 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study assesses the effects of Azotobacter biopriming on the early development of sugar beet. Azotobacter chroococcum F8/2 was screened for plant growth promoting characteristics and biopriming effects were estimated through germination parameters and the structural changes of the root tissues. A. chroococcum F8/2 was characterized as a contributor to nitrogen, iron, and potassium availability, as well as a producer of auxin and 1-aminocyclopropane-1-carboxilic acid deaminase. Applied biopriming had reduced mean germination time by 34.44% and increased vigor I by 90.99% compared to control. Volatile blend comprised 47.67% ethanol, 32.01% 2-methyl-propanol, 17.32% 3-methyl-1-butanol, and a trace of 2,3-butanedione. Root micromorphological analysis of bioprimed sugar beet revealed a considerable increase in primary, secondary xylem area, and vessels size. Obtained results determine A. chroococcum F8/2 as a successful biopriming agent, and active participant in nutrient availability and hormonal status modulation affecting root vascular tissue.
Introduction
Sugar beet (Beta vulgaris L.) is a necessary part of the human diet due to high content of sucrose as the key component (up to 20% of the taproot fresh weight) (Rodrigues et al. Citation2020), vitamins, minerals, phenolics, carotenoids, ascorbic acid, and betalains (Chhikara et al. Citation2019). In 2019 the total world sugar production was 187 Mt, and the share of sugar beet was about 20%. It is projected that sugar production will reach 209.5 Mt by 2026 (OECID-FAO Citation2017). Since the content of sucrose in the root is genetically limited, it is clear that sugar beet production must be directed towards increasing the root yield (Hoffmann and Kenter Citation2018).
The technological yield of sugar is highly influenced by germination and early growth stages (Chomontowski et al. Citation2020), and achieving it needs support from the very beginning of the plant’s life. Successful, uniform, rapid germination as well as the emergence of normal seedlings alleviate adverse impacts and lead to optimization of crop production (Mal et al. Citation2019). Therefore, sugar beet seeds need to be subjected to sophisticated technique such as priming (Chomontowski et al. Citation2020), pelleting, and coating (Jolayemi Citation2019) to promote germination and early growth. Among the new technologies, biopriming and microbiological inoculation as its method are acceptable in terms of sustainability and the reduction of inorganic additives to the environment. Biopriming is conducted through a set of physiological events initiated by hydration (imbibition), and seed inoculation with Plant Growth Promoting Rhizobacteria (PGPR) (Sood et al. Citation2021). PGPR create a tight association with the plant, not only supporting its growth and health (Liu et al. Citation2021), but also the biological integrity and quality of the soil (Khatoon et al. Citation2020). Besides, biostimulants have been shown to improve nutrient uptake, crop tolerance to drought, and pathogens as well as water uptake efficiency by different Plant Growth Promoting (PGP) mechanisms (Jolayemi Citation2019; Khan et al. Citation2020). The rapid growth of sugar beet, its short vegetative cycle, intensive accumulation of dry matter, and extremely high demand on nutrients’ availability as well as their efficient transport (Cardoso et al. Citation2017) could be boosted by activity of PGPR.
One of the most promising PGPR genera is Azotobacter, free-nitrogen fixing bacteria, universally recognized as an inoculant in sustainable agriculture. Nitrogen fixation as a major trait is of particular importance in sugar beet production, whose yields are dependent on nitrogen supply (6–8 pounds/t according to Poindexter Citation2001). Beneficial effect of A. chroococcum on sugar beet yield and technological quality has already been demonstrated (Čačić et al. Citation2003; Mrkovački and Mezei Citation2003; Kuzevski et al. Citation2011; Mrkovacki et al. Citation2016), but without deeper insight into the mechanisms of action. The agronomic significance of Azotobacter sp. is reflected through additional features such as phosphate solubilization (Nosrati et al. Citation2014), synthesis of phytohormones, siderophores, antibiotics, exopolysaccharides, degradation of toxic compounds (Sumbul et al. Citation2020), and contribution to the ecological balance in the agroecosystem (Jiménez et al. Citation2011). All generated knowledge resulted in Azotobacter recognition by The Regulation EU Citation2019/Citation1009 as one of the three bacterial genera that can be used as microbial plant biostimulant/biofertilizer. Consequently, research on Azotobacter is much closer to practical application of the findings, compared to Bacillus and Pseudomonas as widely studied PGPRs. Azotobacter genus is studied as biopriming agent of maize (Sharifi and Khavazi Citation2011), safflower (Sharifi Citation2012), barley (Mirshekari et al. Citation2012), and wheat (Miloševic et al. Citation2012). However, in order to make a better estimation of Azotobacter sp. use in biopriming, further research is needed. Current literature still lacks data regarding Azotobacter sp. volatile organic compounds (VOCs) production and their effects on plants. VOCs play an important role in communication with soil microbes, nematodes, insects, and plants (Xu et al. Citation2015; Schulz-Bohm et al. Citation2017; Calcagnile et al. Citation2019; de Boer et al. Citation2019).
Furthermore, understanding root development at earlier stages is pivotal since it has a huge impact on root function. The root developmental processes in Beta vulgaris are mainly focused on the regulation of cambium activity, which produces precursors for the formation of xylem and phloem elements (Jouannet et al. Citation2015). However, there is not enough evidence showing the effects of priming/biopriming on the root xylem vessels, precisely, on the stimulation of cambium division and differentiation. Such correlation would contribute to understanding the observed effects in a more holistic way.
Taking into account the current state of the art, described above, the main goal of this research was to estimate A. chroococcum F8/2 biopriming effects on the early stages of sugar beet growth and root development. The aim was approached through comprehensive insight into: bacterial PGP mechanisms, and VOCs composition, as well as sugar beet germination parameters and root microstructure.
Materials and methods
Bacterial strain characterization
The bacterial strain was obtained from the collection of Department of Environmental Microbiology, Faculty of Agriculture, Belgrade, Serbia. The strain A. chroococcum F8/2 was previously isolated from the agricultural soil, identified according to morphological traits, and proven to be a potent biopriming agent on several crops (Kerecki et al. Citation2021).
Molecular identification
Genomic DNA was obtained by method of Hopwood et al. (Citation1985). Kapa Taq DNA polymerase (Merck KGaA, Darmstadt, Germany) was used to amplify the 16S rDNA and nifH genes using a Kyratec SuperCycler Trinity (Queensland, 4122 Australia) with specific primers: 16S – Fw (GAATCTTCCACAATGGACG) and 16S – Rev (TGACGGGCGGTGTGTACAAG) (Jovčić et al. Citation2009); nifH-g1 – Fw (GGTTGTGACCCGAAAGCTGA) and nifH – g1 – Rev (GCGTACATGGCCATCATCTC) (Helmut et al. Citation2004). The reaction mixture contained template DNA (100 ng), Taq polymerase buffer (10×), dNTP mix (10 mM), each primer (10 mM), Taq polymerase (2 U/µL). PCR conditions were set as follows: 94°C for 5 min; followed by 30 cycles of denaturation at 94°C for 30 s; annealing 55°C (50°C for nifH-g1 primers) for 30 s.; primer extension at 72°C for 2 min.; at the end final elongation step at 72°C for 7 min. PCR products were sequenced by the Macrogen Sequencing Service (Macrogen Europe, Amsterdam, The Netherlands) and BLAST algorithm was used for analyzing nucleotide sequences (Altschul et al. Citation1997).
Plant growth promoting profiling
Phosphate solubilizing activity was estimated on National Botanical Research Institute’s phosphate growth medium (NBRIP) (Nautiyal Citation1999). Potassium solubilization was determined on the modified Aleksandrov medium with K-Al silicate as the K-bearing mineral (Rajawat et al. Citation2016). Zinc solubilization capacity was performed on the Tris-mineral salt medium supplemented with 1% of ZnO (w/v) (Gandhi and Muralidharan Citation2016). Selective media were spot inoculated with overnight bacterial culture in three replications, and incubated at 28 ± 2°C. The incubation period was two weeks, 72, and 48 h, for NBRIP, Alexander, and Tris-mineral salt medium, respectively. After the incubation period, the appearance of halo zones around the colonies was considered as positive result for the phosphorus, potassium, and zinc solubilization. The solubilization capacity of the strain was expressed through solubilization index (SI) calculated by the formula (Bashir et al. Citation2017):
The potential of dissolving K from K-Al silicate as a K-source in the solid media was classified as low (SI < 2.00), intermediate (SI 2.00–4.00), and high (SI > 4.00) (Setiawati and Mutmainnah Citation2016). Siderophore production was identified using a modified CAS assay (Alexander and Zuberer Citation1991). In brief, the Chrome azurol S (Fluka, USA) agar medium was spot inoculated with the overnight bacterial culture and incubated for 5 days at 28 ± 2°C. The appearance of yellow-orange zones around colonies indicated siderophore production. The diameter of the zone was measured and siderophore production is classified as low (1.5 mm), moderate (6–10 mm), and high (11–15 mm) according to Wani et al. (Citation2007).
Ammonia production was tested in 5 ml peptone water broth (Torlak, Serbia) incubated for 72 h at 28 ± 2°C. After the incubation period, 0.5 ml of Nessler’s reagent (Alfapanonija, Serbia) was added to the tube and the appearance of yellow-brown color indicated ammonia production (Cappuccino and Sherman Citation1996).
The capability to produce acid and alkaline phosphatase was assessed using API ZYM system (Biomerieux, France) according to the manufacturer’s instructions.
The production of exopolysaccharides (EPS) was determined according to Paolo et al. (Citation2012). The sterile filter paper discs (5 mm Ø) were inoculated with 5 μl of the overnight bacterial culture, placed on ATCC No.14 solid media (Arfarita et al. Citation2016), and incubated at 28 ± 2°C for 48 h. The slimy colonies were mixed with 2 ml of absolute alcohol, and the presence of precipitate confirmed EPS production.
The 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity was determined by the 96-well PCR-plate ninhydrin-ACC assay (Li et al. Citation2011). Briefly, the bacterial culture was grown in DF-ACC medium (Penrose and Glick Citation2003), containing 3 mmol ml−1 of ACC (Sigma-Aldrich, USA). The un-inoculated DF-ACC medium was used as a control. After 24 h incubation at 28°C/200 rpm, bacterial supernatant was obtained and added to DF medium in a ratio 1:10. The diluted supernatant (60 µl) was transferred to a 96-well PCR plate with 120 µl of ninhydrin reagent and heated in a boiling water bath for 30 min. Afterwards, 100 µl of the reaction solution was transferred to the flat bottom-96-well microplate. The optical density (OD) was recorded at 570 nm (T70 UV/VIS Spectrometer, PG Instruments LTD). The obtained results were compared to the standard curve prepared with pure ACC (Sigma-Aldrich, USA) in the range of 0.005–0.5 mmol−1.
The indole-3-acetic acid (IAA) production was determined by the colorimetric test (Paten and Glick Citation2002). Minimal salt medium enriched with 100 µg ml−1 of l-tryptophan (Sigma-Aldrich, USA) was inoculated with overnight bacterial culture and incubated for 72 h/30 ± 1°C/150 rpm (KS 260 basic, IKA, Staufen, Germany). The broth was centrifuged at 10,000 g for 5 min (Eppendorf, Germany), 1 ml of collected supernatant was mixed with 2 ml of Salkowski reagent, and incubated in the dark for 20 min. The optical density (OD) was recorded at 540 nm (T70 UV/VIS Spectrometer, PG Instruments LTD). The obtained results were compared to the standard curve prepared with pure IAA (Sigma-Aldrich, USA) in the 1–100 μg ml−1 range.
Seed germination assay
Sugar beet (Beta vulgaris L.) ‘Hurricane’ seeds (SESVanderHave, Belgium) were surface sterilized by immersion in 70% ethanol (v/v) for 2 min, followed by 2 min exposure to 2% NaOCl (v/v). Seeds were carefully washed 5 times with sterile water and air-dried under aseptic conditions in the laminar chamber. The success of the sterilization procedure was confirmed by the absence of bacterial colonies on Nutrient agar (Torlak, Serbia) where treated seeds were placed and incubated for 48 h at 28 ± 2°C.
Seeds were inoculated by immersion into the 48h-old bacterial suspension (107 CFU ml−1, which corresponds to OD 1 at 550 nm; T70 UV/VIS Spectromer, PG Instruments LTD, UK) and incubated for 1 h/28 ± 2°C/130 rpm (KS 260 basic, IKA, Staufen, Germany). The control treatment seeds were immersed in sterile water.
Germination test was carried out by placing 100 of each A. chroococcum-inoculated and control seeds in Petri dishes on sterile filter paper. Each treatment had four replicates. The seeds were subjected to natural light at 25°C for 7 days. The humidity was regularly maintained by adding sterile water. The number of germinated seeds was recorded daily. The final germination percentage (FGP), germination index (GI), mean germination time (MGT), vigor I, and vigor II was calculated by the advanced germination measurement tool (Agron Info-Tech).
The effect of Azotobacter volatile organic compounds (VOCs) on sugar beet seedlings
The closed system (Passive Diffusion System), which involves the use of two-compartment Petri dishes enabling the physical separation of the selected bacterial strain and plant seeds, was used in the study.
A drop of 20 µl 107 CFU ml−1 bacterial suspension was applied on the Fiodorov agar medium (Anderson Citation1985) on one side of the divided Petri dish while the medium was not inoculated for the control treatment. The seeds were sterilized as previously described and placed on moistened Whatman’s filter paper which is placed on the second part of the divided Petri dish. Six seeds were placed per Petri dishes in triplicates. The plates were sealed with Parafilm® (Sigma-Aldrich-USA) to prevent penetration of outside air but allow VOCs exchange between compartments. All boxes were under laboratory conditions for 7 days after which fresh and dry mass of seedlings were measured.
GC-MS analysis of VOCs
The headspace vials (75.5 × 22.5 × 20 ml), (Merck KGaA, Darmstadt, Germany) were filled with 5 ml of Fiodorov medium, sterilized, and inoculated with 20 μl of 107 CFU ml−1 of Azotobacter suspension. The vial with the uninoculated Fiodorov medium represented the control treatment. The incubation regime was at 28 ± 2°C for 3 days, and the samples were subject to GC-MS analysis.
Static HS GC-MS analysis was carried out using the Agilent gas chromatographic system (CA, USA) with an autosampler GC 80, a gas chromatograph 7890A, and a mass detector 5975C MSD. Regarding HS analysis, the regime of the autosampler meant the incubation time of 1200 s, incubation temperature of 120°C, and syringe temperature of 130°C. To perform qualitative and semi-quantitative analysis of volatile components, the gas chromatograph was connected directly to two detectors: flame ionization detector (FID) and mass spectrometric detector (MSD) (chromatograms shown in SM1). The HP-Innowax capillary polar column (30 m × 0.320 mm × 0.25 µm) and helium, as the pure carrier gas, with a constant flow of 3 ml/min were used for the analytical performance. The temperature of the injector was 220°C, and the temperature of the flame ionization detector was 300°C. The regime of the oven was set up as follows: 35°C for 5 min, 65°C a rate of 3°C/min, 225°C at the rate of 10°C/min, and then held for 4 min. The injection was performed in split mode 3:1 and the injected volume of the sample was 2000 µl. The mass spectra were recorded using the electron ionization (EI) mode of 70 eV and the ion source temperature of 230°C, while the mass analyzer (quadrupole) was constantly heated to 150°C.
The relative contents of the identified compounds were calculated from the GC peak areas. Identification and quantification of obtained volatile compounds were confirmed by comparing the mass spectra and retention indices (RI) to those of standard compounds from the NIST WebBook (US National Institute of Standards and Technology).
Assessment of structural changes in the inner tissue of the root
Sugar beet seeds were prepared as previously described in the seed germination assay section. Afterwards, 25 sugar beet seeds per treatment (control/A. chroococcum-inoculated seeds) were sown in plastic containers (500 × 280 × 55 mm) filled with commercial substrate (Klasmann-Dilmann, Germany). The number of emerged plants per treatment was noted and final emergence percentage (FEP) was calculated. The control and A. chroococcum inoculated plants at the stage 21 days after sowing were harvested on the same day. The root samples (with a length of 5 mm), were cut off from the five representative plants, fixed in 50% alcohol (v/v), and embedded in paraffin. Sections 8–12 µm thick were taken by sliding microtome and stained with safranin and alcian blue (Ruzin Citation1999). Transverse sections of five roots of control and five roots of A. chroococcum inoculated beet were observed by bright-field light microscopy in transmitted light (Leica DM2000, Germany), and documented with a Leica DC320 digital camera, and software Leica IM1000 was used for sample capture and measurements. In the case of the mean area of the 10 parenchyma largest cells and vessels as well as the number of parenchyma cells, each recorded measurement is the average of at least 10 or 90 measurements.
Statistical analysis
Data were processed using analysis of variance (ANOVA), and the differences between the means were determined by Tukey’s test at a 5% level of probability.
Results
Molecular identification
According to the results of 16S rDNA and nifH gene sequencing, it was confirmed that the tested strain corresponds to Azotobacter chroococcum. The sequences were submitted to NCBI GenBank database (GenBank accession no. MZ188902 and MZ230598, for 16S and nifH, respectively).
Plant growth promoting assessment
A. chroococcum F8/2 expressed multifunctional PGP traits (). The results of the phosphorus and zinc solubilization assays indicated that the strain had no potential to utilize phosphorus and zinc from inorganic sources, but it is characterized by the production of acid and alkaline phosphatase. A. chroococcum F8/2 had a moderate ability to dissolve K from K-Al silicate (SI(K) 3.01).
Table 1. Characteristics related to plant growth promotion.
A positive test result for siderophore synthesis and average diameter of discoloration zone of 2 cm revealed the strain’s ability to mobilize and bind Fe from the environment. The ammonia production activity was also confirmed as well as the ability to synthesize exopolysaccharides (EPS) by forming mucous colonies on the growing media.
A. chroococcum F8/2 produced 10 µg ml−1 IAA in l-tryptophan supplemented medium, while the ninhydrin test confirmed its capability to utilize ACC. After 16h-incubation in DF-ACC medium, the initial concentration of ACC decreased by 91.67%, which indicates the ACC consumption by the strain.
Germination assay
The FGP of the inoculated treatment was calculated to be lower by 20% in comparison to the control. An interesting observation was the difference in germination after the seeds were planted on the substrate for the assessment of structural changes in the root tissues. The FEP of Azotobacter chroococcum-inoculation treatment was 100%, while it was lower by 7% in the control treatment. The seed inoculation treatment had a significantly lower value of MGT versus control for 34.44% (). The Vigor I value of the inoculated seeds was found to be 90.99% higher compared to control. Analyzing the differences in Vigor II and GI between the treatments, no significant differences were found.
Table 2. Effects of A. chroococcum F8/2 biopriming on germination parameters of sugar beet seeds.
Effect of VOCs on the beetroot seedlings
The exposure of sugar beet seeds to VOCs produced by A. chroococcum F8/2 significantly increased fresh weight (53.24%) compared to the control ().
Table 3. Effects of A. chroococcum F8/2 VOCs on fresh and dry weight of sugar beet seedlings after 7 days of exposure.
The released VOCs are presented in and summarized in .
Figure 1. Chromatographic representation of VOCs realized by Azotobacter grown in Fyodorov medium obtained by: (A) mass spectrometric detector (MSD) and (B) flame ionization detector (FID).
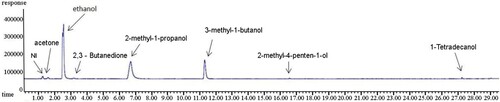
Table 4. Volatile blend metabolized by A. chroococcum F8/2 grown in Fiodorov liquid medium.
The analyzed VOCs spectrum confirmed the production of eight compounds. Alcohols were the dominant component of the blend. The pick of ethyl alcohol is the highest, while 2-methyl-propanol and 3-methyl-1-butanol are in the lower concentration. The retention time ranges from 1 to 27 min. The control treatment showed no presence of volatile compounds.
The microstructure of the sugar beet roots
The examination of transverse root sections of the A. chroococcum-inoculated ((a,c,e)), and control young plants ((b,d,f)) revealed that they have a very similar general microstructure which consists of the common regions, i.e. one layer epidermis, the multi-layer cortex, and the vascular cylinder. The following root tissues could be identified from the outside to the inside: epidermis, cortical parenchyma, endoderm, and pericycle (both hardly visible) (), tertiary phloem, tertiary xylem, primary and secondary phloem, cambium, primary and secondary xylem (). At the stage of a mature young root, the cortical region consisted of five to six layers of thin-walled, isodiametric parenchyma cells with small intercellular spaces ((a–d)). According to measurements of general anatomical root traits (), the average areas of the cortex regions were similar in size in control and A. chroococcum-inoculated plants, as well as the number of parenchyma cells. The mean area of the largest parenchyma cells and vessels, area of primary and secondary xylem was significantly higher in A.chroococcum-inoculated plants (). At the stage of 21 days after emergence ((a–f)), the cambium had been formed, and the massive secondary growth was presented in both root treatments ((e,f)). The increase in beetroot size is mainly due to the activity of the cambium, which throws out cell division and expansion processes. Secondary growth initiated by cambium occurs on the inner parts of the phloem, precisely embedded between the primary, secondary phloem, and primary, secondary xylem ((e,f)). In the very central part of the vascular cylinder remains the rest of the primary tissue, composed of xylem, and represents the remains of a diarch vascular bundle in the beetroots.
Figure 2. Transversal sections of roots of Beta vugaris L. Azotobacter inoculated (a, c, e) and control (b, d, f) root reveals the massive secondary growth, and initiation of tertiary thickening. Detailed view in the cortex (a–d); detailed view in the central cylinder (e, f). e-epidermis; co-cortex, pph-primary phloem; px-primary xylem, sx-secondary xylem, sph- secondary phloem; tx-tertiary xylem, sph- secondary phloem; ca-cambium. Bars on (A, B) indicated 200 µm, on (C–F) indicated 100 µm.
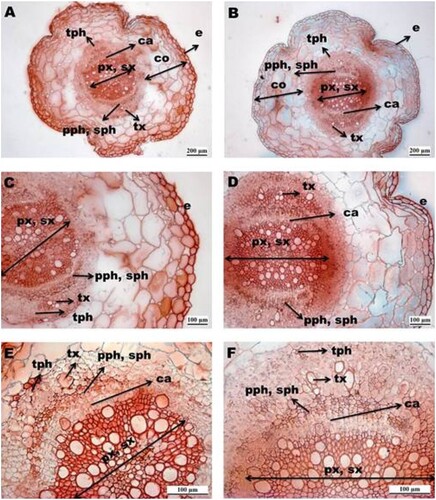
Table 5. Measurements of general anatomical root traits.
Discussion
PGPR properties of A. chroococcum F8/2
The presented study showed the biopriming potential of A. chroococcum, a widespread free-living and nitrogen-fixing bacteria used as a biofertilizer for more than a century. A. chroococcum F8/2 was confirmed as multitasking PGPR capable to regulate the hormonal status of plants through the production of IAA and ACC, solubilize inorganic forms of potassium, and mineralize nitrogen and phosphorus organic compounds. The presence of this isolate with numerous PGP features resulted in increased seedlings length and a significant increase in the mean area of vessels.
In this study, A. chroococcum F8/2 showed an impact on the nutritional cycles of N, P, K, and Fe, elements crucial for plant physiology. Biological nitrogen fixation (BFN) is the crown of its PGPR activity, which ensures the inclusion of atmospheric nitrogen as its cheapest source in sustainable plant production. The isolated strain clearly shows the ability to produce ammonia from organic sources as an additional way of supplying nitrogen available to the plants. The contribution of nitrogen fixation is quantified by Rodrigues et al. (Citation2018) who demonstrated an additional N-fixing amount of 11.40 kg ha−1 by including Azotobacter as a biofertilizer in the field experiment. The presence of nitrogen in the optimal ranges ensures the promotion of the quantitative and qualitative traits of the root, directly affecting the sucrose concentration (Mahmoud et al. Citation2012) while nitrogen input through mineral fertilizer, results in an increase in green mass, adversely affecting the technical quality of raw sugar, and reducing sucrose content (Prvulović et al. Citation2009; Mahmoud et al. Citation2012). Therefore, the use of A. chroococcum F8/2 as a free nitrogen fixer involved in ammonification processes could be a sustainable solution to achieve a balance between the need and availability of nitrogen through all stages of sugar beet development.
Even though Azotobacter species are characterized as phosphate-solubilizing bacteria (Aasfar et al. Citation2021) our strain showed no ability to solubilize tricalcium phosphate in NPRIP medium. Similarly, Husen (Citation2003) reported Azotobacter vinelandii Mac 259 was not able to solubilize tricalcium phosphate. On the other side, the tested strain was confirmed as a producer of acid and alkaline phosphatase (), indicating involvement in the conversion of organic phosphorous compounds to plant-available forms. This is especially important considering the fact that phosphorus deficiency mainly occurs in the early stages of plants’ growth. In the case of sugar beet, it leads to the formation of stunned, stiff plants, and yield reduction (Sims and Smith Citation2001).
Sugar beet, like all ‘potassium-loving’ plants, has high demand for this nutrient (Barłóg et al. Citation2018). Potassium is important for the synthesis, transport of photosynthetic assimilates, and storage of sugar in the sugar beet root (Mubarak et al. Citation2016) and this fact gives special importance to the ability of A. chroococcum F8/2 to solubilize potassium salts. The representatives of Azotobacter sp. are already recognized as potassium-solubilizing bacteria capable to improve potassium absorption by plants (Aasfar et al. Citation2021).
The presence of the iron-chelating feature of A. chroococcum F8/2 raises the value of this priming agent considering iron deficiency as common in plant nutrition since it is present in the soil in poorly soluble forms inaccessible to plants. Azotobacter sp. is known for producing siderophores which are significant not only as iron-chelating compounds but also contribute to antifungal activity of Azotobacter sp. (Aasfar et al. Citation2021).
A comprehensive review regarding Azotobacter sp. (Aasfar et al. Citation2021) provides insight into literature data that confirm stimulation of nutrient uptake of plants after Azotobacter sp. application. Mahato and Kafle (Citation2018) reported an improvement in N, P, Fe, and Zn uptake in wheat after seed inoculation with Azotobacter sp. Also, an increase in protein and carbohydrate content was recorded in two varieties of maize after seeds were inoculated with A. chroococcum (Kizilog et al. Citation2001).
Besides the role of mediator in nutrient cycles, A. chroococcum F8/2 represents a significant factor in phytohormonal modulation thanks to the ability to produce IAA and ACC deaminase. Some authors claim that the stimulation of plant growth is more a result of this feature than N fixation of Azotobacter sp. (Aasfar et al. Citation2021). Rajaee et al. (Citation2007) stated that even though A. chroococcum applied in its study was marked as BNF, HCN, and siderophore producer, the key feature responsible for enhanced nutrient absorption, leaf area, seeds weight, protein content, and yield of wheat was IAA synthesis. The strain tested in this study produced IAA in the concentration of 10 µg ml−1 which is in line with the previous research results. Ahmad et al. (Citation2008) confirmed the ability of Azotobacter strains to produce IAA in a range of 1.27–13.47 µg ml−1. Bjelic et al. (Citation2015) confirmed that all tested strains synthesize IAA in various concentrations up to 50.38 µg ml−1. Microbial phytohormones crucially affect plant physiology and development of not only shoots but also the root architecture and morphology (Egamberdieva et al. Citation2017; Ristova et al. Citation2018; Xu et al. Citation2018). Our results show that A. chroococcum F8/2 modulates the sugar beet root characteristics and increases its functionality. Only small doses of auxin lead to growth promotion (Eliasson et al. Citation1989). Auxin in higher concentrations has a toxic effect since it stimulates the synthesis of ethylene, which in high concentrations inhibits root elongation and sometimes leads to plant death (Glick et al. Citation2007). This is the reason why the PGPR ability to synthesize the ACC deaminase, enzyme decreasing higher concentrations of ethylene, is crucial in the rhizosphere. The ACC test result of A. chroococcum F 8/2 reinforces the view that Azotobacter sp. can modulate ACC concentration, which was also confirmed by previous studies (Omer et al. Citation2016; Viscardi et al. Citation2016). Yousefi et al. (Citation2017) confirmed that inoculating Dodonaea viscosa L. seeds with A. chroococcum resulted in increased germination rates of 88 and 316%, promotion of root and steam fresh and dry weight, as well as the ratio between length and dry mass of root and shoot, emphasizing the role of microbial ACC deaminase in mitigating the negative impact of abiotic stress.
A. chroococcum F8/2 ability to produce EPS implies its potential role in raising the stability of soil aggregates (Arfarita et al. Citation2016), consequently creating a better environment for plant development (Alami et al. Citation2000). This is especially relevant in intensive sugar beet cultivation, where intensive tillage and heavy mechanization have a detrimental impact on soil compaction, and cause soil aggregate disruption. In addition, EPS protect microbial cells from dehydration, antibiotic action, the influence of osmotic stress, bacteriophages, and toxic compounds (Sánchez et al. Citation2006).
Multifunctional PGP activity of A. chroococcum demonstrated in this study is in accordance with previous findings (Ahmad et al. Citation2008; Bjelic et al. Citation2015; Jain et al. Citation2021) confirming exceptional PGPR properties of this genus.
Influence of A. chroococcum F8/2 on sugar beet germination
Considering the significance of germination on final yield, several germination parameters were addressed in this work. FGP, MGT, and Vigor I all demonstrated statistically significant differences between A. chroococcum F8/2 biopriming and control treatment (). The FGP value of the control treatment was higher in comparison to the biopriming treatment. However, both percentages were significantly lower than the germination standard of 96%, set in Serbia. On the contrary, the percentage of emerged plants in the substrate was high. All of the inoculated seeds emerged, while the emergence in the control treatment was lower by 7% (data not shown). Recent studies (El-Keblawy et al. Citation2018; Jovičić-Petrović et al. Citation2021) reported higher FGP in substrate when compared to the filter paper method. The better microclimate of the substrate, primarily the air–water regime, could be an explanation (Jovičić-Petrović et al. Citation2021). Despite being an important parameter of germination, FGP does not ultimately prove the germination success (Kader Citation2005). MGT is a day-based parameter that indicates average lag time between the beginning of imbibition and the appearance of the radicle relating to germination uniformity (Matthews and Khajeh-Hosseini Citation2006). The higher MGT, the worse uniformity is. MGT of the inoculated seeds compared to the control indicated that A. chroococcum F8/2 had beneficial effect (). The increased length of seedlings in the inoculation treatment vs. control resulted in a significant increase in Vigor I of 90.99%. This can be attributed to the bacterial auxin since it promotes cell elongation and division (Baca and Elmerich Citation2007), as well as to the enhanced mobilization of nutrients contained in seed (Bákonyi et al. Citation2013).
Production of VOCs by A. chroococcum F8/2
Many PGPB VOCs were determined during the last decade (Tahir et al. Citation2017; Kumar et al. Citation2020), but little is known about these compounds metabolized by the Azotobacter genus.
Due to their biochemical properties, VOCs easily pass through biological membranes and initiate physiological processes (Ortíz-Castro et al. Citation2009; Cappellari et al. Citation2019). They can serve as plant’s nutrients, promote phytohormone production and contribute to increased tolerance to biotic and abiotic stress (Fincheira and Quiroz Citation2018). The experiment confirmed VOCs production by A. chroococcum F8/2 (Table, ). Ethanol was among the dominant components of the volatile blend (47.67%), which can be explained by high sugar content in medium (20 g/l). Similar effect can be observed as a result of the increase in available sugar in the rizosphere, originated from root exudates (Jones et al. Citation2004), which may affect the properties of VOCs of bacterial strains. Perry and Perry (Citation2019) detected ethanol as an active VOC of Bacillus licheniformis and reviewed its role as a part of the VOCs mixture involved in pathogen inhibition and germination improvement. The authors also emphasized the importance of ethanol as an intermediary in ethylene degradation by soil microbes and as a precursor for the other VOCs. The content of 3-methyl-1-butanol in the A. chroococcum F8/2 metabolized blend was 17.32% (). Fialho et al. (Citation2011) showed that derivatives of 1-butanol, 3-methyl-1-butanol, 2-methyl-1-butanol and 1-butanol, 3-methyl acetate inhibit the growth of Sclerotinia sclerotiorum, contributing to the biocontrol activity. Previous studies identified 3-methyl-butanol and 2-methyl-propanol as the main components of the volatile blend of Bacillus subtilis GB03, Bacillus amyloliquefaciens IN937 (Farag et al. Citation2006), and PGP Fungi Phoma sp. GS8-3 (Naznin et al. Citation2013). Under low-oxygen conditions (i.e. bacteria in the rhizosphere), Proteobacteria and Firmicutes produce short-chain alcohols (Farag et al. Citation2013) to provide an alternative electron sink when aerobic respiration is limited from pyruvate (Xiao and Xu Citation2007). A. chroococcum F8/2 produced 32.01% of 2-methyl-propanol as shown in . In different proportions with other volatile compounds (3-methyl-butanol and 2-methyl-propanol), a positive effect on tobacco growth was achieved (Naznin et al. Citation2013). Acetoin (3-hydroxy-2-butanone) and its oxidized form 2,3-butanedione are responsible for stimulating plant growth (Ryu et al. Citation2004). Only 0.93% of acetoin and 0.56% of 2,3-butanedione was produced by A. chroococcum F8/2 () but it is not neglectable since lower concentrations of VOCs have a greater effect on plant growth than high concentrations (Naznin et al. Citation2013).
Hence, it can be assumed that the VOCs formed by the metabolic activity of A. chroococcum F8/2 may act as plant growth inducers. This study found that the exposure of the sugar beet seedling to the effect of A. chroococcum F8/2 resulted in 53.24% increase in the fresh weight (), but not statistically significant increase in dry weight. Such effect can be the result of better water uptake enabled by promoted development of conductive tissue.
Beneficial effects of the A. chroococcum F8/2 mechanisms on the internal sugar beetroot tissues at the early stages of its development
According to beetroot micromorphological measurements (), the A. chroococcum inoculation treatment did not reveal substantial differences in the total root area and maximum root diameter, as well as the area of the cortical region, probably as a consequence of the higher proportion of cortical region in the whole root compared with vascular tissue. Contrary to that, A. chroococcum inoculated roots bring up the following beneficial points: compared with control roots, there was a significant increase in the whole area of primary and secondary xylem, which means the area of vessels, compared with a significant increase in the mean area of vessels. Only in two studies (El-Afry Citation2012; Hegazi et al. Citation2015), in the case where coriander stem and wheat leaves were inoculated by A. chrocooccum and Pseudomonas sp., the similar results were recorded. El-Afry (Citation2012) noticed an increase in leaf xylem vessel diameter in inoculated wheat, while Hegazi et al. (Citation2015) recorded an increase in stem xylem diameter as well as thickness of phloem tissue and vascular bundle width compared with the control coriander plants. The mechanism of xylogenesis in roots is of specific interest because it determines the diameter of xylem vessels, which are central to the efficiency of water and nutrient transport from roots to the leaves and may strongly determine plant growth and yield (Sorce et al. Citation2013). Among root micromorphological traits, larger xylem vessels and their cross-sectional areas were positively associated with nutrient availability and uptake rates (Comas et al. Citation2002; Bowsher et al. Citation2016) as well as high hydraulic conductivity (Solar et al. Citation2006), which may be particularly important for water and nutrient transport. Considering that Azotobacter improves the availability of iron, potassium, and nitrogen (), the observed effects could be reasons for improved nutritional status. This indicates that A. chroococcum inoculated roots could evolve high nutrient concentrations and high water transport capacity, supporting fast metabolic rates of the plants. Also, several plant growth regulators, such as auxin (IAA), gibberellins mainly together with auxin, cytokinins, brassinosteroids, etc. have a crucial role in the promotion of root overall growth, cambium division, and differentiation in primary and secondary vascular tissues and development, mainly xylem (Sorce et al. Citation2013; Bhalerao and Fischer Citation2014; Immanen et al. Citation2016). Based on micromorphological analysis (), it could be that a higher concentration of IAA intends to stimulate cambium through enhanced cell division and xylem differentiation in A.chroococcum treatment compared to the control plants. The IAA has a role in increasing the incorporation of the radioactive isotope of carbon (known as carbon-14) into the cell wall, which is important during the root maturation stage when there is secondary wall apposition and lignification of cell walls in xylem tissue, a phase known as cell differentiation (Sorce et al. Citation2013; Johnsson et al. Citation2019). Contrary to previous, auxins are also known to inhibit primary root growth by limiting cell elongation (Vaseva et al. Citation2018), an effect that is most likely mediated by ethylene, which has been observed in both roots and shoots (Vanstraelen and Benková Citation2012). The additional exogenous auxin of A. chroococcum F8/2 has an impact on the higher rate of xylem differentiation and its growth compared with control beetroots. An increase in auxins (with or without ethylene increase) could inhibit overall cell expansion in the cortical region both in control and inoculated roots. A. chroococcum F8/2 showed the capability to decrease the concentration of the ethylene precursor ACC, which was previously shown to inhibit root cell elongation (Staal Citation2011). Our results could also be confirmed by an investigation given by Vaseva et al. (Citation2018), where it was found that ethylene restricts plant growth by its effect on auxins in the epidermal and neighboring cortical cells, suggesting that ethylene is capable of inhibiting the growth of the expanding root cells.
Conclusion
The role of an active mediator in the nutritional cycle of N, K, Fe, participation in phytohormone modulation, as well as the promotional effect of synthesized VOCs, confirmed the superior PGPR properties of A. chroococcum F8/2. The early establishment of the plant-microbe interaction during sugar beet biopriming showed beneficial effects on the structural changes at the level of root parenchyma and promoted germination. To the authors’ best knowledge, our study represents the first report on micromorphological examination concerning the early root growth of sugar beet inoculated with Azotobacter. We got evidence that inoculation with A. chroococcum F8/2 raises nutrient availability and its transport through a significant increase in the primary and secondary xylem area and vessel size.
Therefore, as a biopriming agent, it could contribute to solving some of the problems inherent in the intensive production of sugar beet. Obtained results indicate that A. chroococcum F8/2 could be used as an element of a solution for specific challenges in modern agriculture.
Supplemental Material
Download MS Word (88.9 KB)Acknowledgements
The authors would like to express gratitude to Mr Radenko Radošević, from the Faculty of Agriculture, University of Belgrade, for his technical assistance in preparing microslides.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors

Slavica Kerečki
Slavica Kerečki is a PhD student at the Department of Environmental Microbiology,University of Belgrade. Her research interests include plant-PGPR interactions.

Ilinka Pećinar
Ilinka Pećinar is an associate professor at the Department for Agrobotany, Faculty of Agriculture, University of Belgrade. Many of her papers are dealing with macro and micromorphological observation of plant development and different approach to Raman spectroscopy application in plant science, especially the localization of plant metabolites in different plant organs.

Vera Karličić
Vera Karličić is a research associate at the Department for Environmental Microbiology, Faculty of Agriculture, University of Belgrade. The main research interests are plant growth-promoting microorganisms, plant-microbe interactions, biocontrol, and soil science.

Nemanja Mirković
Nemanja Mirković is a teaching assistant at the Department for Industrial Microbiology, Faculty of Agriculture, University of Belgrade. He has theoretical and experimental expertise in the food microbiology and basic methods for detection and characterization of food microorganisms and clinical pathogens.

Igor Kljujev
Igor Kljujev is an associate professor at the Department for Environmental Microbiology, Faculty of Agriculture, University of Belgrade. His main research interests are microbe-plant interaction, colonization plants by human pathogen, food safety.

Vera Raičević
Vera Raičević is a full professor, head of the Department for Environmental Microbiology, Faculty of Agriculture, University of Belgrade. Her main research interests are soil microbial diversity, plant growth-promoting microorganisms, and microbes in sustainable agriculture.

Jelena Jovičić-Petrović
Jelena Jovičić-Petrović is an associate professor at the Department for Environmental Microbiology, Faculty of Agriculture, University of Belgrade. Her main research interests are biological control, plant growth-promoting microorganisms, and plant-microbe interactions.
References
- Aasfar A, Bargaz A, Yaakoubi K, Hilali A, Bennis I, Zeroual Y, Meftah Kadmiri I. 2021. Nitrogen fixing azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front Microbiol. 12:628379.
- Ahmad A, Ahmad I, Khan MS. 2008. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res. 163(2):173–118.
- Alami Y, Achouak W, Marol C, Heulin T. 2000. Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl Environ Microbiol. 66(8):3393–3398.
- Alexander DB, Zuberer DA. 1991. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Ferti Soils. 12(1):39–45.
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25(17):3389–3402.
- Anderson GR. 1985. Ecology of Azotobacter in soils of the Palouse region: I. Occurrence. Soil Sci. 86:57–62.
- Arfarita N, Hidayati N, Rosyidah A, Machfudz M, Higuchi T. 2016. Exploration of indigenous soil bacteria producing-exopolysaccharides for stabilizing of aggregates land potential as biofertilizer. J Degrade Min Lands Manage. 4(1):697–702.
- Baca BE, Elmerich C. 2007. Microbial production of plant hormones. In: Elmerich C, Newton WE, editors. Associative and endophytic nitrogen-fixing bacteria and cyanobacterial association. Vol. 5. Dordrecht: Springer; p. 113–143.
- Bákonyi N, Bott S, Gajdos E, Szabó A, Jakab A, Tóth B, Makleit P, Sz V. 2013. Using biofertilizer to improve seed germination and early development of maize. Pol J Environ Stud. 22(6):1595–1599.
- Barłóg P, Szczepaniak W, Grzebisz W, Pogłodziński R. 2018. Sugar beet response to different K, Na and Mg ratios in applied fertilizers. Plant Soil Environ. 64(4):173–174.
- Bashir Z, Zargar MY, Baba ZA, Mohiddin FA. 2017. Effect of potassium and phosphorus solubilizing bacteria on growth parameters of chilli (Capsicum annuum L.) under Kashmir climatic conditions. Int J Chem Stud. 5(5):692–695.
- Bhalerao RP, Fischer U. 2014. Auxin gradients across wood-instructive or incidental? Physiol Plant. 151(1):43–51.
- Bjelic D, Marinković BJ, Tintor BB, Tančić LJS, Nastasić MA, Mrkovački BN. 2015. Screening of Azotobacter isolates for PGP properties and antifungal activity. J Nat Sci. 129:65–72.
- Bowsher AW, Mason CM, Goolsby EW, Donovan LA. 2016. Fine root tradeoffs between nitrogen concentration and xylem vessel traits preclude unified whole-plant resource strategies in Helianthus. Ecol Evol. 6(4):1016–1031.
- Čačić NA, Mrkovački NB, Mezei M, Kovačev LM. 2003. Efekat primene Azotobacter chroococcum u šećernoj repi. Field Veg Crop Res. 38:271–280.
- Calcagnile M, Tredici SM, Talà A, Alifano P. 2019. Bacterial semiochemicals and transkingdom interactions with insects and plants. Insects. 10(12):441.
- Cappellari DR, Chiappero J, Banchio E. 2019. Invisible signals from the underground: a practical method to investigate the effect of microbial volatile organic compounds emitted by rhizobacteria on plant growth. Biochem Mol Biol Educ. 47(4):388–393.
- Cappuccino JG, Sherman N. 1996. Microbiology – a laboratory manual. Menlo Park (CA): The Benjamin/Cummings Publishing Co. Inc.
- Cardoso AII, Magro FO, Oliveira JMX, Abrahão C, Tavares AEB, Fernandes DM. 2017. Accumulation of macronutrients in beetroot plant. Hortic Bras. 35(3):328–334.
- Chhikara N, Kushwaha K, Sharma P, Gat Y, Panghal A. 2019. Bioactive compounds of beetroot and utilization in food processing industry: a critical review. Food Chem. 272:192–200.
- Chomontowski C, Wzorek H, Podlaski S. 2020. Impact of sugar beet seed priming on seed quality and performance under diversified environmental conditions of germination, emergence, and growth. J Plant Growth Regul. 39(4):183–189.
- Comas LH, Bouma TJ, Eissenstat DM. 2002. Linking root traits to potential growth rate in six temperate tree species. Oecologia. 132(1):34–43.
- de Boer W, Li X, Meisner A, Garbeva P. 2019. Pathogen suppression by microbial volatile organic compounds in soils. FEMS Microbiol Ecol. 95(8):fiz105.
- Egamberdieva D, Wirth SJ, Alqarawi AA, Abd_Allah EF, Hashem A. 2017. Phytohormones and beneficial microbes: essential components for plants to balance stress and fitness. Front Microbiol. 8:2104.
- El-Afry MM. 2012. Anatomical studies on drought-stressed wheat plants (Triticum aestivum L.) treated with some bacterial strains. Acta Biol Szeged. 56(2):165–174.
- Eliasson L, Bertell G, Bolander E. 1989. Inhibitory action of auxin on root elongation not mediated by ethylene. Plant Physiol. 91(1):310–314.
- El-Keblawy A, Gariola S, Elsheikh EAE, Hussain MI, Abhilash PC. 2018. Variability in seed germination behavior of six grasses under laboratory and natural field conditions: implications for restoration of degraded lands in subtropical arid deserts. Trop Ecol. 59(4):715–726.
- Farag MA, Ryu CM, Sumner LW, Paré PW. 2006. GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry. 67(20):2262–2268.
- Farag MA, Zhang H, Ryu CM. 2013. Dynamic chemical communication between plants and bacteria through airborne signals: induced resistance by bacterial volatiles. J Chem Ecol. 39(7):1007–1018.
- Fialho MB, Moraes MHDT, Annelise R, Pascholati SF. 2011. Potential of antimicrobial volatile organic compounds to control Sclerotinia sclerotiorum in bean seeds. Pesqui Agropecu Bras. 46(2):137–142.
- Fincheira P, Quiroz A. 2018. Microbial volatiles as plant growth inducers. Microbiol Res. 208:63–75.
- Gandhi A, Muralidharan G. 2016. Assessment of zinc solubilizing potentiality of Acinetobacter sp. isolated from rice rhizosphere. Euro J Soil Biol. 76:1–8.
- Glick BR, Todorovic B, Czarny J, Chen Z, Duan J, McConkey B. 2007. Promotion of plant growth by bacterial ACC deaminase. Plant Sci. 26(5‒6):227–242.
- Hegazi MA, Metwaly MMS, Belal EB. 2015. Influence of plant growth promoting bacteria (PGPR) on coriander (Coriandrum sativum) and dill (Anethum graveolens L.) plants. J Plant Prod. 6(2):205–218.
- Helmut BF, Widmer W, Sigler V, Zeyer J. 2004. New molecular screening tools for analysis of free-living diazotrophs in sil. Appl Environ Microbiol. 70:240–247.
- Hoffmann CM, Kenter C. 2018. Yield potential of sugar beet – have we hit the ceiling? Front Plant Sci. 9:289.
- Hopwood D, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, Ward JM, Schrempf H. 1985. Genetic manipulation of Streptomyces: a laboratory manual. Norwich: The John Innes Foundation. doi:10.3390/agronomy10111683.
- Husen E. 2003. Screening of soil bacteria for plant growth promotion activities in vitro. Indonesian Agricultural Sciences. 4(1):27–31.
- Immanen J, Nieminen K, Smolander OP, Kojima M, Serra JA, Koskinen P, Helariutta Y. 2016. Cytokinin and auxin display distinct but interconnected distribution and signalling profiles to stimulate cambial activity. Curr Biol. 26(15):1990–1997.
- Jain D, Kaur G, Bhojiya AA, Chauhan SP, Khandelwal SK, Meena RH, Rajpurohit D, Mohanty SR. 2021. Phenetic characterization of nitrogen fixing Azotobacter from rhizospheric soil of Southern Rajasthan. J Pure Appl Microbiol. 15(1):428–436.
- Jiménez DJ, Montaña JS, Martínez MM. 2011. Characterization of free nitrogen fixing bacteria of the genus Azotobacter in organic vegetable-grown Colombian soils. Braz J Microbiol. 42(3):846–858.
- Johnsson C, Jin X, Xue W, Dubreuil C, Lezhneva L, Fischer U. 2019. The plant hormone auxin directs timing of xylem development by inhibition of secondary cell wall deposition through repression of secondary wall NAC-domain transcription factors. Physiol Plant. 165(4):673–689.
- Jolayemi OL. 2019. Enhancing sugar beet’s early growth and establishment by using protein-based biostimulants. Introductory paper at the Faculty of Landscape Architecture Horticulture and Crop Production Science. Swedish University of Agricultural Science. 2019:2.
- Jones DL, Hodge A, Kuzyakov Y. 2004. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 163(3):459–480.
- Jouannet V, Brackmann K, Greb T. 2015. (Pro)cambium formation and proliferation: two sides of the same coin? Curr Opin Plant Biol. 23:54–60.
- Jovčić B, Begović J, Lozo J, Topisirović L, Kojić M. 2009. Dynamics of sodium dodecyl sulfate utilization and antibiotic susceptibility of strain Pseudomonas sp. ATCC1915. Arch Biol Sci. 61(2):159–164.
- Jovičić-Petrović J, Karličić V, Petrović I, Ćirković S, Ristić-Djurović JL, Raičević V. 2021. Biomagnetic priming-possible strategy to revitalize old mustard seeds. Bioelectromagnetics. 42(3):238–249. doi:10.1002/bem.22328.
- Kader MA. 2005. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J Proc R Soc NSW. 138(1):65–75.
- Kerecki S, Jovičić- Petrović j, Kljujev I, Lalević B, Karličić V, Petrović I, Raičević V. 2021. Biopriming: a sustainable support for crop establishment. Proceedings of the XII International Scientific Agricultural Symposium ‘Agrosym 2021’. Jahorina, Bosnia and Herzegovina [accessed 2021 October 7].
- Khan N, Ali S, Tariq H, Latif S, Yasmin H, Mehmood A, Shahid MA. 2020. Water conservation and plant survival strategies of rhizobacteria under drought stress. Agronomy. 10(11):1683.
- Khatoon Z, Huang S, Rafique M, Fakhar A, Kamran MA, Santoyo G. 2020. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J Environ Manage. 273:111118.
- Kizilog IU, Bilen FT, Ataplu N. 2001. Effect of inoculation eight Azotobacter chroococcum and nitrogen fertilizer on plant growth of corn (Zea mays) carbohydrate and protein ccontents. Ziraat Fakultesi Dergisi Ataturk Universitasi. 32:215–221.
- Kumar A, Singh S, Gaurav AK, Srivastava S, Verma JP. 2020. Plant growth-promoting bacteria: biological tools for the mitigation of salinity stress in plants. Front Microbiol. 7(11):1216.
- Kuzevski J, Mrkovački N, Čačić N, Bjelić D, Miljakovic D. 2011. Effect of Azotobacter croococcum on productive traits and microorganisms in sugar beet rhizosphere. Ratar Povrt. 48(2):383–390. doi:10.5937/ratpov1102383K.
- Li Z, Chang S, Lin L, Li Y, An Q. 2011. A colorimetric assay of 1-aminocyclopropane-1-carboxylate (ACC) based on ninhydrin reaction for rapid screening of bacteria containing ACC deaminase. Lett Appl Microbiol. 53(2):178–185.
- Liu Y, Gao J, Bai Z, Wu S, Li X, Wang N, Du X, Fan H, Zhuang G, Bohu T, Zhuang X. 2021. Unraveling mechanisms and impact of microbial recruitment on oilseed rape (Brassica napus L.) and the rhizosphere mediated by plant growth-promoting Rhizobacteria. Microorganisms. 9(1):161.
- Mahato S, Kafle A. 2018. Comparative study of Azotobacter with or without other fertilizers on growth and yield of wheat in Western hills of Nepal. Ann Agrar Sci. 16(3):250–256.
- Mahmoud EA, Hassanin MA, Emara IRE. 2012. Effect of organic and mineral nitrogeneous fertilizers and plant density on yield and quality of sugar beet (Beta vulgaris L.). Egypt J Agron. 34(1):89–103.
- Mal D, Verma J, Levan A, Reddy MR, Avinash AV, Velaga PK. 2019. Seed priming in vegetable crops. Int J Curr Microbiol Appl Sci. 8(6):868–867.
- Matthews S, Khajeh-Hosseini M. 2006. Mean germination time as an indicator of emergence performance in soil of seed lots of maize (Zea mays). Seed Sci Technol. 34(2):339–347.
- Miloševic N, Tintor B, Protic R, Cvijanovic G, Dimitrijevic T. 2012. Effect of inoculation with Azotobacter chroococcum on wheat yield and seed quality. Rom Biotechnol Lett. 17(3):7352–7357.
- Mirshekari B, Hokmalipour S, Sharifi RS, Farahvash F, Gadim A. 2012. Effect of seed biopriming with plant growth promoting rhizobacteria (PGPR) on yield and dry matter accumulation of spring barley (Hordeum vulgare L.) at various levels of nitrogen and phosphorus fertilizers. J Food Agric Environ. 10(3-4):314–320.
- Mrkovački N, Mezei S. 2003. Primena sojeva Azotobacter chroococcum – NS Betafixina u gajenju šećerne repe. Ratar Povrt. 39:49–58.
- Mrkovacki NB, Bijelić DĐ, Maksimovic LL, Čurčić ŽP, Ćirić MZ, Živanov MS. 2016. The effect of inoculation with Azotobacter chroococcum on microorganisms in rhizosphere and sugar beet yield in organic farming. J Nat Sci. 130:45–52.
- Mubarak M, Zahir M, Ahmad S, Wakeel A. 2016. Sugar beet yield and industrial sugar contents improved by potassium fertilization under scarce and adequate moisture conditions. J Inter Agric. 15(11):2620–2626.
- Nautiyal CS. 1999. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 170(1):265–270.
- Naznin A, Kimura M, Miyazawa IM, Hyakumachi M. 2013. Analysis of volatile organic compounds emitted by plant growth-promoting fungus Phoma sp. GS8-3 for growth promotion effects on tobacco. Microbes Environ. 28(1):42–49.
- Nosrati R, Owlia P, Saderi H, Rasooli I, Malboobi MA. 2014. Phosphate solubilization characteristics of efficient nitrogen fixing soil Azotobacter strains. Iran J Microbiol. 6(4):285–295.
- OECD/FAO Agricultural Outlook 2017-2026. 2017. Paris: OECD. doi:10.1787/agr_outlook-2017-en.
- Omer A, Emara H, Zaghloul R, Abdel M, Dawam G. 2016. Potential of Azotobacter salinestris as plant growth promoting rhizobacteria under saline stress conditions. Res J Pharm Biol Chem Sci. 7(6):2572–2583.
- Ortíz-Castro R, Contreras-Cornejo HA, Macías-Rodríguez L, López-Bucio J. 2009. The role of microbial signals in plant growth and development. Plant Signal Behav. 4(8):701–712.
- Patten CL, Glick BR. 2002. Role of Pseudomonas putida indolacetic acid in development of the host plant root system. Appl Environ Microbiol. 68(8):3795–3801.
- Paulo EM, Vasconcelos MP, Oliviera IS, Affe HMJ, Nascimento R, Melo IS, Rogue MRA, Assis SA. 2012. An alternative method for screening lactic bacteria for the production of exopolysacharides with rapid confirmation. Food Sci Technol. 32(4):710–714.
- Penrose DM, Glick BR. 2003. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant. 118(1):10–15.
- Perry G, Perry D. 2019. Bacillus induced to biosintetize VOC and nitriles may benefit in agriculture. Peer J Preprints. doi:10.7287/peerj.preprints.2611v2.
- Poindexter S. 2001. Managing sugar beet cyst nematode. East Lansing: Michigan State University Extension. https://www.canr.msu.edu/news/managing_sugar_beet_cyst_nematode.
- Prvulović D, Popović M, Malenčić D, Marinković B, Jaćimović G. 2009. Effects of nitrogen fertilization on the biochemical and physiological parameters in leaves and root of sugar beet associated with Azotobacter chroococcum. J Plant Nutr. 33(1):15–26.
- Rajaee S, Alikhani HA, Raiesi F. 2007. Effect of plant growth promoting potentials of Azotobacter chroococcum native strains on growth, yield and uptake of nutrients in wheat. JWSS. 11(41):285–297.
- Rajawat MVS, Singh S, Tyagi SP, Saxena AK. 2016. A modifed plate assay for rapid screening of potassium-solubilizing bacteria. Pedosphere. 26(5):768–773.
- Regulation (EU). 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003 (Text with EEA relevance) (OJ L 170) 25.06.2019, p. 1, ELI: http://data.europa.eu/eli/reg/2019/1009.
- Ristova D, Giovannetti M, Metesch K, Busch W. 2018. Natural genetic variation shapes root system responses to phytohormones in Arabidopsis. Plant J. 96(2):468–481.
- Rodrigues CM, Müdsam C, Keller I, Zierer W, Czarnecki O, Corral JM, Reinhardt F, Nieberl P, Fiedler-Wiechers K, Sommer F, et al. 2020. Vernalization alters sink and source identities and reverses phloem translocation from taproots to shoots in sugar beet. Plant Cell. 32(10):3206–3223.
- Rodrigues MA, Ladeira LC, Margarida Arrobas M. 2018. Azotobacter-enriched organic manures to increase nitrogen fixation and crop productivity. Euro J Agron. 93:88–94.
- Ruzin SE. 1999. Plant micro technique and microscopy. Oxford: Oxford University Press.
- Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Paré PW. 2004. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 134(3):1017–1026.
- Sánchez JI, Martínez B, Guillén R, Díaz RJ, Rodríguez A. 2006. Culture conditions determine the balance between two different exopolysaccharides produced by Lactobacillus pentosus LPS26. Appl Environ Microbiol. 72(12):7495–7502.
- Schulz-Bohm K, Martín-Sánchez L, Garbeva P. 2017. Microbial volatiles: small molecules with an important role in intra- and inter-kingdom interactions. Front Microbiol. 8:2484.
- Setiawati TC, Mutmainnah L. 2016. Solubilization of potassium containing mineral by microorganisms from sugarcane rhizosphere. Agric Agric Sci Procedia. 9(2016):108–117.
- Sharifi RS. 2012. Study of nitrogen rates effects and seed biopriming with PGPR on quantitative and qualitative yield of safflower (Carthamus tinctorius L.). Tech J Eng Appl Sci. 2:162–166.
- Sharifi RS, Khavazi K. 2011. Effects of seed priming with plant growth promotion rhizobacteria (PGRP) on yield and yield attribute of maize (Zea mays L.) hybrids. J Food Agric Environ. 9:496–500.
- Sims AL, Smith LJ. 2001. Early growth response of sugarbeet to fertilizer phosphorus in phosphorus deficient soils of the Red River Valley. J Sugar Beet Res. 38(1):1–17.
- Solar LI, Pernice F, De Jong TM. 2006. The relationship of hydraulic conductance to root system characteristics of peach (Prunus persica) rootstocks. Physiol Plant. 128(2):324–333.
- Sood M, Kumar V, Rawa R. 2021. Seed biopriming a novel method to control seed borne diseases of crops. In: Jogaiah S, editor. Biocontrol agents and secondary metabolites. Duxford, Cambridge, MA: Woodhead; p. 118–223.
- Sorce C, Giovannelli A, Sebastiani L, Anfodillo T. 2013. Hormonal signals involved in the regulation of cambial activity, xylogenesis and vessel patterning in trees. Plant Cell Rep. 32(6):885–898.
- Staal M. 2011. Apoplastic alkalinization is instrumental for the inhibition of cell elongation in the Arabidopsis root by the ethylene precursor 1-aminocyclopropane-1- carboxylic acid. Plant Physiol. 155(4):2049–2055.
- Sumbul R, Ansari A, Rizvi R, Mahmood I. 2020. Azotobacter: a potential bio-fertilizer for soil and plant health management. Saudi J Biol Sci. doi:10.1016/j.sjbs.2020.08.004.
- Tahir H, Gu Q, Wu H, Niu Y, Huo R, Gao X. 2017. Bacillus volatiles adversely affect the physiology and ultra-structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Sci Rep. 7:40481.
- Vanstraelen M, Benková E. 2012. Hormonal interactions in the regulation of plant development. Annu Rev Cell Dev Biol. 28:463–487.
- Vaseva II, Qudeimat E, Potuschak T, Du Y, Genschik P, Vandenbussche F, Van Der Straeten D. 2018. The plant hormone ethylene restricts Arabidopsis growth via the epidermis. Proc Natl Acad Sci. 115(17):4130–4139.
- Viscardi S, Ventorino V, Duran P, Maggio A, De Pascale S, Mora ML, Pepe O. 2016. Assessment of plant growth promoting activities and abiotic stress tolerance of Azotobacter chroococcum strains for a potential use in sustainable agriculture. J Soil Sci Plant Nutr. 16(3):848–863.
- Wani PA, Khan MS, Zaidi A. 2007. Co-inoculation of nitrogen fixing and phosphate solubilizing bacteria to promote growth, yield and nutrient uptake in chickpea. Acta Agron Hung. 55(3):315–323.
- Xiao Z, Xu P. 2007. Acetoin metabolism in bacteria. Crit Rev Microbiol. 33(2):127–140.
- Xu L, Wu C, Oelmüller R, Zhang W. 2018. Role of phytohormones in Piriformospora indica-induced growth promotion and stress tolerance in plants: more questions than answers. Front Microbiol. 9:1646.
- Xu YY, Lu H, Wang X, Zhang KQ, Li GH. 2015. Effect of volatile organic compounds from bacteria on nematodes. Chem Biodivers. 12(9):1415–1421.
- Yousefi S, Kartoolinejad D, Bahmani M, Naghdi R. 2017. Effect of Azospirillum lipoferum and Azotobacter chroococcum on germination and early growth of hopbush shrub (Dodonaea viscosa L.) under salinity stress. J Sustain For. 36(2):107–120.
