?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
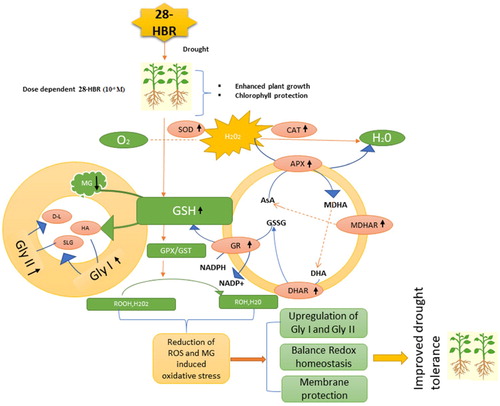
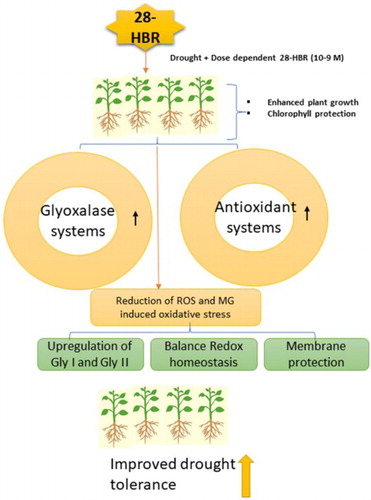
Brassinosteroids (BRs) are well recognized for their defensive role in plants under abiotic stress conditions, but 28-homobrassinolide (HBR)-induced tolerance to drought stress has not been reported in soybean (Glycine max L.). The present study investigated the effect of HBR on soybean seedlings under drought stress. Drought stress suppressed growth and photosynthetic systems while increased the proline, glycine betaine (GB), anthocyanin, total phenolic (TP), and total flavonoid (TF) levels in soybean seedlings. HBR restricted reactive oxygen species (ROS) accumulation and decreased the hydrogen peroxide (H2O2) and malondialdehyde (MDA) content by triggering the antioxidant systems. HBR acts as a shield in soybean, protecting the plant against the harmful effects of methylglyoxal (MG) effects by upregulating the enzymes glyoxalase I, (Gly I;15%) and glyoxalase II (Gly II;29.1%) compared to the levels in drought stressed seedlings. Overall, HBR improved drought tolerance in soybean seedlings by modulating osmolytes, the AsA–GSH cycle, and enzyme activities.
GRAPHICAL ABSTRACT
Introduction
Soybean (Glycine max L.) is one of the most important edible oil crops. It is an oleaginous crop with a high capacity to yield grains with high protein content (Bamji and Corbitt Citation2017), and thus, there is great demand for improved soybean growth and yield to maintain food security. Soybean plants are often subjected to several abiotic stresses or natural climatic stresses e.g. chilling, drought, extreme temperature, heavy metals, high radiation, salinity, and flooding.
Drought negatively influences plant growth and yield, especially in drought-prone areas of the world (Hasan et al. Citation2020). The prolongation of drought may lead to critical physiological and developmental changes in plants (Khan et al. Citation2017; Hasan et al. Citation2019). Drought causes various metabolic and physiological abnormalities in plants such as loss of cell turgor, cell shrinkage, photosynthetic reduction, and imbalance in mineral content (Choudhury et al. Citation2017; Dubey et al. Citation2018; Yang et al. Citation2019). Plants undergo several physiological and morphological adaptations to survive during prolonged water shortage. Small and succulent leaves help to minimize transpirational water loss, and a vigorous root system improves the water uptake capacity as well as nutrient uptake (Li et al. Citation2015; Hughes et al. Citation2017; Dunn et al. Citation2019). To sustain cellular functions, proline, other amino acids, and water-soluble proteins act as osmoprotectants, which play a significant role in plants (Singh et al. Citation2015; Sami et al. Citation2016; Ahammed et al. Citation2020a).
Furthermore, drought stress causes imbalance in cell homeostasis due to excessive generation of reactive oxygen species (ROS), including hydrogen peroxide (H2O2) and superoxide radical (), which are responsible for membrane lipid peroxidation, leading to cell death (Mittler Citation2020). Plants possess an impressive antioxidant defense system, including superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), dehydroascorbate reductase (DHAR), and monodehydroascorbate reductase (MDHAR), that protects membrane components. Such enzymes can help the ascorbate–glutathione (AsA–GSH) cycle to maintain redox homeostasis through avoiding cellular and oxidative damage (Ahmad et al. Citation2018a; Ahmad et al. Citation2018b). The AsA–GSH cycle is regarded as a key player involved in avoiding oxidative and cellular damage (Noctor and Foyer Citation1998). APX plays a vital role in the detoxification of ROS by reducing H2O2 to H2O (Asada Citation1999). MDHAR regenerates ascorbate (AsA) and dehydroascorbate (DHA) by catalyzing the monodehydroascorbate reduction (Sano et al. Citation2005; Asada Citation2006). The DHA reaction can occur with DHAR and GSH through enzymatic and non-enzymatic reactions (Foyer and Noctor Citation2011). Glutathione (GSH) recycling is catalyzed by GR through the reduction of glutathione disulfide (GSSG) by using NADPH as an electron donor (Foyer and Noctor Citation2011).
Methylglyoxal (MG) accumulates in plants during abiotic stress. Excessive formation of MG in leaves could lead to damaged membrane proteins and lipids (Liu et al. Citation2016; Li Citation2019; Rai et al. Citation2019). Gly I and Gly II are glyoxalase enzymes that are upregulated to scavenge accumulated MG (Mostofa et al. Citation2014). The enzymatic reactions are indistinguishably linked with phytohormones, which are essential for plant management under abiotic stress (Pacifici et al. Citation2015). However, the harmful effects of several abiotic stressors, including drought stress, can be mitigated by using potential bio-stimulators such as brassinosteroids (BRS); a well-known group of endogenous plant hormones that regulate abiotic stress tolerance in crops (Ahammed et al. Citation2020b; Serna et al. Citation2015). BRs play a vital role in cell elongation, cell differentiation, gene modulation, protein synthesis, photosynthesis, antioxidant and glyoxalase systems (Talaat and Shawky Citation2013; Li et al. Citation2017; Ahammed et al. Citation2020b). In addition, BRs have a critical role in flower and fruit development, leaf senescence, and abscission (Clouse Citation2003; Müssig Citation2005). Serna et al. (Citation2015) suggested that BRs reduced salt-induced oxidative damage in lettuce plants. In Lycopersicum esculentum, BRs mitigated the heat-induced photosynthetic damage and upregulated the antioxidant system (Ogweno et al. Citation2007). Approximately, 70 different forms of BRs have been identified in plants to date (Bajguz and Hayat Citation2009; Alyemeni and Al-Quwaiz Citation2016).
In recent years, two polyhydroxylated plant steroid hormones, known as 24-epibrassinolide (EBR), and 28-homobrassinolide (HBR) have been extensively studied (Talaat and Shawky Citation2013; Fariduddin et al. Citation2014; Abd_Allah et al. Citation2017; Kaur et al. Citation2018; Alam et al. Citation2019). Previous studies reported that HBR could mitigate a variety of abiotic stresses, such as temperature and salinity (Ali et al. Citation2007; Kaur et al. Citation2014; Sirhindi et al. Citation2017). However, there are few studies about the effect of HBR on soybean antioxidant and glyoxalase systems under drought stress. How HBR interacts within soybean plants and how it copes with the stress associated with the AsA–GSH cycle during drought conditions remain unknown. In this context, the study of the effects of drought on soybean and the amelioration or mitigation of these effects by HBR is very important. In view of the positive impacts of HBR on several crops under various stresses, we hypothesized that HBR might reduce oxidative stress in soybean seedlings. Therefore, to verify this hypothesis, we focused on physiological and biochemical parameters such as (i) photosynthetic pigments and chlorophyll fluorescence, (ii) gas exchange, (iii) osmoregulation, and (iv) antioxidant and glyoxalase systems, (v) MG detoxification in soybean exposed to drought stress. The study indicated that HBR defends against oxidative stress and improves osmolyte metabolism and defense mechanisms.
Materials and methods
Plant material and experimental treatments
The experiments were conducted at the Cairo University greenhouse (30.0228° N, 31.2073° E), Giza, Egypt. Healthy soybean (Glycine max; cv. BARI Soybean-6) seeds were collected from Bangladesh Agricultural Research Institute (BARI), Gazipur, Bangladesh. For sterilization, seeds were soaked in 2.5% sodium hypochlorite with 2.0% Tween-20 solution for 15 min. All seeds were washed with distilled water four times. Sterilized soybean seeds were germinated (10 seeds per pot) following the recent methods of Antoniou et al. (Citation2020). The germinated soybean seedlings were grown in plastic pots (3 seedlings per pot, pot size- height × diameter = 16 cm × 19 cm), which were filled with clay soil and peat. Growing soybean seedlings were watered twice per week until experimental treatments were applied. Commercial nutrient solution (Plant-Prod 20-20-20 Fertilizer, Lambrou Agro, Cyprus) was used every two weeks according to the protocol of Antoniou et al. (Citation2020). We carried out several trial experiments before starting the main experiment. We used different concentrations of HBR to treat drought stress. Based on the best results obtained in the carefully conducted trial experiments, which were conducted in soybean plants by considering the protective effect against drought stress, we chose 10−9 M as the concentration for foliar application. Our results are consistent with those of Sirhindi et al. (Citation2017) and Kaur et al. (Citation2018), who performed their experiments with the same concentration (10−9 M) of HBR. Drought stress was imposed on 23-day-old seedlings (fully developed first trifoliate leaf) by withholding water (resulting in phenotypic foliar damage like chlorosis and leaf wilting) for 10 days. Soybean seedlings were categorized into four groups, well-watered (WW) as a control, drought stressed (DS), well-watered with HBR (HBR + WW), drought stressed with HBR (HBR + DS). During the experimental period, the day/night temperature was 22/160C, with 60–70% relative humidity (RH), and the photoperiod was 16/8-h. The first trifoliate leaves were sampled at 33 days for measuring biochemical, physiological, and morphological parameters.
Growth estimation
Shoot lengths were measured by a measuring tape after harvest. The samples were weighed, and fresh weight (FW) was recorded. The fresh samples were transferred to an oven and kept for 72 h at 700C, and the dry weight (DW) was measured.
Photosynthetic pigment content and chlorophyll fluorescence
The photosynthetic pigment content was determined based on the methods of Arnon (Citation1949) by homogenizing 0.5 g of leaves with 10 ml of acetone (80%, v/v) followed by centrifugation for 10 min at 9000 × g. For chlorophyll (Chl a, Chl b, total Chl) and carotenoid estimation, the absorbance was measured at 645, 663, 510, and 480 nm, respectively. A CIRAS 3 portable photosynthesis system (PP Systems, Amesbury, MA, USA) was used to determine the chlorophyll fluorescence parameters (PSII efficiency, Fv /Fm; quantum yield of PSII, ΦPSII; photochemical quenching, qp; and non-photochemical quenching, NPQ).
Macro element contents
The macro element content was assayed in shoots and roots of soybean seedlings according to the protocols of Chapman and Pratt (Citation1978). Phosphorous (P) and nitrogen (N) were estimated by using a Spekol Spectro Colorimeter, VEB Carl Zeiss. Calcium (Ca) and potassium (K) levels were determined using a flame photometer. Magnesium (Mg) levels were measured by using an atomic absorption spectrophotometer.
Gas exchange estimation
Fresh, healthy leaves were chosen from the soybean seedlings to determine the CO2 assimilation rate (A) and stomatal conductance (gs), and gas exchange data were recorded between 11:00 AM and 12:30 PM. Both parameters were estimated by the CIRAS 3 portable photosynthesis system (PP Systems, Amesbury, MA, USA).
Proline, glycine betaine (GB) content and root activity
The proline content was measured using the methods of Bates et al. (Citation1973). Half a gram of leaves was homogenized in 3% (w/v) sulfosalicylic acid (5 ml). The sample was centrifuged at 40°C for 20 min at 12,000 × g. Then, 2 ml of supernatant was reacted with 2 ml of glacial acetic acid and 2 ml of ninhydrin reagent in a water bath for 1 h at 100°C. The tubes were kept in an ice bath immediately, and 4 ml of toluene was added. The upper toluene phase was discarded, and the optical density (OD) of the extract was measured at 520 nm and expressed as µmolg−1 FW.
The glycine betaine (GB) content was estimated by a technique described by Grieve and Grattan (Citation1983). Half a gram of leaves was homogenized in 5 ml of toluene (0.05%, v/v) and distilled water mixture. The extract was mixed with 2 N H2SO4 followed by the addition of cold KI-I2 reagent. The mixture was centrifuged at 10,000 × g for 15 min. The OD was measured at 365 nm.
The triphenyltetrazolium chloride (TTC) protocol was used for the determination of root activity (Comas et al. Citation2000; He et al. Citation2020). A 0.5 g root sample was dipped in an equal amount of TTC (0.4%, w/v). Phosphate buffer (10 ml) was added to the solution. The mixed solution was kept for 1–3 h at 37°C. Two ml of 1 M H2SO4 was added to stop the reaction. Finally, the dipped roots were mixed and transferred into a new tube containing 10 ml of ethyl acetate solution. The absorbance was measured at 485 nm, and root activity was expressed as mg g−1 h−1.
Estimation of total phenols, flavonoids, and anthocyanins
The total phenolic (TP) content was determined following the procedures described by Velioglu et al. (Citation1998). The total flavonoid (TF) content was measured by the procedure of Zhishen et al. (Citation1999) and expressed as mg catechin g−1 FW. The anthocyanin content was estimated based on the Mancinelli (Citation1984) protocol.
Determination of hydrogen peroxide (H2O2), malondialdehyde (MDA), electrolyte leakage (EL), and ROS production
The H2O2 content was measured according to the protocol of Velikova et al. (Citation2000). The procedure of Rao and Sresty (Citation2000) was used for determination of the malondialdehyde (MDA) content and electrolyte leakage (EL). EL was calculated according to the procedure of Dionisio-Sese and Tobita (Citation1998) by using the following equation:
Thiobarbituric acid reactive substances (TBARS) were determined according to the method of Cakmak and Horst (Citation1991). The O2- production rate was determined by using the protocol of Elstner and Heupel (Citation1976).
Enzyme extractions and assays
Soluble proteins were extracted by using the protocol of Antoniou et al. (Citation2020). From each sample, a 0.1 g leaf sample was collected and homogenized in ice-cold extraction buffer (0.1 M phosphate buffer (pH 7.5), 0.5 mM EDTA, 1 mM PMSF). Centrifugation was performed at 16000 × g for 20 min at 4°C. The collected samples were used for the analysis of enzyme activity and protein content. The protein concentration was estimated by a technique described by Bradford (Citation1976). Bovine serum albumin (BSA) was used as a standard for the determination of protein concentration.
SOD (EC1.15.1.1) activity was determined by the method of Hasanuzzaman et al. (Citation2011). The absorbance was measured at 560 nm and expressed as EU mg−1 protein.
For assessing the CAT (EC1.11.1.6) activity, the protocol of Aebi (Citation1984) was used. The OD was noted at 240 nm, and CAT activity was expressed as EU mg−1 protein.
The activity of APX (EC 1.11.1.11) was estimated by measuring the mixture solution of H2O2 and ascorbic acid (Nakano and Asada Citation1981). The OD was measured at 290 nm and revealed as EU mg−1.
GR (EC 1.6.4.2) activity was measured by using the procedure of Foyer and Halliwell (Citation1976), and expressed as EU mg−1 protein.
Glutathione S-transferase (GST; EC 2.5.1.18) activity was assayed according to methods of Hossain et al. (Citation2006). The absorbance was recorded at 340 nm. The extinction coefficient was 9.6 mM−1 cm−1.
Glutathione peroxidase (GPX, EC:1.11.1.9) activity was determined based on the protocol of Elia et al. (Citation2003). The extinction coefficient was 6.62 mM−1 cm−1, and the OD was recorded at 340 nm.
MDHAR (EC1.6.5.4) activity was determined as described by Miyake and Asada (Citation1992). The OD was noted at 340 nm for 3 min and expressed as EU mg−1 protein.
DHAR (EC 1.8.5.1) activity was measured by the method of Nakano and Asada (Citation1981). The absorbance was measured at 265 nm and expressed as EU mg−1 protein.
Lipoxygenase (LOX, EC:1.13.11.12) activity was estimated by a technique described by Doderer et al. (Citation1992). The extinction coefficient was 25 mM−1 cm−1, and the OD was noted at 234 nm.
Ascorbate and glutathione redox determination
AsA levels were measured as suggested by Yu (Citation2003) and Huang et al. (Citation2005). The reduced and total AsA content were determined spectrophotometrically at 265nm. The DHA content was determined by using the equation DHA = total AsA–reduced AsA. The GSH content was determined based on the Griffiths (Citation1980) method and calculated by using the following equation: GSH = total GSH– glutathione disulfide, GSSG.
Determination of methylglyoxal content and activity of Gly I and Gly II
MG accumulation was measured by using the procedure of Wild et al. (Citation2012). Both Gly I and Gly II enzyme activities were measured by the procedures of Hasanuzzaman et al. (Citation2011).
Statistical analysis
A heatmap was generated in R version 3.6.3 using the ggplot2 package. Principal component analysis (PCA) was performed to show the treatment-variable relationships. To analyze the treatment-variable relationships, PCA was performed by OriginPro 2018. Fisher’s LSD test was conducted by Minitab 17.0 software to test the significance between mean values (p < 0.05). Each treatment was replicated 3 times.
Results
Growth and biomass yield
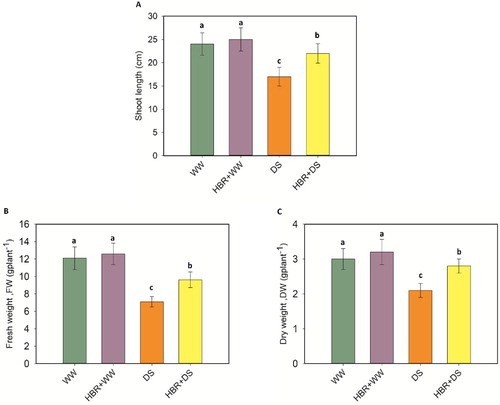
Drought stress caused a drastic decline in growth as well as biomass yield. Drought stress decreased the shoot length by 29.1% compared to the control. When drought stress was applied in combination with HBR, the shoot length was enhanced by 22.7% (A). Exogenous application of HBR increased the shoot FW and DW by 41.3% and 30%, respectively, under drought stress (B, C).
Chlorophyll contents, gas exchange, and chlorophyll fluorescence
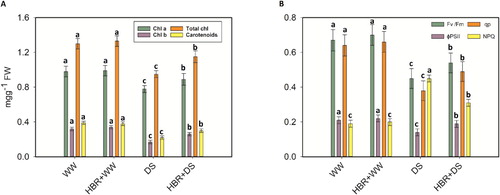
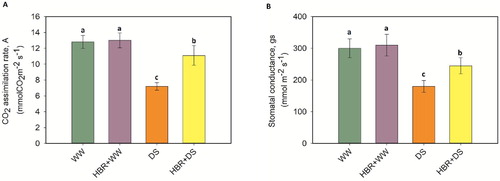
The Chl a, Chl b, total Chl and carotenoid content in soybean seedlings declined by 20.4%, 46.8%, 26.9%, and 43.5%, respectively, under drought stress compared with the levels in the control seedlings. However, application of HBR to drought-treated soybean plants improved the Chl a, Chl b, total Chl, and carotenoid content by 12.3%, 34.6%, 17.3%, and 26.6%, respectively (A). Soybean plants under drought stress showed a 32.8% decrease in the Fv /Fm, a 33.3% decrease in the ΦPSII, and a 40.6% decrease in qp, while the NPQ increased by 57.7% under the same conditions, compared to the control. Applying HBR increased the Fv /Fm, ΦPSII, and qp by 16.6%, 25.9%, and 22.4%, respectively, and decreased the NPQ by 53.3% in the HBR + WW group compared to the HBR + DS group. The A and gs values declined abruptly and significantly in drought-affected soybean seedlings. The decreases were 43.7% and 40% in the DS treatments compared to the control. However, HBR supplementation in drought-treated plants led to an increase of 35.1% in A and of 26.5% in gs relative to the values in drought-treated plants (A, B).
Macro element content
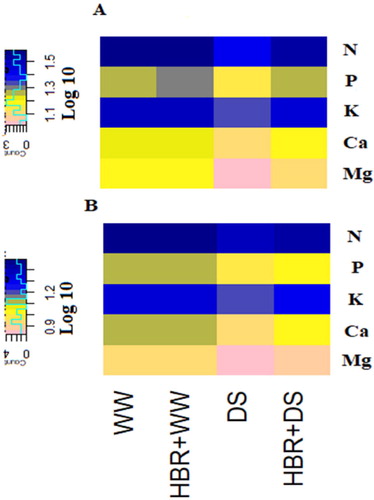
The macro element content decreased under drought stress, which is shown as a heatmap in . In the drought-treated seedlings, the N, P, K, Ca, and Mg levels in shoots decreased by 31.2%, 23.5%, 24.6%, 22.3%, and 30.6%, respectively, and those in roots decreased by 23%, 27.6%, 20.4%, 34.6%, and 25.5%, respectively, compared to the control. HBR supplementation in drought-stressed soybean seedlings improved the N, P, K, Ca, and Mg levels in the shoots and roots ().
Figure 4. Heat map showing the effects of HBR macro element contents (N, P, K Ca, Mg) in the shoot (A) and root (B) of soybean seedlings under different treatments (WW, HBR +WW, DS, HBR +DS). Logarithmic transformation (log10) values shown in color scale (higher levels are displayed in dark blue, lower levels in pink and intermediate levels in yellow colors).
Proline and GB content
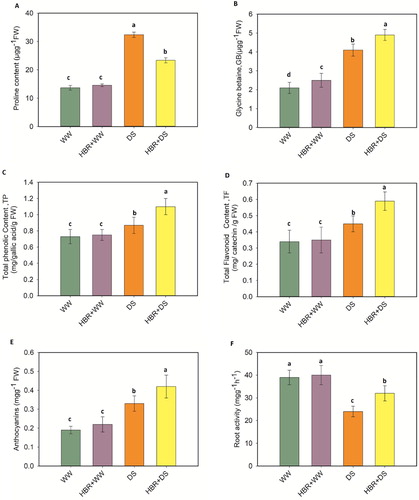
The proline content improved markedly, by 57.7%, in the drought-treated seedlings compared with the control (A). However, exogenous application of HBR decreased the proline content in leaves by 27.7% compared to that in plants treated with drought alone. Drought-stressed soybean seedlings showed a profound increase in the GB content, by 48.8%, compared to the control (B). Nonetheless, HBR application further increased the GB content by 16.32% compared with the level in drought-stressed soybean plants that did not receive HBR.
Figure 5. Effect of HBR on proline content (A), glycine betaine (B), total phenolic content (C), total flavonoid content (D), Anthocyanins (E), and Root activity (F) in the leaf of soybean seedlings under drought stress. Data presented are mean (±SE) of three replicates, and bars with dissimilar letters are significantly different at the P ≤ 0.05 level.
Total phenolic and flavonoid content
The secondary metabolites such as total phenolic (TP) and flavonoid content (TF) increased by 16.09% and 24.4%, respectively, in drought treated soybean plants compared to the control. The TP and TF content further increased by 20.9% and 23.7%, respectively, when HBR was applied under drought stress (C, D).
Anthocyanin and root activity
Drought stress increased the anthocyanin content in soybean seedlings by 42.4% compared with that in the control plants, while the root activity decreased by 38.4% under drought stress over the control. Strikingly, HBR application, along with drought stress showed more increase in anthocyanin and root activity by 17.5% and 25%, respectively, compared to drought treated seedlings (E, F).
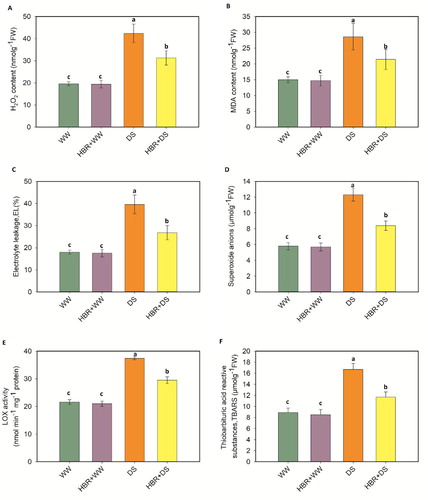
ROS production and LOX activity
In the drought-treated soybean plants, the H2O2 content increased by 53.7%, the MDA content increased by 47.5%, EL (%) increased by 54.5%, the O2- content increased by 52.8%, the TBARS content increased by 46.7%, and LOX activity increased by 42.1% over the respective controls (A-F). Compared with only drought-stressed plants, drought-stressed plants supplied with HBR exhibited 26.1%, 25.1%, 32.32%, 31.7%, 29.9%, and 21.1% decreases in H2O2 content, MDA content, EL, O2- content, TBARS content, and LOX activity, respectively.
Figure 6. Exogenous application of HBR on H2O2 content (A), lipid peroxidation (MDA content) (B), Electrolyte leakage (C), superoxide anions (D), LOX activity (E), and Thiobarbituric acid reactive substances, TBARS (F) in leaf of soybean seedlings under drought stress. Data presented are mean (±SE) of three replicates, and bars with dissimilar letters are significantly different at the P ≤ 0.05 level.
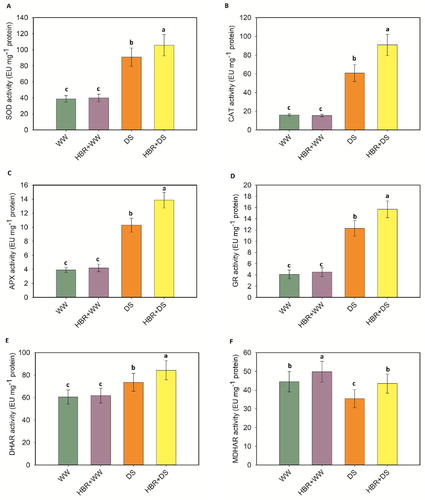
Activities of antioxidant enzymes
The activities of SOD, CAT, APX, GR, and DHAR were enhanced by 57.2%, 73.7%, 61.9%, and 66.6%, 17.7%, respectively, over the control seedlings (). However, the MDHAR activity surprisingly decreased by 31.3% compared to the control. HBR supplementation in drought-treated seedlings increased the activities of SOD, CAT, APX, GR, DHAR, and MDHAR by 14%, 32.9%, 25.7%, 21.6%, 12.6%, and 19.3%, respectively, compared with the activities in seedlings treated with drought alone (A-F).
Figure 7. Exogenous application of HBR on SOD activity (A), CAT activity (B), APX activity (C), GR activity (D), DHAR activity (E), and MDHAR activity (F) in the leaf of soybean seedlings under drought stress. Data presented are mean (±SE) of three replicates, and bars with dissimilar letters are significantly different at the P ≤ 0.05 level.
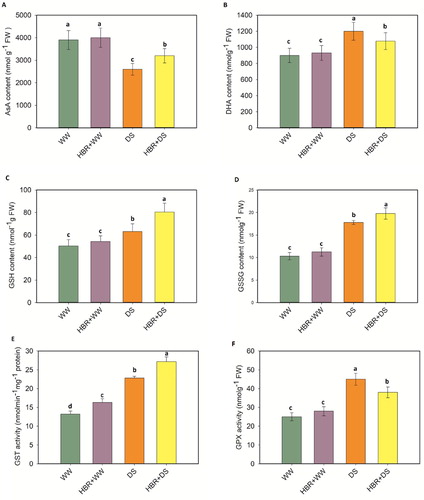
Ascorbate and glutathione redox system
In drought-stressed seedlings, the AsA content decreased by 33.3%, which was then enhanced by HBR application (A). The DHA content increased by 25% in drought-treated seedlings and decreased with HBR supplementation (B). The GSH and GSSG levels improved significantly by 20.3% and 42.02%, respectively, in the drought-stressed soybean seedlings compared with the control (C, D). HBR application further enhanced the GSH and GSSG levels by 21.4% and 10.1%, respectively, compared to the levels in drought-treated seedlings. The greatest increase was recorded in the drought-treated soybean seedlings, with values 44.4% (GPX) and 42.1% (GST) higher than those in the control. HBR increased the GST activity by 16.17% compared to that in plants treated with drought alone. However, exogenous application of HBR decreased the GPX activity by 15.5%, respectively, compared to that in drought-treated seedlings (E, F).
Figure 8. Effect of HBR on AsA content (A), DHA content (B), GSH content (C), GSSG content (D), GST activity (E), and GPX activity (F) in the leaf of soybean seedlings under drought stress. Data presented are mean (±SE) of three replicates, and bars with dissimilar letters are significantly different at the P ≤ 0.05 level.
Glyoxalase systems
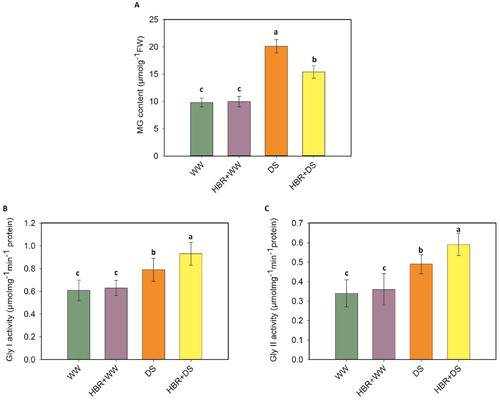
The effects of drought stress on the glyoxalase systems are shown in A-C. Drought treatment increased MG accumulation by 51.2% compared to the control. However, HBR + DS lowered MG accumulation by 23.3%. Under drought stress, the Gly I and Gly II activities increased by 22.7% and 33.3%, respectively. However, HBR supplementation improved the Gly I and Gly II activities.
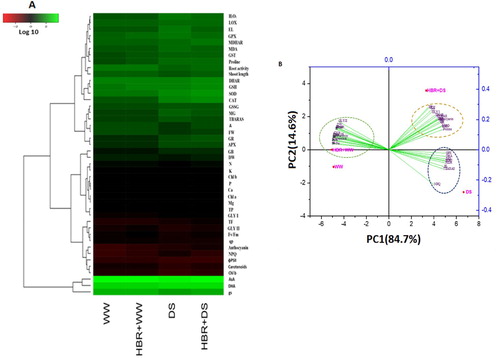
Heat map and PCA analysis of HBR treated soybean seedlings under drought stress
The mean values of the parameters were used to generate the heatmap, which clearly shows the log value data with color intensities ranging from low to high (A). The heatmap clearly shows the drought-treated plants separated from the control seedlings as well as the HBR-treated plants. PCA was performed to determine the morphological, physiological, and biochemical parameters of the four treatment groups (WW, WW + HBR, DS, and HBR + DS). The PCA biplot analysis clearly shows the separation of the treatments and their biological replicates, which were divided into three clustering groups. PC1 and PC2 both elucidated 99.3% of the variability of the data (B).
Figure 10. The Heatmap and PCA display the variable-treatment relationships. In Heatmap, logarithmic transformation (log10) values shown in color scale (lower values to higher values revealed as red to green). All dataset was performed using PCA. The variables include shoot length, FW,DW, Chl a, Chl b, carotenoid, qp, Fv/Fm, ɸPSII, NPQ, N, P, K Ca, Mg content, proline content, glycine betaine (GB),total flavonoid content (TF), total phenolic contents (TP), H2O2 content, malondialdehyde (MDA),Electrolyte leakage (EL), LOX activity, Anthocyanins, Root activity, SOD activity, CAT activity, APX activity, GR activity, DHAR activity, and MDHAR activity, and Thiobarbituric acid reactive substances, TBARS (F), AsA content, DHA content, GSH content, GSSG content, GST activity,GPX activity, MG content, GLY I activity, and GLY II activity.
Discussion
Prolongation of drought hampers major crop production and leads to imbalance in antioxidant and glyoxalase systems (Nahar et al. Citation2016). Plants have antioxidant defense mechanisms to adapt during drought stress. BRs are phytohormones that can regulate the protective responses of crops (Sirhindi et al. Citation2009; Sharma et al. Citation2014). In the present study, drought-affected soybean seedlings exhibited growth retardation in terms of shoot length, FW, and DW, but these traits were improved by HBR application. A similar outcome was found in cucumber plants during salt stress, as reported by Ahmad et al. (Citation2017). The membrane protein complex was destabilized by drought stress resulting in the reduction of photosynthetic pigments (Hasanuzzaman et al. Citation2014; Elkeilsh et al. Citation2019). Soybean seedlings exposed to drought had lower photosynthesis or chlorophyll levels (A). We found that HBR application in drought-treated soybean plants improved the photosynthetic pigment levels. Our result is similar to previous results in which exogenously applied BRs improved photosynthetic pigment levels during salt and drought stress in chickpea and Chorispora bungeana (Ali et al. Citation2007; Li et al. Citation2012).
Drought stress negatively impacts chlorophyll fluorescence parameters such as ɸPSII, the Fv/Fm ratio, qP, and NPQ in soybean (B), while HBR improved these photosynthetic traits. HBR might maintain the electron pool, resulting in a decrease in PSII photoinhibition and preservation of the FV/FM ratio, ɸPSII, and qP during the drought stress response, which has been documented by Nahar et al. (Citation2016) and Li et al. (Citation2012). Hayat et al. (Citation2010) suggested that HBR improves the maximum quantum yield of PSII under salt and temperature stress. Drought-stressed plants displayed decreased chlorophyll fluorescence parameters compared to those documented by Rahman et al. (Citation2020) in soybean plants under acetic acid application. Drought stress reduced the gas exchange parameters such as A and gs, while the result was reversed when the drought-treated plants were subjected to HBR application. HBR application is believed to improve the activities of an antioxidant enzyme that helps restore protein function. This might be caused by the increase in the A and gs parameters during drought stress. A similar finding was obtained in Cucumis sativus seedlings under HBR treatment during chilling stress (Fariduddin et al. Citation2011).
Macro element levels decreased significantly under drought stress in soybean seedlings, and our results are similar to those of several authors, including those of Arjenaki et al. (Citation2012) for wheat plants under drought stress, Ramadan et al. (Citation2019) for sunflower plants under salt stress, and Sadak et al. (Citation2020) for Moringa oleifera under drought stress. The macro elements decrease might be due to the decline in photosynthetic pigment levels, which is supported by the findings of Hasan et al. (Citation2019). In the current study, the application of HBR to drought-treated soybean seedlings restored the macro element levels, possibly due to the increased absorption and accumulation of these elements (). This result is consistent with previous research outcomes in which supplementation with other brassinolides, such as 28-epibrassinolide, increased mineral element levels under salt stress conditions (Alam et al. Citation2019).
Drought stress conditions led to overproduction of ROS ( and H2O2) in G. max seedlings, which consequently caused oxidative damage to lipids, resulting in an increase in the MDA content, EL (%), and the H2O2 content. The H2O2 increase in G. max during drought stress was consistent with a study on the response of Triticum aestivum to drought stress (Elkeilsh et al. Citation2019). EL (%) was markedly increased in G. max under drought stress, which has been widely reported in crops (Assaha et al. Citation2016; Khan et al. Citation2017). In previous studies, changes in root activity were not reported with HBR application under drought stress. We have demonstrated that root activity decreased under drought stress, while exogenous HBR application restored root damage and might help with maintenance of suitable root function.
MDA is considered an end product of the lipid peroxidation reaction and acts as a signal of oxidative stress (Geetika et al. Citation2014; Kaur et al. Citation2014). Ogweno et al. (Citation2007) and Li et al. (Citation2008) stated that HBR minimizes the MDA content during temperature stress in tomato seedlings and Zea mays, which is similar to our outcome. In Chorispora bungeana, it was evident that BRs suppressed the overproduction of MDA under chilling stress (Liu et al. Citation2009). In addition, MDA content mitigation was observed under BR treatment in Rubinia pseudoacacia seedlings (Li et al. Citation2008). HBR reduced H2O2 and ROS production in G. max seedlings. HBR can increase the ROS scavenging capacity, which mitigates oxidative stress and membrane lipid peroxidation or induces endogenous phytohormone such as abscisic acid (ABA) (Bajguz and Hayat Citation2009).
Proline acts as an osmoprotectant and plays a crucial role in stress tolerance in crops (Hasanuzzaman et al. Citation2014). Soybean plants exposed to drought stress showed increased proline accumulation, exhibiting drought-induced water imbalance. A similar drought-induced increase in proline accumulation was observed in maize (Anjum et al. Citation2017). Nevertheless, HBR decreased proline accumulation in drought-affected soybean seedlings. GB is considered to be another osmoprotectant similar to proline and improves growth, decreases oxidative damage, and restores photosynthetic efficiency. In our study, HBR supplementation increased the GB content, similar to what was suggested by Alam et al. (Citation2019) for soybean treated with 24-epibrassinolide.
Secondary metabolites such as TP and TF play a role in defense mechanisms via efficient scavenging of free radicals (Hasan et al. Citation2018). In soybean plants, the TP and TF levels increased under drought stress, possibly due to augmented activities of enzymes that are thought to be linked with the biosynthesis of these compounds (Bhattacharya et al. Citation2010). Hasan et al. (Citation2019) also reported similar results, showing that the TP and TF levels increased in Moringa species under drought stress. TP and TF accumulation in plants provides tolerance against abiotic stress, protects the cell membrane and decreases lipid peroxidation (Potapovich and Kostyuk Citation2003). HBR further enhanced the TP and TF levels under drought stress, and an analogous outcome was observed under 24-epibrassinolide treatment in soybean (Alam et al. Citation2019).
Plants have developed an antioxidant defense system against drought stress to decrease ROS accumulation (Kaur et al. Citation2018). Stress tolerance induced by brassinolide is linked with improved SOD, CAT, APX, DHAR, MDHAR, and GR activities (Vardhini and Anjum Citation2015). Exogenous application of brassinolide could affect the activities of antioxidant enzymes to mitigate ROS generation in crops (Kaur et al. Citation2018). Brassinolide application in plants could induce endogenous production of nitric oxide (NO), commonly known as a signaling molecule, which activates ROS scavenging enzymes under oxidative stress. Our results suggested that detoxification pathways might be associated with several metabolic processes in treated seedlings, including antioxidant machinery activation. The improved activities of SOD and CAT in HBR-treated seedlings might lead to efficient scavenging of ROS in soybean seedlings; a similar result was observed by Ahmad et al. (Citation2017) in cucumber seedlings under salt stress with HBR application. SOD plays a key role against ROS production, catalyzing the conversion of O2− to O2 and H2O2, whereas CAT is responsible for the conversion of H2O2 (Asada Citation1992). The AsA-GSH cycle is an essential pathway for the mitigation of drought-induced oxidative stress (Talaat Citation2014). APX converts H2O2 to H2O through oxidation of AsA to DHA. To maintain the redox cellular balance, the enzymes MDHAR and DHAR together with GR generate AsA and GSH (Mishra et al. Citation2013; Srivastava et al. Citation2014). Exogenous HBR application increased APX, MDHAR, DHAR, and GR activities in drought treated G. max seedlings. The enhancement of antioxidant enzyme activities plays a key role in eliminating ROS generation and preventing lipid peroxidation. For efficient detoxification, antioxidant enzymes help to maintain the AsA and GSH pool in plants. The enzymes GPX and GST generate water-soluble conjugates to defend against oxidative stress (Hossain et al. Citation2019). GPX and GST activities are increased in soybean seedlings under drought stress. The GPX and GST activities further increased when HBR was applied to drought-stressed seedlings, which might play a significant role in H2O2 detoxification.
Upregulation of the glyoxalase system or MG detoxification is vital for elimination of MG overproduction (). MG is mainly detoxified by two types of enzymes, namely, Gly I and Gly II, which are the main components of the glyoxalase system (Mostofa et al. Citation2018). In plants, overexpression of Gly I and Gly II improved drought stress tolerance (Singla-Pareek et al. Citation2008). The drought-stressed soybean seedlings exhibited overaccumulation of MG along with increased Gly I and Gly II activities, possibly due to an incompetent glyoxalase system. These results are consistent with Rahman et al. (Citation2016) findings. Higher Gly I and Gly II activities were observed with lower MG production when drought-treated seedlings were supplemented with HBR. Similar results were observed by Nahar et al. (Citation2016), who showed that exogenous pretreatment with spermine improved the detoxification of MG under drought-induced oxidative stress by increasing glyoxalase enzyme activity.
Conclusions
Drought stress decreased growth, gas exchange, chlorophyll fluorescence, and the chlorophyll content and increased the proline, GB content, oxidative stress biomarkers, and MG level in G. max. The levels of secondary metabolites, including the TP, TF, and anthocyanin levels, increased under drought stress. However, all these parameters were rescued by exogenous application of HBR. Antioxidant enzymes prevent the excessive formation of ROS during drought stress conditions. The antioxidant systems participate in the Asc-Glu cycle and glyoxalase system, which are enhanced by HBR. The cellular damage in soybean plants was less marked under supplementation with HBR. However, further physiological and molecular studies are needed to investigate the mechanisms of action of HBR action to reveal the actual molecular pathways.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2020/134), King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Md. Mahadi Hasan
Dr. Md. Mahadi Hasan is a postdoc fellow from State Key Laboratory of Grassland Agro-Ecosystems, School of Life Sciences, Lanzhou University, Lanzhou 730000, China. He has completed his PhD from King Abdulaziz University, Saudi Arabia. Experience in work in the field of plant stress physiology. Moreover, he is experience in working on ABA regulations, leaf hydraulics, photosynthetic safety, and efficiency of plants under drought stress.
Md. Arfan Ali
Dr. Md. Arfan Ali is an assistant professor of Department of Horticulture, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Dhaka, Bangladesh. His specialization on plant molecular biology and postharvest physiology.
Mona H. Soliman
Dr. Mona H. Soliman is a professor from Botany and Microbiology Department, Faculty of Science, Cairo University, 12613, Giza, Egypt. Her work focuses on plant physiology and molecular biology
Abdulaziz A. Alqarawi
Dr. Abdulaziz A. Alqarawi from Plant Production Department, College of Food and Agricultural Sciences, King Saud University. He has made significant contributions in crop improvement under abiotic stress conditions.
Elsayed Fathi Abd_Allah
Dr. Elsayed Fathi Abd_Allah from Plant Production Department, College of Food and Agricultural Sciences, King Saud University. His work focuses on investigations of chemical amendments or other beneficial compounds impact on plant growth, development, genetic changes under adverse conditions.
Xiang-Wen Fang
Dr. Xiang-Wen Fang is a professor of State Key Laboratory of Grassland Agro-Ecosystems, School of Life Sciences, Lanzhou University, Lanzhou 730000, China. His work emphases on plant ecophysiology, drought, leaf hydraulics, gas exchange, and hormonal regulations etc.
References
- Abd_Allah EF, Alqarawi AA, Hashem A, Wirth S, Egamberdieva D. 2017. Regulatory roles of 24-epibrassinolide in tolerance of Acacia gerrardii Benth to salt stress. Bioengineered. 9:61–71.
- Aebi H. 1984. Catalase in vitro. Methods Enzymol. 105:121–126.
- Ahammed GJ, Li X, Liu A, Chen S. 2020b. Brassinosteroids in plant tolerance to abiotic stress. J Plant Growth Regul. doi:10.1007/s00344-020-10098-0.
- Ahammed GA, Li X, Wan H, Zhou G, Cheng Y. 2020a. SlWRKY81 reduces drought tolerance by attenuating proline biosynthesis in tomato. Sci Hortic. 270:23–38.
- Ahmad P, Ahanger MA, Egamberdieva D, Alam P, Alyemeni MN, Ashraf M. 2018a. Modification of osmolytes and antioxidant enzymes by 24-epibrassinolide in chickpea seedlings under mercury (Hg) toxicity. J Plant Growth Regul. 37:309–322.
- Ahmad P, Alyemeni MN, Ahange MA, Wijaya L, Alam P, Kumar A, Ashraf M. 2018b. Upregulation of antioxidant and glyoxalase systems mitigates NaCl stress in Brassica juncea by supplementation of zinc and calcium. J Plant Interactions. 13:151–162.
- Ahmad H, Hayat S, Ali M, Ghani MI, Zhihui C. 2017. Regulation of growth and physiological traits of cucumber (Cucumis sativus L.) through various levels of 28-homobrassinolide under salt stress conditions. Canadian J Plant Sci. 98:132–140.
- Alam P, Albalawi TH, Altalayan FH, Bakht MA, Ahanger MA, Raja V, Ashraf M, Ahmad P. 2019. 24-Epibrassinolide (EBR) confers tolerance against NaCl stress in soybean plants by up-regulating antioxidant system, ascorbate-glutathione cycle, and glyoxalase system. Biomolecules. 9:640. doi:10.3390/biom9110640.
- Ali B, Hayat S, Ahmad A. 2007. 28-Homobrassinolide ameliorates the saline stress in chickpea (Cicer arietinum L.). Environ Exp Bot. 59:217–223.
- Alyemeni MN, Al-Quwaiz SM. 2016. Effect of 28-homobrassinolide on the performance of sensitive and resistant varieties of Vigna radiata. Saudi J Biol Sci. 23:698–705.
- Anjum SA, Ashraf U, Tanveer M, Khan I, Hussain S, Shahzad B, Zohaib A, Abbas F, Saleem MF, Ali I, Wang LC. 2017. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front Plant Sci. 8:69. doi:10.3389/fpls.2017.0006.
- Antoniou C, Xenofontos R, Chatzimichail G, Christou A, Kashfi K, Fotopoulos V. 2020. Exploring the potential of nitric oxide and hydrogen sulfide (NOSH)-releasing synthetic compounds as novel priming agents against drought stress in Medicago sativa plants. Biomolecules. 10:120.
- Arjenaki FG, Jabbari R, Morshedi A. 2012. Evaluation of drought stress on relative water content, chlorophyll content and mineral elements of wheat (Triticum aestivum L.) varieties. Int J Agric Crop Sci. 4:726–729.
- Arnon DT. 1949. Copper enzymes in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol. 24:1–15.
- Asada K. 1992. Ascorbate peroxidase-a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant. 85:235–241. doi:10.1111/j.1399-3054.1992.tb04728.x.
- Asada K. 1999. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Biol. 50:601–639.
- Asada K. 2006. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 141:391–396. doi:10.1104/pp.106.082040.
- Assaha DVM, Liu L, Ueda A, Nagaoka T, Saneoka H. 2016. Effects of drought stress on growth, solute accumulation and membrane stability of leafy vegetable, huckleberry (Solanum scabrum mill.). J Environ Biol. 37:107.
- Bajguz A, Hayat S. 2009. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem. 47:1–8.
- Bamji SF, Corbitt C. 2017. Glyceollins: soybean phytoalexins that exhibit a wide range of health promoting effects. J Funct Foods. 34:98–105.
- Bates LS, Waldren RP, Teare ID. 1973. Rapid determination of proline for water-stress studies. Plant Soil. 39:205–207.
- Bhattacharya A, Sood P, Citovsky V. 2010. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol Plant Pathol. 11:705–719.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 72:248–254.
- Cakmak I, Horst JH. 1991. Effects of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant. 83:463–468. doi:10.1111/j.1399-3054.1991.tb00121.x.
- Chapman HD, Pratt PF. 1978. Methods of analysis for soils, plants and waters. Division of agricultural sciences. Berkeley, USA: University of California. pp: 3043.
- Choudhury FK, Rivero RM, Blumwald E, Mittler R. 2017. Reactive oxygen species, abiotic stress and stress combination. Plant J. 90:856–867.
- Clouse SD. 2003. Recent advances in brassinosteroid research: from molecular mechanisms to practical applications. J Plant Growth Regul. 22:273–275.
- Comas LH, Eissenstat DM, Lakso AN. 2000. Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytol. 147:171–178.
- Dionisio-Sese ML, Tobita S. 1998. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 135:1–9.
- Doderer A, Kokkelink I, VanderVeen S, Valk B, Schram A, Douma A. 1992. Purification and characterization of two lipoxygenase iso enzymes from germinating barley. Biochim Biophys Acta. 1120:97–104. doi:10.1016/0167-4838(92)90429-H.
- Dubey A, Kumar A, Abd-Allah EF, Hashem A, Khan ML. 2018. Growing more with less: breeding and developing drought resilient soybean to improve food security. Ecol Indic. 105:425–437.
- Dunn J, Hunt L, Afsharinafar M, Meselmani MA, Mitchell A, Howells R, Wallington E, Fleming AJ, Gray JE. 2019. Reduced stomatal density in bread wheat leads to increased water-use efficiency. J Exp Bot. 70:4737–4748.
- Elia AC, Galarini R, Taticchi MI, Dorr AJM, Mantilacci L. 2003. Antioxidant responses and bioaccumulation in Ictalurus melasunder mercury exposure. Ecotoxicol Environ Saf. 55:162–167. doi:10.1016/S0147-6513(02)00123-9.
- Elkeilsh A, Awad YM, Soliman MH, Abu-Elsaoud A, Abdelhamid MT, El-Metwally IM. 2019. Exogenous application of β-sitosterol mediated growth and yield improvement in water-stressed wheat (Triticum aestivum) involves up-regulated antioxidant system. J Plant Res. 132:881–901. doi:10.1007/s10265-019-01143-5.
- Elstner FF, Heupel C. 1976. Inhibition of nitrite formation from hydroxyl ammonium chloride: a simple assay for superoxide dismutase. Anal Biochem. 70:616–620. doi:10.1016/0003-2697(76)90488-7.
- Fariduddin Q, Mir BA, Yusuf M, Ahmad A. 2014. 24-epibrassinolide and/or putrescine trigger physiological and biochemical responses for the salt stress mitigation in Cucumis sativus L. Photosynthetica. 52:464–474.
- Fariduddin Q, Yusuf M, Chalkoo S, Hayat S, Ahmad A. 2011. 28-homobrassinolide improves growth and photosynthesis in Cucumis sativus L. through an enhanced antioxidant system in the presence of chilling stress. Photosynthetica. 49:55–64.
- Foyer CH, Halliwell B. 1976. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 133:21–25.
- Foyer CH, Noctor G. 2011. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 155:2–18.
- Geetika S, Harpreet K, Renu B, Spal KN, Poonam S. 2014. Thermo-protective role of 28-homobrassinolide in Brassica juncea plants. American J Plant Sci. 5:2431–2439. doi:10.4236/ajps.2014.515257.
- Grieve CM, Grattan SR. 1983. Rapid assay for determination of water-soluble quaternary ammonium compounds. Plant Soil. 70:303–307.
- Griffiths OW. 1980. Determination of glutathione and glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 106:207–212. doi:10.1016/00032697(80)90139-6.
- Hasan MM, Alharby HF, Hajar AS, Hakeem KR, Alzahrani T. 2018. Effects of magnetized water on phenolic compounds, lipid peroxidation and antioxidant activity of Moringa species under drought stress. J Animal Plant Sci. 28:803.
- Hasan MM, Alharby HF, Hajar AS, Hakeem KR, Alzahrani Y. 2019. The effect of magnetized water on the growth and physiological conditions of Moringa species under drought stress. Pol J Environ Stud. 28:1145–1155.
- Hasan MM, Alharby HF, Uddin MN, Ali MA, Anwar Y, Fang XW, Hakeem KR, Alzahrani Y, Hajar AS. 2020. Magnetized water confers drought stress tolerance in Moringa biotype via modulation of growth, gas exchange, lipid peroxidation and antioxidant activity. Pol J Environ Stud. 29:1625–1636.
- Hasanuzzaman M, Alam MM, Rahman A, Nahar K, Fujita M. 2014. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Biomed Res Int. 757219,1-17.
- Hasanuzzaman M, Hossain MA, Fujita M. 2011. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep. 5:353–365.
- Hayat S, Hasan SA, Yusuf M, Hayat Q, Ahmad A. 2010. Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ Exp Bot. 69:105–112.
- He M, Jahan MS, Wang Y, Sun J, Shu S, Guo S. 2020. Compost amendments based on vinegar residue promote tomato growth and suppress bacterial wilt caused by Ralstonia solanacearum. Pathogens. 9:227.
- Hossain MS, Hasanuzzaman M, Sohag MMH, Bhuyan MHMB, Fujita M. 2019. Acetate-induced modulation of ascorbate: glutathione cycle and restriction of sodium accumulation in shoot confer salt tolerance in Lens culinaris Medik. Physiol Mol Biol Plants. 25:443–455.
- Hossain MZ, Hossain MD, Fujita M. 2006. Induction of pumpkin glutathione S-transferase by different stresses and its possible mechanisms. Biol Plant. 50:210–218. doi:10.1007/s10535-006-0009-1.
- Huang C, He W, Guo J, Chang X, Su P, Zhang L. 2005. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J Exp Bot. 56:3041–3049.
- Hughes J, Hepworth C, Dutton C, Dunn JA, Hunt L, Stephens J, Waugh R, Cameron DD, Gray JE. 2017. Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant Physiol. 174:776–787.
- Kaur H, Sirhindi G, Bhardwaj R, Alyemeni MN, Siddique KHM, Ahmad P. 2018. 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt and temperature-induced oxidative stress in Brassica juncea. Sci Rep. 8:1–13.
- Kaur H, Sirhindi G, Bhardwaj R, Sharma P, Mudasir M. 2014. 28-homobrassinolide modulate antenna complexes and carbon skeleton of Brassica juncea L. under temperature stress. J Stress Physiol Biochem. 10:186–196.
- Khan A, Anwar Y, Hasan MM, Iqbal A, Ali M, Alharby HF, Hakeem KR, Hasanuzzaman M. 2017. Attenuation of drought stress in Brassica seedlings with exogenous application of Ca2+ and H2O2. Plants. 6:20.
- Li ZG. 2019. Methylglyoxal: A novel signaling molecule in plant responses to abiotic stresses. In: Khan MIR, Reddy PS, Ferrante A, Khan NA., editors. Plant signaling molecules. New Delhi: Woodhead Publishing; p. 219–233.
- Li YH, Liu YJ, Xu XL, Jin M, An LZ, Zhang H. 2012. Effect of 24-epibrassinolide on drought stress-induced changes in Chorispora bungeana. Biol Plant. 56:192–196. doi:10.1007/s10535-012-0041-2.
- Li C, Tan DX, Liang D, Chang C, Jia D, Ma F. 2015. Melatonin mediates the regulation of ABA metabolism, free- radical scavenging, and stomatal behaviour in two Malus species under drought stress. J Exp Bot. 66:669–680.
- Li K, Wang H, Han G, Wang Q, Fan J. 2008. Effects of brassinolide on the survival, growth and drought resistance of Robinia pseudoacacia seedlings under water-stress. New For. 35:255–266.
- Li X, Zhang L, Ahammed GJ, Li ZX, Wei JP, Shen C, Yan P, Zhang LP, Han WY. 2017. Nitric oxide mediates brassinosteroid-induced flavonoid biosynthesis in Camellia sinensis L. J Plant Physiol. 214:145–151. doi:10.1016/j.jplph.2017.04.005.
- Liu Z, Ding Y, Wang F, Ye Y, Zhu C. 2016. Role of salicylic acid in resistance to cadmium stress in plants. Plant Cell Rep. 35:719–731.
- Liu L, Zhao Z, Si J, Di C, Han J, An L. 2009. Brassinosteroids alleviate chilling-induced oxidative damage by enhancing antioxidant defense system in suspension cultured cells of Chorispora bungeana. Plant Growth Regul. 59:207–214. doi:10.1007/s10725-009-9405-9.
- Mancinelli AL. 1984. Photoregulation of anthocyanin synthesis: VIII. Effect of light pretreatments. Plant Physiol. 75:447–453.
- Mishra P, Bhoomika K, Dubey RS. 2013. Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive indica rice (Oryza sativa L.) seedlings. Protoplasma. 250:3–19. doi:10.1007/s00709-011-0365-3.
- Mittler R. 2020. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7:405–410. doi:10.1016/s1360-1385(02)02312-9.
- Miyake C, Asada K. 1992. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol. 33:541–553.
- Mostofa MG, Ghosh A, Li ZG, Siddiqui MN, Fujita M, Tran LSP. 2018. Methylglyoxal—a signaling molecule in plant abiotic stress responses. Free Radic Biol Med. 122:96–109.
- Mostofa MG, Seraj ZI, Fujita M. 2014. Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma. 251:1373–1386.
- Müssig C. 2005. Brassinosteroid-promoted growth. Plant Biol. 7:110–117.
- Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M. 2016. Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol Environ Saf. 126:245–255.
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22:867–880.
- Noctor G, Foyer CH. 1998. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol. 49:249–279.
- Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH, Yu JQ, Nogués S. 2007. Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul. 27:49–57. doi:10.1007/s00344- 007-9030-7.
- Pacifici E, Polverari L, Sabatini S. 2015. Plant hormone cross-talk: the pivot of root growth. J Exp Bot. 66:1113–1121.
- Potapovich A, Kostyuk V. 2003. Comparative study of antioxidant properties and cytoprotective activity of flavonoids. Biochem (Mosc). 68:514–519. doi:10.1023/a:1023947424341.
- Rahman MM, Mostofa MG, Keya SS, Rahman MM, Das AK, Islam MR, Abdelrahman MM, Bhuiyan SU, Naznin T, Ansary MMU, Tran LP. 2020. Acetic acid improves drought acclimation in soybean: an integrative response of photosynthesis, osmoregulation, mineral uptake and antioxidant defense. Physiol Plantarum. doi:10.1111/ppl.13124.
- Rahman A, Nahar K, Hasanuzzaman M, Fujita M. 2016. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front Plant Sci. 7:609. doi:10.3389/fpls.2016.00609.
- Rai S, Yadav S, Rai R, Chatterjee A, Singh S, Rai LC. 2019. Molecular and biochemical characterization of All0580 as a methylglyoxal detoxifying glyoxalase II of Anabaena sp. PCC7120 that confers abiotic stress tolerance in E. coli. Int J Biol Macromol. 124:981–993.
- Ramadan AA, Elhamid EMA, Sadak MS. 2019. Comparative study for the effect of arginine and sodium nitroprusside on sunflower plants grown under salinity stress conditions. Bull Nat Res Centre. 43:118.
- Rao KV, Sresty TVS. 2000. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L. Millspaugh) in Response to Zn and Ni Stresses. Plant Sci. 157:113–128.
- Sadak MS, Abdalla AM, Elhamid EMA, Ezzo MI. 2020. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull Nat Res Centre. 44:18.
- Sami F, Yusuf M, Faizan M, Faraz A, Hayat S. 2016. Role of sugars under abiotic stress. Plant Physiol Biochem. 109:54–61.
- Sano S, Tao S, Endo Y, Inaba T, Hossain MA, Miyake C, Matsuo M, Aoki H, Asada K, Saito K. 2005. Purification and cDNA cloning of chloroplastic monodehydroascorbate reductase from Spinach. Biosci Biotechnol Biochem. 69:762–772. doi:10.1271/bbb.69.762.
- Serna M, Coll Y, Zapata PJ, Botella MA, Pretel MT, Amorós A. 2015. A brassinosteroid analogue prevented the effect of salt stress on ethylene synthesis and polyamines in lettuce plants. Sci Hort. 185:105–112. doi:10.1016/j.scienta.2015.01.005.
- Sharma N, Hundal GS, Sharma I, Bhardwaj R. 2014. 28-Homobrassinolide alters protein content and activities of glutathione-S transferase and polyphenol oxidase in Raphanus sativus L. plants under heavy metal stress. Toxicol Int. 21:44–50.
- Singh M, Kumar J, Singh S, Singh VP, Prasad SM. 2015. Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev Environ Sci Biotechnol. 14:407–426.
- Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK. 2008. Enhancing salt tolerance in a crop plant by over expression of glyoxalase II. Transgenic Res. 17:171–180. doi:10.1007/s11248-007-9082-2.
- Sirhindi G, Kaur H, Bhardwaj R, Sharma P, Mushtaq R. 2017. 28-Homobrassinolide potential for oxidative interface in Brassica juncea under temperature stress. Acta Physiol Plant. 39:228.
- Sirhindi G, Kumar S, Bhardwaj R, Kumar M. 2009. Effects of 24-epibrassinolide and 28-homobrassinolide on the growth and antioxidant enzyme activities in the seedlings of Brassica juncea L. Physiol Mol Biol Plants. 15:335–341. doi:10.1007/s12298-009-0038-2.
- Srivastava RK, Pandey P, Rajpoot R, Rani A, Gautam A, Dubey RS. 2014. Exogenous application of calcium and silica alleviates cadmium toxicity by suppressing oxidative damage in rice. Protoplasma. 252:959–975. doi:10.1007/s00709-014-0731-z.
- Talaat NB. 2014. Effective microorganisms enhance the scavenging capacity of the ascorbate–glutathione cycle in common bean (Phaseolus vulgaris L.) plants grown in salty soils. Plant Physiol Biochem. 80:136–143. doi:10.1016/j.plaphy.2014.03.035.
- Talaat NB, Shawky BT. 2013. 24-Epibrassinolide alleviates salt-induced inhibition of productivity by increasing nutrients and compatible solutes accumulation and enhancing antioxidant system in wheat (Triticum aestivum L.). Acta Physiol Plant. 35:729740.
- Vardhini BV, Anjum NA. 2015. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front Environ Sci. 2:67.
- Velikova V, Yordanov I, Edreva A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 151:59–66.
- Velioglu YS, Mazza G, Gao L, Oomah BD. 1998. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 46:4113–4117.
- Wild R, Ooi L, Srikanth V, Münch G. 2012. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: the N-acetyl-l-cysteine assay. Anal Bioana Chem. 403:2577–2581.
- Yang Z, Liu J, Poree F, Schaeufele R, Helmke H, Frackenpohl J, Lehr S, von Koskull-Döring P, Christmann A, Schnyde H, Schmidhalter U. 2019. Abscisic acid receptors and coreceptors modulate plant water use efficiency and water productivity. Plant Physiol. 180:1066–1080.
- Yu JQ. 2003. A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J Exp Bot. 55:1135–1143.
- Zhishen J, Mengcheng T, Jianming W. 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64:555–559.