage by stimulating antioxidant systems (such as ascorbate and glutathione) under various environmental stresses, such as salt stress (Liu et al. Citation2015), cold stress (Turk et al. Citation2014) and drought stress (Wang, Sun, Li et al. Citation2013) has been reported.
Based on our results in this study, it seems that melatonin could protect pistachio seedlings against oxidative stress through detoxifying ROS directly and indirectly with maintaining the ascorbate and reduced glutathione content than the control plants.
Soluble carbohydrates piled up in plants function as a cytoprotective combination, which is able to avoid or slow ice crystal formation (Karimi and Ershadi Citation2015). Apart from carbohydrates, trees accumulate proline during exposure to cold, which provokes osmotic regulation, maintains turgor pressure in cells during drought stress, and enables plants to endure dehydrative stresses (Zhang, Wu et al. Citation2012). There are contradictory reports regarding the function of proline in chilling tolerance. In a low-temperature-stressed plant, the high content level of proline had been reported as a factor responsible for conferring chilling tolerance (Chen et al. Citation1999). In contrary, accumulation of proline has also been regarded as an indication of stress-induced injury to plant tissue (Upadhyaya et al. Citation1989). In this research, we observed the high content of soluble sugars and proline in freezing-stressed pistachio. When the plants were pretreated with MEL, the levels of soluble sugars and proline were decreased. Similar effects of melatonin treatment have been reported by Sarropoulou et al. (Citation2012) that they showed melatonin treatments significantly drop the levels of proline in the root of the cherry rootstock PHL-C (Prunus avium × Prunus cerasus) compared to those of the control. Also, Wang, Sun, Chang et al. (Citation2013) have found that significant inhibition of hexose (fructose and glucose) accumulation and delaying leaf senescence of Malus hupehensis followed by exogenous application of melatonin. In contrast, it has been reported that melatonin elevates levels of proline and carbohydrates (glucose, fructose, maltose, sucrose), which brings about resistance to stresses in plant (Fan et al. Citation2015; Qian et al. Citation2015; Shi et al. Citation2015). It might be concluded that melatonin has a regulatory function in carbohydrate metabolism of plants. However, additional studies will be required to ascertain the association between melatonin, proline, and carbohydrates.
Chlorophyll is vulnerable and can be simply injured by ROS which is produced during the environmental changes such as cold or hot temperatures and UV or visible light irradiation (Triantaphylides and Havaux Citation2009). Our data for chlorophyll a, chlorophyll b, and total chlorophyll amount demonstrated that chlorophyll degraded in freezing-stressed leaves and MEL application increased the chlorophyll content (). It seems that MEL can be protected chlorophylls as an antioxidant. The chlorophyll-protective efficacy of melatonin was also reported in seedling of cucumber exposed to heat stress (Xu et al. Citation2010) and wheat seedlings subjected to cold stress (Turk et al. Citation2014).
GABA is a metabolite that has public cryoprotective characteristics and have been proposed to have a function in responses to stress signaling (Kaplan et al. Citation2007). In this experiment, GABA content increased significantly during freezing stress however it decreased in MEL pretreatment plants compared to the non-pretreated plants. We also found that the effect of MEL on the relation of GABA content was dose-dependent. It has been reported that under different adverse environmental conditions, high levels of GABA were accumulated (Kim et al. Citation2015; Shelp et al. Citation2012; Xing et al. Citation2007; Zhang et al. Citation2015). Our results showed that GABA may act as cold stress signaling which increased under stress condition and decreased in MEL-pretreated plants because of MEL alleviation effects.
Different degrees of cold tolerance can be found among plant genotypes possessing same levels of unsaturated fatty acids which confirm that other components of plant metabolism are also necessary for whole cold stress resistance in plants (Ariizumi et al. Citation2002).
The effect of melatonin in model membranes (lipid vesicles) has been demonstrated that melatonin increases fluidity and lipid dynamics (Severcan et al. Citation2005). Our results showed that fatty acid desaturase (FAD2) expression increased remarkably in plants under freezing stress. By contrast, in melatonin pretreated plants, the FAD2 expression decreased when compared with non-pretreated plants. It should be noted that the lower transcription levels were recorded in plant treated with higher concentration of melatonin (0.5 µM). It has been proposed that the positioning of melatonin in the bilayers might be responsible for the reduction of lipid peroxidation and increasing of membrane fluidity (García et al. Citation2014).
The findings of the current study verified that melatonin and its related catabolites might act as excellent free radical scavengers directly and by maintaining the fluidity of cell membrane, plays protective roles in pistachio seedlings under freezing stress. it seems that MEL concentration in plant tissues might influence antioxidant system homeostasis and potential resistance to chilling stress. Considering that MEL is economical and safe for animals and humans, its utilization as a bio stimulator could be an invaluable and affordable method in agriculture. It seems that by scavenging ROS through the cell, melatonin.
Conclusion
The results of this study showed that MEL could alleviate the oxidative damages induced by freezing and to maintain the structure and mobility of cell membrane in pistachio seedlings under freezing stress. These results were also confirmed by analysis of FAD gene expression. Considering that MEL is an inexpensive and, safe bio-stimulator, it could be applied in the food and agriculture industry. According to recent reports on the effect of abiotic stresses such as salinity, freezing and, drought on growth parameters and bud abscission in pistachio, it seems that MEL is a potential candidate that could be used to increase the resistant pistachio trees against variety of biotic and abiotic stresses in the field conditions.
Acknowledgements
The authors thank Shahid Bahonar University of Kerman and Pistachio Research Center for supporting this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Adeleh Barand
Adeleh Barand is a PhD student in plant physiology, major studies in the field of abiotic stresses, especially cold.
Fatemeh Nasibi
Fatemeh Nasibi is a Associate Professor of Plant Physiology. Fields of research are stress physiology in plants, medicinal plants and secondary metabolites, conducting 4 research projects, supervising 5 PhD students, 22 postgraduate students and publishing 30 international articles in reliable journals and 26 articles in Persian in national journals of Iran.
Khosrow Manouchehri Kalantari
Khosrow Manouchehri Kalantari is a Professor of Plant Physiology. Fields of research are stress physiology in plants, supervising 12 PhD students, 30 postgraduate students and publishing 50 international articles in reliable journals and 40 articles in Persian in national journals of Iran.
Mohammad Moradi
Mohammad Moradi is a PhD in Plant Pathology, have 40 research articles, Performing 14 research projects and having 4 volumes of books and fluent in of DNA and RNA based molecular and PCR, RFLP, AFLP and Real-Time PCR techniques.
References
- Afshari H, Hokmabadi H, Ebadi A, Laee G. 2010. Measurement of chemical and non-chemical parameters of three native pistachio cultivars of Damghan region (Iran) for studying spring frost. Asian J Chem. 22:2356–2366.
- Aghdam MS, Fard JR. 2017. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria× anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 221:1650–1657.
- Akçay N, Bor M, Karabudak T, Özdemir F, Türkan I. 2012. Contribution of Gamma amino butyric acid (GABA) to salt stress responses of Nicotiana sylvestris CMSII mutant and wild type plants. J Plant Physiol. 169:452–458.
- Akula R, Ravishankar GA. 2011. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 6:1720–1731.
- Alexieva V, Sergiev I, Mapelli S, Karanov E. 2001. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 24:1337–1344.
- Ariizumi T, Kishitani S, Inatsugi R, Nishida I, Murata N, Toriyama K. 2002. An increase in unsaturation of fatty acids in phosphatidylglycerol from leaves improves the rates of photosynthesis and growth at low temperatures in transgenic rice seedlings. Plant Cell Physiol. 43:751–758.
- Arnao MB, Hernández-Ruiz J. 2014. Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 19:789–797.
- Arnao MB, Hernández-Ruiz J. 2015. Chapter 11 – Phytomelatonin: searching for plants with high levels for use as a natural nutraceutical. In: Atta-ur-Rahman FRS, editor. Studies in natural products chemistry. Netherlands: Elsevier Science Publishers; p. 519–545.
- Bałabusta M, Szafrańska K, Posmyk MM. 2016. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front Plant Sci. 7:1–12.
- Bano-Otalora B, Madrid JA, Rol MA. 2020. Melatonin alleviates circadian system disruption induced by chronic shifts of the light-dark cycle in Octodon degus. J Pineal Res. 68:1–13.
- Bates LS. 1973. Rapid determination of free proline for water stress studies. Plant Soil. 39:205–207.
- Bizzarri M, Proietti S, Cucina A, Reiter RJ. 2013. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: a review. Expert Opin Ther Tar. 17:1483–1496.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal Biochem. 72:248–254.
- Cen H, Wang T, Liu H, Tian D, Zhang Y. 2020. Melatonin application improves salt tolerance of alfalfa (Medicago sativa L.) by enhancing antioxidant capacity. Plants. 9:1–17.
- Ceraulo L, Ferrugia M, Tesoriere L, Segreto S, Livrea MA, Liveri VT. 1999. Interactions of melatonin with membrane models: portioning of melatonin in AOT and lecithin reversed micelles. J Pineal Res. 26:108–112.
- Chen J, Xu C, Liang L. 1999. Effect of low temperature on protein and proline in banana (Musa spp.) leaves. J South China Agric Univ. 20:54–58.
- Chinnusamy V, Zhu JK, Sunkar R. 2010. Gene regulation during cold stress acclimation in plants. In: Sunkar R, editor. Plant stress tolerance: methods and protocols. Totowa: Humana Press; p. 39–55.
- D´cunha GB, Satyanarayan V, Nair PM. 1996. Purification of phenylalanine ammonialyase from Rhodotorula glutinis. Phytochem. 42:17–20.
- Dhindsa RS, Dhindsa P, Thorpe AT. 1981. Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation and decrease levels of superoxide dismutase and catalase. J Exp Bot. 32:93–101.
- Ellman GL. 1959. Tissue sulfhydryl groups. Arch Biochem Bioph. 82:70–77.
- Erdal S. 2012. Androsterone-induced molecular and physiological changes in maize seedlings in response to chilling stress. Plant Physiol Biochem. 57:1–7.
- Fan J, Hu Z, Xie Y, Chan Z, Chen K, Amombo E, Chen L, Fu J. 2015. Alleviation of cold damage to photosystem II and metabolisms by melatonin in Bermudagrass. Front Plant Sci. 6:1–14.
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. 1997. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant. 100:241–254.
- Franco R, Cidlowski J. 2009. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 16:1303–1314.
- García JJ, López-Pingarrón L, Almeida-Souza P, Tres A, Escudero P, García-Gil FA, Tan DX, Reiter RJ, Ramírez JM, Bernal-Pérez M. 2014. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J Pineal Res. 56:225–237.
- Griffith M, Antikainen M. 1996. Extracellular ice formation in freezing-tolerant plants. Adv Low Temp Biol. 3:107–139.
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplast, kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 125:189–198.
- Hossain Z, Nouri MZ, Komatsu S. 2012. Plant cell organelle proteomics in response to abiotic stress. J Proteome Res. 11:37–48.
- Janas KM, Posmyk MM. 2013. Melatonin, an underestimated natural substance with great potential for agricultural application. Acta Physiol Plant. 35:3285–3292.
- Jannatizadeh A, Aghdam MS, Luo Z, Razavi F. 2019. Impact of exogenous melatonin application on chilling injury in tomato fruits during cold storage. Food Bioproc Tech. 12:741–750.
- Kampfenkel K, Vanmontagu M, Inze D. 1995. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem. 225:165–167.
- Kaplan F, Kopka J, Sung DY, Zhao W, Popp M, Porat R, Guy CL. 2007. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 50:967–981.
- Karimi R, Ershadi A. 2015. Role of exogenous abscisic acid in adapting of ‘Sultana’ grapevine to low temperature stress. Acta Physiol Plant. 37:1–11.
- Kim HS, Lee EJ, Lim S-T, Han J-A. 2015. Self-enhancement of GABA in rice bran using various stress treatments. Food Chem. 172:657–662.
- Kolodziejczyk I, Dzitko K, Szewczyk R, Posmyk MM. 2016. Exogenous melatonin improves corn (Zea mays L.) embryo proteome in seeds subjected to chilling stress. J Plant Physiol. 193:47–56.
- Kostopoulou Z, Therios I, Roumeliotis E, Kanellis AK, Molassiotis A. 2015. Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings. Plant Physiol Biochem. 86:155–165.
- Kratsch H, Wise R. 2000. The ultrastructure of chilling stress. Plant Cell Environ. 23:337–350.
- Lado J, Rodrigo MJ, López-Climent M, Gómez-Cadenas A, Zacarías L. 2016. Implication of the antioxidant system in chilling injury tolerance in the red peel of grapefruit. Postharvest Biol Tec. 111:214–223.
- Lee HY, Byeon Y, Back K. 2014. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J Pineal Res. 57:262–268.
- Lee DH, Lee CB. 2000. Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays. Plant Sci. 159:75–85.
- Li T, Jiang S, Lu C, Yang W, Yang Z, Hu W, Xin Z, Yang Y. 2019. Melatonin: another avenue for treating osteoporosis? J Pineal Res. 66:1–12.
- Li C, Wang P, Wei ZW, Liang D, Liu CH, Yin LJ DF, Fu MY, Ma FW. 2012. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J Pineal Res. 53:298–306.
- Lichtenthaler HK. 1987. Chlorophyll and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148:350–383.
- Liu N, Gong B, Jin Z, Wang X, Wei M, Yang F, Li Y, Shi Q. 2015. Sodic alkaline stress mitigation by exogenous melatonin in tomato needs nitric oxide as a downstream signal. J Plant Physiol. 186-187:68–77.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408.
- Mannino G, Caradonna F, Cruciata I, Lauria A, Perrone A, Gentile C. 2019. Melatonin reduces inflammatory response in human intestinal epithelial cells stimulated by interleukin-1β. J Pineal Res. 67:1–11.
- Mannino G, Gentile C, Maffei ME. 2019. Chemical partitioning and DNA fingerprinting of some pistachio (Pistacia vera L.) varieties of different geographical origin. Phytochem. 160:40–47.
- Meir S, Philosoph-Hadas S, Aharoni N. 1992. Ethylene-increased accumulation of fluorescent lipid-peroxidation products detected during senescence of parsley by a newly developed method. J Am Soc Hortic Sci. 117:128–132.
- Mittler R, Vanderauwer S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Breusegem FV. 2011. ROS signaling: the new wave? Trends Plant Sci. 16:300–309.
- Moustafa-Farag M, Almoneafy A, Mahmoud A, Elkelish A, Arnao MB, Li L, Ai S. 2020. Melatonin and its protective role against biotic stress impacts on plants. Biomolecules. 10:1–12.
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22:867–880.
- Palma F, Carvajal F, Ramos JM, Jamilena M, Garrido D. 2015. Effect of putrescine application on maintenance of zucchini fruit quality during cold storage: Contribution of GABA shunt and other related nitrogen metabolites. Postharvest Biol Technol. 99:131–140.
- Plewa MJ, Smith SR, Wanger ED. 1991. Dieth yldithiocarbamate suppresses the plant activation of aromatic amines into mutagens by inhibiting tobacco cell peroxidase. Mutat Res. 247:57–64.
- Posmyk MM, Kuran H, Marciniak K, Janas KM. 2008. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J Pineal Res. 45:24–31.
- Potters G, De Gara L, Asard H, Horemans N. 2002. Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol Biochem. 40:537–548.
- Pourhanifeh M, Sharifi M, Reiter R, Abdolhossein D, Asemi Z. 2019. Melatonin and non-small cell lung cancer: New insights into signaling pathways. Cancer Cell Int. 19:1–7.
- Qian Y, Tan D-X, Reiter RJ, Shi H. 2015. Comparative metabolomic analysis highlights the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in Arabidopsis. Sci Rep. 5:1–11.
- Roe JH. 1955. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 212:335–343.
- Sarropoulou VN, Therios IN, Dimassi-Theriou KN. 2012. Melatonin promotes adventitious root regeneration in in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P (Prunus cerasus L.), Gisela 6 (P. cerasus× P. canescens), and MxM 60 (P. avium× P. mahaleb). J Pineal Res. 52:38–46.
- Severcan F, Sahin I, Kazancı N. 2005. Melatonin strongly interacts with zwitterionic model membranes—evidence from Fourier transform infrared spectroscopy and differential scanning calorimetry. Biochim Biophys Acta. 1668:215–222.
- Shao HB, Chu LY, Shao MA, Jaleel CA, Mi HM. 2008. Higher plant antioxidants and redox signaling under environmental stresses. C R Biol. 331:433–441.
- Shelp BJ, Bozzo GG, Trobacher CP, Zarei A, Deyman KL, Brikis CJ. 2012. Hypothesis/review: contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 193-194:130–135.
- Shi H, Jiang C, Ye T, Tan D-x, Reiter RJ, Zhang H, Liu R, Chan Z. 2015. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J Exp Bot. 66:681–694.
- Singh RP, Chidambara Murthy KN, Jayaprakasha GK. 2002. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem. 50:81–86.
- Singleton VL, Orthofer R, Lamuela-Raventós RM. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 299:152–178.
- Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, Reiter RJ. 2012. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J Exp Bot. 63:577–597.
- Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ. 2013. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes. J Pineal Res. 54:127–138.
- Triantaphylides C, Havaux M. 2009. Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci. 14:219–228.
- Turi CE, Axwik KE, Murch SJ. 2014. In vitro conservation, phytochemistry, and medicinal activity of Artemisia tridentata Nutt.: metabolomics as a hypothesis-generating tool for plant tissue culture. Plant Growth Regul. 74:239–250.
- Turk H, Erdal S, Genisel M, Atici O, Demir Y, Yanmis D. 2014. The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedlings. Plant Growth Regul. 74:139–152.
- Upadhyaya A, Davis T, Walser R, Galbraith A, Sankhla N. 1989. Uniconazole-induced alleviation of low-temperature damage in relation to antioxidant activity. HortScience. 24:955–957.
- Wan YY, Zhang Y, Zhang L, Zhou ZQ, Li X, Wang XJ, Bai JG. 2015. Caffeic acid protects cucumber against chilling stress by regulating antioxidant enzyme activity and proline and soluble sugar contents. Acta Physiol Plant. 37:1–10.
- Wang GJ, Miao W, Wang JY, Ma DR, Li JQ, Chen WF. 2013. Effects of exogenous abscisic acid on antioxidant system in weedy and cultivated rice with different chilling sensitivity under chilling stress. J Agro Crop Sci. 199:200–208.
- Wang P, Sun X, Chang C, Feng F, Liang D, Cheng L, Ma F. 2013. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J Pineal Res. 55:267–274.
- Wang P, Sun X, Li C, Wei Z, Liang D, Ma F. 2013. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J Pineal Res. 54:292–302.
- Wang P, Yin L, Liang D, Li C, Ma F, Yue Z. 2012. Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate–glutathione cycle. J Pineal res. 53:11–20.
- Wei S, Smits MG, Tang X, Kuang L, Meng H, Ni S, Xiao M, Zhou X. 2020. Efficacy and safety of melatonin for sleep onset insomnia in children and adolescents: a meta-analysis of randomized controlled trials. Sleep Med. 68:1–8.
- Xing SG, Jun YB, Hau ZW, Liang LY. 2007. Higher accumulation of γ-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. roots. Plant Physiol Biochem. 45:560–566.
- Xu XD, Sun Y, Guo XQ, Sun B, Zhang J. 2010. Effects of exogenous melatonin on ascorbate metabolism system in cucumber seedlings under high temperature stress. Yingyong Shengtai Xuebao. 21:2580–2586.
- Yu X, Peng Y, Zhang M, Shao YJ, Su W, Tang Z. 2006. Water relations and expression analysis of plasma membrane in trinsic proteins in sensitive and tolerant rice during chiling and recovery. Cell Res. 16:599–608.
- Zamani Bahramabadi E, Rezanejad F, Sasan H. 2011. Sequensing and expression study of dehydrin gene under cold and short day treatments in seedlings and reganerated shoots of pistachio (Pistacia vera L.). Kerman: Shahid Bahonar University of Kerman.
- Zhang L, Jia J, Xu Y, Wang Y, Hao J, Li T. 2012. Production of transgenic Nicotiana sylvestris plants expressing melatonin synthetase genes and their effect on UV-B-induced DNA damage. In Vitro Cell Dev Biol Plant. 48:275–282.
- Zhang N, Sun Q, Zhang H, Cao Y, Weeda S, Ren S, Guo YD. 2015. Roles of melatonin in abiotic stress resistance in plants. J Exp Bot. 66:647–656.
- Zhang J, Wu X, Niu R, Liu Y, Liu N, Xu W, Wang Y. 2012. Cold-resistance evaluation in 25 wild grape species. Vitis. 51:153–160.
- Zhang HJ, Zhang N, Yang RC, Wang L, Sun QQLDB, Cao YY, Weeda S, Zhao B, Ren S, Guo YD. 2014. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J Pineal Res. 57:269–279.
- Zhang N, Zhao B, Zhang HJ, Weeda S, Yang C, Yang ZC, Ren S, Guo YD. 2013. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J Pineal Res. 54:15–23.