ABSTRACT
Arbuscular mycorrhizal fungi (AMF) was considered as a biotechnological tool for plant stress tolerance improvement and degraded ecosystem restoration. However, the variations in the effects of AMF species and abundance on plant performance under stress condition have to be still investigated. The study was conducted to evaluate the mechanisms of five AMF species, single or mixture, on Leymus chinensis stress tolerance along a saline-alkaline gradient. The results showed that AMF enhanced plant stress tolerance by promoting plant growth, enhancing nutrient absorption, maintaining ion balance, and improving photosynthetic efficiency. Funneliformis mosseae, Rhizophagus intraradices and the mixture treatments had more beneficial effects than Diversispora versiformis and Acaulospora scrobiculata inoculations. Plant grown under high stress level exhibited more benefits from AMF symbiosis. Our study suggests that not only presence of AMF, but also the species and abundance should be considered to reveal the exact effects of AMF on plant saline-alkaline tolerance and degraded grassland restoration.
Introduction
Soil salinization and alkalization is one of the most serious environmental issues throughout the world, which cause major reductions in agricultural production, terrestrial biodiversity, and ecological health (Rao et al. Citation2013). The USDA Salinity Laboratory defines saline-alkaline soil as having an electrical conductivity of the saturation extract (ECc) higher than 4 dS m−1, and an exchangeable sodium percentage (ESP) greater than 15, while the pH can be variable and usually above 8.5 depending on the relative amounts of exchangeable sodium and soluble salts. According to the FAO Land and Nutrition Management Service (Citation2008), over 6% of the world’s land has been affected by either salinity or alkalinity which accounts for more than 800 million ha of land, and the area of saline and alkaline soil continues to increase each year due to introduction of irrigation in new areas (Shrivastava and Kumar Citation2015). Salinization and alkalization occur through natural processes due to a high salt content of the parent material or in groundwater and/or caused by anthropogenic activities such as inappropriate irrigation practices, fertilizer application and irrational land use (Wang et al. Citation2009).
Soil saline-alkaline stress has been considered one of the most important environmental factors limiting plant growth and development resulting from osmotic stress and ion-induced injury (Yang et al. Citation2009). However, plants have evolved a variety of complex adaptive mechanisms to cope with this abiotic stress such as associating symbiosis with arbuscular mycorrhizal fungi (AMF) for a better exploration of water and mineral nutrients. AMF are ubiquitous soil microorganisms that form mutual symbiotic association with over 80% terrestrial plants (Smith and Read Citation2008). Several studies have indicated that AMF have the ability to enhance plant tolerance to saline-alkaline stress through promoting plant growth, improving gas exchange, enhancing nutrient uptake and maintaining ion balance (Evelin et al. Citation2009; Tisarum et al. Citation2020). Mycorrhizal plants had higher net photosynthetic rate, stomatal conductance and chlorophyll content compared to non-mycorrhizal plants under saline-alkaline stress (Lin et al. Citation2017; Kong et al. Citation2020). AMF inoculation improves nutritional status of plants by absorbing and translocating mineral nutrients beyond rhizospheric zone (Rouphael et al. Citation2015). Al-Karaki (Citation2006) found high Na+ accumulation in roots, without transport to shoots in mycorrhizal plants, and suggested that the Na+ could be retained in AMF hyphae or compartmentalized in AMF vesicles. The molecular mechanisms further showed that the protective effect of AMF on maize and rice plants under salinity was mediated by upregulating several well-known transporters involved in Na+/K+ homeostasis, such as ZmAKT2, ZmSKOR, OsNHX3 and OsSOS1 (Estrada et al. Citation2013a; Porcel et al. Citation2016). Gong et al. (Citation2014) suggested that the expression of FcSISP gene from Meiwa kumquat (Fortunella crassifolia) can also develop an increase salinity resistance in plants. Therefore, the optimum combination of AMF-plant has prompted to be an ideal biotechnology for plant tolerance improvement and degraded grassland restoration (Asmelash et al. Citation2016).
Although many studies have indicated the beneficial effects of AMF on salt stress alleviation in various plants (Shi-chu et al. Citation2019; Tisarum et al. Citation2020; Kong et al. Citation2020), but the magnitudes of effect greatly differ among these studies. Simultaneously, the different impacts of AMF inoculation on the alleviation of salt stress have been also found in citrus seedlings with differential salinity tolerance (Navarro et al. Citation2014). The differences might be attributed to AMF species, AMF abundance, plant-AMF interaction, and soil conditions (Bompadre et al. Citation2013). Our previous study demonstrated that both Funneliformis mosseae and Rhizophagus intraradices had beneficial effects on plant growth, photosynthesis and nutrient content of black locust seedlings under drought stress, and no significant difference was found between the two AMF species (Yang et al. Citation2014). But other researchers found that Glomus versiforme had better effect than R. irregularis in terms of the growth, gas exchange and chlorophyll fluorescence of black locust seedlings (Zhu et al. Citation2014). Wang et al. (Citation2008) reported a positive stimulatory influence of F. mosseae on the growth of cucumber seedlings, whereas G. versiforme presented a negative effect. Chen et al. (Citation2017a) revealed that inoculation with multiple AMF species had relatively better effect on growth of cucumber seedling compared with that of single AMF species inoculation. Estrada et al. (Citation2013a; Citation2013b) showed that AMF isolated from saline areas confer higher salinity tolerance to maize compared with AMF species from a culture collection. More broadly, Crossay et al. (Citation2019) suggested that co-inoculation of multiple AMF belonging to different families might be more beneficial when plants face multiple abiotic stresses. However, the study using a Bangladeshi rice (Oryza sativa) cultivar as the host plant indicated that adding inocula which contain several AMF species may not always be the best approach to increase plant performance under salt stress (Parvin et al. Citation2020). However, the influences of AMF from different families on plant saline-alkaline resistance in clonal grass species have not been well studied.
L. chinensis, a dominant or co-dominant perennial rhizomatous clonal C3 grass, is widely distributed across the eastern region of Eurasian Steppe, from western part of the Northeast Plain to the eastern part of the Mongolian Plateau in China (Wang and Ba Citation2008). The plant offers various important economic and ecological values because it not only contains relatively large amounts of trace minerals, vitamins, high-quality protein, and carbohydrates, but also grows rapidly with high biomass (Wang et al. Citation2017). The grass, with a strong resistant capability to soil salinization, has been considered the most valuable plant species for grassland restoration in the Northeast of China (Wang and Ba Citation2008). Our previous investigation indicated that F. mosseae, R. intraradices, Diversispora versiformis, Acaulospora scrobiculata, Claroideoglomus etunicatum were the most dominant AMF species in the rhizosphere soil of L. chinensis (Figure S1), but there still remains a lack of information and understanding about the mechanisms and how much benefit can be gained when the plants are inoculated with these different AMF species along saline-alkaline stress gradient, and whether the multiple effects of AMF on host stress tolerance depends on AMF species, AMF abundance and stress level. Therefore, the objectives of the current study were (1) to reveal the mechanisms of AMF L. chinensis saline-alkaline stress tolerance through determining plant morphology, photosynthetic pigments, gas exchange, nutrient uptake and ion balance, etc.; (2) to exam whether the effects of AMF on saline-alkaline tolerance of L. chinensis depends on AMF species, AMF abundance and stress level. The study will provide insights into the effectiveness of using an appropriate AMF for exploiting the benefits of these microorganisms in restoration of degraded grassland.
Materials and methods
Growth substrate
Growth substrate consisted of soil and sand (1:1, v/v). Soil samples were collected from the top layer (0-15 cm) in Grassland Ecological Research Station, Northeast Normal University, Jilin Province, China (123o44’E, 44o44’N). The district has a temperate continental arid climate and a mixed salt-alkali meadow soil, with an annual rainfall of 300–450 mm and a mean annual temperature of 4.6-6.4°C. The obtained soil samples were air-dried for 20 days, then passed through a 2-mm sieve to remove root-stone residue and ensure them homogeneity. The sieved soils used for growth substrate has the following properties: pH 7.3 (1:2.5, soil: water, m/v); 21.3 mg g−1 of soil organic matter; 0.41 mg g−1 of total phosphorus; 5.13 mg kg−1 of available phosphorus; 1.18 mg g−1 of total nitrogen; 72.5 mg kg−1 of available nitrogen; and 131.6 mg kg−1 of available potassium; 219.7 μS cm−1 of electrical conductivity. The mixture of sieved soil and washed sand (1:1, v/v) were then placed into a clean cloth bag and autoclaved at 121°C for 1 h to eliminate all possible mycorrhizal propagules and other soil microorganisms, which was used as the growth substrate in this study.
Plant and fungal inoculum
Seeds of L. chinensis were obtained from the Grassland Ecological Research Station of Northeast Normal University, Jilin Province, China (123o44'E, 44o44'N) in July. The seeds were surface sterilized with sodium hypochlorite (0.5%, v/v) for 5 min, washed three times with sterile water, treated with 70% ethanol for 5 min, and then washed three times again with sterile water. Surface sterilized seeds were pre-germinated on moist filter paper petri dishes (9 cm in diameter) in the dark for 2 days at 28°C before they were transferred into plastic pots.
Three AMF strains, F. mosseae (T.H. Nicolson & Gerd.) C. Walker & A. Schüßler, R. intraradices (N.C. Schenck & G.S. Sm.) C. Walker & A. Schüßler and D. versiformis (P. Karst.) Oehl, G.A. Silva & Sieverd were kindly provided by Professor Qiangsheng Wu (Wu et al. Citation2016), while A. scrobiculata Trappe (BGC HK02A) and C. etunicatum (W.N. Becker & Gerd.) C. Walker & A. Schüßler (BGC HEB07A) were obtained from Beijing Academy of Agriculture and Forestry Sciences, Beijing, China. The fungus was propagated on maize (Zea mays L.) and white clover (Trifolium repens L.) grown in pot cultures for 4 months. The live spores of different AMF species were extracted by wet sieving procedures from air-dried substrate and then used as AMF inoculum in the pot culture experiment.
Experimental design
The experiment was conducted in the greenhouse of Northeast Normal University, Changchun, Jilin Province, China (125°25'36"E, 43°49'30"N) for 95 days. The average temperature was 20-35°C and the relative air humidity was 55-87%. The experiment consisted of a randomized complete block design with seven inoculation treatments: (1) control plants inoculated without AMF (NM), (2) plants inoculated with AMF F. mosseae strain (FM), (3) plants inoculated with AMF R. intraradices strain (RI), (4) plants inoculated with AMF D. versiformis strain (DV), (5) plants inoculated with AMF A. scrobiculata strain (AS), (6) plants inoculated with AMF C. etunicatum strain (CE), (7) plants inoculated with a mixture of the five AMF strains (MIX). Two neutral salts (NaCl: Na2SO4) and two alkaline salts (NaHCO3: Na2CO3) were both mixed in a 9: 1 molar ratio to simulate a range of mixed saline-alkaline stress conditions according to the ion composition of the salt-alkali soil in Northeast China. Five saline-alkaline concentrations were applied: (I) plants grow under the control (0 mM) (NS), (II) plants grow under light saline-alkaline stress (50 mM); (III) plants grow under moderate saline-alkaline stress (100 mM); (IV) plants grow under high saline-alkaline stress (200 mM); (V) plants grow under severe saline-alkaline stress (300 mM). Each treatment had five replicates for a total of 175 plastic pots.
The pre-germinated seeds of L. chinensis were transplanted into each plastic pot (15 cm upper diameter, 12-cm lower diameter, and 15-cm depth) filled with 2.5 kg sterilized growth substrate. In each pot of AMF inoculation treatment, AMF inoculum (500 spores) were placed 2-cm below the seeds and covered with substrate. After emergence, seedlings were thinned to a final density of 5 plants per pot. During the first 20 days, seedlings were grown without addition of neutral and alkaline salts in order to obtain plants with functional mycorrhizas and avoid stress effects on AMF symbiosis establishment. To avoid osmotic shock, four saline-alkaline levels (50, 100, 200 and 300 mM) were introduced gradually by successively adding 100 mL of prescribed solution of neutral and alkaline salts in distilled water every 2 days starting at day 20 after sowing. An equivalent volume of distilled water was added to the non-stress control pots in a way that ensured that no excess leaching from the pots occurred. A saucer was placed under each pot to retain salt and other nutrients. A total volume of 400 mL of the corresponding saline-alkaline solution or distilled water was added per pot in this experiment. The plants were watered every 2 days and each pot was irrigated with Hoagland’s nutrient solution (Hoagland and Arnon Citation1950) every month throughout the experiment.
Mycorrhizal colonization
After 95 days of growth, the plant roots were harvested to stain following the methods described by Phillips and Hayman (Citation1970). The roots were cut into 1-cm long fragments, cleared in 10% KOH at 90°C for 30 min, bleached in alkaline hydrogen peroxide for 20 min, acidified with 1% lactic acid for 5 min, and finally stained with 0.05% trypan blue in lactoglycerol solution (lactic acid: glycerol: water = 1: 1: 1) overnight. The Staining quality was substantially improved by destaining roots in lactic acid and glycerol solution (lactic acid: glycerol = 1: 1) for several days prior to observation. Slides were then prepared by squashing the stained root samples with a cover glass onto a glass slide. Mycorrhizal colonization was assessed using Biermann and Linderman (Citation1981) method where the colonization is assessed as proportion of root length colonized by AMF using a Nikon Eclipse Ni light microscopy (Tokyo, Japan) under bright-field illumination.
Photosynthetic pigments and leaf gas exchange
Before harvest, fresh leaf samples (0.25 g) were cleaned with deionized water to remove any surface contamination, and then thoroughly homogenized in chilled acetone (80%) in a mortar and pestle in the dark at 4°C. The homogenates were centrifuged at 10,000 g for 20 min, and the supernatants were collected to determine the absorbances of the acetone extracts using a UV/Vis spectrophotometer (UV-5500PC, Shanghai Metash Instruments Co., Ltd., China) at 663 and 646 nm. The contents of chlorophyll (Chl) a, Chl b, Chl a + b, and Chl a/b ratios were calculated according to the method described by Wellburn (Citation1994).
The net photosynthetic rate by unit of leaf area (Pn), transpiration rate (Tr), intercellular CO2 concentration (Ci), and stomatal conductance (Gs) were measured by a portable gas exchange system (Li-6400, Li-COR, USA) between 9:00 AM and 11:00 AM before harvest according to our previous study (Yang et al. Citation2014). During the period of measurements, a 6400-02B LED source provided a photosynthetic photon flux density of 1,300 μmol m−2 s−1, and the CO2 concentration in the growth chamber was maintained at 400 g m−3. The photosynthetic water use efficiency (WUE) was determined by the ratio of net photosynthesis rate (Pn) to transpiration rate (Tr) (WUE = Pn/Tr).
Biomass production, nutrient accumulation and ion balance
During harvest, plants were separated into shoots and roots. The samples were dried at 80°C to constant weight, and finally weighed by a digital balance (PTX-FA2015, Fuzhou Huazhi).
Dry shoot and root samples (1 g) were ground to pass through a 0.5 mm sieve in a ball mill and stored for determination of mineral nutrients. Phosphorus content was measured by the molybdovanado phosphate method (Kitson and Mellon Citation1944). Nitrogen (N) was determined by micro-Kjeldahl method (Bremner Citation1965).
Dry shoot and root samples (100 mg) were treated with 20 mL deionized water at 100°C for 60 min, and the extract was used to determine the contents of free inorganic ions. The concentration of Cl− was determined by ion chromatography (DX300, Sunnyvale, CA, USA). An atomic absorption spectrophotometer (TAS-990 Super AFG, Purkinje General, Beijing, China) was used to determine contents of Na+ and K+.
Alkaline phosphatase (ALP) activity
Fresh root samples of L. chinensis were washed with cold distilled water to remove soil particles, ground in 29 mM 4-aminoantypirine buffer in 3 M aminomethyl propanol (pH = 10), and then incubated at 37°C for 10 min. Potassium ferricyanide (10 mM) was added and the homogenate was centrifuged at 2000 g for 10 min. The supernatant was used for ALP activity determination using the kit from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) under the instructions of the manufacturer. ALP activity was expressed in µg g−1 h−1.
Statistical analysis
The mycorrhizal dependency (MD) presents the degree to which a plant relies upon the mycorrhizal condition to produce its maximum growth or yield at a given level of soil fertility (Plenchette et al. Citation1983).
Data analysis was performed using the Statistical Package for the Social Sciences (Version 16.0, SPSS Inc., Chicago, IL, USA). Before analysis for treatment differences, the normality and homogeneity of the date were checked using the nonparametric Kolmogorov–Smirnov test and the Levene test, respectively. When necessary, dependent variables were transformed using the natural logarithmic, arcsine or Box–Cox functions to achieve requirements of normality and homogeneity of variance (P > 0.05). Potential differences among different treatments were analyzed using one-way ANOVA followed by Duncan’s multiple-comparison tests (P < 0.05). Two-way ANOVA was used to determine the significance of the effects of AMF inoculation, saline-alkaline stress and their interactions on morphological and physiological parameters. Bivariate relationships between variables for different treatments were conducted using Pearson correlation. All tests were two-tailed and significance of the obtained results was judged at the 5% level. All data in the figures and tables are presented with original data, and they are presented as mean ± SD (standard deviation).
Results
Mycorrhizal colonization, spore density, and hyphal length density
Microscope assessment confirmed that the non-inoculation treatment plants were not colonized by AMF. The plants inoculated with F. mosseae, R. intraradices, D. versiformis, A. scrobiculata, C. etunicatum and mixture of five AMF species had mycorrhizal colonization (MC) ranging from 28.8% to 58.2%, 32.6% to 56.0%, 24.1% to 51.2%, 20.5% to 55.8%, 26.8% to 56.1%, and 31.1% to 57.7%, respectively (). The MC was greatly inhibited by saline-alkaline stress and the significantly lower value could be detected at 200 and 300 mM stress levels compared with that of other three stress levels (Table S1). The plants colonized by F. mosseae, R. intraradices and mixture of five AMF species had much higher MC than the plants inoculated by D. versiformis, A. scrobiculata or C. etunicatum at 300 mM stress level (P < 0.05), but no significance in MC could be detected among mycorrhizal plants at 50 mM stress level (P > 0.05).
Figure 1. Mycorrhizal colonization (MC) in roots, spore density (SPD) and hyphal length density (HLD) in rhizosphere soils of L. chinensis under saline-alkaline stress. FM, RI, DV, AS, CE and MIX represent plants inoculated without AMF, or with F. mosseae, R. intraradices, D. versiformis, A. scrobiculata, C. etunicatum and mixture of five AMF species, respectively. Plants were subjected to 0, 50, 100, 200, and 300 mM saline-alkaline stress. The results are presented as the mean ± SD of five replicates. Different letters indicate significant differences in parameters among different AMF inoculation treatments based on Duncan’s test (P < 0.05) within AMF inoculation treatment.
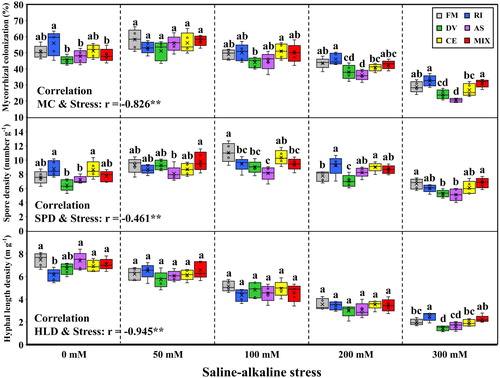
Spore density (SPD) in rhizosphere soil of L. chinensis rose with increasing saline-alkaline stress level, and the mycorrhizal plants exposed to 100 mM stress level had much higher SPD compared with 0 mM and 300 mM stress treatment (P < 0.05), except for the plants colonized by R. intraradices and C. etunicatum (Table S1). The rhizosphere soils of plants inoculated by F. mosseae and R. intraradices had the largest SPD at 300 mM stress level, but no significant difference in SPD of single AMF inoculated plants at 50 mM stress level (P > 0.05).
Hyphal length density (HLD) was significantly declined with increasing saline-alkaline stress, and the significantly negative correlation was observed between HLD and stress (r = −0.945, P < 0.001). The highest HLD (7.47 m/g) was found in the rhizosphere soil of plants inoculated by F. mosseae at 0 mM saline-alkaline stress, and the lowest (1.46 m/g) in the rhizosphere soil of plants inoculated by D. versiformis at 300 mM stress level ().
Biomass production and root/shoot ratio
Saline-alkaline stress increased root dry weight and total dry weight of plants at low stress level (50 mM) except for C. etunicatum inoculated plants (Table S1). Nevertheless, the shoot dry weight, root dry weight, total dry weight, and shoot/root ratio were significantly reduced by the highest saline-alkaline stress level (300 mM) for all plants. Mycorrhizal plants had much higher shoot dry weight, root dry weight and total dry weight compared with non-mycorrhizal plants at 50, 100, and 200 mM saline-alkaline stress levels (). However, no significance was found in root/shoot ratio among plants at 100 mM stress level (P > 0.05). The root dry weight, total dry weight and root/shoot ratio of plants inoculated by F. mosseae, R. intraradices and mixed AMF strains were significantly higher compared with plants colonized by A. scrobiculata and C. etunicatum at the highest saline-alkaline stress level (P < 0.05) ().
Figure 2. Shoot dry weight, root dry weight and root/shoot ratio of L. chinensis under saline-alkaline stress. NM, FM, RI, DV, AS, CE and MIX represent plants inoculated without AMF, or with F. mosseae, R. intraradices, D. versiformis, A. scrobiculata, C. etunicatum and mixture of five AMF species, respectively. Plants were subjected to 0, 50, 100, 200, and 300 mM saline-alkaline stress. The results are presented as the mean ± SD of five replicates. Different letters indicate significant differences in parameters among different AMF inoculation treatments based on Duncan’s test (P < 0.05).
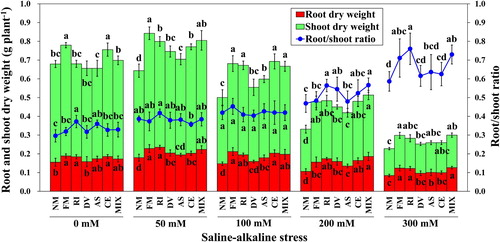
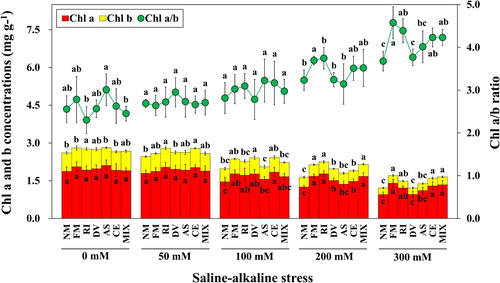
Figure 3. Chlorophyll a, chlorophyll b and chlorophyll a/b ratio of L. chinensis under saline-alkaline stress. NM, FM, RI, DV, AS, CE and MIX represent plants inoculated without AMF, or with F. mosseae, R. intraradices, D. versiformis, A. scrobiculata, C. etunicatum and mixture of five AMF species, respectively. Plants were subjected to 0, 50, 100, 200, and 300 mM saline-alkaline stress. The results are presented as the mean ± SD of five replicates. Different letters indicate significant differences in parameters among different AMF inoculation treatments based on Duncan’s test (P < 0.05).
Photosynthetic pigments
Under saline-alkaline stress conditions, L. chinensis exhibited considerable reductions in chlorophyll (Chl) a, Chl b and total Chl content by 39.9%, 62.3%, and 46.2% at the severe stress level (300 mM), respectively (Table S2). However, no significant difference in Chl a and total Chl contents of non-mycorrhizal and mycorrhizal plants was found between 0 mM and 50 mM stress levels (P > 0.05). Plants inoculated by F. mosseae, R. intraradices, C. etunicatum or mixture of five AMF species had significantly higher Chl a, Chl b and total Chl contents compared with non-mycorrhizal plants at 200 and 300 mM stress levels. Whereas, no significant difference in Chl a and total Chl contents between mycorrhizal and non-mycorrhizal plants was detected under control stress treatment (P > 0.05). The Chl a/b ratio increased with increasing saline-alkaline stress level, and a significantly negative correlation could be observed between Chl a/b ratio and saline-alkaline stress (r = −0.816, P < 0.001). On the other hand, the Chl a/b ratio in the leaves of plants colonized by F. mosseae, R. intraradices, C. etunicatum or mixture of five AMF species was larger than non-mycorrhizal plants at 300 mM stress level, while no significant difference at 50 mM and 100 mM stress levels (P > 0.05).
Gas exchange
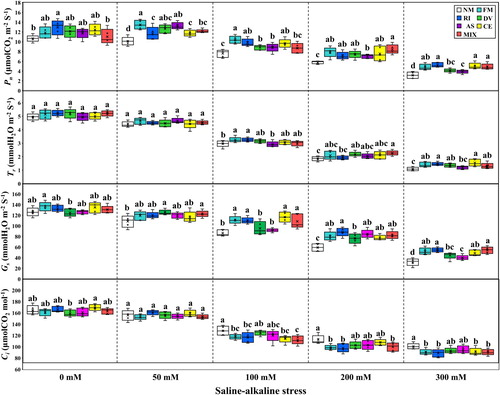
The net photosynthetic rate (Pn) and intercellular CO2 concentration (Ci) of non-mycorrhizal and mycorrhizal plants were significantly lower under 100, 200, and 300 mM treatments compared with these at 0 mM and 50 mM stress levels (Table S3), whereas no significant difference in Pn and Ci could among control, R. intraradices and D. versiformis inoculated plants at 0 mM and 50 mM stress levels (P > 0.05). The stomatal conductance (Gs) and transpiration rate (Tr) of all plants were significantly inhibited by saline-alkaline stress (P < 0.05), but there was no significant difference in Gs among D. versiformis, A. scrobiculata and mixed AMF strains colonized plants at 0 mM and 50 mM stress levels (P > 0.05). Mycorrhizal plants had greatly higher Pn and Gs compared with non-mycorrhizal plants at 200 and 300 mM stress treatments (), but no significant difference in Tr between non-mycorrhizal and mycorrhizal plants at 0 mM and 50 mM stress levels (P > 0.05). The plants inoculated by D. versiformis or A. scrobiculata had much lower Gs compared with other mycorrhizal plants at both 100 mM and 300 mM stress levels (P < 0.05). The plants exposed to the highest stress level (300 mM) had the lowest Pn, Gs, Tr and Ci compared with the control treatment, while the Pn decreased by 70.5%, 59.3%, 59.3%, 65.9%, 66.9%, 59.6% and 55.6% for non-mycorrhizal, F. mosseae, R. intraradices, D. versiformis, A. scrobiculata and mixed AMF strains inoculated plants, respectively, at 300 mM stress level.
Figure 4. The net photosynthetic rate (Pn), stomatal conductance (Gs) transpiration rate (Tr) and intercellular CO2 concentration (Ci) of L. chinensis under saline-alkaline stress. NM, FM, RI, DV, AS, CE and MIX represent plants inoculated without AMF, or with F. mosseae, R. intraradices, D. versiformis, A. scrobiculata, C. etunicatum and mixture of five AMF species, respectively. Plants were subjected to 0, 50, 100, 200, and 300 mM saline-alkaline stress. The results are presented as the mean ± SD of five replicates. Different letters indicate significant differences in parameters among different AMF inoculation treatments based on Duncan’s test (P < 0.05).
Nutrient accumulation
The effects of saline-alkaline stress and AMF strains on N concentration, P concentration and N/P ratio in shoot and root of L. chinensis seedlings are shown in and Table S4. Nutrient concentration under saline-alkaline stress in shoot and root of plants was significantly decreased compared with the control treatment, and the markedly lower N and P concentrations in both shoot and root at 200 and 300 mM stress levels compared with 0 mM and 50 mM were observed in this study (P < 0.05). However, N/P ratio in both shoot and root of plants increased with increasing saline-alkaline stress levels, and there was significantly higher N/P ratio in shoot and root of plants at high and severe stress levels than these under control treatment. No significant difference in shoot N and root N concentrations were found between 0 mM and 50 mM saline-alkaline stress levels (P > 0.05). Whereas, shoot P concentration was significantly higher under control treatment than that at 50 mM stress level (P < 0.05).
Figure 5. The N concentration, P concentration and N/P ratio in shoot and root of L. chinensis under saline-alkaline stress. NM, FM, RI, DV, AS, CE and MIX represent plants inoculated without AMF, or with F. mosseae, R. intraradices, D. versiformis, A. scrobiculata, C. etunicatum and mixture of five AMF species, respectively. Plants were subjected to 0, 50, 100, 200, and 300 mM saline-alkaline stress. The results are presented as the mean ± SD of five replicates. Different letters indicate significant differences in parameters among different AMF inoculation treatments based on Duncan’s test (P < 0.05).
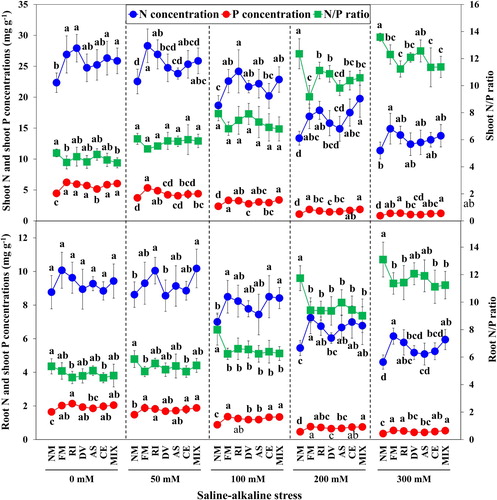
Mycorrhizal plants had greatly higher shoot P and root P concentrations compared with non-mycorrhizal plants under all stress treatments (P < 0.05). However, root N concentrations of F. mosseae, R. intraradices and mixed AMF strains inoculated plants were markedly higher than non-mycorrhizal plants at 100, 200, and 300 mM stress levels, whereas no significant difference was observed among non-mycorrhizal and mycorrhizal plants at 0 mM stress level. The shoot and root N/P ratio of mycorrhizal plants were much lower than non-mycorrhizal plants at 200 and 300 mM stress levels, but no significant difference for shoot N/P ratio at 50 mM stress level (P > 0.05).
Ion concentration
Saline-alkaline stress induced accumulation of Na+ and Cl− concentrations but its distribution was different in non-mycorrhizal and mycorrhizal plants (, Table S5). Shoot and root concentrations of Na+ and Cl− at 200 and 300 mM stress levels were significantly higher compared with these under control treatment (P < 0.05), while the plants exposed to high and severe saline-alkaline stress levels had much lower shoot and root K+ concentration and K+/Na+ ratio than these of the plants grown at 0 mM and 50 mM stress levels (P < 0.05).
Figure 6. The Na+ concentration, Cl− concentration, K+ concentration and K+/Na+ ratio in shoot and root of L. chinensis under saline-alkaline stress. NM, FM, RI, DV, AS, CE and MIX represent plants inoculated without AMF, or with F. mosseae, R. intraradices, D. versiformis, A. scrobiculata, C. etunicatum and mixture of five AMF species, respectively. Plants were subjected to 0, 50, 100, 200, and 300 mM saline-alkaline stress. The results are presented as the mean ± SD of five replicates. Different letters indicate significant differences in parameters among different AMF inoculation treatments based on Duncan’s test (P < 0.05).
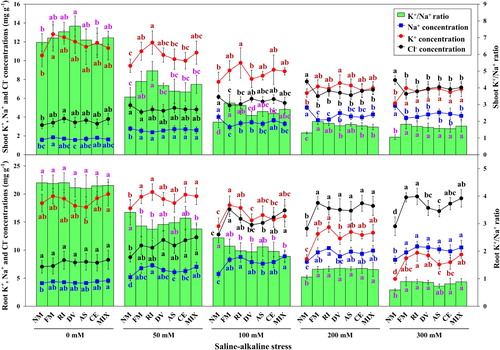
Mycorrhizal plants had significantly lower shoot Na+ and Cl− concentrations, but higher K+ concentration and K+/Na+ ratio compared with non-mycorrhizal plants at 200 and 300 mM saline-alkaline stress levels (P > 0.05). However, no significant difference in shoot K+ concentration was found between non-mycorrhizal plants and mycorrhizal plants under control treatment except for F. mosseae inoculated plants.
The K+/Na+ ratio in both shoot and root of plants had the same decreased trend with increasing stress level. However, different changing trend in K+/Na+ ratio of non-mycorrhizal and mycorrhizal plants between shoot and root was observed in the current study. The K+/Na+ ratio in mycorrhizal plants was higher than non-mycorrhizal plants in shoot but the lower value could be detected in root at 50 and 100 mM stress levels (). Under heavy and severe stress levels, K+/Na+ ratio in mycorrhizal plants was significantly higher compared with non-mycorrhizal plants in both shoot and root tissue (P < 0.05).
Discussion
Effects of AMF inoculation and saline-alkaline stress on mycorrhizal colonization, spore density, and hyphal length density
The addition of various salts and alkali to soil can significantly inhibit AMF hyphal growth with a subsequent decrease in the mycorrhizal root colonization and the spread of mycorrhizal hyphal network (Lin et al. Citation2017). Our results confirmed these findings and demonstrated that the mycorrhizal colonization in plant roots and hyphal length density in rhizosphere soil greatly decreased with increase of saline-alkaline stress levels. It is interesting to observed that the spore density of AMF in rhizosphere soils at 50 and 100 mM saline-alkaline stress levels were significantly higher compared with the controls. The high accumulation of AMF spores in saline-alkaline soil may be an effectively reproductive strategy for AMF to successfully survive in stress environment (Aliasgharzadeh et al. Citation2001).
According to Clark (Citation1997) and Oehl et al. (Citation2010), some ubiquitous AMF species, F. mosseae, R. intraradices and C. etunicatum, can be considered as ‘generalists’ because they are generally associated with highly disturbed sites and have the potential to colonize a range of plants. The larger MC, SP and HLD in F. mosseae, R. intraradices and C. etunicatum plants compared with other mycorrhizal plants may contribute to their high adaption to saline-alkaline soil in this study. On the other hand, F. mosseae had the ability to propagate by mycelial fragments and mycorrhizal root fragments, unlike other AMF species that relay on spore germination (Klironomos and Hart Citation2002).
Effects of AMF inoculation and saline-alkaline stress on plant biomass production
Saline-alkaline stress caused a significant decrease in shoot and root dry weight only at a relatively high stress level (Table S1). Such stimulation in dry matter production under the impacts of salinity and alkalinity (50 mM) probably due to the accumulation of inorganic ions and essential nutrients for osmotic adaptation, whereas a reduction in dry matter production at the highest stress level (>50 mM) could be attributed to the inhibition in nutrients uptake and their translocation to plant aerial parts (Xu et al. Citation2008). In the present study, the root system of L. chinensis was less affected by saline-alkaline stress than shoot system, and the root/shoot ratio increased with increasing stress level because the declines in shoot development were not matched by an equivalent loss of root growth. The increase of root/shoot ratio was probably due to the rapid reduction of shoot dry matter production, indicating that the response of plant root to salt and alkali stresses were more sensitive than that of shoot. In salinity and alkalinity stress conditions, this response strategy may therefore present the advantage of limiting the capacity of the plant to accumulate toxic ions in the aerial parts (Munns and Tester Citation2008).
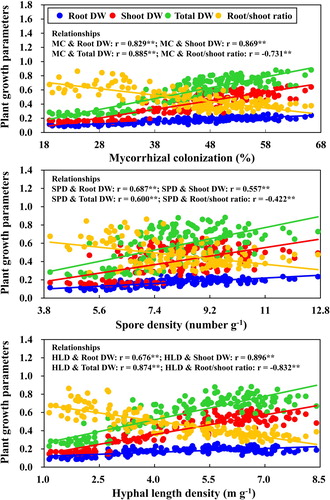
The beneficial effects of AMF on plant growth have been well reported and mainly related to increasing P accumulation (Smith and Read Citation2008), being more efficiency in soils with low nutrient availability. As for grasses, L. chinensis lacks a taproot, and their adventitious root system is comprised chiefly of tissues with primary development (Kong et al. Citation2010), resulting in a very limited intrinsic ability to absorb nutrients from salt and alkalic stress soils (). Therefore, direct nutrient uptake through taproots might not meet the demands of plant growth, while the extraradical mycelium (ERM) of AMF is able to take up more P and N resources from soil and then transfer them to the host via indirect uptake pathway (Smith and Read Citation2008). This finding can be supported by our study where the positive correlations were observed between HLD and N, P concentration in both shoots and roots (P < 0.05).
Effects of AMF inoculation and saline-alkaline stress on plant gas exchange system
The reduction in leaf chlorophyll content was an important factor connected with decreased photosynthesis under abiotic stress conditions as shown in . Salt and alkali induced osmotic effect could lead to a gradual reduction in photosynthesis due to stomata closure under saline-alkaline regimes (Muranaka et al. Citation2002). On the other hand, an uptake and accumulation of excessive amounts of Na+ and Cl- may directly influence the electron transport and cause a significant decline in photosynthetic (). Besides, the adverse effect of salt and alkali induced osmotic stress on Pn was greatly related to decreased production of ATP due to the impaired electron transport (Asrar et al. Citation2017).
AMF inoculation was able to improve the gas exchange capacity of the L. chinensis seedlings most likely by maintaining stomatal opening, reducing stomatal resistances and increasing transpiration fluxes (Evelin et al. Citation2019). Our results demonstrated that the positive effect of AMF on net photosynthetic rate improvement was associated with an increase in stomatal conductance resulted in a noticeable promotion in photosynthesis efficiency which is in line with reports by Shi-chu et al. (Citation2019). In this study, the improvement in leaf gas processes could be attributed to the increasing nutrient uptake, chlorophyll content and water use efficiency (WUE) caused by AMF inoculation ( and Figure S2). The stimulation in Calvin cycle by AMF could promote export of triose P to the root that reduced limitation on photosynthesis, resulting in the enhancement in the rate of CO2 fixation (Kaschuk et al. Citation2009). Higher Pn in mycorrhizal plant is possible due to enhanced RuBisCO activity (Chen et al. Citation2017b) owing to higher expression of the large subunit of RuBisCO, RprbcL gene. Our study showed that AMF colonization increased Pn, Gs and Tr, but decreased Ci (), which agrees with previous studies conducted by Sheng et al. (Citation2008) who indicated that the increase in Ci could lead to the reduction in leaf photosynthesis under stress condition.
Effects of AMF inoculation and saline-alkaline stress on photosynthetic pigment
L. chinensis seedlings exhibited a drastic reduction of photosynthetic pigment contents under saline-alkaline stress (), which was accompanied with a decreased Pn in the plant leaves (). Saline-alkaline stress could destroy the thylakoid membrane structure, declined the affinity between the chlorophyll and the chloroplast protein, and reduced the activity of the chlorophyll enzyme, which in turn, improved chlorophyll degradation and destroyed leaf photosynthesis (Wang et al. Citation2013). Relatively high photosynthetic pigment contents were found in mycorrhizal plants under saline-alkaline stress condition in this study (). The promoted synthesis of chlorophyll is an important adaptive mechanism for plants under salt stress because it can lead to increases in photosynthetic efficiency (Elhindi et al. Citation2017). In the current study, mycorrhizal inoculation improved the chlorophyll concentration, which agrees with several studies which have indicated that AMF can enhance chlorophyll synthesis in host plants under stress, and thereby promoting the potential capacity of carbon fixation (Porcel et al. Citation2015).Moreover, AMF may provide host better protection against oxidative stress and reduce plasma membrane lipid peroxidation of plant leaf, and thereby protecting the chlorophyll pigment from degradation under stressful conditions (Colla et al. Citation2008).
Effects of AMF inoculation and saline-alkaline stress on ion balance
Plant absorbed more Na+ and simultaneously inhibit K+ absorption, resulting in the increase of Na+ concentration and the decline of K+ concentration in both root and shoot of L. chinensis under saline-alkaline stress treatment (). The phenomenon was agreement with Gao et al. (Citation2008) who suggested that plant attended to accumulate greater amounts of Na+, while K+ absorption is inhibited to avoid Na+ toxicity in the cytosol under saline stress. The compartmentalization of more Na+ was probably an important mechanism for L. chinensis to survive in saline-alkaline stress environments. Our results also found a decrease of K+/Na+ ratio with increasing Na+ concentration in both plant shoot and root (), indicating that the saline-alkaline stress interfered the selective absorption of K+ and Na+ in plant tissues, and then led to imbalance of intracellular K+-Na+ (Yang et al. Citation2008). Plants generally accumulated inorganic anions, such as Cl–, into vacuoles to maintain ionic balance (Yang et al. Citation2009). This observation is supported by our study and increased Cl− concentration in plant tissues was found with the increasing intensity of saline-alkaline stress ().
The lower concentration of Na+ in mycorrhizal plants may also be explained by the dilution effect because of the growth improvement of plant by AMF inoculation (Evelin et al. Citation2009). According to Evelin et al. (Citation2012), a relatively high K+/Na+ plasma ratio was essential to increase the plant tolerance to salinity, enhance enzymatic processes, and promote the metabolism associated with alleviation of salt stress in a variety of physiological mechanisms, including chlorophyll and compatible solute synthesis. Moreover, mycorrhizal plants compartmentalized larger amount of Na+ and Cl− into roots compared with aerial part of plant to prevent ion toxicity in leaves, resulting in high photosynthetic efficiency of mycorrhizal plants even under saline-alkaline stress condition (Evelin et al. Citation2019). This phenomenon has been previously studied, indicating that the AMF acts as a primary barrier to Na+ ions when they reach toxic levels, by increasing the concentration in the root and decreasing its translocation towards the shoot (Santander et al. Citation2019). The higher K+ concentration and K+/Na+ ratio in mycorrhizal plants under stress could alleviate the harmful influences by the ionic balance of the cytoplasm or the Na+ efflux from the plant (Giri et al. Citation2007).
Effects of AMF inoculation and saline-alkaline stress on plant nutrient accumulation
The reduction in N concentration in plants was associated with more accumulation of toxic ions as shown in Figure S2, where the significantly negative correlations could be found between N and Na+ (P < 0.001), N and Cl− (P < 0.001) in both plant shoots and roots (Gomathi and Thandapani Citation2005). Botella et al. (Citation1994) indicated that Cl− could exert a negative effect on NO3− uptake in wheat seedlings, but we still could not tell whether the observed interaction was due to competitive inhibition or not. The lower N concentration in plant shoots and roots probably was the consequence of a generally lower rate of solute flow in the xylem as a result of a reduced transpiration rate for NO3− or amino acids (Rubinigg et al. Citation2003). Saline-alkaline stress had effect on decrease of P uptake because of the possible competition between P and Cl− absorption (Bui Citation2013), which could be partly supported by our results (Figure S2). Plants can only uptake P as the free orthophosphate ions H2PO4− and HPO42−. However, as pH increases above 7.0, most of the dissolved phosphorus will react with calcium forming calcium phosphates that cause phosphate to become unavailable for plants (Siebielec et al. Citation2015). It has been widely accepted that N and P concentrations are related to the photosynthesis rate, therefore, decrease of N and P in plant leaves will inhibit the plant growth under saline-alkaline conditions ().
Figure 7. Relationships between AMF status and plant biomass production and allocation of L. chinensis under saline-alkaline stress. NM, FM, RI, DV, AS, CE and MIX represent plants inoculated without AMF, or with F. mosseae, R. intraradices, D. versiformis, A. scrobiculata, C. etunicatum and mixture of five AMF species, respectively.
The observed relatively lower pH in the rhizosphere soil of mycorrhizal plants compared with non-mycorrhizal plants probably another mechanism of AMF on improvement of plant N and P uptake under saline-alkaline stress condition (Figure S3). It has been reported that the application of AMF had more N, P, and S contents in the shoots of mycorrhizal Lallemantia iberica compared with non-mycorrhizal plants (Heydari and Pirzad Citation2020). The increased nutrient uptake observed may be explained by the fact that the extraradical hyphae of AMF is able to explore several meters away from the nutrient depletion zone, increase root surface area and facilitate nutrient absorption by the plant (Schnepf et al. Citation2008). AMF mycelia can also mineralize and enhance utilization of soil N and P by stimulating the gene expression of phosphate, NH4+ and NO3− transporters (Harrison et al. Citation2002; Heike and Arjun Citation2015). A significantly higher ALP activity in the roots of mycorrhizal plant than that of non-mycorrhizal plants was detected (Figure S4), which supported the assumed that the alkaline phosphatase (ALP) of the intraradical mycelium was beneficial to the transfer of P from AMF to the plant (Tisserant et al. Citation1993).
Beneficial effects of AMF on L. chinensis depend on AMF species, AMF abundance and stress level
Mycorrhizal dependency (MD) refers to the degree of plant benefit from AMF inoculations, generally defined by comparing mean plant dry mass for a given plant species with AMF to uninoculated control plants (Graham et al. Citation1991). A MD > 0 represents that a plant benefits from AMF, while a MD < 0 represents that AMF decrease the growth of a plant (van der Heijden Citation2002). MD can vary greatly because of differential growth responses of plant to specific AMF (Boller et al. Citation1998). In the current study, the growth of L. chinensis benefited from F. mosseae, C. etunicatum and mixed AMF strains, while was inhibited by D. versiformis and A. scrobiculata under control treatment (). The results show that the magnitude of plant growth response can be very high according to the AMF species present, ranging from negative to positive. A model summarizing the effects of AMF inoculation on plant tolerance to saline-alkaline stress depending on AMF species and stress level are presented in .
Figure 8. Mycorrhizal dependency (MD) of L. chinensis under saline-alkaline stress. NM, FM, RI, DV, AS, CE and MIX represent plants inoculated without AMF, or with F. mosseae, R. intraradices, D. versiformis, A. scrobiculata, C. etunicatum and mixture of five AMF species, respectively. Plants were subjected to 0, 50, 100, 200, and 300 mM saline-alkaline stress. The results are presented as the mean ± SD of five replicates. Different black and blue letters indicate significant differences in parameters among different AMF inoculation treatments based on Duncan’s test (P < 0.05).
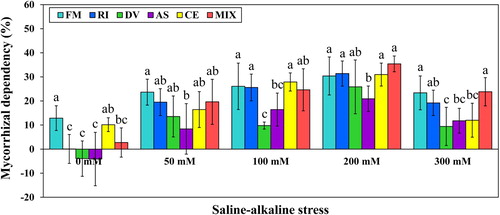
Figure 9. Pathway proposed for improving stress tolerance in L. chinensis through AMF inoculation under saline-alkaline stress condition. AMF facilitates nutrient uptake and ion balance in both root and shoot, which contribute to the increase in photosynthetic pigment and efficiency. Increased nutrient accumulation and photosynthesis improvement provide high biomass production. The degree of plant benefit from AMF associations varied among AMF species, AMF abundance and stress level. The upward and downward short arrows represent the positive and negative effects of AMF on physiological and biochemical parameters of L. chinensis or the rhizosphere soil, respectively.
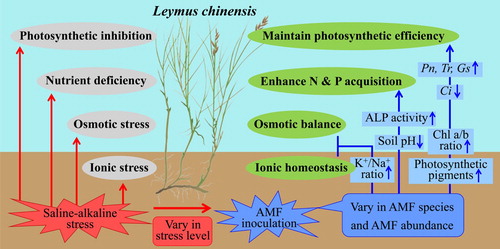
Besides, the significantly positive correlation could be observed between MD and saline-alkaline stress level (r = −0.382; P < 0.001), indicating that AMF inoculation had the tendency to be more beneficial to plant growth under stress condition. The concentrations of P in the soil and the ability of the plant roots to acquire P from the soil play a very important role in determining the MD of a plant species (van der Heijden Citation2002). From the present results, the significantly negative correlations were detected between MD and shoot P (r = −0.464; P < 0.001), MD and root P (r = −0.421; P < 0.001). The observed decline in P uptake could be attributed to decreased HLD under saline-alkaline stress condition. AMF inoculation with higher extraradical mycelium (ERM) generally had better P uptake efficiencies due to the large surface area of the mycelium used for exploring the soil (Heike Bücking and Shachar-Hill Citation2005). It is, therefore, possible that the higher HLD of F. mosseae, R. intraradices and mixed AMF strains may have contributed to the greater P concentrations in both root and shoot of L. chinensis under stress condition ( and ). Some previous studies had shown that the morphology of the root system was an indicator of MD (Reynolds et al. Citation2006), but others did not (Guissou et al. Citation1998).
Additionally, the differential efficiency and behavior of AMF mainly depend on the source of their isolation (Feddermann et al. Citation2010). For example, Estrada et al. (Citation2013b) reported that the higher plant performance could be observed when plants were inoculated with AMF isolated from saline conditions. This may be attributed to the high functional diversity in nutritional benefit, not only among different fungal morphospecies but also among isolates within one morphospecies (Santander et al. Citation2019). Specific mechanisms that result in functional differences between AMF could be expected because of differences in fungal characteristics such as length of external mycelia, distribution of hyphae, and/or nutrient translocation capacity (Porras-Soriano et al. Citation2009). On the other hand, this phenomenon can be also due to the intrinsic characteristics of the isolates used, where the association with the host is more compatible and therefore lead to greater benefits. The study in this field requires to be further investigated because the possibility of using efficient AMF isolate under saline-alkaline conditions is emerging as a promising biotechnological tool for plant production improvement and degraded ecosystem restoration.
Conclusions
Overall, soil salinity and alkalinity are one of the most severe abiotic stresses affecting plant establishment, growth, and production worldwide. Our results demonstrate that mixed saline-alkaline stress induced nutritional disorders, ion imbalance, and photosynthesis decline, thereby altering the major metabolic processes of L. chinensis. However, the development and growth responses induced by AMF inoculation not only relied on combination between a specific AMF and L. chinensis, but depended on saline-alkaline stress level. AMF species with greater HLD and P absorption capacity preferentially benefited the plant species with a high MD (F. mosseae, R. intraradices, and mixed AMF strains), while the AMF species with the lowest HLD and P absorption capacity benefited the plant species with a low MD (D. versiformis and A. scrobiculata). Our study can provide powerful insights into the feedback mechanisms between AMF and host plants, and also practical implications to grassland restoration using specific combination of plant-AMF under multiple stress environments.
Author contributions
ZL and YY participated in the design and coordination of the study. YC, XW, AZ, YW and YY carried out the experiment. XW, ZL and YY performed the statistical analysis. ZL and YY prepared the draft for the manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download MS Word (53.6 KB)Acknowledgements
We would like to express our special thanks to Dr. Yanhong Xiao, Dr. Songtao Yang and Lingyue Yang (Northeast Normal University) for taking care of the pot culture experiment and assisting in the determination of various parameters.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Yaping Cao
Yaping Cao and Yue Weng are postgraduate students from Northeast Normal University (China), and have vast research experience in discipline of Plant Science and Microbiology.
Xuefeng Wu
Xuefeng Wu and Anastasiia Zhukova are doctoral candidates, majoring in Grassland Science and Microbiology.
Anastasiia Zhukova
Xuefeng Wu and Anastasiia Zhukova are doctoral candidates, majoring in Grassland Science and Microbiology.
Zhanhui Tang
Zhanhui Tang and Zhenxin Li are working as Associate Professors in School of Environment, Northeast Normal University (China).
Yue Weng
Yaping Cao and Yue Weng are postgraduate students from Northeast Normal University (China), and have vast research experience in discipline of Plant Science and Microbiology.
Zhenxin Li
Zhanhui Tang and Zhenxin Li are working as Associate Professors in School of Environment, Northeast Normal University (China).
Yurong Yang
Yurong Yang is an Assistant Professor in Heavy metal Pollution Science and Microbiology. Authorship of one book and more than 15 academic papers make it prominent in his field.
References
- Al-Karaki GN. 2006. Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci Hortic. 109:1–7.
- Aliasgharzadeh N, Rastin SN, Towfighi H, Alizadeh A. 2001. Occurrence of arbuscular mycorrhizal fungi in saline soils of the Tabriz Plain of Iran in relation to some physical and chemical properties of soil. Mycorrhiza. 11:119–122.
- Asmelash F, Bekele T, Birhane E. 2016. The potential role of arbuscular mycorrhizal fungi in the restoration of degraded lands. Front Microbiol. 7:1095.
- Asrar H, Hussain T, Hadi SMS, Gul B, Nielsen BL, Khan MA. 2017. Salinity induced changes in light harvesting and carbon assimilating complexes of Desmostachya bipinnata (L.) staph. Environ Exp Bot. 135:86–95.
- Biermann B, Linderman RG. 1981. Quantifying vesicular-arbuscular mycorrhizae: A proposed method towards standardization. New Phytol. 87:63–67.
- Boller T, Wiemken A, Sanders IR. 1998. Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology. 79:2082–2091.
- Bompadre MJ, de Molina MR, Colombo RP, Bidondo LF, Silvani VA, Pardo AG, Ocampo JA, Godeas AM. 2013. Differential efficiency of two strains of the arbuscular mycorrhizal fungus Rhizophagus irregularis on olive (Olea europaea) plants under two water regimes. Symbiosis. 61:105–112.
- Botella MA, Cerdá A, Lips SH. 1994. Kinetics of NO3− and NH4+ uptake by wheat seedlings. effect of salinity and nitrogen source. J Plant Physiol. 144:53–57.
- Bremner JM. 1965. Organic forms of nitrogen. In: Black CA, editor. Methods of soils analysis. Madison: American Society of Agronomy; p. 1238–1255.
- Bücking H, Shachar-Hill Y. 2005. Phosphate uptake, transport and transfer by the arbuscular mycorrhizal fungus Glomus intraradices is stimulated by increased carbohydrate availability. New Phytol. 165:899–912.
- Bui E. 2013. Soil salinity: a neglected factor in plant ecology and biogeography. J Arid Environ. 92:14–25.
- Chen J, Zhang H, Zhang X, Tang M. 2017b. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front Plant Sci. 8:1739.
- Chen S, Zhao H, Zou C, Li Y, Chen Y, Wang Z, Jiang Y, Liu A, Zhao P, Wang M, Ahammed GJ. 2017a. Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front Microbiol. 8:2516.
- Clark RB. 1997. Arbuscular mycorrhizal adaptation, spore germination, root colonization, and host plant growth and mineral acquisition at low pH. Plant Soil. 192:15–22.
- Colla G, Rouphael Y, Cardarelli M, Tullio M, Rivera CM, Rea E. 2008. Alleviation of salt stress by arbuscular mycorrhizal in zucchini plants grown at low and high phosphorus concentration. Biol Fert Soils. 44:501–509.
- Crossay T, Majorel C, Redecker D, Gensous S, Medevielle V, Durrieu G, Cavaloc Y, Amir H. 2019. Is a mixture of arbuscular mycorrhizal fungi better for plant growth than single-species inoculants? Mycorrhiza. 29(4):325–339.
- Elhindi KM, El-Din AS, Elgorban AM. 2017. The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L.). Saudi J Biol Sci. 24(1):170–179.
- Estrada B, Aroca R, Barea JM, Ruiz-Lozano JM. 2013b. Native arbuscular mycorrhizal fungi isolated from a saline habitat improved maize antioxidant systems and plant tolerance to salinity. Plant Sci. 201:42–51.
- Estrada B, Aroca R, Maathuis FJ, Barea JM, Ruiz-Lozano JM. 2013a. Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell Environ. 36(10):1771–1782.
- Evelin H, Devi TS, Gupta S, Kapoor R. 2019. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: current understanding and new challenges. Front Plant Sci. 10:470.
- Evelin H, Giri B, Kapoor R. 2012. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza. 22(3):203–217.
- Evelin H, Kapoor R, Giri B. 2009. Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot. 104:1263–1280.
- FAO. 2008. Land and plant nutrition management service. http://www.fao.org/ag/agl/agll/spush.
- Feddermann N, Finlay R, Boller T, Elfstrand M. 2010. Functional diversity in arbuscular mycorrhiza–the role of gene expression, phosphorous nutrition and symbiotic efficiency. Fungal Eco. 3(1):1–8.
- Gao Y, Wang D, Ba L, Bai Y, Liu B. 2008. Interactions between herbivory and resource availability on grazing tolerance of Leymus chinensis. Environ Exp Bot. 63:113–122.
- Giri B, Kapoor R, Mukerji KG. 2007. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb Ecol. 54:753–760.
- Gomathi R, Thandapani TV. 2005. Salt stress in relation to nutrient accumulation and quality of sugarcane genotypes. Sugar Tech. 7:39–47.
- Gong X, Zhang J, Liu JH. 2014. A stress responsive gene of Fortunella crassifolia FcSISP functions in salt stress resistance. Plant Physiol Bioch. 83:10–19.
- Graham JH, Eissenstat DM, Drouillard DL. 1991. On the relationship between a plant’s mycorrhizal dependency and rate of vesicular-arbuscular mycorrhizal colonization. Funct Ecol. 5:773–779.
- Guissou T, Ba AM, Ouadba JM, Guinko S, Duponnois R. 1998. Responses of Parkia biglobosa (Jacq.) Benth, Tamarindus indica L. and Zizyphus mauritiana Lam. to arbuscular mycorrhizal fungi in a phosphorus-deficient sandy soil. Biol Fer Soils. 26:194–198.
- Harrison MJ, Dewbre GR, Liu J. 2002. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell. 14:2413–2429.
- Heike B, Arjun K. 2015. Role of arbuscular mycorrhizal fungi in the nitrogen uptake of plants: current knowledge and research gaps. Agron J. 5:587–612.
- Heydari S, Pirzad A. 2020. Mycorrhizal fungi and Thiobacillus co-inoculation improve the physiological indices of Lallemantia iberica under salinity stress. Curr Microbiol. doi:10.1007/s00284-020-02034-y.
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. California Agricultural Experiment Station, Circular-347.
- Kaschuk G, Kuyper TW, Leffelaar PA, Hungria M, Giller KE. 2009. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol Biochem. 41:1233–1244.
- Kitson RE, Mellon MG. 1944. Colorimetric determination of phosphorus as molybdivanadophosphoric acid. Ind Eng Chem. 16:379–383.
- Klironomos JN, Hart MM. 2002. Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza. 12:181–184.
- Kong L, Gong X, Zhang X, Zhang W, Sun J, Chen B. 2020. Effects of arbuscular mycorrhizal fungi on photosynthesis, ion balance of tomato plants under saline-alkali soil condition. J Plant Nutr. 43(5):682–698.
- Kong D, Wu H, Wang M, Simmons M, Lü X, Yu Q, Han X. 2010. Structural and chemical differences between shoot-and root-derived roots of three perennial grasses in a typical steppe in Inner Mongolia China. Plant Soil. 336:209–217.
- Lin J, Wang Y, Sun S, Mu C, Yan X. 2017. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci Total Environ. 576:234–241.
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Ann Rev Plant Biol. 59:651–681.
- Muranaka S, Shimizu K, Kato M. 2002. A salt-tolerant cultivar of wheat maintains photosynthetic activity by suppressing sodium uptake. Photosynthetica. 40:505–515.
- Navarro JM, Pérez-Tornero O, Morte A. 2014. Alleviation of salt stress in citrus seedlings inoculated with arbuscular mycorrhizal fungi depends on the rootstock salt tolerance. J Plant Physiol. 171(1):76–85.
- Oehl F, Laczko E, Bogenrieder A, Stahr K, Bösch R, van der Heijden M, Sieverding E. 2010. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol Biochem. 42:724–738.
- Parvin S, Van Geel M, Yeasmin T, Verbruggen E, Honnay O. 2020. Effects of single and multiple species inocula of arbuscular mycorrhizal fungi on the salinity tolerance of a Bangladeshi rice (Oryza sativa L.) cultivar. Mycorrhiza. 30:431–444.
- Phillips JM, Hayman DS. 1970. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. T Brit Mycol Soc. 55:158–161.
- Plenchette C, Fortin JA, Furlan V. 1983. Growth responses of several plant species to mycorrhizae in a soil of moderate P-fertility: II. Soil fumigation induced stunting of plants corrected by reintroduction of the wild endomycorrhizal flora. Plant Soil. 1:211–217.
- Porcel R, Aroca R, Azcon R, Ruiz-Lozano JM. 2016. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza. 26(7):673–684.
- Porcel R, Redondo-Gómez S, Mateos-Naranjo E, Aroca R, Garcia R, Ruiz-Lozano JM. 2015. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J Plant Physiol. 185:75–83.
- Porras-Soriano A, Soriano-Martín ML, Porras-Piedra A, Azcón R. 2009. Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J Plant Physiol. 166(13):1350–1359.
- Rao PS, Mishra B, Gupta SR. 2013. Effects of soil salinity and alkalinity on grain quality of tolerant, semi-tolerant and sensitive rice genotypes. Rice Sci. 20:284–291.
- Reynolds HL, Vogelsang KM, Hartley AE, Bever JD, Schultz PA. 2006. Variable responses of old-field perennials to arbuscular mycorrhizal fungi and phosphorus source. Oecologia. 147:348–358.
- Rouphael Y, Franken P, Schneider C, Schwarz D, Giovannetti M, Agnolucci M, de Pascale S, Bonini P, Colla G. 2015. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci Hortic. 196:91–108.
- Rubinigg M, Posthumus F, Ferschke M, Elzenga JTM, Stulen I. 2003. Effects of NaCl salinity on 15N-nitrate fluxes and specific root length in the halophyte Plantago maritima L. Plant Soil. 250:201–213.
- Santander C, Sanhueza M, Olave J, Borie F, Valentine A, Cornejo P. 2019. Arbuscular mycorrhizal colonization promotes the tolerance to salt stress in lettuce plants through an efficient modification of ionic balance. J Soil Sci Plant Nut. 19(2):321–331.
- Schnepf A, Roose T, Schweiger P. 2008. Impact of growth and uptake patterns of arbuscular mycorrhizal fungi on plant phosphorus uptake-a modelling study. Plant Soil. 312:85–99.
- Sheng M, Tang M, Chen H, Yang B, Zhang F, Huang Y. 2008. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza. 18:287–296.
- Shi-chu L, Yong J, Ma-bo L, Wen-xu Z, Nan X, Hui-hui Z. 2019. Improving plant growth and alleviating photosynthetic inhibition from salt stress using AMF in alfalfa seedlings. J Plant Interact. 14(1):482–491.
- Shrivastava P, Kumar R. 2015. Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci. 22:123–131.
- Siebielec G, Ukalska-Jaruga A, Kidd P. 2015. Bioavailability of trace elements in soils amended with high-phosphate materials. In: Phosphate in soils: interaction with micronutrients, radionuclides and heavy metals. Boca Raton: CRC Press; p. 237–260.
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis. New York, NY: Academic Press.
- Tisarum R, Theerawitaya C, Samphumphuang T, Polispitak K, Thongpoem P, Singh HP, Cha-um S. 2020. Alleviation of salt stress in upland rice (Oryza sativa L. ssp. indica cv. Leum Pua) using arbuscular mycorrhizal fungi inoculation. Front Plant Sci. 11:348.
- Tisserant B, Gianinazzi-Pearson V, Gianinazzi S, Gollotte A. 1993. In planta histochemical staining of fungal alkaline phosphatase activity for analysis of efficient arbuscular mycorrhizal infections. Mycol Res. 97:245–250.
- van der Heijden MGA. 2002. Arbuscular mycorrhizal fungi as a determinant of plant diversity. In: Search of underlying mechanisms and general principles. Berlin: Springer; p. 243–265.
- Wang D, Ba L. 2008. Ecology of meadow steppe in northeast China. Rangeland J. 30:247–254.
- Wang D, Du J, Zhang B, Ba L, Hodgkinson KC. 2017. Grazing intensity and phenotypic plasticity in the clonal grass Leymus chinensis. Rangeland Ecol Manag. 70:740–747.
- Wang C, Li X, Zhou J, Wang G, Dong Y. 2008. Effects of arbuscular mycorrhizal fungi on growth and yield of cucumber plants. Commun Soil Sci Plan. 39:499–509.
- Wang L, Seki K, Miyazaki T, Ishihama Y. 2009. The causes of soil alkalinization in the Songnen Plain of Northeast China. Paddy Water Environ. 7:259–270.
- Wang X, Wang J, Liu H, Zou D, Zhao H. 2013. Influence of natural saline-alkali stress on chlorophyll content and chloroplast ultrastructure of two contrasting rice (Oryza sativa L. japonica) cultivars. Aust J Crop Sci. 7:289–292.
- Wellburn AR. 1994. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 144:307–313.
- Wu QS, Liu CY, Zhang DJ, Zou YN, He XH, Wu QH. 2016. Mycorrhiza alters the profile of root hairs in trifoliate orange. Mycorrhiza. 26:237–247.
- Xu X, Xu H, Wang Y, Wang X, Qiu Y, Xu B. 2008. The effect of salt stress on the chlorophyll level of the main sand-binding plants in the shelterbelt along the Tarim Desert Highway. Chinese Sci Bull. 53:109–111.
- Yang Y, Tang M, Sulpice R, Chen H, Tian S, Ban Y. 2014. Arbuscular mycorrhizal fungi alter fractal dimension characteristics of Robinia pseudoacacia L. seedlings through regulating plant growth, leaf water status, photosynthesis, and nutrient concentration under drought stress. J Plant Growth Regul. 33:612–625.
- Yang CW, Wang P, Li CY, Shi DC, Wang DL. 2008. Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat. Photosynthetica. 46:107–114.
- Yang C, Xu H, Wang L, Liu J, Shi D, Wang D. 2009. Comparative effects of salt-stress and alkali-stress on the growth, photosynthesis, solute accumulation, and ion balance of barley plants. Photosynthetica. 47:79–86.
- Zhu XQ, Wang CY, Chen H, Tang M. 2014. Effects of arbuscular mycorrhizal fungi on photosynthesis, carbon content, and calorific value of black locust seedlings. Photosynthetica. 52:247–252.
