ABSTRACT
The Hessian fly causes severe economic losses in host wheat. The genome complexity of hexaploid wheat makes functional characterization of candidate defense genes extremely challenging. Kitaake rice, a model and simpler genome, exhibits responses resembling nonhost resistance to Hessian fly. Larvae feeding on Kitaake rice plants do not develop beyond first-instars similar to resistant host wheat, although, they show prolonged survival. Kitaake nonhost differs from nonhost Brachypodium, where some larvae develop into second-instars. Kitaake rice plants exhibit a molecular response similar to not only resistant but also susceptible host wheat for six Hessian fly-responsive biomarker genes assayed. Further, in Kitaake, lectins and secondary metabolites may play an important role in early defense preventing the larvae from developing. The phenotypic and molecular characterization of Kitaake rice reveals its suitability as a surrogate model genome for undertaking downstream functional genomics studies of candidate wheat genes that respond to Hessian fly larval attack.
Introduction
The Hessian fly, Mayetiola destructor (Say), belonging to the gall midge family (Cecidomyiideae) is an obligate Dipteran pest of hexaploid bread wheat (Triticum aestivum L.) in the United States and worldwide (Flanders et al. Citation2013; Schmid et al. Citation2018), leading to economic losses (Smiley et al. Citation2004). The life cycle of Hessian fly begins with mated adult females laying eggs on the leaf surface. The newly hatched first-instar larvae (neonates) crawl to the base (crown) of the seedling where they probe the plants to establish permanent feeding sites. Larval attack on host wheat plants yields an incompatible or compatible interaction. In the former, the Hessian fly resistance (H) gene-mediated defense responses result in larval death within five days after egg hatch (5 DAH), leading to a resistant plant showing normal growth and larvae being avirulent. However, in the latter, the larvae are virulent and within three days of attack establish permanent feeding sites and alter the host plant physiology (Zhu et al. Citation2008) to provide a diet rich in nutrients (Harris et al. Citation2006; Saltzmann et al. Citation2008; Subramanyam et al. Citation2015; Subramanyam et al. Citation2018), allowing the larvae to develop completely, resulting in a susceptible plant with stunted height (Byers and Gallun Citation1972; Stuart et al. Citation2012).
Deploying resistant wheat cultivars harboring H genes is the most effective and economical way to manage this insect pest (Berzonsky et al. Citation2002; Schmid et al. Citation2018). To date, 37 genes (H1–H36 plus Hdic) have been identified (Liu et al. Citation2005; Sardesai et al. Citation2005; Li et al. Citation2013; Subramanyam et al. Citation2016; Zhao et al. Citation2020). However, extensive deployment of resistant wheat lines exerts strong selection pressure on Hessian fly populations resulting in the development of virulent biotypes (Johnson et al. Citation2017) posing a severe threat to wheat production, thereby necessitating the need to identify and employ alternate strategies that can enhance and complement native or introgressed H gene resistance.
An alternate, or complementary, effective strategy to H genes is employing forward genetics that leads to the development of wheat lines ectopically expressing Hessian fly resistance-associated defense response genes and negatively regulating susceptibility-associated genes. However, the challenge to such a strategy in hexaploid wheat can be attributed to its huge genome (∼17 Gb) and associated complexities (Gupta et al. Citation2008) associated with the presence of three sets of genomes (AABBDD), and highly repetitive (85%) genomic sequences (IWGSC Consortium Citation2018). To circumvent this issue, we have recently explored the possibility of using surrogate model organisms like Brachypodium distachyon (Bd), which have a smaller genome size (355 Mb), shorter life cycle and availability of greater genetic resources, for undertaking functional analysis of candidate Hessian fly-responsive genes. Bd plants exhibit a nonhost response with physical and molecular responses being intermediate between resistant and susceptible wheat host lines (Hargarten et al. Citation2017; Subramanyam et al. Citation2019). However, using the Bd system may have some limitations as well, since mutants for all genes are not easily available yet for carrying out functional analyses.
In the current study, we explore yet another model genome, Oryza sativa variety Kitaake (377.6 Mb genome size), for functional genomics of candidate Hessian fly-responsive genes. Unlike other O. sativa varieties, the Kitaake variety offers unique advantages such as rapid life cycle (9 weeks), ease of transformation and propagation (Jung et al. Citation2008; Jain et al. Citation2019) and the availability of a large collection of fast-neutron (FN) mutant populations (Li et al. Citation2017). Further, Kitaake genome has been explored to understand various aspects of rice biology including disease resistance (Liu et al. Citation2015; Zhou et al. Citation2018), as well as CRISPR-Cas9 and TALEN technologies (Li et al. Citation2012; Xie et al. Citation2015) for genome editing. Thus O. sativa Kitaake variety has emerged as an ideal model genome for downstream functional applications. Although, a previous study reported the interaction of Biotype L Hessian fly with four rice varieties, including Kitaake (Chen et al. Citation2009), in-depth analyses of physical and molecular responses were not undertaken. Here, we characterize the phenotypic and molecular responses of Kitaake rice variety to Biotype L Hessian fly larval attack and discuss the suitability of utilizing this rice variety for functional genomic studies of candidate Hessian fly-responsive genes.
Materials and methods
Plant and insect material
Oryza sativa Kitaake (referred to as Kitaake from here on) seeds were provided by Dr Faik Ahmad (Ohio University). The Hessian fly (Mayetiola destructor) Biotype L used in this study were maintained in diapause at 4°C at the USDA-ARS Crop Production and Pest Control Research Unit in West Lafayette, IN as per Sosa and Gallun (Citation1973).
Plant growth and infestation
Pots (4-inch) were prepared one day prior to planting seeds by placing a paper towel in the bottom of each pot, covering with ProMix BX Mycorrhizae growing medium (Premier Horticulture Inc., Quakertown, PA) and watering thoroughly with reverse osmosis water. The following day, 10 dehusked Kitaake rice seeds were sown in each pot. The pots were then placed in a cold chamber at 4°C for five days, and then moved to a Conviron growth chamber (Controlled Environment Ltd., Winnipeg, Manitoba, Canada) set at 26°C with 80% humidity and 20°C with 60% humidity for 12 h day and night cycle, respectively. Pots were placed in a tray filled with water and were watered daily. After the seeds germinated, plants were fertilized with Peters Excell 15-5-15 solution (ICL Fertilizers, St. Louis, MO) once a week and with Sprint 330 iron solution (BASF Corporation, Florham Park, NJ) twice per week. Trays were replaced periodically to limit algal growth. When Kitaake seedlings reached the 2-leaf stage, pots were covered with vented plastic cup covers and 8 females plus 2 male adult Biotype L Hessian flies were released in each pot.
For undertaking larval size comparisons, host wheat lines ‘Iris’ (resistant) and ‘Newton’ (susceptible) and nonhost Brachypodium distachyon (Bd) seedlings were also grown and infested with Biotype L Hessian flies as per Hargarten et al. (Citation2017).
Larval development and leaf measurement
To document larval development, Biotype L Hessian fly larvae feeding on Kitaake rice seedlings, resistant Iris wheat and susceptible Newton wheat were photographed at 5, 7, and 12 DAH using the DP27 camera system on a SZX2 stereomicroscope (Olympus, Center Valley, PA). The larvae developing on Bd nonhost plants were photographed on 9 and 36 DAH as described above.
Leaf measurements (from soil level to leaf blade tips) were taken from a set consisting of 23 Biotype L-infested or uninfested Kitaake rice seedlings at 9 DAH time point. Measurements were taken for leaf 1 (L1), 2, (L2), 3 (L3), and 4 (L4). Significant differences in leaf growth between infested and uninfested Kitaake seedlings were determined using one-sided Tukey pairwise comparison (JMP Pro ver. 15, SAS Institute Inc.). Differences were considered statistically significant at p < .05.
Neutral red staining
To determine if the epidermal cell wall integrity is disrupted by Hessian larval feeding on Kitaake seedling, the crown tissue (feeding site) was stained with neutral red (NR) as described in Nemacheck et al. (Citation2019). Cell wall permeability was assessed in Biotype-L infested Kitaake seedlings at 1, 4, and 9 DAH time points. At each time point, the first leaf and sometimes second leaf from Hessian fly-infested rice seedlings were carefully peeled off to expose the crown tissue (feeding site). Care was taken to avoid injuring or wounding the tissue during the dissection process. The uninfested Kitaake seedlings were also dissected in a similar manner and injured with 0.2 mm minuten pin prior to staining, to mimic wounding, as positive controls. The plant tissues were placed in 0.1% w/v NR stain (Sigma-Aldrich, St. Louis, MO) for 10 min, following which they were removed from the stain solution and thoroughly rinsed with water. Degree of staining was scored using the scale established as per Williams et al. (Citation2011). The scale ranged from 0 (indicating no stain) to 7 (completely dark red crown). Representative stained plants at each time point were photographed using a SZX2 stereomicrosope with a DP27 camera (Olympus).
Tissue collection and RNA extraction
For gene expression studies Kitaake seedlings were grown and infested as described above. For tissue collections, the seedlings were dissected to expose the second leaf sheath. If the larvae were present on this leaf, the bottom 1.5 cm of infested crown tissue (feeding site) were collected and immediately frozen in liquid nitrogen. If no larvae were present on sheath 2, the second leaf was peeled back to confirm the presence of larvae on sheath 3, before harvesting and freezing in a similar manner. Tissue collections from uninfested plants were also performed in a similar fashion to maintain consistency in the manner of collection. Collections from 10 infested and uninfested plants were done on 1, 3, 5, and 9 DAH time points per replicate. Tissues were collected from three biological replicates. RNA was extracted from the frozen harvested tissue using TRIzol reagent (Life Technologies Corporation, Carlsbad, CA) as per manufacturer’s instructions.
Transcript profiling
To quantify mRNA abundance, quantitative real-time reverse transcription PCR (qRT-PCR) was performed for a select set of genes. These included genes encoding (i) Hessian fly-responsive biomarker proteins that were previously documented to be associated with either resistance or susceptibility in wheat, (ii) cell wall-associated proteins, and (iii) enzymes involved in the biosynthesis of phenylpropanoids and lignins. Rice orthologs of wheat genes were identified by BLASTp searches against the NCBI nr database and primers were designed (Table S1) using Primer Express 3.0 software (Applied Biosystems, Foster City, CA). First-strand cDNA was synthesized using oligo (dT) primers as described in Subramanyam et al. (Citation2015). The qRT-PCR was performed on a LightCycler 480 II instrument (Roche Diagnostics Corporation, Indianapolis, IN) with three biological replicates and three technical replicates for each reaction. Reaction volume of 10 µl contained 5 µl of 2× SYBR Green I Master mix (Roche), forward and reverse primers at a final concentration of 0.5 µM each, and 20 ng cDNA template. The PCR cycling parameters were as follows: 45 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 10 s. Melt-curve analysis was performed to determine the amplification of a single product for each target gene. The housekeeping gene OsGAPDH (Table S1) was used as an endogenous control gene to normalize the cDNA levels. Transcript abundance was determined using Relative Standard Curve method (Applied Biosystems User Bulletin 2) as described in Subramanyam et al. (Citation2015). Significant differences in the log2-transformed data were determined by analysis of variance (ANOVA) using the PROC Mixed procedure (SAS version 0.4) as described in Subramanyam et al. (Citation2015). The ANOVA model included treatments, time-points, biological replicates, and the interaction between treatments and time points as fixed effects. Data from the biological and technical replicates were combined and included as a random effect in the analysis model and orthogonal contrasts were used to evaluate differences in treatments at each time point. Differences in transcript levels in the infested plants as compared to the uninfested controls at the same time point were considered statistically significant if the contrast was p < .05.
Results
Kitaake rice seedlings display resistance phenotype to Hessian fly larval feeding
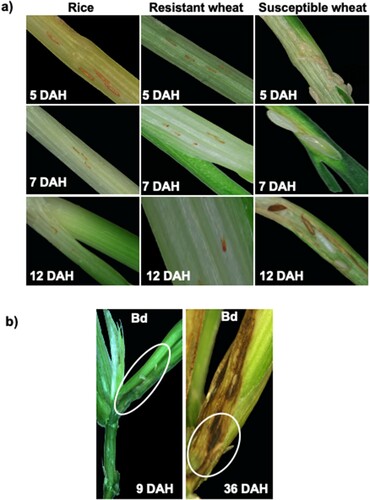
By 5 DAH, Biotype L larvae feeding on Kitaake rice seedlings were in the first-instar larval development stage and appeared as red larvae ((a); rice 5 DAH). This response was very similar to the phenotypic reaction observed for the avirulent larvae feeding on resistant host wheat ((a), resistant wheat 5 DAH) and unlike the virulent white, second-instars feeding on the susceptible host wheat ((a); susceptible wheat 5 DAH). However, by 7 DAH while all avirulent larvae on wheat were dead and shrivelled, not all larvae feeding on Kitaake rice seedlings were dead or shrivelled ((a), rice, resistant wheat 7 DAH). The larvae on susceptible wheat continued development as second-instars ((a), susceptible wheat 7 DAH). By 12 DAH, larvae on Kitaake rice seedlings also appeared shrivelled, resembling the avirulent larvae feeding on resistant wheat, appearing as dead reds ((a); rice and resistant wheat 12 DAH). In contrast, most of the larvae feeding on susceptible host wheat developed into third-instars (green gut white larvae) with a few of them pupating by 12 DAH ((a); susceptible wheat 12 DAH). By 26 DAH, no larvae were found on Kitaake rice plants, unlike the phenotypic response observed for larvae feeding on another nonhost, Bd plants. In Bd plants, many of the larvae were avirulent (appearing as dead reds) by 9 DAH, similar to the response observed in resistant host wheat and Kitaake rice seedlings, while some of the larvae developed into small white larvae ((b)). Some of the larvae on nonhost Bd plants were able to survive as small, white larvae until 36 DAH ((b)) but did not complete their development, unlike the virulent larvae feeding on susceptible host wheat (this study and Hargarten et al. Citation2017).
Figure 1 Comparative phenotypic response of Kitaake rice seedlings to Hessian fly larval feeding. (a) Representative plants of Kitaake rice plants showing resistance response having first-instar larvae at the base of the crown tissue (the larval feeding site); resistant host wheat Iris showing dead first-instar larvae; and susceptible host wheat Newton showing larvae at the second-instar (white) and third-instar developmental stage at 5, 7, and 12 DAH time points. (b) Nonhost Bd (Brachypodium distachyon), where most larvae are dead first-instars, however, there are some larvae that have developed into second-instars at 9 and 36 DAH, but the larvae are much smaller in size as compared to virulent larvae feeding on susceptible host wheat, at 7 DAH.
Leaf length does not differ between infested and uninfested rice seedlings
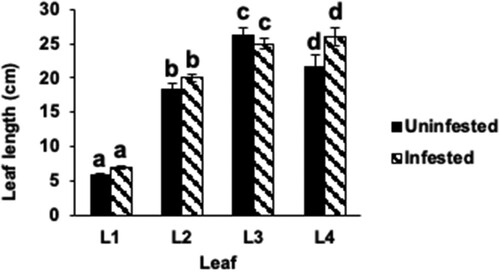
To assess whether Hessian fly infestation affects the development of the leaves in Kitaake rice seedlings, the length of leaves 1 (L1), 2 (L2), 3 (L3), and 4 (L4) were measured in Hessian fly-infested and uninfested plants at 9 DAH (). The differences in the lengths of L1, L2, L3, and L4 leaves from infested plants as compared to the uninfested control leaves were insignificant (p > .05 for all pairs). The plant growth was comparable between uninfested and infested Kitaake rice seedlings.
Figure 2 Leaf and plant growth in Hessian fly-infested Kitaake rice seedlings. (a) Nondestructive leaf (L1: leaf 1; L2: leaf 2; L3: leaf 3; L4: leaf 4) length measurements were taken in Biotype L-infested and uninfested Kitaake rice seedlings at 9 DAH. Measurements were taken from soil level to the tips of the leaf blades. Data are represented as means from 23 biological replicates. Error bars represent the standard error of the mean. Letters at the top of bars indicate significant differences determined using Tukey’s HSD test (p < .05). Same letters indicate no difference in lengths.
Cell wall permeability is localized in Kitaake seedlings
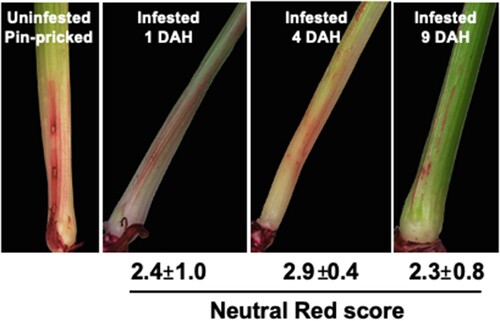
Induced cell wall permeability was assessed by staining Biotype L-infested Kitaake rice seedlings with neutral red (NR) stain and the scores compared with those obtained for resistant and susceptible host wheat lines, and the nonhost Bd, documented previously (Williams et al. Citation2011; Subramanyam et al. Citation2019). Resembling host wheat and nonhost Bd plants, Hessian fly larval feeding induced epidermal cell wall permeability in rice plants as evident by NR stained crown tissue where the larvae were present, but not in uninfested seedlings, unless wounded by piercing with a minuten pin as a positive control (). The NR staining appeared as a smear, blush or solid lines, and, similar to staining in resistant wheat, was restricted to the larval feeding site at the base of the crown tissue. The average NR score remained the same, temporally, with score intensity of 2.4 ± 1.0, 2.9 ± 0.4 and 2.3 ± 0.8, at 1, 4 and 9 DAH, respectively ().
Figure 3. Changes in plant cell wall permeability in Kitaake rice seedlings. Permeability was determined by neutral red (NR) staining. Representative uninfested control rice plant was pin-pricked and stained to differentiate staining caused by physical damage and larval feeding. Representative NR stained Hessian fly-infested rice seedlings at 1, 4, and 9 DAH time points show NR staining as blush and solid lines that are restricted at the larval feeding site. Scores for the staining are given below the images as means of staining scores from 7 biological replicates ± standard error of mean.
Molecular responses of Kitaake rice seedlings to Hessian fly larval feeding
Transcript profile of Hessian fly-responsive biomarker genes
The expression of a set of genes that serve as key biomarkers during wheat resistance (incompatible interaction) and susceptibility (compatible interactions), were profiled in Hessian fly-infested rice seedlings over a time-course. The resistance-associated biomarker genes included OsHfr-1 (Hessian fly response gene 1), OsHfr-3 (Hessian fly response gene 3), and OsHfrDrd (Hessian fly-responsive dirigent-like defense protein). In Hessian fly-infested Kitaake plants, there was significant increase in accumulation of OsHfr-1 and OsHfr-3 transcripts as compared to the uninfested control plants ((a and b)). While OsHfr-1 transcript accumulation occurred at 1 (43.4-fold; p < .0001), 3 (5.0-fold; p < .0001) and 9 (2.8-fold; p < .001) DAH time points in the infested rice plants, the transcripts of OsHfr-3 increased only at 1 DAH (5.3-fold, p < .008) and were not significantly different on 3, 5, and 9 DAH time points ((b)). OsHfrDrd, unlike in resistant host wheat, was down-regulated at 1 DAH (1.5-fold; p < .011), in Kitaake rice plants, and lacked significant difference in expression at other time points as compared to uninfested control plants ((c)). The susceptibility-associated biomarker genes included OsMds-1 (Mayetiola destructor susceptibility gene 1), and OsOdc (ornithine decarboxylase). Both the susceptibility-associated genes showed increased transcripts in Hessian fly-infested Kitaake plants as compared to the uninfested plants ((d and e)). While the transcripts for OsMds-1 were significantly up only at 1 DAH ((d)), OsOdc transcripts increased significantly at 1 and 3 DAH time points ((e)). OsMds-1 and OsOdc increased as high as 5.7-fold (p < .0001) and 28.9-fold (p < .001), respectively, at 1 DAH as compared to the uninfested controls ((d and e)). The biomarker gene that is associated with both resistance and susceptibility included Ltp1 (lipid transfer protein 1). In Hessian fly-infested Kitaake rice plants.
Figure 4 Expression of Hessian fly-responsive biomarker genes in Kitaake rice seedlings in response to Hessian fly larval feeding. Transcript levels of (a) OsHfr-1 (Hessian fly response gene 1), (b) OsHfr-3 (Hessian fly response gene 3), (c) OsHfrDrd (Hessian fly responsive disease resistance dirigent-like protein), (d) OsMds-1 (Mayetiola destructor susceptibility gene 1), (e) OsOdc (ornithine decarboxylase), and (f) OsLtp1 (lipid transfer protein 1) were quantified by qRT-PCR in Hessian fly-infested and uninfested Kitaake rice seedlings at 1, 3, 5, and 9 DAH time points. Values are plotted as the log2 fold change of infested compared to uninfested control plants with standard error bars for three biological replicates. Asterix (*) above each bar indicates statistically significant differences (p < .05). Linear fold change values are also indicated above each bar.
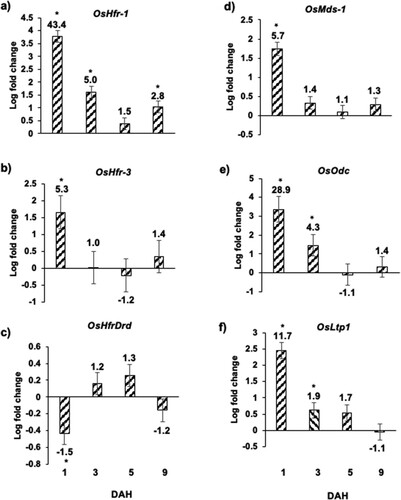
OsLtp1 transcripts increased significantly at 1 (11.7-fold; p < .0001) and 3 (1.9-fold; p < .001) DAH time points as compared to uninfested control but did not change at 5 and 9 DAH time points ((f)).
Cell wall-associated genes are differentially regulated minimally in infested Kitaake rice
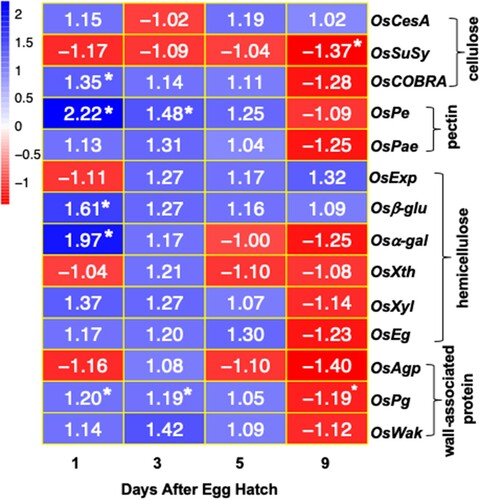
To better understand how larval feeding affects the cell wall integrity at the feeding sites, temporal changes in the expression of 14 genes involved in cell wall metabolism (cellulose, hemicellulose, pectin synthesis, and wall-associated proteins) in Kitaake rice seedlings were determined by qRT-PCR (). Except for a few, most cell wall genes were not significantly expressed as compared to the uninfested controls, temporally (). While genes encoding OsCOBRA, Osβ-glu and Osα-gal were significantly upregulated at 1 DAH time point, OsPe and OsPg genes were significantly upregulated at 1 and 3 DAH time points (). Although these genes were upregulated, the increase in transcripts was marginal ranging from 1.2- to 2.2-fold (p < .05) as compared to the uninfested controls. Genes encoding OsSuSy and OsPg were significantly downregulated by 9 DAH time point ().
Figure 5 Expression of cell wall-associated proteins in Kitaake rice seedlings in response to Hessian fly larval feeding. Heatmap depicting the ratio of transcript levels quantified by qRT-PCR in Hessian fly-infested and uninfested Kitaake rice seedlings at 1, 3, 5, and 9 DAH time points. Blue and red represent up-regulated and down-regulated genes, respectively. Values shown within each cell is the mean fold change of infested compared to uninfested control plants for three biological replicates. Asterix (*) within a cell indicates statistically significant difference (p < .05). OsCesA (cellulose synthase), OsSuSy (sucrose synthase), OsCOBRA (glycosyl-phosphatidyl inositol-anchored protein), OsPe (pectin esterase), OsPae (pectin acetyl esterase), OsExp (expansin), Osβ-glu (beta-glucosidase), Osα-gal (alpha-galactosidase), OsXth (xyloglucan endotransglucosylase/hydrolase), OsXyl (xylanase), OsEg (endoglucanase), OsAgp (arabinogalactan protein), OsPg (polygalactouronase), and OsWak (wall-associated kinase).
Secondary metabolite genes are upregulated in Hessian fly-infested Kitaake rice
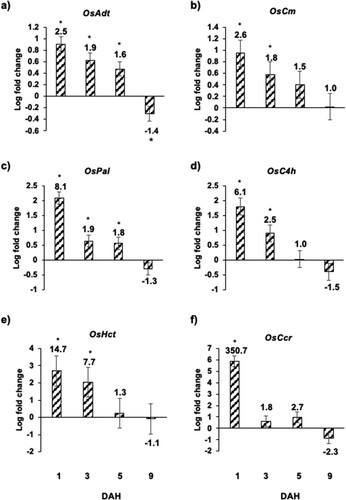
To determine if secondary metabolites are involved in Kitaake resistance to Hessian fly, we looked at the expression of genes involved in the biosynthesis of phenylpropanoids including arogenate dehydratase (OsAdt), chorismate mutase (OsCm), phenylalanine-ammonia lyase (OsPal), cinnamate 4-hydroxylase (OsC4 h), hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase (OsHct), and in the biosynthesis of lignins including cinnamoyl CoA reductase (OsCcr). Kitaake rice plants showed significant upregulation of OsAdt, OsCm, OsPal, OsC4h, OsHct and OsCcr (). The highest increase in mRNA levels for these genes was observed at 1 DAH then gradually decreased by 5 DAH as compared to the uninfested controls (). While the transcripts of OsAdt ((a)) and OsCm ((b)) increased only marginally to 2.5-fold (p<0.01), the transcripts for OsPal, OsC4 h and OsHct increased as high as 8.1 – (p < .0001), 6.1 – (p = .0002), and 14.7 – (p = .015) folds, respectively at 1 DAH as compared to uninfested controls ((c–e)). On the other hand, the transcripts of OsCcr, in the lignin biosynthetic pathway, increased dramatically to 350.7-fold (p < .0001) at 1 DAH time point ((f)). By 3 and 5 DAH time points, OsCcr showed a sharp decrease in transcript accumulation and was not significantly different as compared to the uninfested controls ((f)). By 9 DAH time point, the transcripts for these genes were either downregulated or showed no significant difference as compared to the uninfested controls ().
Figure 6 Expression of genes involved in biosynthesis of phenylpropanoids and lignins in Kitaake rice seedlings in response to Hessian fly larval feeding. Transcript levels of (a) OsAdt (arogenate dehydratase), (b) OsCm (chorismate mutase), (c) OsPal (phenylalanine-ammonia lyase), (d) OsC4 h (cinnamate 4-hydroxylase), (e) OsHct (hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase, and (f) OsCcr (cinnamoyl CoA reductase) were quantified by qRT-PCR in uninfested and Hessian fly-infested Kitaake rice seedlings at 1, 3, 5, and 9 DAH time points. Values are plotted as the log2 fold change of infested compared to uninfested control plants with standard error bars for three biological replicates. Asterix (*) above each bar indicates statistically significant differences (p < .05). Linear fold change values are also indicated above each bar.
Discussion
The classical host wheat gene-for-gene interaction with Hessian fly larvae yields an incompatible or compatible interaction, with the plants being resistant and larvae dying in the first-instar developmental stage in the former, and plants being susceptible and larvae completing their development, in the latter. However, in the nonhost Bd-Hessian fly interaction, while most larvae do not develop beyond the first-instars (dead reds), similar to the resistant host wheat, some larvae continue to develop into small second-instars (white larvae) that are able to prolong their survival as long as 46 DAH (Hargarten et al. Citation2017). Unlike the virulent larvae feeding on susceptible host wheat, none of the small second-instars on nonhost Bd complete their development and they disintegrate, eventually (Hargarten et al. Citation2017). Hessian fly larvae feeding on Kitaake rice seedlings do not develop beyond the first-instar developmental stage, resembling the phenotypic response of host resistant wheat. However, not all larvae feeding on Kitaake are dead and shrivelled by 7 DAH, a response distinct from that observed in resistant host wheat, where all larvae are dead by this time point. Thus the larvae feeding on Kitaake plants have a prolonged first-instar developmental stage before eventually dying by 12 DAH. A similar response has been seen in Kitaake rice and three other rice varieties (Azucena, Nipponbare and IR64) infested with two Hessian fly populations, Scott-KS-05 from Kansas and Kay-OK-06 from Oklahoma, that are composed of a mix of biotypes (Chen et al. Citation2009). The phenotypic response of Kitaake rice plants indicates that they are nonhost to Hessian fly and lack the classical H gene-mediated resistance as seen in resistant host wheat. Nonhost resistance is the most common form of plant resistance against pests and pathogens, in which all genotypes of a plant species are resistant against species-specific parasite or pathogens (Heath Citation2000). Nonhost resistance is more robust and durable than host-plant resistance as they can adapt an array of defense mechanisms not found in the host species (Heath Citation2000; Gill et al. Citation2015). These observations are similar to those documented previously for Hessian fly larvae feeding on four rice varieties (Chen et al. Citation2009). However, by 26 DAH, no larvae were seen on Kitaake rice seedlings, thus suggesting that the mechanism of nonhost resistance observed in Kitaake differs from the nonhost resistance observed in Bd plants to Hessian fly larval attack, where some larvae can survive till 46 DAH (Hargarten et al. Citation2017). At no point do the phenotypic responses of Kitaake share any resemblances with the true compatible interaction in which the virulent larvae feeding on host wheat complete their development making the plant susceptible.
Leaf lengths in Hessian fly-infested Kitaake rice seedlings were comparable to leaves in uninfested control plants. Hessian fly larval feeding on susceptible host wheat rapidly inhibits leaf elongation, with leaf 3 being significantly shorter than the uninfested control plants by 3 DAH (Hargarten et al. Citation2017). The susceptible plants have dark leaves and show stunted growth (Schmid et al. Citation2018). In contrast, although the leaves on Hessian fly-infested resistant host hexaploid and diploid wheats, and nonhost Bd, exhibit some measure of initial leaf stunting on leaves that are actively growing during larval feeding, once the larvae die, there is an accelerated growth of leaves which end up having the same leaf length as compared to the uninfested controls (Hargarten et al. Citation2017; Nemacheck et al. Citation2019). None of the four leaves in Kitaake seedlings exhibited the initial stunting observed in leaves of resistant host wheat and Bd nonhost plants, suggesting that there are no physical consequences to the Kitaake rice plants in response to attempted larval feeding. The infested and uninfested Kitaake rice plants showed comparable plant growth at 9 DAH.
The epidermal cell wall layer is considered as the first line of plant defense against herbivory (Schönherr Citation1982; Javelle et al. Citation2011). Previous studies using neutral red, a stain that enters cuticular gaps or damaged cell walls and spreads mainly in the major vasculature (Joel and Juniper Citation1982), revealed a two-way exchange of molecules during host wheat- and nonhost Bd-Hessian fly interactions (Williams et al. Citation2011; Subramanyam et al. Citation2019; Nemacheck et al. Citation2019). The increased and sustained wall permeability in susceptible wheat has been attributed to the delivery of salivary effectors from the virulent Hessian fly larvae, altering host plant physiology and offering a nutrient-rich environment conducive to the developing larvae (Williams et al. Citation2011). In contrast to the permeability levels observed in susceptible host wheat, the resistant host plant and nonhost Bd plants show transient and limited permeability at the early stages of larval development, allowing the delivery of defense proteins that prevent the larvae from establishing permanent feeding sites and completing their development (Williams et al. Citation2011; Subramanyam et al. Citation2019). In Hessian fly-infested Kitaake rice plants, NR staining appeared as lines or blush and was restricted to the larval feeding sites, with NR scores remaining the same, temporally. The staining pattern resembled that observed in resistant host wheat (Williams et al. Citation2011) but differed from nonhost Bd plants which exhibit staining intensity intermediate between resistant and susceptible host wheat, with NR scores increasing over time from 1 to 9 DAH (Subramanyam et al. Citation2019). The differences in temporal NR scores and stain intensity between the two nonhosts, Kitaake and Bd, may be attributed to the fact that while larvae do not develop beyond first-instars on Kitaake rice plants, some larvae on Bd plants develop into second-instars and survive as long as 46 DAH (Hargarten et al. Citation2017). It may be noted here that although Bd plants showed higher NR scores as compared to Kitaake rice or host wheat plants, the intensity of NR staining and scores were not comparable with those observed in susceptible host wheat with intense NR stain covering the entire crown tissue (Williams et al. Citation2011). The limited permeability in Kitaake rice seedlings possibly allows the delivery of defense proteins and toxins to the larval feeding sites thereby preventing the larvae from establishing permanent feeding sites and developing beyond the first-instar stage.
To better understand cell wall integrity in rice plants infested with Hessian fly we quantified the expression of genes encoding wall-associated polymers (cellulose, pectin and hemicellulose) and proteins. In Hessian fly-infested Kitaake rice plants, most cell wall-associated genes, barring a few that showed marginal increase in transcripts, were not significantly expressed as compared to the uninfested control plants. Our results were comparable with expression profiles of wall-associated genes observed in resistant host wheat (Subramanyam et al. unpublished) but differed from the profile seen in susceptible host wheat (Subramanyam et al. unpublished data) and nonhost Bd plants (Subramanyam et al. Citation2019), where most cell wall genes are significantly down-regulated as compared to their uninfested controls. These results clearly suggest that there is minimal cell wall damage caused by larvae feeding on Kitaake rice plants. Further, the little damage caused by larval probing is very restricted and does not spread possibly due to induced early defense responses in Kitaake rice plants following larval attack. In contrast, the entire crown tissue of a susceptible host plant becomes a nutrient sink (Williams et al. Citation2011) due to suppression of cell wall genes.
Since the phenotypic responses observed in Kitaake rice seedlings were similar to resistant host wheat but different from susceptible host wheat, we expected them to show comparable expression profiles with respect to Hessian fly-responsive resistance-associated biomarker genes documented previously (Hargarten et al. Citation2017; Subramanyam et al. Citation2019; Nemacheck et al. Citation2019). The resistance-associated genes included two genes, Hfr-1 and Hfr-3, that encode for mannose and chitin-binding lectins, respectively, and HfrDrd, a gene encoding a dirigent-like protein. While lectins possess antifeedant and insecticidal properties (Subramanyam et al. Citation2008; Pyati et al. Citation2012), the dirigent proteins are involved in cell wall fortification (Subramanyam et al. Citation2013). Resembling the resistant host wheat, transcripts for orthologous genes encoding both the lectins, OsHfr-1 and OsHfr-3, increased in Kitaake rice plants. However, unlike the resistant host wheat, OsHfrDrd was not significantly expressed at any of the time points in Kitaake rice seedlings following larval attack. These results suggest the involvement of rice lectins as antifeedants that prevent the Hessian fly larvae from establishing permanent feeding sites, making them unable to develop beyond the first-instar developmental stage. Unlike the resistant wheat, dirigent proteins do not appear to play a role in Kitaake defense against Hessian fly. The susceptibility-associated biomarker genes in hexaploid wheat include genes (i) encoding a heat shock protein (Mayetiola destructor susceptibility gene, Mds-1), and (ii) involved in polyamine biosynthetic pathway (ornithine decarboxylase, Odc). The transcripts for Mds-1 and Odc increase dramatically in susceptible host wheat in response to Hessian fly larval attack (Liu et al. Citation2013; Subramanyam et al. Citation2015). Interestingly, transcripts for these susceptibility-associated genes, OsMds-1 and OsOdc, showed increased accumulation in Kitaake rice seedlings as early as 1 DAH time point, resembling the susceptible host wheat. However, the levels of elevation of transcripts for these genes were much lower than in the susceptible host wheat (Liu et al. Citation2013; Subramanyam et al. Citation2015). Further, in the susceptible wheat, the transcripts for both Mds-1 and Odc stayed elevated till 8 DAH time points unlike the Kitaake rice plants where transcripts for OsMds-1 and OsOdc did not stay elevated beyond 1 and 3 DAH time points, respectively. Ltp1, encoding lipid transfer protein 1, is expressed at high levels in both the resistant and susceptible host wheat at early time points (Kosma et al. Citation2010). Resembling this response, OsLtp1 transcripts increased in Hessian fly-infested Kitaake rice seedlings at 1 and 3 DAH time points. These results indicate that Kitaake plants exhibit a molecular response, with respect to the expression of Hessian fly-responsive biomarker genes, that is intermediate between resistant and susceptible host wheat, despite resembling the phenotypic reaction of resistant host wheat. Unlike the phenotypic response, the molecular response observed in Kitaake rice is very similar to that observed in nonhost Bd (Hargarten et al. Citation2017). The accumulation of susceptibility-associated biomarker genes in nonhost plants could possibly explain the larval survival for a prolonged period of time followed by gradual death, as compared to larvae on resistant host wheat. It appears the larvae are attempting to induce susceptibility-related pathways, suppress the plant defense pathways and establish a feeding site, however, the early nonhost defense mechanisms, including insecticidal and antifeedant lectins, likely prevent the larvae from further feeding which ultimately leads to their death.
In response to insect herbivory, plants produce secondary metabolites that play an important role in defense to counter biotic stress (War et al. Citation2012; Gols Citation2014; Huang et al. Citation2015). Feeding by Hessian fly triggers increased expression of key genes involved in the biosynthesis of phenylpropanoids and lignins including genes encoding OsAdt, OsCm, OsPal, OsC4h, OsHct and OsCcr, in Kitaake rice seedlings. Peak expression of all the secondary metabolite pathway genes was observed as early as 1 DAH and gradually decreased by 5 DAH. This early increase in transcripts suggests that secondary metabolites may be involved in an early defense strategy. This defense is maintained until day 5 after egg hatch to prevent the larvae from establishing permanent feeding sites. Secondary metabolites are documented to play a significant role in wheat resistance to Hessian fly (Liu et al. Citation2007; Nemacheck et al. Citation2019). A similar increase in transcripts encoding secondary metabolites has been previously documented in nonhost Bd plant defenses against Hessian fly (Subramanyam et al. Citation2019). These results clearly indicate the role of secondary metabolites as a key defense strategy in nonhost Kitaake resistance against Hessian fly attack.
Conclusion
Genome complexity in hexaploid wheat makes functional characterization of candidate Hessian fly-responsive genes extremely challenging. Identification and characterization of less complex model genomes that show phenotypic and molecular responses resembling host wheat could serve as ideal surrogates and can be utilized for downstream functional genomics. The Kitaake cultivar has recently emerged as a model genome owing to its favorable characteristics including easy propagation, shorter life cycle, amenability to transformation, and availability of diverse genetic and genomic mutant lines. Our study revealed that Kitaake rice exhibits nonhost resistance to Hessian fly at the phenotypic and molecular level thus making it a suitable surrogate genome to functionally characterize candidate Hessian fly-responsive genes by mutation and complementation studies, and deciphering plant-Hessian fly molecular interactions. Additionally, Kitaake molecular responses revealed the significance of lectins and secondary metabolites as an early defense strategy in nonhost resistance. Although both Kitaake and Bd plants show nonhost responses, they differ in their phenotypic response to Hessian fly larval attack. Further, genetic manipulation of genes encoding lectins and enzymes in the secondary metabolite biosynthesis pathway in nonhosts like Kitaake rice and Bd plants, could reveal the mechanisms of nonhost resistance to this and other important cereal pests.
Supplemental Material
Download MS Word (17.1 KB)Acknowledgements
The authors thank Sue Cambron (USDA-ARS) for maintaining fly stocks. Mention of a commercial or a proprietary product does not constitute endorsement or recommendation for its use by the USDA.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Subhashree Subramanyam
Subhashree Subramanyam is a Research Molecular Biologist in USDA-ARS, Crop Production & Pest Control Research Unit, and an Adjunct Assistant Professor in the Department of Entomology, Purdue University, West Lafayette, IN, USA.
Jill A. Nemacheck
Jill A. Nemacheck is a Biological Science Technician in USDA-ARS, Crop Production & Pest Control Research Unit, and the Department of Entomology, Purdue University, West Lafayette, IN, USA.
References
- Berzonsky WA, Ding H, Haley SD, Harris MO, Lamb RJ, McKenzie RIH, Ohm HW, Patterson FL, Peairs FB, Porter DR, et al. 2002. Breeding wheat for resistance to insects. Plant Breed Rev. 22:221–296.
- Byers RA, Gallun RL. 1972. Ability of the Hessian fly to stunt winter wheat. 1. Effect of larval feeding on elongation of Leaves12. J Econ Entomol. 65:955–958. doi:10.1093/jee/65.4.955.
- Chen M-S, Liu X, Wang H, El-Bouhssini M. 2009. Hessian fly (Diptera: Cecidomyiidae) interactions with barley, rice, and wheat seedlings. J Econ Entomol. 102:1663–1672. doi:10.1603/029.102.0434.
- Flanders KL, Reisig DD, Buntin GD, Winslow M, HerbertJr.DA, Johnson DW. 2013. Biology and management of Hessian fly in the southeast. Alabama Cooperative Extension System, ANR1069.
- Gill US, Lee S, Mysore KS. 2015. Host versus nonhost resistance: distinct wars with similar arsenals. Phytopathol. 105:580–587. doi:10.1094/PHYTO-11-14-0298-RVW.
- Gols R. 2014. Direct and indirect chemical defences against insects in a multitrophic framework. Plant Cell Environ. 37:1741–1752. doi:10.1111/pce.12318.
- Gupta PK, Mir RR, Mohan A, Kumar J. 2008. Wheat genomics: present status and future prospects. Int J Plant Genom. 2008:1–36.
- Hargarten AM, Nemacheck JA, Subramanyam S, Xiao X, Schemerhorn BJ, Williams CE. 2017. Physical and metabolic consequences of Hessian fly infestation are more severe on nonhost Brachypodium distachyon than on host-plant resistant wheat. Arthropod Plant Interact. 11:767–783. doi:10.1007/s11829-017-9542-4.
- Harris MO, Freeman TP, Rohfritsch O, Anderson KG, Payne SA, Moore JA. 2006. Virulent Hessian fly (Diptera: Cecidomyiidae) larvae induce a nutritive tissue during compatible interactions with wheat. Ann Entomol Soc Am. 99:305–316. doi:10.1603/0013-8746(2006)099[0305:VHFDCL]2.0.CO;2.
- Heath MC. 2000. Nonhost resistance and nonspecific plant defenses. Curr Opin Plant Biol. 3:315–319. doi:10.1016/S1369-5266(00)00087-X.
- Huang X-Z, Chen J-Y, Xiao H-J, Xiao Y-T, Wu J, Wu J-X, Zhou J-J, Zhang Y-J, Guo Y-Y. 2015. Dynamic transcriptome analysis and volatile profiling of Gossypium hirsutum in response to the cotton bollworm Helicoverpa armigera. Sci Rep. 5:11867. doi:10.1038/srep11867.
- International Wheat Genome Sequencing Consortium (IWGSC). 2018. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. 361:661. doi: 10.1126/science.aar7191.
- Jain R, Jenkins J, Shu S, Chern M, Martin JA, Copetti D, Duong PQ, Pham NT, Kudrna DA, Talag J, et al. 2019. Genome sequence of the model rice variety KitaakeX. BMC Genomics. 20:905. doi:10.1186/s12864-019-6262-4.
- Javelle M, Vernoud V, Rogowsky PM, Ingram GC. 2011. Epidermis: the formation and functions of a fundamental plant tissue. New Phytol. 189:17–39. doi:10.1111/j.1469-8137.2010.03514.x.
- Joel DM, Juniper BE. 1982. Cuticular gaps in carnivorous plant glands. In: Cutler DF, Alvin KL, Price CE, editor. The plant cuticle. London: Academic Press; p. 121–130.
- Johnson AJ, Abdel Moniem HEM, Flanders KL, Buntin GD, Reay-Jones FPF, Reisig D, Stuart JJ, Subramanyam S, Shukle RH, Schemerhorn BJ. 2017. A novel, economical way to assess virulence in field populations of Hessian fly (Diptera: Cecidomyiidae) utilizing wheat resistance gene H13 as a model. J Econ Entomol. 110:1863–1868. doi:10.1093/jee/tox129.
- Jung K-H, An G, Ronald PC. 2008. Towards a better bowl of rice: assigning function to tens of thousands of rice genes. Nat Rev Genet. 9:91–101. doi:10.1038/nrg2286.
- Kosma DK, Nemacheck JA, Jenks MA, Williams CE. 2010. Changes in properties of wheat leaf cuticle during interactions with Hessian fly. Plant J. 63:31–43.
- Li C, Chen M-S, Chao S, Yu J, Bai G. 2013. Identification of a novel gene, H34, in wheat using recombinant inbred lines and single nucleotide polymorphism markers. Theor Appl Genet. 126:2065–2071. doi:10.1007/s00122-013-2118-5.
- Li G, Jain R, Chern M, Pham NT, Martin JA, Wei T, Schackwitz WS, Lipzen AM, Duong PQ, Jones KC, et al. 2017. The sequences of 1504 mutants in the model rice variety Kitaake facilitate rapid functional genomic studies. Plant Cell. 29:1218–1231. doi:10.1105/tpc.17.00154.
- Li T, Liu B, Spalding MH, Weeks DP, Yang B. 2012. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat Biotechnol. 30:390–392. doi:10.1038/nbt.2199.
- Liu X, Bai J, Huang L, Zhu L, Liu X, Weng N, Reese JC, Harris M, Stuart JJ, Chen M-S. 2007. Gene expression of different wheat genotypes during attack by virulent and avirulent Hessian fly (Mayetiola destructor) larvae. J Chem Ecol. 33:2171–2194. doi:10.1007/s10886-007-9382-2.
- Liu XM, Brown-Guedira GL, Hatchett JH, Owuoche JO, Chen M-S. 2005. Genetic characterization and molecular mapping of a Hessian fly-resistance gene transferred from T. turgidum ssp. dicoccum to common wheat. Theor Appl Genet. 111:1308–1315. doi:10.1007/s00122-005-0059-3.
- Liu X, Khajuria C, Li J, Trick HN, Huang L, Gill BS, Reeck GR, Antony G, White FF, Chen M-S. 2013. Wheat Mds-1 encodes a heat-shock protein and governs susceptibility towards the Hessian fly gall midge. Nat Commun. 4:2070. doi:10.1038/ncomms3070.
- Liu Y, Wu H, Chen H, Liu Y, He J, Kang H, Sun Z, Pan G, Wang Q, Hu J, et al. 2015. A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat Biotechnol. 33:301–305. doi:10.1038/nbt.3069.
- Nemacheck JA, Schemerhorn BJ, Scofield SR, Subramanyam S. 2019. Phenotypic and molecular characterization of Hessian fly resistance in diploid wheat, Aegilops tauschii. BMC Plant Biol. 19:439. doi:10.1186/s12870-019-2058-6.
- Pyati P, Chellamuthu A, Gatehouse AMR, Fitches E, Gatehouse JA. 2012. Insecticidal activity of wheat Hessian fly responsive proteins HFR-1 and HFR-3 towards a non-target wheat pest, cereal aphid (Sitobion avenae F.). J Insect Physiol. 58:991–999. doi:10.1016/j.jinsphys.2012.05.003.
- Saltzmann KD, Giovanini MP, Zheng C, Williams CE. 2008. Virulent Hessian fly larvae manipulate the free amino acid content of host wheat plants. J Chem Ecol. 34:1401–1410. doi:10.1007/s10886-008-9544-x.
- Sardesai N, Nemacheck JA, Subramanyam S, Williams CE. 2005. Identification and mapping of H32, a new wheat gene conferring resistance to Hessian fly. Theor Appl Genet. 111:1167–1173. doi:10.1007/s00122-005-0048-6.
- Schmid RB, Knutson A, Giles KL, McCornack BP. 2018. Hessian fly (Diptera: Cecidomyiidae) biology and management in wheat. J Integr Pest Manag. 9:1–12.
- Schönherr J. 1982. Resistance of plant surfaces to water loss: transport properties of cutin, suberin and associated lipids. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editor. Physiological plant ecology II. encyclopedia of plant physiology. Berlin: Springer; p. 153–179.
- Smiley RW, Gourlie JA, Whittaker RG, Easley SA, Kidwell KK. 2004. Economic impact of Hessian fly (Diptera: Cecidomyiidae) on spring wheat in Oregon and additive yield losses with fusarium crown rot and lesion nematode. J Econ Entomol. 97:397–408. doi:10.1093/jee/97.2.397.
- Sosa O, Gallun RL. 1973. Purification of races B and C of the Hessian Fly by genetic manipulation. Ann Entomol Soc Am. 66:1065–1070. doi:10.1093/aesa/66.5.1065.
- Stuart JJ, Chen M-S, Shukle RH, Harris MO. 2012. Gall midges (Hessian flies) as plant pathogens. Annu Rev Phytopathol. 50:339–357. doi:10.1146/annurev-phyto-072910-095255.
- Subramanyam S, Nemacheck JA, Hargarten AM, Sardesai N, Schemerhorn BJ, Williams CE. 2019. Multiple molecular defense strategies in Brachypodium distachyon surmount Hessian fly (Mayetiola destructor) larvae-induced susceptibility for plant survival. Sci Rep. 9:2596. doi:10.1038/s41598-019-39615-2.
- Subramanyam S, Nemacheck JA, Xiao X, McDonald MJ, Williams CE. 2016. Targeted discovery of single-nucleotide polymorphisms in an unmarked wheat chromosomal region containing the Hessian fly resistance gene H33. Crop Sci. 56:1106–1114. doi:10.2135/cropsci2015.10.0630.
- Subramanyam S, Sardesai N, Minocha SC, Zheng C, Shukle RH, Williams CE. 2015. Hessian fly larval feeding triggers enhanced polyamine levels in susceptible but not resistant wheat. BMC Plant Biol. 15:3. doi:10.1186/s12870-014-0396-y.
- Subramanyam S, Shreve JT, Nemacheck JA, Johnson AJ, Schemerhorn BJ, Shukle RH, Williams CE. 2018. Modulation of nonessential amino acid biosynthetic pathways in virulent Hessian fly larvae (Mayetiola destructor), feeding on susceptible host wheat (Triticum aestivum). J Insect Physiol. 105:54–63. doi:10.1016/j.jinsphys.2018.01.001.
- Subramanyam S, Smith DF, Clemens JC, Webb MA, Sardesai N, Williams CE. 2008. Functional characterization of HFR1, a high-mannose N-glycan-specific wheat lectin induced by Hessian fly larvae. Plant Physiol. 147:1412–1426. doi:10.1104/pp.108.116145.
- Subramanyam S, Zheng C, Shukle JT, Williams CE. 2013. Hessian fly larval attack triggers elevated expression of disease resistance dirigent-like protein-encoding gene, HfrDrd, in resistant wheat. Arthropod Plant Interact. 7:389–402. doi:10.1007/s11829-013-9253-4.
- War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC. 2012. Mechanisms of plant defense against insect herbivores. Plant Signaling & Behavior. 7:1306–1320. doi:10.4161/psb.21663.
- Williams CE, Nemacheck JA, Shukle JT, Subramanyam S, Saltzmann KD, Shukle RH. 2011. Induced epidermal permeability modulates resistance and susceptibility of wheat seedlings to herbivory by Hessian fly larvae. J Exp Bot. 62:4521–4531. doi:10.1093/jxb/err160.
- Xie K, Minkenberg B, Yang Y. 2015. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc Natl Acad Sci USA. 112:3570–3575. doi:10.1073/pnas.1420294112.
- Zhao L, Abdelsalam NR, Xu Y, Chen M-S, Feng Y, Kong L, Bai G. 2020. Identification of two novel Hessian fly resistance genes H35 and H36 in a hard winter wheat line SD06165. Theor Appl Genet. 133:2343–2353. doi:10.1007/s00122-020-03602-3.
- Zhou X, Liao H, Chern M, Yin J, Chen Y, Wang J, Zhu X, Chen Z, Yuan C, Zhao W, et al. 2018. Loss of function of a rice TPR-domain RNA-binding protein confers broad-spectrum disease resistance. Proc Natl Acad Sci USA. 115:3174–3179. doi:10.1073/pnas.1705927115.
- Zhu L, Liu X, Liu X, Jeannotte R, Reese JC, Harris MO, Stuart JJ, Chen M-S. 2008. Hessian fly (Mayetiola destructor) attack causes a dramatic shift in carbon and nitrogen metabolism in wheat. Mol Plant-Microbe Interact. 21:70–78. doi:10.1094/MPMI-21-1-0070.
