ABSTRACT
‘Sushuai’ is a disease-resistant apple variety. Transcriptome sequencing revealed differentially expressed genes mainly in the metabolism, biosynthesis of secondary metabolites, plant–pathogen interactions, and plant signaling transduction pathways. The jasmonate/ethylene signaling pathway and WRKY transcription factors (especially WRKY75 genes) played important roles in improving Alternaria alternata resistance in ‘Sushuai’. An investigation of the effect of biotic and abiotic stresses on the expression of MdWRKY75s revealed that MdWRKY75d and MdWRKY75e expression was upregulated by A. alternata and methyl jasmonate treatments, while their expression was downregulated by salicylic acid treatment. Transient MdWRKY75d and MdWRKY75e expression improved apple resistance to A. alternata. The analysis, prediction, and validation of the transcriptome data revealed that MdLAC7, MdRFK1, and MdWFK1 are the most likely target genes of MdWRKY75d and MdWRKY75e. Our study provides a basis for investigating mechanisms of resistance to A. alternata.
1. Introduction
In production practice, most cultivated apples are susceptible to Alternaria alternata (apple pathotype), while most wild apple germplasm resources are resistant to it. However, owing to the complex pathogenesis of A. alternata disease, little research has been conducted to elucidate its mechanisms. Most studies on the interactions between apples and A. alternata have focused on apple defenses against A. alternata, such as the discovery and functional identification of the MD-NBS (Ma et al. Citation2014) and CPK genes (Wei et al. Citation2016) for resistance to A. alternata. Recently, high-throughput sequencing technology has been available to study A. alternata resistance mechanisms. Zhang et al. (Citation2014) used re-sequencing technology to re-sequence the genomes of the disease-resistant apple variety ‘Sushuai’ and the disease-susceptible apple variety ‘Indo’; Zhu et al. (Citation2017) performed transcriptome sequencing analysis through the inoculation of the susceptible variety ‘Red Delicious’ with A. alternata; Ni et al. (Citation2017) conducted a proteomic study on the interaction between apples and A. alternata, using bidirectional electrophoresis and iTRAQ technology; and Zhang et al. (Citation2017) used whole genomic miRNA sequencing technology to analyze the miRNAs of A. alternata-infected or uninfected ‘Golden Delicious’ apples. These sequencing technologies provide the basis for a more in-depth investigation of disease-resistance-related genes. Therefore, this study mainly focuses on the functional identification of disease-resistance-related genes and regulatory networks.
Plants have evolved multiple resistance mechanisms to defend themselves against invasion by external pathogens. In particular, many members of the transcription factor family form a complex regulatory pathway for plant defense responses that perceives upstream protein kinase modulation and regulates downstream defense genes (van Verk et al. Citation2009). Among these, the WRKY transcription factor extensively regulates the expression of defense- and stress-response-related genes and plays a critical role in plant stress response (Jiang et al. Citation2017; Finatto et al. Citation2018). Numerous studies have shown that WRKY transcription factors mainly regulate the expression of downstream genes by binding to their own target genes through W-box cis-acting elements or other cis-acting elements to achieve their functions. Such studies have mainly focused on the model plants Arabidopsis, rice, and tobacco, while few have been conducted on non-model plants (with especially few on fruit trees). For example, the Arabidopsis AtWRKY18 protein has been found to regulate NPR1 gene expression by binding to the NPR1 through the W-box (Chen and Chen Citation2002). Gene function verification is usually accomplished by transgenic technology. However, some orchard tree species have low genetic efficiency compared to transgenic model plants such as Arabidopsis and tobacco. Thus, transient expression is sometimes used to verify gene functions for faster and more efficient verification; for example, apple MicroRNA397b was verified for disease resistance using transient expression (Yu et al. Citation2020).
In this study, the experimental material was a new disease-resistant apple variety ‘Sushuai’ and its parents, which were selected from a cross between ‘Indo’ as the mother and ‘Golden Delicious’ as the father at Nanjing Agricultural University. ‘Sushuai’ is extremely resistant to A. alternata, which is a distinctive feature of this variety (Zhang et al. Citation2014). A transcriptome sequencing-based study of ‘Sushuai’ apples for resistance to A. alternata revealed that MdWRKY75s expression was significantly upregulated in response to A. alternata infection, indicating that MdWRKY75s responds to apple defenses against A. alternata. The transient expression of MdWRKY75d and MdWRKY75e improved disease resistance in tobacco and apples, respectively. This study will provide a basis for further analysis of apple resistance to A. alternata.
2. Materials and methods
2.1. Plant and pathogen infection analysis
The study was conducted in the Life Science Building of the University. Potted, grafted, three-year-old apple seedlings of the cultivars ‘Golden Delicious’, ‘Sushuai’, and ‘Indo’ (rootstock: Malus × robusta) were used as test materials. Tobacco seeds (Nicotiana benthamiana) and Gala-3 (Malus domestica) seedlings were sourced from the laboratory conservatory. Botrytis cinerea and A. alternata were inoculated in potato dextrose agar medium and cultivated in a mycobacterial incubator at 25 °C for 1 week. At the beginning of May, the third and fourth leaves of the annual branches of the ‘Golden Delicious’, ‘Sushuai’, ‘Indo’, tobacco, and apple seedlings were cut for pathogen inoculation, and the inoculation was performed according to Wei et al. (Citation2016). Treated leaves were collected separately at each time point after inoculation, rapidly frozen in liquid nitrogen, and stored at −80 °C for subsequent use. Photographs of the leaves inoculated with the pathogens were taken with a camera (Nikon D5200) at each time point. ImageJ software was used to count the percentage of disease-spotted area to whole leaf area (Zhou et al. Citation2019). The experiment contained three replicates of 15 leaves each replicate.
2.2. Transcriptome sequencing analysis
Firstly, high-quality cDNA libraries were constructed from ‘Sushuai’ 24 h after inoculation with A. alternata (SSL24C) and the corresponding control (SSL24D)by referring to the test reported method (Zhu et al. Citation2017). These libraries were then sequenced using an Illumina HiSeqTM 4000 platform. The obtained clean reads were used to control the data quality by mapping the composition and quality distribution of the bases. The clean reads were compared to a ‘Golden Delicious’ apple reference genome using Burrows-Wheeler Aligner software and to ‘Golden Delicious’ apple reference genes using Bowtie software. Gene annotations for the ‘Golden Delicious’ genome and the related genes were obtained from the Phytozome V10.0 database (ftp://ftp.jgi-psf.org/pub/compgen/phytozome/v9.0/Mdomestica/assembly and ftp://ftp.jgi-psf.org/pub/compgen/phytozome/v9.0/Mdomestica/annotation). Wang et al. (Citation2009) used the distribution of reads across the genes to evaluate interrupted randomness. Exon coverage was calculated based on reads that were uniquely compared to corresponding exons, to ensure sequencing accuracy.
We calculated gene expression using the reads per kilobase per million reads algorithm (Mortazavi et al. Citation2008). Following the Poisson distribution analysis method of Audic and Claverie (Citation1997), we strictly filtered the differentially expressed genes between two samples (SSL24D and SSL24C). Gene ontology (GO) and pathway enrichment analyses were performed for the differentially expressed genes, based on the public GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases.
2.3. Biotic and abiotic stress treatments on apple leaves
The following treatments were carried out in May of the year. ‘Sushuai’, ‘Golden Delicious’, and ‘Indo’ apple leaves were inoculated with A. alternata and samples were taken after 0, 6, 12, 24, 36, 48, 60, and 72 h, respectively. ‘Sushuai’ apple leaves were also treated with 0.1 mM methyl jasmonate (MeJA), 0.1 mM salicylic acid (SA), 200 mM NaCl, low temperature (4 °C), high temperature (40 °C), trauma, and dehydration, and samples were taken after 0, 1, 3, 6, 12 and 24 h, respectively. All samples were rapidly frozen with liquid nitrogen and stored at –80 °C for later use.
2.4. MdWRKY75d and MdWRKY75e gene cloning and expression vector construction
The MdWRKY75d and MdWRKY75e gene sequences were downloaded from a website (https://phytozome-next.jgi.doe.gov/info/Mdomestica_v1_1) as templates for designing specific amplification primers (Table S5). Total RNA was extracted from young ‘Sushuai’ leaves, and the cDNA obtained by reverse transcription was used as a template for cloning the CDS sequences of MdWRKY75d and MdWRKY75e. The PCR products were collected, using a TaKaRa gel recovery kit, and ligated into a 007-Blunt (TsingKe) cloning vector. The single colonies were sequenced after DH5α transformation and kanamycin resistance screening. Specific primers (Table S5), with SacI and BamHI restriction enzymatic sites, were designed to amplify the MdWRKY75d and MdWRKY75e gene sequences from the cloning vector. The purified target sequences were recovered and inserted into the pCAMBIA1301 expression vector using a double digestion system. Finally, the constructed 35S-MdWRKY75d and 35S-MdWRKY75e vectors were verified by double digestion and sequencing.
2.5. Transient expression of MdWRKY75d and MdWRKY75e in tobacco and apples
First, pCAMBIA1301-MdWRKY75d, pCAMBIA1301-MdWRKY75e, and pCAMBIA1301 were transferred into Agrobacterium EHA105 by hot excitation. A single EHA105 colony was picked and incubated with shaking until the optical density at 600 nm (OD600) was about 1.0. It was then diluted with resuspension fluid until the OD600 was about 0.8. The tobacco and apple leaves were transiently expressed by Agrobacterium-mediated transformation. The tobacco transient transformation was performed by pressure injection and the apple transient transformation was performed using the vacuum method (Yu et al. Citation2020). the reliability of the transient transformation system was demonstrated by GUS staining, qRT-PCR, and the amplification of MdWRKY75d and MdWRKY75e after transient transformation in tobacco and apple, which can be used to verify gene function. Consistent and well-grown leaves were collected from well-cultured wild-type, empty vector, and MdWRKY75d and MdWRKY75e gene vector tobacco and apple, for B. cinerea and A. alternata infection. Photographs and samples were collected for analysis and further experimentation at specific time points. GUS staining was conducted as described previously (Guo et al. Citation2017).
2.6. Prediction and analysis of MdWRKY target genes
It is well known that WRKY genes regulate the expression of target genes by binding to their target gene promoter sequences with W-box cis-acting elements, which in turn regulate downstream genes. Based on existing transcriptome sequencing data, we selected differentially expressed upregulated genes (|log2Ratio| ≥ 4) and downloaded their 2000-bp promoter sequences upstream of the ATG from the National Center for Biotechnology Information (NCBI). The promoter sequences, including the cis-acting elements and transcription start sites, were analyzed using the websites PLACE (http://www.dna.affrc.go.jp/htdocs/PLACE/) and Plant-CARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). We then identified the gene promoter sequences containing W-box cis-acting elements as candidate MdWRKY target genes for further analysis and investigation.
2.7. qRT-PCR
Total RNA extraction from the leaves was carried out according to the RNA extraction kit instructions (Fuji, China). The cDNA was synthesized according to the Takara Reverse Transcription Kit instructions. The qRT-PCR primers (Table S4) were designed using Beacon Designer v 7.0, based on the gene sequences published in the Apple Genome Database. All primers were prepared using NCBI primer-BLAST (https://www.ncbi.nlm.nih.gov /tools/primer-blast). Experiments were performed using an ABI 7500 real-time fluorescence quantitative PCR system (Applied Biosystems). qRT-PCR was conducted with SYBR-Green PCR kit (TakaRa, Tokyo, Japan) according to the manufacturer’s instructions. Tubulin was used as the reference gene for M. domestica and tobacco to normalize gene expression levels. Each reaction was repeated four times and its Ct value was averaged across three biological replicates. The data obtained were analyzed using the 2−ΔΔCt method (Livak and Schmittgen Citation2011).
2.8. Measurement of enzyme activity and MDA content
The enzyme activity of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), polyphenol oxidase (PPO), and phenylalanine aminolysis enzyme (PAL), and the malondialdehyde (MDA) content, were determined according to Huang et al. (Citation2016).
2.9. Statistical analysis
The experiments were repeated at least three times for each stress treatment. All data were analyzed by one-way ANOVA in SPSS, and data pairs were analyzed using Tukey's test. * P < 0.05, ** P < 0.01, and *** P < 0.001 were considered significant.
3. Results
3.1. Assessment of disease resistance in the leaves of different apple varieties after infection with A. alternata
There were significant differences in the disease incidence of ‘Sushuai’, ‘Golden Delicious’, and ‘Indo’ apple leaves inoculated with A. alternata. The results are shown in Figures S1–S3, the phenotypes and disease indices of the inoculated leaves were statistically analyzed. The disease incidence in ‘Sushuai ‘ leaves was significantly lower than that in ‘Golden Delicious’ and ‘Indo’ leaves. The SOD, POD, CAT, PPO, and PAL enzyme activities and the MDA content were also measured in the inoclulated ‘Sushuai’, ‘Golden Delicious’, and ‘Indo’ apple leaves. There was no significant change in SOD activity in the ‘Sushuai’ apple leaves over time, compared to that in the ‘Golden Delicious’ and ‘Indo’ apple leaves. The POD, CAT, PPO, and PAL enzyme activities, and the MDA content, of the ‘Sushuai’, ‘Golden Delicious’, and ‘Indo’ apple leaves showed a trend of increasing and then decreasing over the infection period. The POD, CAT, PPO, and PAL enzyme activities in the ‘Sushuai’ leaves were higher than those in the ‘Golden Delicious’ and ‘Indo’ leaves. The MDA content of the ‘Sushuai’ leaves was lower than that of the ‘Golden Delicious’ and ‘Indo’ leaves. We further the analyzed MdSOD, MdPOD, MdCAT, MdPPO, and MdPAL expression in ‘Sushuai’ and ‘Indo’ apple leaves inoculated with A. alternata. The results show that MdSOD, MdPOD, MdCAT, MdPPO, and MdPAL expression in the ‘Sushuai’ and ‘Indo’ apple leaves increased and then decreased with the increasing infection time, peaking at 24 h (MdCAT and MdPPO) and 36 h (MdSOD, MdPOD, and MdPAL). The peak expression of ‘Sushuai’ was significantly higher than that of ‘Indo’. In summary, the results show that ‘Sushuai’ was the most disease-resistant variety, and ‘Indo’ the most susceptible.
3.2. Transcriptome sequencing data analysis results
We obtained two libraries, SSL24D and SSL24C, from high-throughput RNA sequencing of ‘Sushuai’ apple leaves 24 h after being inoculated with A. alternata and the corresponding control, respectively. The clean reads were compared with apple reference genes and a reference genome, and the results are shown in Table S1. Furthermore, we conducted statistical analysis on the distribution, uniformity, exon coverage, base composition balance, and base quality exon coverage of the clean reads. The results show that the sequencing quality was reliable (Figure S4–S5).
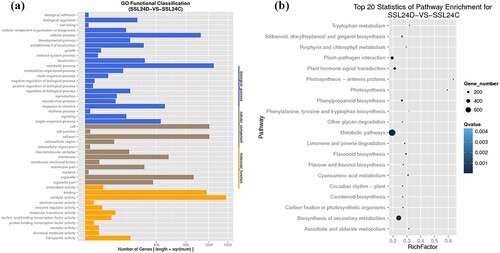
The number and expression level of the differentially expressed genes were obtained by comparing the two libraries (SSL24D: control, SSL24C: treatment) (). There were 3909 differentially expressed genes in SSL24C, of which 1715 were upregulated and 2194 were downregulated (Figure S6). There was no significant difference between the number of upregulated and downregulated genes. The differential genes were mainly concentrated in the cellular, metabolic, and catalytic activity processes in the GO enrichment analysis. Moreover, they were mainly concentrated in the metabolism pathways, biosynthesis of secondary metabolites, plant–pathogen interactions, and plant hormone signaling transduction pathways in the KEGG pathway analysis, accounting for 74.04% of the differentially expressed genes, and most of these were upregulated.
Figure 1. Gene ontology functional classification (a) and pathway-enrichment analysis (b) of differentially expressed genes in ‘Sushuai’ apple leaves.
As shown in Table S2, the five disease-related protein genes, two PR1, PR4, and two PR17, were detected among the differentially expressed genes, and some resistance genes (NBS-LRR, chitinase genes, β-1,3-glucanase, and Mald1), including all the differential Mald1 genes, were upregulated. Some genes related to calcium signaling, MAPK protein kinases, and cell wall receptor kinases were also upregulated. The differentially expressed genes involved in ethylene (ET) signaling (e.g. ERF, EIN, and ETR) were upregulated and most of the differentially expressed genes in jasmonic acid (JA) signaling (e.g. JAZ and MYC2), SA signaling (NPR1 and TGA), and abscisic acid signaling were upregulated. There were 29 differentially expressed WRKY transcription factors (28 upregulated and one downregulated), 17 differentially expressed NAC genes (15 upregulated and two downregulated), and 26 differentially expressed MYB genes (14 upregulated and 12 downregulated) detected; among them, the WRKY75 genes MDP0000154734 and MDP0000792088 were significantly induced. In conclusion, most of the disease-resistance-related differentially expressed genes were upregulated, and these genes may play an important role in A. alternata resistance in ‘Sushuai’ apples, providing a basis for the future research.
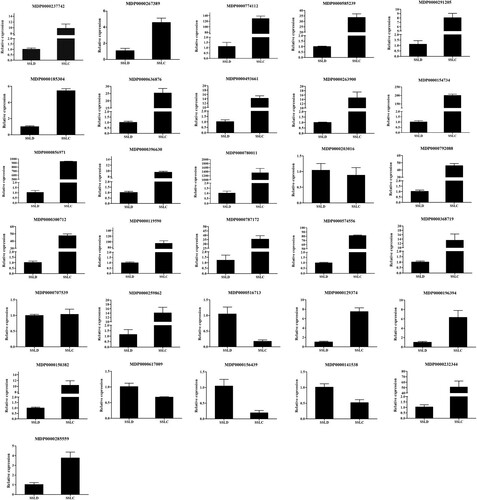
3.3. qRT-PCR validation of differentially expressed genes
To verify the accuracy of the transcriptome results and to select key genes associated with disease resistance, we used qRT-PCR to validate the differentially expressed genes from the RNA-seq data. The qRT-PCR results are shown in . Consistent expression trends between the qRT-PCR data and the RNA-seq data were found for 30 of the 31 selected differential expressed genes (concordance rate: 96.7%). Even among the genes with consistent expression trends, the increases and decreases in expression were not identical. These differences in expression may be the result of the different sensitivities of the two technologies. The functional annotations and expression statistics of these differentially expressed genes are shown in Table S2. To further verify the functions of these differentially expressed genes, those with > 10-fold differences were selected from the qRT-PCR results, and gene expression analysis was performed on samples collected 12, 36, 48, 60, and 72 h after inoculation. The results are shown in Figure S7. The upregulated genes showed a trend of increasing and then decreasing during the infection period and reached peak expression at 24 h. In contrast, the downregulated gene expression was consistently lower than that at 0 h. These results further demonstrate the accuracy of the sequencing data and provide a basis for future studies.
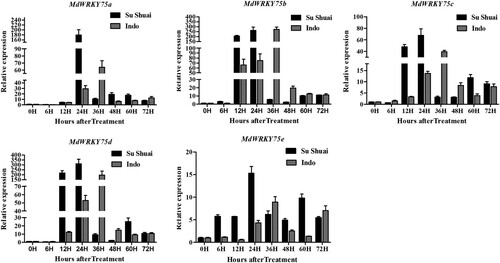
3.4. MdWRKY75s expression in ‘Sushuai’ and ‘Indo’ apples after different treatments
The ‘Sushuai’ transcriptome sequencing data revealed that the MdWRKY75s genes may play an important role in apple resistance to A. alternata. In our study, MDP0000123888, MDP0000142583, MDP0000792088, MDP0000154734, and MDP0000263768 are referred to as MdWRKY75a, MdWRKY75b, MdWRKY75c, MdWRKY75d and MdWRKY75e. They are all genes belonging to the IIc subfamily (Dong et al. Citation2003; Lui et al. Citation2017). To further verify the function of the MdWRKY75s gene, we inoculated the disease-resistant variety ‘Sushuai’ and the disease-susceptible variety ‘Indo’ with A. alternata, and then treated the ‘Sushuai’ leaves with abiotic stress treatments (SA, MeJA, NaCl, high temperature, low temperature, and trauma). The results are shown in . MdWRKY75s genes were significantly upregulated in the ‘Sushuai’ and ‘Indo’ apple leaves inoculated with A. alternata. However, ‘Sushuai’ MdWRKY75s gene expression peaked at 24 h, while ‘Indo’ MdWRKY75s gene expression peaked at 36 h. The peak expression values of MdWRKY75a, c, d, and e were higher in the ‘Sushuai’ leaves than in the ‘Indo’ leaves (2.8, 1.71, 1.72, and 1.58-fold, respectively). The peak expression of MdWRKY75b in the ‘Sushuai’ leaves was similar to that in the ‘Indo’ leaves (only 1.04-fold higher). We speculate that, as MdWRKY75s expression in the ‘Sushuai’ apple leaves was higher than that in the ‘Indo’ apple leaves, the response to A. alternata injury was faster, and the downstream expression of disease resistance genes was stimulated earlier. Thus, the ‘Sushuai’ variety showed higher disease resistance.
As shown in Figures S10–S16, MdWRKY75s was significantly upregulated by the MeJA treatment. MdWRKY75s was significantly downregulated by the SA and high temperature (40 °C) treatments and MdWRKY75a, b, c, and d were significantly downregulated by the low temperature (4 °C) treatment. However, MdWRKY75e expression was significantly upregulated by the low temperature (4 °C) treatment. MdWRKY75b, d, and e were significantly upregulated by the NaCl treatment, while MdWRKY75a and c were significantly downregulated. MdWRKY75a, b, c, and d were significantly downregulated in response to the trauma treatment, while MdWRKY75e was significantly upregulated. MdWRKY75a and c were significantly downregulated in response to the dehydration treatment, while MdWRKY75b, d, and e were significantly upregulated. These findings reveal that both MdWRKY75d and MdWRKY75e are upregulated by A. alternata, MeJA, NaCl, and dehydration, and MdWRKY75e is also upregulated by low temperatures and trauma. In addition, MdWRKY75d and MdWRKY75e are downregulated by SA. This indicates that the same WRKY gene can respond variably to different stresses and plays multiple stress-response roles, as shown in Figures S8–S9 and Tables S6–S7. Further analysis of the promoter sequences of MdWRKY75d and MdWRKY75e showed that they have cis-acting elements that respond to SA, JA, and low temperatures. MdWRKY75d has specific cis-acting elements that respond to drought and growth hormones, and MdWRKY75e has specific cis-acting elements that respond to injury and stress, and gibberellins. Comparison with the qRT-PCR results showed that MdWRKY75d and MdWRKY75e were downregulated by SA treatment, and it was hypothesized that other regulatory mechanisms might be involved in this response. Meanwhile, MdWRKY75d and MdWRKY75e were functionally specific. Therefore, the MdWRKY75d and MdWRKY75e genes will be further investigated as candidate genes.
3.5. Transient expression of MdWRKY75d and MdWRKY75e improved B. cinerea resistance in tobacco
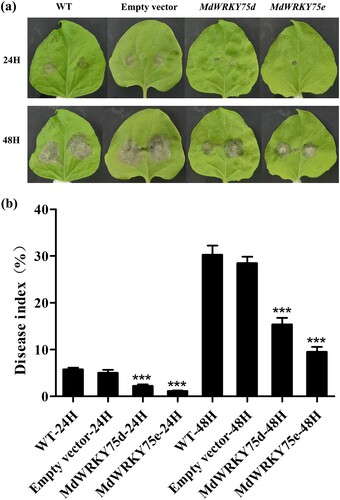
To further validate the functions of MdWRKY75d and MdWRKY75e, an Agrobacterium-mediated method was used to induce transient MdWRKY75d and MdWRKY75e expression in tobacco. First, the reliability of the transient transformation system was demonstrated by GUS staining, qRT-PCR, and the amplification of MdWRKY75d and MdWRKY75e after transient transformation in tobacco, which can be used to verify gene function (Figure S17). As shown in , the phenotypic analysis and disease index statistics revealed that the transient transformations of the pCAMBIA1301-35SN-MdWRKY75d and pCAMBIA1301-35SN-MdWRKY75e vectors showed lower B. cinerea disease frequencies than the wild-type and empty vectors at 24 and 48 h.
Figure 4. Transient expression of MdWRKY75d and MdWRKY75e enhances B. cinerea infection resistance in tobacco. (a) Time-course of B. cinerea infection phenotypes in WT, empty vector, MdWRKY75d, and MdWRKY75e leaves over 48 h. (b) Time-course of B. cinerea infection disease index in WT, empty vector, MdWRKY75d, and MdWRKY75e leaves over 48 h. WT: wild tobacco; empty vector: pCAMBIA1301-35SN plasmid; MdWRKY75d: pCAMBIA1301-35SN-MdWRKY75d plasmid; MdWRKY75e: pCAMBIA1301-35SN-MdWRKY75e plasmid.
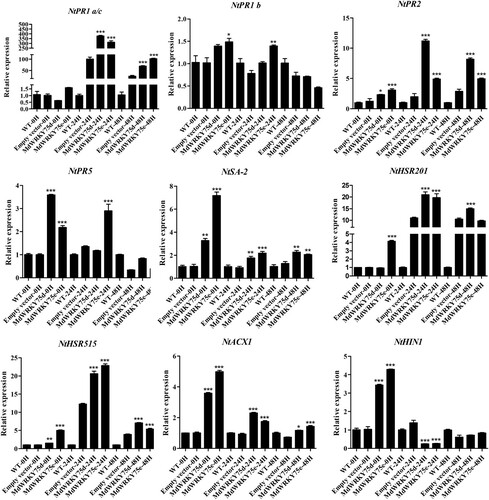
To further investigate the regulatory mechanism of the transient expression of MdWRKY75d and MdWRKY75e in improving resistance to B. cinerea in tobacco, we examined the expression patterns of some genes associated with disease resistance using qRT-PCR. As shown in , in infected tobacco leaves expressing transient MdWRKY75d and MdWRKY75e (0, 24, and 48 h after infection), NtPR1a/c, NtPR2, NtSA-2, NtHSR515, and NtACX1 expression was significantly higher than that in the wild-type and empty-vector transformed tobacco leaves. In tobacco leaves expressing transient MdWRKY75d, NtPR5 expression was significantly higher than in the wild-type and empty-vector transformed leaves 0 h after infection. Similarly, NtHSR201 expression was significantly higher 24 h and 48 h after infection. In tobacco leaves expressing transient MdWRKY75e, NtPR1b, NtPR5, and NtHSR201 expression was significantly higher than in the wild-type and empty-vector transformed leaves 0 and 24 h after infection. In tobacco leaves expressing transient MdWRKY75d and MdWRKY75e, NtHIN1 expression was significantly higher than that in the wild-type and empty-vector transformed leaves 0 and 24 h after infection. In conclusion, the transient expression of MdWRKY75d and MdWRKY75e improved B. cinerea resistance in tobacco, probably by regulating the expression of downstream disease-resistance-related genes.
3.6. Transient expression of MdWRKY75d and MdWRKY75e improved A. alternata resistance in apples
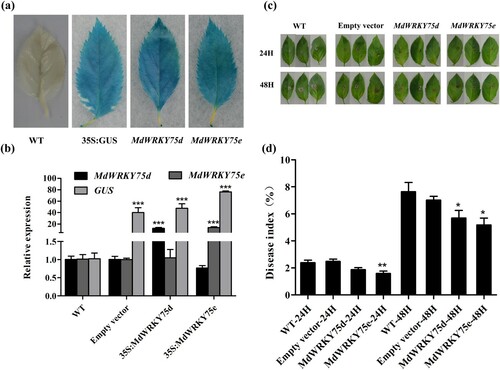
To further verify the functions of MdWRKY75d and MdWRKY75e, they were transiently expressed in ‘Gala-3’ apple leaves using an Agrobacterium-mediated method. First, the reliability of the apple transient transformation system was demonstrated by GUS staining and qRT-PCR ( a,b). As shown in (c,d), the phenotype analysis and disease index statistics revealed that the transient transformations of the pCAMBIA1301-35SN-MdWRKY75d and pCAMBIA1301-35SN-MdWRKY75e vectors showed less disease than the wild-type and empty vector apple leaves 24 and 48 h after A. alternata infection.
Figure 6. Verification of the transient expression of MdWRKY75d and MdWRKY75e enhancing A. alternata infection resistance in apples. (a) CUS staining of apple leaves transiently transformed with the empty vector, and the MdWRKY75d and MdWRKY75e expression vectors. (b) qRT-PCR for transient expression of MdWRKY75d and MdWRKY75e in apples. (c) Time-course of A. alternata infection phenotypes in WT, empty vector, MdWRKY75d, and MdWRKY75e apple leaves over 48 h. (d) Time-course of A. alternata infection disease index in WT, empty vector, MdWRKY75d, and MdWRKY75e apple leaves over 48 h. WT: wild GL-3; empty vector: pCAMBIA1301-35SN plasmid; MdWRKY75d: pCAMBIA1301-35SN-MdWRKY75d plasmid; MdWRKY75e: pCAMBIA1301-35SN-MdWRKY75e plasmid.
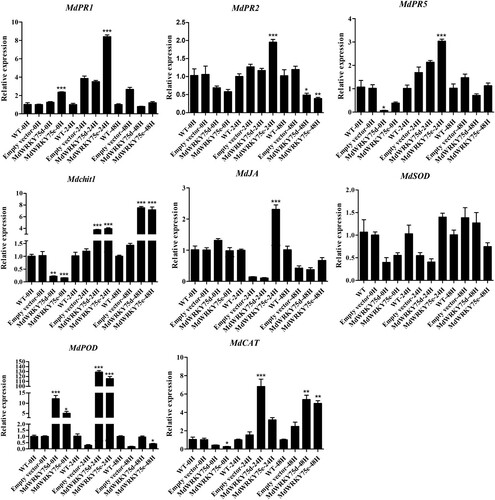
To further investigate the regulatory mechanisms of the transient expression of MdWRKY75d and MdWRKY75e in improving A. alternata resistance in apples, we examined the expression patterns of some genes related to disease resistance using qRT-PCR, as shown in . In apple leaves expressing transient MdWRKY75d and MdWRKY75e, MdPOD expression was significantly higher than that in the wild-type and empty-vector transformed leaves 24 h after infection. Similarly, Mdchit1 expression was significantly higher 48 h after infection. However, MdSOD expression was not significantly different. In apple leaves expressing transient MdWRKY75d, MdCAT expression was significantly higher than that in the wild-type and empty-vector transformed apple leaves 24 and 48 h after infection. In apple leaves expressing transient MdWRKY75e, MdPR1 expression was significantly higher than that in the wild-type and empty-vector transformed leaves 0 h after infection, while MdCAT expression was significantly lower. MdPR1, MdPR2, MdPR5, and MdJA expression was significantly higher than that in the wild-type and empty-vector transformed apple leaves 24 h after infection, and MdPR2 expression was significantly lower than that in the wild-type and empty-vector transformed apple leaves 48 h after infection. In contrast, MdCAT expression was significantly higher than that in the wild-type and empty-vector transformed apple leaves 48 h after infection. In conclusion, the transient expression of MdWRKY75d and MdWRKY75e improved A. alternata resistance in apples, probably by regulating the expression of downstream disease-resistance-related genes.
3.7. Prediction study of MdWRKY target genes based on transcriptome sequencing data
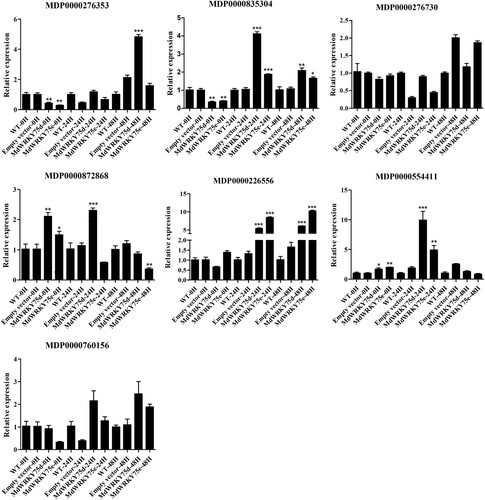
Based on the analysis of the transcriptome sequencing data, we obtained 301 differentially upregulated genes with |log2Ratio| ≥ 4, and 49 genes whose promoter sequences contained W-box elements (Table S8). The following seven predicted MdWRKY target genes (MDP0000276353, MDP0000835304, MDP0000276730, MDP0000872868, MDP0000226556, MDP0000554411, MDP0000760156) were screened for preliminary validation based on their functions and research progress (Table S9). As shown in Figures S18–S20, the predicted MdWRKY target genes were significantly upregulated by the A. alternata and MeJA treatments in ‘Sushuai’ apple leaves. The predicted MdWRKY target genes were also significantly upregulated by the SA treatment, except for MDP0000276730. However, their peak expression values under MeJA treatment were higher than their peak expression values under SA treatment. The results show that the screened MdWRKY target genes responded to the A. alternata, SA, and MeJA treatments through upregulated expression.
To further investigate the regulatory mechanisms of the transient expression of MdWRKY75d and MdWRKY75e in improving apple resistance to A. alternata, we examined the expression of the abovementioned predicted MdWRKY target genes using qRT-PCR. The results are shown in . In apple leaves expressing transient MdWRKY75d and MdWRKY75e, MDP0000835304 and MDP0000226556 expression was significantly higher than that in the wild-type and empty-vector transformed apple leaves 24 and 48 h after infection, and MDP0000554411 expression was significantly higher 0 and 24 h after infection. In apple leaves expressing transient MdWRKY75d, MDP0000872868 expression was significantly higher than that in the wild-type and empty-vector transformed apple leaves 0 and 24 h after infection. In apple leaves expressing transient MdWRKY75e, MDP0000872868 expression was significantly higher than that in the wild-type and empty-vector transformed apple leaves 0 h after infection, and MDP0000276353 expression was significantly higher 48 h after infection. Expression analysis revealed that MDP0000226556 (MdLAC7), MDP0000835304 (MdRFK1), and MDP0000554411 (MdWFK1) are the most likely potential target genes for apple MdWRKY75d and MdWRKY75e.
4. Discussion
Transcription factor gene families, such as WRKY (Rushton et al. Citation2010), NAC (Nuruzzaman et al. Citation2015), and MYB (Vimolmangkang et al. Citation2013), play important roles in disease resistance in plants. WRKY transcription factors are particularly important for plant disease resistance. In this study, 29 differentially expressed WRKY genes (28 upregulated and one downregulated), 17 differentially expressed NAC genes (15 upregulated and two downregulated), and 26 differentially expressed MYB genes (14 upregulated and 12 downregulated) were detected. For example, the expression of the WRKY75 genes MDP0000154734 and MDP0000792088 was significantly induced. Four members of the IIc subfamily, AtWRKY8, AtWRKY28, AtWRKY57, and AtWRKY75, were found to be involved in osmotic stress in Arabidopsis; therefore, IIc genes may be key regulators in drought and salt stress responses (Chen et al. Citation2018). In addition to the abovementioned functions, the Arabidopsis AtWRKY75 gene enhances disease resistance, inhibits lateral root growth and root hair number, participates in phosphorus stress signaling, and suppresses leaf senescence in Arabidopsis, indicating that AtWRKY75 is extensively involved in plant stress responses and growth and development processes (Johnson et al. Citation2002; Devaiah et al. Citation2007; Li et al. Citation2012; Chen et al. Citation2013). In the study, we found that MdWRKY75d and MdWRKY75e expression was upregulated by A. alternata, MeJA, NaCl, and dehydration, and MdWRKY75e expression was also upregulated by low temperatures and trauma. Moreover MdWRKY75d and MdWRKY75e expression was downregulated by SA. We analyzed the promoter sequences of MdWRKY75d and MdWRKY75e and found that they contained SA- and JA-responsive cis-acting elements; however, they were downregulated by SA treatment, and presumably regulated by other mechanisms. Meanwhile, MdWRKY75d contained cis-acting elements specific to drought and growth hormone, and MdWRKY75e contained cis-acting elements specific to injury and stress, and gibberellin. This suggests that MdWRKY75d and MdWRKY75e are functionally specific.
WRKY transcription factors are widely involved in various biotic, abiotic, and hormonal signaling pathways, and in plant growth and development, which in turn regulate the molecular mechanisms of plant stress responses (Jiang et al. Citation2017; Finatto et al. Citation2018). Furthermore, the same transcription factor can multiply in response to different stressors. For example, Arabidopsis AtWRKY70 responds to both the SA and JA pathways to regulate plant defensive responses, but plays opposite roles in these two processes (Li et al. Citation2004). The grape VpWRKY2 gene is upregulated under SA, MeJA, ethephon, and pathogen treatments, indicating that it responds to all of these stressors (Li et al. Citation2010). In this study, all the differential genes involved in ET signaling (e.g. ERF, EIN, and ETR) and most of the differential genes involved in JA (e.g. JAZ and MYC2) and SA signaling (NPR1 and TGA) were upregulated by A. alternata in ‘Sushuai’ apple leaves. We found that MdWRKY75d and MdWRKY75e expression was upregulated by MeJA treatment, while it was downregulated by SA treatment. Therefore, we hypothesize that MdWRKY75d and MdWRKY75e are positively regulated by the JA pathway and negatively regulated by the SA pathway during plant response to pathogen infection.
Various phytohormones, such as JA and SA, enhance plant defense responses to biotic and abiotic stresses (De Vleesschauwer et al. Citation2013; Ruan et al. Citation2019; Zhang and Li Citation2019). It is commonly believed that SA mediates defense signals against biotrophic and hemibiotrophic pathogens, while JA and ET are associated with defense responses against necrotrophic pathogens (Glazebrook Citation2005). Both the SA and JA pathways are activated by pathogen infection, leading to the accumulation of endogenous SA and JA. These act as signaling molecules to regulate the transcriptional response of pathogenesis-related genes (van Loon et al. Citation2006; Robert-Seilaniantz et al. Citation2011). In this study, five disease-related genes, two PR1, PR4, and two PR17, were detected. Some disease resistance genes (NBS-LRR, Chitinase genes, β-1,3-glucanase, and Mald1), including all the differential Mald1 genes, were upregulated. Transiently expressing MdWRKY75d and MdWRKY75e improved resistance to B. cinerea and A. alternata in tobacco and apple leaves, respectively. In tobacco and apple leaves expressing transient MdWRKY75d, the NtPR1a/c, NtPR2, NtSA-2, NtHSR201, NtHSR515, NtACX1, Mdchit1, MdPOD, and MdCAT expression levels were significantly higher than those in the wild-type and empty-vector transformed leaves. In tobacco and apple leaves expressing transient MdWRKY75e, the NtPR1a/c, NtPR1b, NtPR2, NtPR5, NtSA-2, NtHSR201, NtHSR515, NtACX1, MdPR1, MdPR2, MdPR5, MdJA, Mdchit1, MdPOD, and MdCAT expression levels were significantly higher than those in the wild-type and empty-vector transformed leaves. Previous studies have identified PR1, PR2, PR5, and SA-2 as disease resistance marker genes associated with the SA pathway; chit1, PR1b, ACX1, and JA as disease resistance marker genes associated with the JA pathway; and HSR201, HSR515, and HIN1 as marker genes associated with hypersensitive response (Loake and Grant Citation2007; Liu et al. Citation2019). The abovementioned disease-resistance-related gene expression indicates that the transient expression of MdWRKY75d and MdWRKY75e in tobacco and apple leaves inoculated with pathogens activated the SA and JA disease resistance pathways, which together regulated the expression of downstream disease-resistance-related genes, enhancing plant disease resistance.
At the transcriptional level, WRKY transcription factors can regulate the expression of target genes by specifically binding to the W-box (T)TGACC (A/T) elements in the promoters of their target genes (Rushton et al. Citation2010). For example, MdWRKY9 promoted dwarfing of M26 through W-box binding to MdDWF4 and MdWRKY79 improved disease resistance through W-box binding to MdNLR16 in apples (Zheng et al. Citation2018; Meng et al. Citation2018). The transcriptome data and expression analysis of the predicted WRKY target genes indicate that MDP0000226556 (MdLAC7), MDP0000835304 (MdRFK1), and MDP0000554411 (MdWFK1) are the most likely potential target genes of MdWRKY75d and MdWRKY75e in apples, and their expression was significantly upregulated under the A. alternata, MeJA, and SA treatments. Taken together, the target genes of MdWRKY75d and MdWRKY75e may be cooperatively regulated by both the SA and JA pathways to improve disease resistance in plants.
5. Conclusion
In summary, analysis of transcriptome sequencing data from the disease-resistant apple variety ‘Sushuai’ revealed that the JA/ET signaling pathway and the WRKY75 gene play important roles in improving resistance to A. alternata in ‘Sushuai’ apple leaves. Transient MdWRKY75d and MdWRKY75e expression was induced by the A. alternata and MeJA treatments, while MdWRKY75d and MdWRKY75e were downregulated by the SA treatment. Transient MdWRKY75d and MdWRKY75e expression may regulate downstream disease resistance genes associated with the SA and JA pathways, and their target genes, to improve resistance to B. cinerea and A. alternata in tobacco and apple leaves, respectively. Our study provides a basis for exploring mechanisms of resistance to A. alternata.
Additional information
Supporting Information is available for this article.
Acknowledgements
We thank Dr. Zhihong Zhang, Shenyang Agricultural University, for providing tissue-cultured GL-3 plants, Yunzhong Guo, Northwest A&F University, for providing the A. alternata.
Data availability
The data used to support the findings of this study are available from the corresponding author on reasonable request. All sequence data were deposited in GenBank under SRA accession number PRJNA749394.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author’s contribution
Shenchun Qu designed the research. Yingjun Hou performed experiments. Shenchun Qu and Yingjun Hou wrote the manuscript. Xinyi Yu and Weiping Chen provided technical support. Lifang Cao, Xiaoyue Geng and Chao Sun helped to analyze data.
Additional information
Funding
References
- Audic S, Claverie J. 1997. M. The significance of digital gene expression profiles. Genome Res. 7(10):986–995.
- Chen C, Chen Z. 2002. Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol. 129:706–716.
- Chen F, Hu Y, Vannozzi A, Wu K, Cai H, Qin Y, Mullis A, Lin Z, Zhang L. 2018. The WRKY transcription factor family in model plants and crops. Crit Rev Plant Sci. 36:311–335.
- Chen X, Liu J, Lin G, Wang A, Wang Z, Lu G. 2013. Overexpression of AtWRKY28 and AtWRKY75 in Arabidopsis enhances resistance to oxalic acid and Sclerotinia sclerotiorum. Plant Cell Rep. 32:1589–1599.
- Devaiah BN, Karthikeyan AS, Raghothama KG. 2007. WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol. 143:1789–1801.
- De Vleesschauwer D, Gheysen G, Hofte M. 2013. Hormone defense networking in rice: tales from a different world. Trends Plant Sci. 18:555–565.
- Dong J, Chen C, Chen Z. 2003. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol. 51:21–37.
- Finatto T, Viana VE, Woyann LG, Busanello C, Maia LCD, Oliveira AC. 2018. Can WRKY transcription factors help plants to overcome environmental challenges? Genet Mol Biol. 41:533–544.
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 43:205–227.
- Guo P, Li Z, Huang P, Li B, Fang S, Chu J, Guo H. 2017. A tripartite amplification loop involving the transcription factor WRKY75, Salicylic acid, and reactive oxygen species accelerates leaf senescence. Plant Cell. 11(29):2854–2870.
- Huang K, Zhong Y, Li Y, Zheng D, Cheng ZM. 2016. Genome-wide identification and expression analysis of the apple ASR gene family in response to Alternaria alternata f. sp. Mali. Genome. 59:866–878.
- Jiang J, Ma S, Ye N, Jiang M, Cao J, Zhang J. 2017. WRKY transcription factors in plant responses to stresses. J Integr Plant Biol. 59:86–101.
- Johnson CS, Kolevski B, Smyth DR. 2002. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell. 14:1359–1375.
- Kuusik I, Tarkanovskaja M, Kruusma J, Kisand V, Tõnisoo A, Lust E, Nõmmiste E. 2016. Valence band photoelectron spectra of [EMIM][BF4 ] ionic liquid vapor: evidences of electronic relaxation. J Mol Liq. 223:939–942.
- Li H, Xu Y, Xiao Y, Zhu Z, Xie X, Zhao H, Wang Y. 2010. Expression and functional analysis of two genes encoding transcription factors, VpWRKY1 and VpWRKY2, isolated from Chinese wild Vitis pseudoreticulata. Planta. 232:1325–1337.
- Li J, Brader G, Palva ET. 2004. The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell. 16:319–331.
- Li Z, Peng J, Wen X, Guo H. 2012. Gene network analysis and functional studies of senescence-associated genes reveal novel regulators of Arabidopsis leaf senescence. J Integr Plant Biol. 54:526–539.
- Liu Y, Liu Q, Tang Y, Ding W. 2019. NtPR1a regulates resistance to Ralstonia solanacearum in Nicotiana tabacum via activating the defense-related genes. Biochem Biophys Res Commun. 508:940–945.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408.
- Loake G, Grant M. 2007. Salicylic acid in plant defence–the players and protagonists. Curr Opin Plant Biol. 10:466–472.
- Lui S, Luo C, Zhu L, Sha R, Qu S, Cai B, Wang S. 2017. Identification and expression analysis of WRKY transcription factor genes in response to fungal pathogen and hormone treatments in apple (Malus domestica). J Plant Biol. 60:215–230.
- Ma C, Lu Y, Bai S, Zhang W, Duan X, Meng D, Wang Z, Wang A, Zhou Z, Li T. 2014. Cloning and characterization of miRNAs and their targets, including a novel miRNA-targeted NBS-LRR protein class gene in apple (Golden Delicious). Mol Plant. 7:218–230.
- Meng D, Li C, Park HJ, Gonzalez J, Wang J, Dandekar AM, Turgeon BG, Cheng L. 2018. Sorbitol modulates resistance to Alternaria alternata by regulating the expression of an NLR resistance gene in apple. Plant Cell. 30:1562–1581.
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 5:621–628.
- Ni W, Zhu L, Sha R, Tao J, Cai B, Wang S. 2017. Comparative iTRAQ proteomic profiling of susceptible and resistant apple cultivars infected by Alternaria alternata apple pathotype. Tree Genet Genomes. 13:23.
- Nuruzzaman M, Sharoni AM, Satoh K, Karim MR, Harikrishna JA, Shimizu T, Sasaya T, Omura T, Haque MA, Hasan SM, et al. 2015. NAC transcription factor family genes are differentially expressed in rice during infections with Rice dwarf virus, Rice black-streaked dwarf virus, Rice grassy stunt virus, Rice ragged stunt virus, and Rice transitory yellowing virus. Front Plant Sci. 6:676.
- Robert-Seilaniantz A, Grant M, Jones JD. 2011. Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol. 49:317–343.
- Ruan J, Zhou Y, Zhou M, Yan J, Khurshid M, Weng W, Cheng J, Zhang K. 2019. Jasmonic acid signaling pathway in plants. Int J Mol Sci. 20:2479.
- Rushton PJ, Somssich IE, Ringler P, Shen QJ. 2010. WRKY transcription factors. Trends Plant Sci. 15:247–258.
- van Loon LC, Rep M, Pieterse CM. 2006. Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol. 44:135–162.
- van Verk MC, Gatz C, Linthorst HJM. 2009. Transcriptional regulation of plant defense responses. Adv Bot Res. 51:397–438.
- Vimolmangkang S, Han Y, Wei G, Korban SS. 2013. An apple MYB transcription factor, MdMYB3, is involved in regulation of anthocyanin biosynthesis and flower development. BMC Plant Biol. 13(1):1–13.
- Wang Z, Gerstein M, Snyder M. 2009. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 10(1):57–63.
- Wei M, Wang S, Dong H, Cai B, Tao J. 2016. Characterization and comparison of the CPK gene family in the apple (Malus x domestica) and other rosaceae species and its response to Alternaria alternata infection. PLoS One. 11:e0155590.
- Yu X, Gong H, Cao L, Hou Y, Qu S. 2020. MicroRNA397b negatively regulates resistance of Malus hupehensis to Botryosphaeria dothidea by modulating MhLAC7 involved in lignin biosynthesis. Plant Sci. 3(292):110390.
- Zhang Q, Li Y, Zhang Y, Wu C, Wang S, Hao L, Wang S, Li T. 2017. Md-miR156ab and Md-miR395 target WRKY transcription factors to influence apple resistance to leaf spot disease. Front Plant Sci. 8:526.
- Zhang S, Chen W, Xin L, Gao Z, Hou Y, Yu X, Zhang Z, Qu S. 2014. Genomic variants of genes associated with three horticultural traits in apple revealed by genome re-sequencing. Hortic Res. 1:14045.
- Zhang Y, Li X. 2019. Salicylic acid: biosynthesis, perception, and contributions to plant immunity. Curr Opin Plant Biol. 50:29–36.
- Zheng X, Zhao Y, Shan D, Shi K, Wang L, Li Q, Wang N, Zhou J, Yao J, Xue Y, et al. 2018. MdWRKY9 overexpression confers intensive dwarfing in the M26 rootstock of apple by directly inhibiting brassinosteroid synthetase MdDWF4 expression. New Phytol. 217:1086–1098.
- Zhou K, Hu L, Li Y, Chen X, Zhang Z, Liu B, Li P, Gong X, Ma F. 2019. MdUGT88F1-mediated phloridzin biosynthesis regulates apple development and valsa canker resistance. Plant Physiol. 180:2290–2305.
- Zhu L, Ni W, Liu S, Cai B, Xing H, Wang S. 2017. Transcriptomics analysis of apple leaves in response to Alternaria alternata apple pathotype infection. Front Plant Sci. 8:22.