ABSTRACT
Cu and Zn are common and potentially harmful heavy metals to plants, animals and humans. Herein, we investigated the effects of Cu and Zn stress on the photosynthesis and tolerance mechanism of alfalfa plants to ROS using fluorescence and biochemical methods. The results showed that Cu stress significantly reduced the chlorophyll content of the leaves, while Zn stress only reduced the Chl a content. The Fv/Fm decreased significantly under Cu stress but was not affected by Zn treatment. However, the PIABS of the leaves were sensitive to Cu and Zn stress. Both Cu and Zn stress resulted in the weakening of the ability of PQ library to accept electrons, the damage of OEC and the inhibition of the electron transfer from QA- to QB. Moreover, Cu stress also dissociated the thylakoids of leaves, but Zn stress did not significantly damage it. In Cu and Zn stressed leaves, the reduction of RC/CSm significantly increased the ABS/RC and TRo/RC values. When the stress intensified, the value of DIo/RC increased indicated a plant self-protection mechanism that eliminates excess energy in the PSII reaction center and increases the energy for heat dissipation per unit reaction center. Cu stress significantly increased the O2- production rate, H2O2 content, and MDA accumulation in the leaves. However, Zn stress exhibited a minimal effect on the ROS production and oxidative damage in the alfalfa leaves but increased the O2- production rate at the concentration of 800 μmol·L−1. Cu stress increased the activities of SOD, POD, CAT, APX, and GPX in the leaves; however, leaves adapts to Zn stress by enhancing the activities of SOD and GPX. Thus under Cu stress, the degree of photoinhibition and oxidative damage in alfalfa leaves were significantly higher than under Zn stress.
1. Introduction
Heavy metals are elements whose density exceeds 4.5 kg/dm3, including chromium (Cr), mercury (Hg), lead (Pb), copper (Cu), zinc (Zn), cadmium (Cd), arsenic (As), etc (Kavamura and Esposito Citation2010). The common heavy metal pollutants worldwide are Cr3+, Cd2+, Hg2+, As3+, Cu2+, and Zn2+ (Su et al. Citation2014; Wjcik et al. Citation2015), mainly resulting from rock weathering and human activities. With the rapid development of mining, metallurgy, textile, and other manufacturing industries, several heavy metals are being discharged into the atmosphere, water sources, and soil (Facchinelli et al. Citation2001; Li Citation2019), resulting in water pollution (Montalvo et al. Citation2014), air pollution (Popov et al. Citation2014) and soil pollution (Manu et al. Citation2016). The misuse of drugs and fertilizers in modern agriculture has also contributed to soil pollution with heavy metals (Zhang et al. Citation2020). These soil-polluting heavy metals can be enriched by plants, eventually causing damage to plants (Schwalbert et al. Citation2021). Heavy metals damage plants in multiple steps; they destroy the chlorophyll making the plants lose the green leaf coloration, which subsequently affects photosynthesis and leads to malnutrition and even death of plants (Liu and Hallenbeck Citation2016). Moreover, heavy metal ions can capture the channel position of essential element ions in plants, disrupting the normal physiological and biochemical processes. Heavy metal ions can also destroy plant cell DNA, cellular membrane structures, electron transport chain, and other physiological activities, causing irreversible damage to plants (Chaabene et al. Citation2018; He et al. Citation2021).
Generally, plants can metabolize reactive oxygen species (ROS) and maintain a dynamic balance (Baxter et al. Citation2014). However, when plants are severely stressed with heavy metals, the ROS accumulate in large quantities, damaging various physiological functions, such as enzyme activities, photosynthetic pigments synthesis, and accelerating the photosynthetic pigments degradation (Martin and Sies Citation2017). The ROS also attacks the plant biofilm system and induces peroxidation of the unsaturated fatty acids, leading to the destruction of the membrane structure. This increases the non-selective permeability of the cell membrane, extravasation of cellular ions, and disruption of the cellular metabolic process (Scandalios Citation2002). Thus, plants activate the antioxidant system when the ROS exceeds a certain limit to protect their cells and tissues from the oxidative damage of ROS. The antioxidant defense system is a precise and efficient ROS scavenging system composed of various antioxidant substances, including enzymes such as SOD, CAT, POD, and APX (Miller et al. Citation2008). Several studies have shown that plant adaptability and tolerance to heavy metals are closely associated with the plant antioxidant system, and strong antioxidant capacity can improve plant adaptability (Halliwell and Gutteridge Citation1985; Ali et al. Citation2014; Baxter et al. Citation2014). For example, Cu, an essential trace element and a cofactor of many enzymes such as ascorbic acid oxidase and SOD (Fan et al. Citation2011), induces a high accumulation of ROS. This significantly increases the malondialdehyde content, damaging the leaf cells (Deng et al. Citation2013; Leitao et al. Citation2021), but improves the activities of antioxidant enzymes such as SOD, GPX, APX, and CAT (Buapet et al. Citation2018; Gong et al. Citation2019). Additionally, Zn, a trace metal necessary for the normal growth and development of plants (Broadley et al. Citation2007), can also cause poisoning and affect the normal ROS balance in plants. It has been reported that Zn stress increases POD activity but inhibits CAT activity in pigeon pea (Cajanus cajan (L.) Millspaugh) leaves (Madhava Rao Citation2000). Zn stress also inhibits APX activity in kidney bean (Phaseolus vulgaris) (Ann et al. Citation2001); however, some studies have reported that it enhances APX activity in mustard (Brassica juncea) (Prasad et al. Citation1999) and pigeon pea (Cajanus cajan (L.) Millspaugh) (Madhava Rao Citation2000) leaves.
Alfalfa (Medicago sativa L.) is a perennial leguminous forage widely cultivated because of its high yield and good quality and strong tolerance to abiotic stresses such as drought (Huang et al. Citation2018), low temperatures (Xu et al. Citation2020), and saline-alkali (Zhang et al. Citation2018). However, there are few studies on the photosynthetic function and antioxidant mechanisms of alfalfa under Cu and Zn stress. In-depth studies on the antioxidant mechanisms of plants under different heavy metal stress can provide basic theoretical data for improving plant resistance to heavy metal stress. Therefore, this study evaluated the chlorophyll fluorescence parameters and the activities of enzymes related to ROS production and scavenging in Cu- and Zn-stressed alfalfa leaves to reveal the toxic and adaptive mechanisms of alfalfa under heavy metal stress through photosynthesis, ROS metabolism, and antioxidant mechanism.
1. Materials and methods
1.1. Experimental materials and treatments
We selected mature and plump seeds of Medicago sativa cv. Zhaodong, with relatively consistent sizes, for germination in culture dishes. After the embryos grew to about 0.5 cm, we selected the germinating seeds with relatively similar growth rates and sowed them in a 1:1:1 mixture of peat soil, perlite and vermiculite. Each seedling was planted per bowl by covering the seed surface about 1 cm with soil, followed by culturing at room temperature under 400 μmol·m−2·s−1 of light intensity and 12/12 h photoperiod (light/dark) using LED lamps. The seedlings were carefully removed from the culture medium when their height reached approximately 20 cm, and the culture medium attached to the root surface was washed off. Thereafter, the seedlings were fixed on a black foam board with a sponge and incubated in water-tight culture boxes (width of 25 cm and a height of 30 cm) containing a half-strength Hoagland nutrient solution. Each water culture box (hydroponic incubator) contained 10 L of the nutrient solution and was continuously aerated using electric air pumps. Ten seedlings were cultured in each hydroponic incubator, and the nutrient solution was changed every 3 days. After 30 days of the seedlings culturing, CuSO4 and ZnSO4 were added to the nutrient solution to the final Cu2+ and Zn2+ concentrations of 100, 200, 400, and 800 μmol·L−1, each. The l half-strength Hoagland nutrient solution without Cu2+ and Zn2+ was used as the control (represented by CK). The photosynthetic parameters and physiological indexes of the alfalfa leaves were measured on the 7th-day post-treatment.
1.2. Determination of photosynthetic parameters and physiological indexes
Determination of chlorophyll content: Fresh leaves (about 0.5 g) without main vein from each group were immersed in 2 mL of the mixture of acetone and ethanol (V:V = 1:1) and oscillated in the dark until the green completely faded. Absorbance values at 665 and 649 nm were measured using a spectrophotometer (Agilent Technologies, China). The content of chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Chl a + b), and chlorophyll a/b (Chl a/b) were measured via spectrophotometry (Pirie and Mullins Citation1976).
Physiological indicators related to the ROS metabolism: Thiobarbituric acid colorimetry was used for MDA content determination (Ernster and Nordenbrand Citation1977), superoxide anion (O2-) production rate was evaluated using the method by Elstner and Heupel (Citation1976). Hydrogen peroxide (H2O2) was extracted using 5% (w/v) trichloroacetic acid and measured according to the method by Patterson et al. (Citation1984). Conversely, SOD, POD, and CAT activities were determined using the method by Wang and Huang (Citation2015). The activity (1U) of SOD is defined as the amount of enzymes required to reduce NBT to half of that of the control group. The activity (1U) of CAT is defined as the reduction of an absorbance at 240 nm (A240) by 0.1, and the activity (1U) of POD is defined as the reduction of an absorbance at 470 nm (A470) by 0.01. APX and GPX activities were measured using their respective assay kits (Suzhou Comin Biotechnology Co., Ltd).
Evaluation of the OJIP curve: The fully expanded and functional alfalfa leaves were selected from each treatment, and their dark adaptation was measured for 30 min using a dark adaptation clip. Subsequently, the OJIP curves of the leaves were measured, five times each, by an Hansatech multifunctional plant efficiency instrument (M-PEA), and the OJIP curves were analyzed using a JIP-test. A method by Strasser (Citation1997) was then used to calculate the following parameters: 1) maximum photochemical efficiency of PSII (Fv/Fm), 2) photosynthetic performance indexes based on absorbed light energy (PIABS), 3) absorbed light energy per unit reaction center (ABS/RC), 4) electron transfer energy per unit reaction center (ETo/RC), 5) dissipated energy per unit reaction center (DIo/RC), 6) absorbed energy per unit area (ABS/CSm), 7) electron transfer energy per unit area (ETo/CSm), 8) heat-dissipated energy per unit area (DIo/CSm), and 9) the number of active reaction centers per unit area (RC/CSm).
The OJIP curves were further normalized to O-P, O-J, and O-K using the formulae VO-P = (Ft-Fo)/(Fm-Fo), VO-J = (Ft-Fo)/(FJ-Fo), and VO-K = (Ft-Fo)/(FK-Fo), respectively, generating the VO-P, VO-J, and VO-K curves, respectively. The FJ and FK represented the relative fluorescence intensities at 2.0 and 0.3 ms on the OJIP curves, respectively. In contrast, Ft represented the relative fluorescence intensity at each time point on the OJIP curves. Moreover, the relative variable fluorescence of Point J at 2 ms of the VO-P curve was represented by VJ. The VK and VL denoted the relative variable fluorescence of point K at 0.3 ms of the VO-J curve and point L at 0.15 ms of the VO-K curve, respectively. The VO-P, VO-J, and VO-K curves of the leaves from each treatment were determined by obtaining their differences with the CK and were expressed as △VO-P, △VO-J, and △VO-K, respectively. This was to analyze the variation amplitude of each characteristic point of the curve.
1.3. Data processing
The obtained data were statistically analyzed in Excel and Statistical Package for the Social Sciences (SPSS, version 22.0) software, and the variation between the different data groups was compared using one-way analysis of variance (ANOVA) and the least significant difference (LSD).
2. Results
2.1. Plant phenotype
The 100 and 200 μmol·L−1 concentrations of Cu treatment had little effect on the alfalfa phenotype, as shown in . Upon increasing the concentration to 400 and 800 μmol·L−1, the alfalfa leaves turned yellow, while a concentration of 800 μmol·L−1 resulted in darkening and abscission of alfalfa roots leaves, respectively. Meanwhile, the different concentrations of Zn treatment had significantly lesser effects on the alfalfa phenotype than Cu. Although alfalfa roots were also darkened by a Zn concentration of 800μmol·L−1, the leaves did not show pronounced chlorosis.
2.2. Chlorophyll content
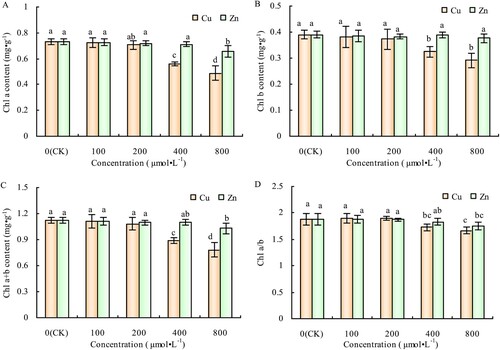
The Cu concentrations of 100 and 200 μmol·L−1 Cu had no significant effects on Chl a, Chl b, Chl a + b, and Chl a/b contents of alfalfa leaves; however, when the concentrations increased to 400 and 800 μmol·L−1, Chl a, Chl b, and Chl a + b contents decreased significantly (-A, 2-B, 2-C). The decrease in Chl a was greater than in Chl b; therefore, higher Cu concentrations also reduced the Chl a/b content of alfalfa leaves (-D). Similar to its phenotypic effects, Zn treatment had significantly reduced effects on chlorophyll content of alfalfa leaves compared to Cu. It significantly decreased the Chl a, Chl a + b, and Chl a/b contents of the treated plants, only at a concentration of 800 μmol·L−1 (-A, 2-C), but had no significant effect on Chl b content compared to CK plants (-B, 2-D).
Figure 2. Effects of Cu and Zn treatment on Chl a content (A), Chl b content (B), Chl a + b content (C) and Chl a/b (D) in alfalfa leaves. Note: The data in the figure are from three biological repeats (n = 3), and represent means ± standard error (SE). Significant differences were expressed by different letters (p< 0.05).
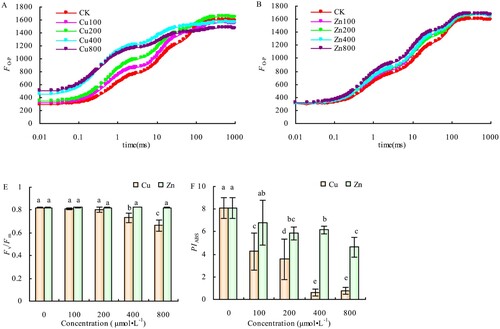
2.3. OJIP curve and photochemical activity of PSII
The relative fluorescence intensities (Fo and Fm) points O and P of the OJIP curve of the alfalfa leaves did not change significantly. However, the relative fluorescence intensity (FJ) of the J point showed a significant increase compared with CK, at Cu concentrations of 100 and 200 μmol·L−1. The FJ and Fo of the treated plants increased significantly compared with CK when Cu concentration increased to 400 and 800 μmol·L−1. Conversely, 800 μmol·L−1 of Cu also significantly reduced the Fm of alfalfa leaves (-A). There were no significant differences in the Fo of the OJIP curve compared with CK; however, the Fm was slightly higher than that of the CK at different Zn concentrations, while FJ increased greatly with Zn concentration (-B). The Fv/Fm did not significantly change, but PIABS significantly decreased compared to CK, at Cu concentrations of 100 and 200 μmol·L−1, as shown in -C and 3-D. Additionally, the Fv/Fm decreased by 10.59% (P < 0.05) and 19.64% (P < 0.05) at the Cu concentrations of 400 and 800 μmol·L−1, respectively, while PIABS reduced by 92.57% (P < 0.05) and 90.44% (P < 0.05), the same Cu concentrations, respectively. There were no significant differences in Fv/Fm among the different Zn concentrations, but PIABS was significantly lower than the CK when Zn concentration reached 200 μmol·L−1.
2.4. Normalized OJIP curves and their relative variable fluorescence at the characteristic points
Under different Cu and Zn concentrations, the relative variable fluorescence of VJ at 2 ms on standardized O-P curve (-A, 4-B), VK at 0.3 ms on standardized O-J curve (-C, 4-D), and VL at 0.15 ms on standardized O-K curve (-E, 4-F) were significantly different compared to CK. Moreover, the relative variable fluorescence of VJ, VK, and VL were significantly greater in Cu than in Zn treatment at each characteristic point.
Figure 4. Effects of Cu and Zn treatment on standardized O-P curve (A, B), standardized O-J curve (C, D), standardized O-K curve (E, F), VJ (G), VI(H), VK(I) and VL (J) in alfalfa leaves. Note: The data in the figure are from three biological repeats (n = 3), and represent means ± standard error (SE). Significant differences were expressed by different letters (p< 0.05).
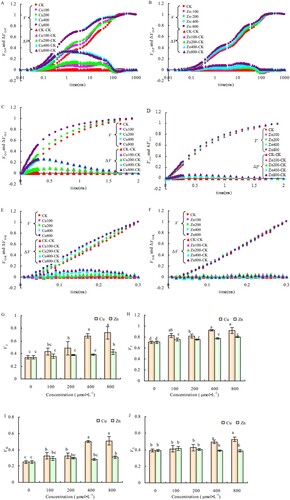
Compared with CK, the VJ, VI, and VK of the alfalfa leaves increased significantly with the different Cu concentrations, as shown in -G, 4-H, and 4-I. Meanwhile, the VI increased significantly under different concentrations of Zn, but VJ increased significantly only at the Zn concentration of 800 μmol·L−1, compared to CK. Although the VK increased to varying degrees under different concentrations of Zn compared to CK, the difference was significant only at Zn concentrations of 400 and 800 μmol·L−1(-I). The VL increased significantly at Cu concentrations of 400 and 800 μmol·L−1 but had no significant changes at different concentrations of Zn treatment (-J).
2.5. PSII per unit reaction center and the energy distribution parameters and number of active reaction centers per unit area
The ABS/RC, TRo/RC, DIo/RC, and DIo/CSm of the alfalfa leaves increased with the increase of Cu concentration; however, there were no significant differences among parameters at concentrations 100 and 200 μmol·L−1 of the Cu treatment compared with CK. However, the parameters increased significantly compared with CK at Cu concentrations of 400 and 800 μmol·L−1. The different concentrations of Cu treatment did not change the ETo/RC and TRo/CSm of the alfalfa leaves, but ETo/CSm was significantly reduced compared to CK at Cu concentrations of 400 and 800 μmol·L−1 (-A). The range of energy allocation parameters of the alfalfa leaves exhibited minimal changes at different concentrations of Zn treatment, as shown in -B. The other parameters did not change significantly except for the significant increase in ABS/RC, TRo/RC, DIo/RC, and DIo/CSm at different Zn concentrations.
Figure 5. Effects of Cu (A) and Zn (B) treatment on energy distribution parameters of PSII reaction center and RC/CSm (C) in alfalfa leaves. Note: The data in the figure are from three biological repeats (n = 3), and represent means ± standard error (SE). Significant differences were expressed by different letters (p< 0.05).
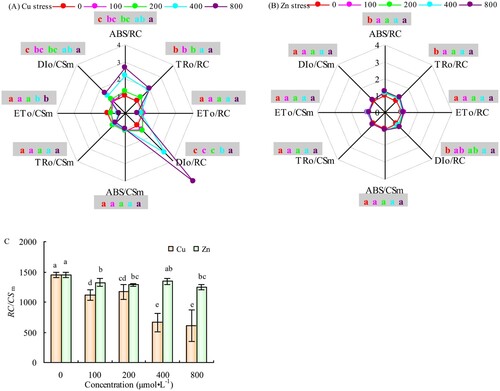
Additionally, the RC/CSm decreased by 54.18% (P < 0.05) and 58.01% (P < 0.05) at Cu concentrations of 400 and 800 μmol·L−1, respectively, compared with CK (-C). The different concentrations of Zn also reduced RC/CSm to varying degrees; however, differences were not significant, and the extent of reduction was significantly lower than that of Cu treatment (-C).
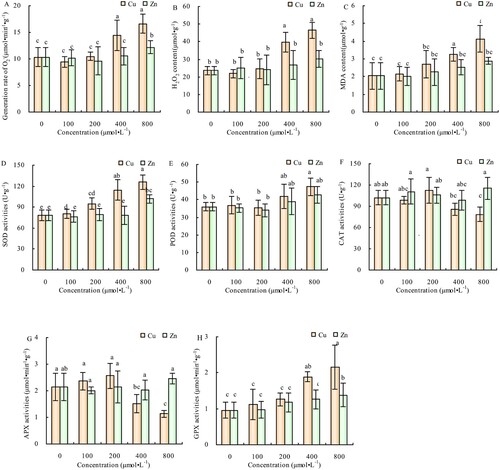
2.6. Physiological analysis
The generation rate and contents of O2-, H2O,2 c and MDA in alfalfa leaves did not change significantly compared with CK at 100 and 200 μmol·L−1 of Cu treatment but increased significantly when Cu concentration increased to 400 and 800 μmol·L−1. Conversely, except for the increase in the generation rate of O2- at 800 μmol·L−1, the other parameters did not change significantly at different concentrations of Zn treatment (-A, 6-B, 6-C). The activities of SOD, POD and GPX in the alfalfa leaves increased with the increase of Cu concentration, had no significant differences compared to CK at 100 and 200 μmol·L−1 of Cu treatment (-D, 6-E, 6-H). Moreover, the CAT and APX activities showed no significant differences in compared with CK at 100 and 200 μmol·L−1 of Cu treatment but decreased significantly when Cu concentrations increased to 400 and 800 μmol·L−1 (-F, 6-G). There were minimal changes in the activities of SOD, POD, CAT, APX, and GPX in alfalfa leaves at different Zn concentrations, and at 800 μmol·L−1 of Zn concentration, the SOD and GPX activities were significantly higher than in CK (-D, 6-H).
Figure 6. Effects of Cu and Zn treatment on generation rate of O2- (A), H2O2 content (B), MDA content (C), SOD activities (D), POD activities (E), CAT activities (F), APX activities (G), GPX activities (H) in alfalfa leaves. Note: The data in the figure are from three biological repeats (n = 3), and represent means ± standard error (SE). Significant differences were expressed by different letters (p< 0.05).
3. Discussion
Chlorophyll Is an important component of photosynthesis that reflects the photosynthetic strength indexes of plants. Its higher contents in plants are essential for maintaining normal photosynthesis processes under stressful conditions (Zhang et al. Citation2020). The decrease in chlorophyll content has been reported to inhibit the capture and utilization of light energy (Zhang et al. Citation2016). In this study, the Cu concentration of 400 μmol·L−1significantly decreased the chlorophyll contents of the alfalfa leaves. Similarly, the Zn concentration of 800 μmol·L−1 significantly decreased the Chl a content but did not significantly change the Chl b content. This suggests that the alfalfa leaves are more sensitive to Cu than Zn stress and that Chl a is more sensitive to Zn stress than Chl b. Excessive Cu2+ and Zn2+ enter chloroplasts and replace Mg2+ in the chlorophyll molecules, disrupting photosynthesis (Kupper et al. Citation1998; Ambrosini et al. Citation2018; Schwalbert et al. Citation2019). It is reported that low concentrations of Cu2+ (<300 mg/kg) increase the chlorophyll contents of ‘Hanfu’ apple seedlings, thus keeping their leaves healthy. However, the Cu2+ concentration exceeding 300 mg/kg stresses the plants and reduces their chlorophyll content and photosynthetic rate (Bu Citation2019). When Chen et al. (Citation2015) applied 600 mg/kg of Cu2+ to Phyllostachys edulis (moso), the photosynthetic efficiency index of Chl a/b decreased significantly in its leaves, indicating that high Cu concentrations inhibits chlorophyll synthesis and reduces the photosynthetic efficiency of the plant. Consistently, Sagardoy et al. (Citation2010) found that excessive Zn reduces the content of photosynthetic pigments such as Chl a and Chl b and affects the electron transfer of PSII in sugar beet (Beta vulgaris L.). The inhibition of chlorophyll synthesis turns the leaves yellow and destroys the photosynthesis of plants, inhibiting their normal growth, development, and metabolism (Brune et al. Citation2010).
Chlorophyll fluorescence kinetics is considered a fast and non-invasive technique for studying plant photosynthetic functions. It plays a unique role in measuring the absorption, transmission, dissipation, and distribution of light energy during the photosynthesis process (Govindjee and Papageorgiou Citation2004; Baker Citation2008). Since photosynthesis inhibition first affects the PSII, the response mechanism of PSII to the stress is considered the most important survival strategy by which plants adapt to photosynthetic stress. Moreover, the decline of photosynthetic rate affects the absorption, transmission, and transformation of light energy, and the photochemical activity in plants (Zhang et al. Citation2013). The photochemical efficiency of PSII (Fv/Fm) and the photosynthetic performance index (PIABS) are important parameters for studying the photosynthetic physiological state of plants (Maxwell and Johnson Citation2000). In this experiment, the Fv/Fm significantly decreased when the concentration of Cu reached 400 μmol·L−1. Similarly, the 100 μmol·L−1 of Cu and 200 μmol·L−1 of Zn treatments also significantly reduced the PIABS. These results show that Cu and Zn stress damaged the photosynthetic performance of the alfalfa leaves. Tuba et al. (Citation2010) and Baycu et al. (Citation2016) also demonstrated that heavy metal stress inhibits the photosynthetic electron transfer rate and PSII activity, thus hindering photosynthesis.
Heavy metals have multiple action points for damaging the electron transport chain (Vasi et al. Citation2007; Zhang et al. Citation2020); therefore, we specifically analyzed the relative variable fluorescence changes at points L, K, J, and I after standardizing the original OJIP curves according to O-P. The VJ of the leaves treated with 200, 400 and 800 μmol·L−1 of Cu, and 800 μmol·L−1 of Zn increased significantly compared with CK, indicating that the electron transfer from QA- to QB was inhibited after its transfer from Pheo to QA- (Strasser Citation1997; Haldimann and Strasser Citation1999). Consequently, it is postulated that the change in VI reflects the heterogeneity of the PQ library during the electron transfer process from QA to QB (Govindje Citation1995; Li et al. Citation2005). We demonstrated that 100 μmol·L−1 of Cu or Zn significantly increased the VI value was, indicating that the electron acceptance of PQ is sensitive to Cu and Zn metal stress. The VK value of the leaves increased at 100, 200, 400, and 800 μmol·L−1 of Cu, and 800 μmol·L−1 Zn treatments, suggesting damage of the oxygen releasing complex (OEC). The OEC damage limits electron transfer from the electron donor side to the secondary electron donor YZ, resulting in an electron imbalance between the donor and the reaction center and between the reaction center and the receptor side (Strasser Citation1997; Zhang et al. Citation2012; Salau et al. Citation2015). Moreover, the increase in the relative variable fluorescence (VL) at the I point of the OJIP curve indicates thylakoid dissociation (Essemine et al. Citation2012). The thylakoid membrane contains photosynthetic pigments and electron transport chain components where the light reaction occurs; therefore, thylakoids dissociation disrupts the function of the leaf photosystem, thus reducing photosynthesis. In this experiment, leaf thylakoids were damaged at 400 and 800 μmol·L−1 of Cu treatment but were not affected by Zn stress. Muzammal et al. (Citation2019) also reported that high concentrations of Cu destroy the structure and function of thylakoids and eventually inhibit photosynthesis.
Changes in the specific activity parameters of the unit reaction center determine the absorption and utilization of light energy and the reaction center activity (Strasserf et al. Citation2008). Normally, the PSII reaction center captures light energy for the next-stage energy transfer, and the remaining energy undergoes heat dissipation (Wang et al. Citation2019). Our results show that ABS/RC and TRo/RC increased significantly in the leaves treated with 400 and 800 μmol·L−1 of Cu and 100 μmol·L−1 of Zn. This is because reducing the number of active reaction centers per unit area after Cu and Zn stress increases the function of the remaining active reaction centers, enhancing specific activity parameters per unit reaction center. A phenomenon also supported by the decrease in RC/CSm values. Furthermore, the increase in DIo/RC value when the concentration of Cu and Zn reached 400 μmol·L−1 indicated a plant self-protection mechanism that reduces excess energy in the PSII reaction center and increases the energy for heat dissipation per unit reaction center.
Excess electron leakage accumulates in the photosynthetic electron transport chain when the photosynthesis is inhibited and convert the free cellular O2 to O2-, which is then reduced to H2O2 by SOD. Thereafter, the inhibition of the photosynthetic oxygen releases the OEC complex, which catalyzes the incomplete cleavage of water to produce H2O2 (Noctor et al. Citation2017; Foyer Citation2018). The accumulation of ROS, such as O2- and H2O2, lead to the peroxidation of the plant cell membrane to produce malondialdehyde (MDA), aggravating membrane lipid peroxidation and reducing the integrity of the plant membrane system (Deng et al. Citation2013; Zhang et al. Citation2020; Yang et al. Citation2021). At 400 and 800 μmol·L−1 of Cu treatment, we observed a significant increase in the O2- production rate, H2O2 content, and MDA accumulation; however, Zn treatment had fewer effects on ROS production and oxidation in alfalfa leaves. The O2- production rate increased, while H2O2 and MDA contents had no significant changes at the Zn concentration of 800 μmol·L−1. Excessive cellular O2- is eliminated by SOD scavenging (Lee et al. Citation2021), which was enhanced by the excessive Cu and Zn in our experiment because Cu and Zn are essential metal auxiliary groups of the Cu/Zn-SOD that maintain the SOD functions (Wang et al. Citation2004). Boojar MMA (Citation2007) and Pandey et al. (Citation2002) reported that SOD activity increases significantly in plant leaves under high concentrations of Cu2+ and Zn2+. Hammerschmitt et al. (Citation2020) also found that SOD activity was usually increased with high Cu and Zn concentrations. Furthermore, O2- can also be removed by POD and CAT reduced to H2O2 by SOD (Mi and Shin Citation2003). In this study, POD and CAT activities increased in alfalfa leaves at 800 μmol·L−1 of Cu treatment but exhibited no significant changes under Zn treatment. Wang et al. (Citation2004) demonstrated that high concentrations of Cu induced several O2- and inhibited CAT activity. Similar to APX, GPX also reduces H2O2 during the oxidation of GSH to GSSG (Stasolla and Yeung Citation2010). We found that 400 and 800 μmol·L−1 of Cu treatment increased the activities of APX and GPX in the leaves, except for the 800 μmol·L−1 of Zn treatment which increased the GPX activity, the other Zn concentrations resulted in no significant changes. A study by Yang et al. (Citation2021) reported that excessive Zn inhibits the function of GPX in tobacco leaves.
4. Conclusion
Cu stress significantly reduced the chlorophyll content of the leaves, while Zn stress only reduced the Chl a content. The Fv/Fm decreased significantly under Cu stress but was not affected by Zn treatment. However, the PIABS of the leaves were sensitive to Cu and Zn stress. Both Cu and Zn stress resulted in the weakening of the ability of PQ library to accept electrons, the damage of OEC and the inhibition of the electron transfer from QA- to QB. Moreover, Cu stress also dissociated the thylakoids of leaves, but Zn stress did not significantly damage it In Cu and Zn stressed leaves, the reduction of RC/CSm significantly increased the ABS/RC and TRo/RC values. Cu stress significantly increased the O2- production rate, H2O2 content, and MDA accumulation in the leaves. However, Zn stress exhibited a minimal effect on the ROS production and oxidative damage in the alfalfa leaves but increased the O2- production rate at the concentration of 800 μmol·L−1. Cu stress increased the activities of SOD, POD, CAT, APX, and GPX in the leaves; however, leaves adapts to Zn stress by enhancing the activities of SOD and GPX. Thus under Cu stress, the degree of photoinhibition and oxidative damage in alfalfa leaves were significantly higher than under Zn stress.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Hongzhi Chen
Hongzhi Chen is a Lecturer in Xinxiang Institute of Engineering. Her research interests lie in the area of Application of plant resources.
Linlin Song
Linlin Song is a Lecturer in Henan Institute of Science and Technology. Her research interests lie in the area of Research and development of medicinal plant resources.
Hongbo Zhang
Hongbo Zhang is a Postgraduate Student in Northeast Forestry University. His research interests lie in the area of Plant physiology and molecular biology.
Jiechen Wang
Jiechen Wang is a Doctoral Student in Northeast Forestry University. Her research interests lie in the area of Plant physiology and molecular biology.
Yue Wang
Yue Wang is a Doctoral Student in Northeast Forestry University. Her research interests lie in the area of Plant physiology and molecular biology.
Huihui Zhang
Huihui Zhang is an Associate Professor in Northeast Forestry University. His research interests lie in the area of Plant physiology and molecular biology.
References
- Ali B, Song WJ, Hu WZ, Luo XN, Gill RA, Wang J, Zhou WJ. 2014. Hydrogen sulfide alleviates lead-induced photosynthetic and ultrastructural changes in oilseed rape. Ecotoxicol Environ Saf. 102:25–33.
- Ambrosini VG, Rosa DJ, Melo GWB, Zalamena J, Cella C, Simao DG, Silva LS, Santos HP, Toselli M, Tiecher TL, Brunetto G. 2018. High copper conten in vineyard soils promotes modifications in photosynthetic parameters and morphological changes in the root system of ‘Red niagara’ plantlets. Plant Physiol Biochem. 128:89–98.
- Ann C, Jaco V, Herman C. 2001. The redox status of plant cells (AsA and GSH) is sensitive to zinc imposed oxidative stress in roots and primary leaves of Phaseolus vulgaris. Plant Physiol Biochem. 39(7):657–664.
- Baker NR. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol. 59(1):89–113.
- Baxter A, Mittler R, Suzuki N. 2014. ROS as key players in plant stress signalling. J Exp Bot. 65:1229–1240.
- Baycu G, Gevrek-Kurum N, Moustaka J, Csatari I, Rognes SE, Moustakas M. 2016. Cadmium-zinc accumulation and photosystem II responses of noccaea caerulescens to Cd and Zn exposure. Environmental Science and Pollution Research. 24(3):2840–2850.
- Boojar MMA G. 2007. The copper tolerance strategies and the role of antioxidative enzymes in three plant species grown on copper mine. Chemosphere. 67(11):2138–2147.
- Broadley MR, Hammond JP, Zelko I, Lux A, White PJ. 2007. Zinc in plants. New Phytol. 173(4):677–702.
- Brune A, Urbach W, Dietz KJ. 2010. Compartmentation and transport of zinc in barley primary leaves as basic mechanisms involved in zinc tolerance. Plant Cell Environ. 17(2):153–162.
- Bu F. 2019. Physiological and biochemical response of ‘hanfu’ apple seedlings to heavy metal copper stress. Shenyang Agricultural University.
- Buapet P, Mohammadi NS, Pernice M, Kumar M, Kuzhiumparambil U, Ralph PJ. 2018. Excess copper promotes photoinhibition and modulates the expression of antioxidant-related genes in zostera muelleri. Aquat Toxicol. 207:91–100.
- Chaabene Z, Hakim IR, Rorat A, Elleuch A, Mejdoub H, Vandenbulcke F. 2018. Copper toxicity and date palm (phoenix dactylifera) seedling tolerance: Monitoring of related biomarkers. Environ Toxicol Chem. 37(3):797–806.
- Chen JR, Shafi M, Li S, Wang Y, Wu JS, Ye ZQ, Peng DL, Yan WB, Liu D. 2015. Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of moso bamboo (Phyllostachys pubescens). Sci Rep. 5:13554.
- Deng F, Yamaji N, Xia J, Ma JF. 2013. A member of the heavy metal P-type ATPase OsHMA5 is involved in xylem loading of copper in rice. Plant Physiol. 163(3):1353–1362.
- Elstner EF, Heupel A. 1976. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem. 70(2):616–620.
- Ernster L, Nordenbrand K. 1977. Microsomal lipid peroxidation. Methods in Enzymol. 52(11):302–310.
- Essemine J, Govindachary S, Ammar S, Bouzid S, Carpentier R. 2012. Enhanced sensitivity of the photosynthetic apparatus to heat stress in digalactosyl-diacylglycerol deficient arabidopsis. Environ Exp Bot. 80:16–26.
- Facchinelli A, Sacchi E, Mallen L. 2001. Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ Pollut. 114(3):313–324.
- Fan LM, Ma ZQ, Liang JQ, Li HF, Wang ET, Wei GH. 2011. Characterization of a copper-resistant symbiotic bacterium isolated from Medicago lupulina growing in mine tailings. Bioresour Technol. 102(2):703–709.
- Foyer CH. 2018. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ Exp Bot. 154:134–142.
- Gong Q, Wang L, Dai TW, Zhou JY, Kang Q, Chen HB, Li K, Li ZH. 2019. Effects of copper on the growth, antioxidant enzymes and photosynthesis of spinach seedlings. Ecotoxicol Environ Saf. 171:771–780.
- Govindje E. 1995. Sixty-three years since kautsky: chlorophyll a fluorescence. Aust J Plant Physiol. 22(2):131–160.
- Govindjee G, Papageorgiou G. 2004. Chlorophyll a Fluorescence: A Signature of Photosynthesis.
- Haldimann P, Strasser RJ. 1999 Effects of anaerobiosis as probed by the polyphasic chlorophyll a fluorescence rise kinetic in pea (pisum sativum L.). Photosynth Res. 62(1):67–83.
- Halliwell B, Gutteridge JMC. 1985. Free radicals in biology and medicine. Journal of Free Radicals in Biology and Medicine. 1(4):331–332.
- Hammerschmitt RK, Tiecher TL, Facco DB, Silva LOS, Schwalbert R, Drescher GL, Trentin E, Somavilla LM, Kulmann MSS, Silva ICB, et al. 2020. Copper and zinc distribution and toxicity in ‘jade’/ ‘genovesa’ young peach tree. Sci Hortic. 259:1–9.
- He GQ, Zhang HB, Liu SQ, Li HQ, Huo YZ, Guo KW, Xu ZS, Zhang HH. 2021. Exogenous γ-glutamic acid (GABA) induces proline and glutathione synthesis in alleviating Cd-induced photosynthetic inhibition and oxidative damage in tobacco leaves. Journal of Plant Interactions. 16(1):296–306.
- Huang Z, Yu L, Zeng C, Yan F, Wu GL. 2018. Soil water storage deficit of alfalfa (Medicago sativa) grasslands along ages in arid area (China). Field Crops Res. 221:1–6.
- Kavamura VN, Esposito E. 2010. Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnol Adv. 28(1):61–69.
- Kupper H, Kupper FC, Spiller M. 1998. In situ detection of heavy metal substituted chlorophylls in water plants. Photosynth Res. 58(2):123–133.
- Lee HJ, Lee JH, Wi S, Jang Y, An S, Choi CK, Jang S. 2021. Exogenously applied glutamic acid confers improved yield through increased photosynthesis efficiency and antioxidant defense system under chilling stress condition in solanum lycopersicum L. cv. dotaerang Dia. Sci Hortic. 277(5):109817.
- Leitao I, Sales J, Martins LL, Mourato MP. 2021. Response to stress induced by different potentially toxic elements (As, Cd, Cu and Na) in rapeseed leaves. Plant Physiology Reports. 26(3):478–490.
- Li L. 2019. Physio-biochemical and molecular mechanism of exogenous brassinosteroids in regulating growth of Brassica napus under copper and chromium stress. Zhejiang University.
- Li PM, Gao HY, Strasser RJ. 2005. Application of the fast chlorophyll fluorescence induction dynamics analysis in photosynthesis study. Acta Photophysiologica Sinica. 31(6):559.
- Liu Y, Hallenbeck PC. 2016. A kinetic study of hydrogen production by a calvin-benson-bassham cycle mutant, PRK (phosphoribulose kinase), of the photosynthetic bacterium rhodobacter capsulatus. Int J Hydrogen Energy. 41(26):11081–11089.
- Madhava Rao KS. 2000. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 157(1):113–128.
- Manu M, Bancila RI, Iordache V. 2016. Impact assessment of heavy metal pollution on soil mite communities (acari: mesostigmata) from zlatna depression - transylvania. transactions of The institution of chemical engineers. Process Safety and Environmental Protection, Part B. 108:121–134.
- Martin WF, Sies H. 2017. Genomic redox footprints. Nat Plants. 3(6):17071.
- Maxwell K, Johnson GN. 2000. Chlorophyll fluorescence–a practical guide. J Exp Bot. 51(345):659–668.
- Mi YL, Shin HW. 2003. Cadmium-induced changes in antioxidant enzymes from the marine alga nannochloropsis oculata. J Appl Phycol. 15(1):13–19.
- Miller G, Shulaev V, Mittler R. 2008. Reactive oxygen signaling and abiotic stress. Physiol Plant. 133(3):481–489.
- Montalvo C, Aguilar CA, Amador LE, Ceron JG, Ceron RM, Anguebes F, Cordova AV. 2014. Metal contents in sediments (Cd, Cu, Mg, Fe, Mn) as indicators of pollution of palizada river, Mexico. Environment and Pollution. 3(4):89–98.
- Muzammal R, Liu LJ, Bashir S, Saleem MH, Chen C, Peng DX, Siddique KHM. 2019. Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant Physiol Biochem. 138(1):121–129.
- Noctor G, Reichheld JP, Foyer CH. 2017. ROS-related redox regulation and signaling in plants. Seminars in Cell and Developmental Biology. 80:3–12.
- Pandey N, Pathak GC, Singh AK, Sharma CP. 2002. Enzymic changes in response to zinc nutrition. J Plant Physiol. 159(10):1151–1153.
- Patterson BD, Macrae EA, Ferguson IB. 1984. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem. 139(2):487–492.
- Pirie A, Mullins MG. 1976. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic acid. Plant Physiol. 58(4):472–486.
- Popov BB, Hristova VK, Ahmad MA, Petrovska M. 2014. Monitoring of heavy metals and trace elements contamination in the soil and vegetables and air pollution in the Republic of Macedonia. Chemistry. 3:205–214.
- Prasad KVSK, Saradhi PP, Sharmila P. 1999. Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea. Environ Exp Bot. 42(1):1–10.
- Sagardoy R, Morales F, Lopez-Millan AF, Abadia A, Abadia J. 2009. Effects of zinc toxicity on sugar beet (Beta vulgaris L.) plants grown in hydroponics. Plant Biology. 11(3):339–350.
- Salau AW, Olasantan FO, Bodunde JG, Makinde EA. 2015. Soil temperature and moisture content changes with growth and yield of cassava/vegetable intercrops. Archives of Agronomy and Soil Science. 61(4):447–460.
- Scandalios JG. 2002. The rise of ROS. Trends Biochem Sci. 27(9):483–486.
- Schwalbert R, Silva LOS, Schwalbert RA, Tarouco CP, Fernandes GS, Marques ACR, Costa CC, Hammerschmitt RK, Brunetto G, Nicoloso FT. 2019. Physiological responses of soybean (glycine max (L.) merrill) cultivars to copper excess. Anais da Academia Brasileira de Ciências. 91(4):1–15.
- Schwalbert R, Stefanello OL, Schwalbert RA, Tarouco CP, Drescher GL, Trentin E, Tassinari A, Silva IB, Brunetto G, Nicoloso FT. 2021. Soil tillage affects soybean growth and promotes heavy metal accumulation in seeds. Ecotoxicol Environ Saf. 216:112191.
- Stasolla C, Yeung EC. 2010. Ascorbic acid metabolism during white spruce somatic embryo maturation and germination. Physiol Plant. 111(2):196–205.
- Strasser BJ. 1997. Donor side capacity of photosystem II probed by chlorophyll a fluorescence transients. Photosynth Res. 52(2):147–155.
- Strasserf RJ, Srivastava A, Govindjee G. 2008. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol. 61(1):32–42.
- Su C, Jiang LQ, Zhang WJ. 2014. A review on heavy metal contamination in the soil worldwide: situation, impact and remediation techniques. Environmental Skeptics and Critics. 3(2):24–38.
- Tuba Z, Saxena DK, Kajal SR, Shiv OS, Czobel S, Kalaji MH. 2010. Chlorophyll a fluorescence measurements for validating the tolerant bryophytes for heavy metal (Pb) biomapping. Curr Sci. 98(11):1505–1508.
- Vasi V, Koji D, Krinulovi K, olovi M, Stoji D. 2007. Time-dependent inhibition of Na+/K+-ATPase induced by single and simultaneous exposure to lead and cadmium. Russ J Phys Chem A. 81(9):1402–1406.
- Wang SH, Yang ZM, Yang H, Lu B, Li SQ, Lu YP. 2004. Copper-induced stress and antioxidative responses in roots of Brassica juncea L. Botanical Bulletin of Academia Sinica. 45(3):203–212.
- Wang XK, Huang JL. 2015. Principle and technology of plant physiological and biochemical experiment. Higher Education Press.
- Wang Y, Zhang XL, Hu YB, Teng ZY, Zhang SB, Chi Q, Sun GY. 2019. Phenotypic response of tobacco leaves to simulated acid rain and its impact on photosynthesis. International Journal of Agriculture and Biology. 21:391–398.
- Wjcik M, Dresler S, Tukiendorf A. 2015. Physiological mechanisms of adaptation of dianthus carthusianorum L. to growth on a Zn-Pb waste deposit - the case of chronic multi-metal and acute Zn stress. Plant Soil. 390(1-2):237–250.
- Xu HY, Tong ZY, He F, Li XL. 2020. Response of alfalfa (Medicago sativa L. to Abrupt Chilling as Reflected by Changes in Freezing Tolerance and Soluble Sugars. Agronomy. 10(2):255.
- Yang FW, Zhang HB, Wang Y, He GQ, Wang JC, Guo DD, Li T, Sun GY, Zhang HH. 2021. The role of antioxidant mechanism in photosynthesis under heavy metals Cd or Zn exposure in tobacco leaves. Journal of Plant Interactions. 16(1):354–366.
- Zhang HH, Li X, Guan YP, Li MB, Wang Y, An MJ, Zhang YH, Liu GJ, Xu N, Sun GY. 2020a. Physiological and proteomic responses of reactive oxygen species metabolism and antioxidant machinery in mulberry (Morus alba L.) seedling leaves to NaCl and NaHCO3 stress. Ecotoxicol Environ Saf. 193:110259.
- Zhang HH, Li X, Xu N, Sun GY, Sun ML, Cai DJ, Gu SY. 2018. Alkalinity and salinity tolerance during seed germination and early seedling stages of three alfalfa (Medicago sativa L. cultivars. Legume Research. 40(5):853–858.
- Zhang HH, Li X, Xu ZS, Wang Y, Teng ZY, An MJ, Zhang YH, Zhu WX, Xu N, Sun GY. 2020b. Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotoxicol Environ Saf. 195:110469.
- Zhang HH, Zhong HX, Wang JF, Sui X, Xu N. 2016. Adaptive changes in chlorophyll content and photosynthetic features to low light in Physocarpus amurensis maxim and Physocarpus opulifolius “diabolo”. PeerJ. 4(3):e2125.
- Zhang LY, Wen X, Lin YM, Jian L, Chen C, Zhen WC. 2013. Effect of salt stress on photosynthetic and chlorophyll fluorescent characteristics in alnus formosana seedlings. Journal of Fujian College of Forestry. 33(3):193–199.
- Zhang ZS, Li G, Gao HY, Zhang LT, Yang C, Liu P, Meng QW. 2012. Characterization of photosynthetic performance during senescence in stay-green and quick-leaf-senescence Zea mays L. inbred lines. Plos One. 7(8):e42936.