 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We used chlorophyll fluorescence technology and biochemical methods to analyze the effects of wood vinegar (WV) on the photosynthetic mechanism and reactive oxygen species metabolism of tobacco (Nicotiana tabacum L.) leaves infected by Pseudomonas syringae pv. tabaci (Pst). The results showed that Pst infection reduced the chlorophyll content and the activities of PSII and PSI, which inhibited the normal photosynthesis of tobacco leaves. However, pretreatment with WV alleviated the degradation of chlorophyll. Treatment with WV alleviated the downregulation of core gene expression in PSII and PSI and improved the photosynthetic electron transfer in Pst-infected leaves. The levels of expression of PetE, ATPa and ATPc in the Pst-infected leaves were significantly upregulated when pretreated with WV. Pst infection increased the rate of production of superoxide anions and the contents of hydrogen peroxide. WV pretreatment could eliminate the oxidative damage of Pst-infected leaves by enhancing the activities of peroxidase (POD) and glutathione peroxidase (GPx) and upregulating the levels of expression of the POD2 and GPX2 genes. In conclusion, pretreatment with WV can alleviate the photosynthetic inhibition and oxidative damage of tobacco leaves caused by Pst infection.
1. Introduction
Plant-pathogen interactions are precise and complex processes. The host plant leaf is the most important part of photosynthesis and the primary target of many pathogenic microorganisms (Steffens and Sauter Citation2009). Infection of plants with pathogens usually causes changes in physiological functions, such as the inhibition of energy metabolism, including photosynthesis (Garry et al. Citation2007; Xu et al. Citation2019; Wang, Li, et al. Citation2019) and respiration (Xue et al. Citation2018), outbreaks of reactive oxygen species (ROS) (Miguel et al. Citation2006; Camejo et al. Citation2016), damage to cellular and membrane structures (Zurbriggen et al. Citation2009; Chaves-Gómez et al. Citation2019), and the disruption of plant hormone metabolism (Kunkel and Brooks Citation2002; Jia et al. Citation2020).
The plant-pathogen Pseudomonas syringae can be divided into more than 50 pathogenic variants based on its host specificity (Cheng Citation2017). Pseudomonas syringae pv. tabaci (Pst) is one of the pathogenic variants that can induce the occurrence of tobacco wildfire. At the initial stage of infection, brown water stains appear on the leaves, which look like dots. The toxin secreted by Pst can form an obvious yellow halo around the disease spot. Bacterial discharge is produced on the disease spot under high humidity (Wang Citation2012), which will lead the plants to close their stomata (Lee et al. Citation2013) and initiate programmed cell death (Ito et al. Citation2014). At the initial stage of infection, Pst produces virulence factors, including effector proteins and secondary metabolites, which inactivate the early defense responses of plants (Turner and Taha Citation1984).
Photosynthetic inhibition is one of the main hazards caused by pathogen infections (Kottapalli et al. Citation2009). Leaf chlorosis and plant tissue necrosis eventually lead to a reduction in the accumulation of carbon assimilation products (Kocal and Sonnewald Citation2008; Kim et al. Citation2010), which manifests in the reduction of crop yield in agricultural production (Roloff et al. Citation2004; Berger et al. Citation2007; Ficke et al. Citation2018). Studies by Chen et al. (Citation2015) and Li et al. (Citation2015) showed that Pst infection reduced the photosynthetic activity of wheat (Triticum aestivum L.). Lee et al. (Citation2013) found that Pst metabolites induced stomatal closure in tobacco (Nicotiana benthamiana) leaves. Cheng et al. (Citation2016) used western blot technology to show that Pst infection significantly reduced the expression of proteins related to photosynthetic electron transport in tobacco leaves, which is also an important mechanism used by Pst to inhibit photosynthetic electron transport and even photoinhibition in tobacco leaves. ROS are inevitable by-products of biochemical processes, such as glycolysis and photosynthesis. They are considered to be important stress signals that regulate the pathway of programmed cell death (Mittler Citation2016). Under normal conditions, these molecules can be eliminated through different mechanisms of antioxidant defense at specific sites (Apel and Hirt Citation2004). However, in biotic and abiotic stresses, such as pathogen invasion, high temperature and drought and mechanical damage, the balance between plant production and removal is often disturbed, resulting in a rapid increase in the levels of ROS, such as superoxide anion () and hydrogen peroxide (H2O2) (Singh et al. Citation2016; Zhou et al. Citation2017; Chen et al. Citation2019). In the symbiotic relationship and pathogen relationship, the production of ROS is one of the earliest responses of plant tissues to pathogen attack (Doke Citation1983). Previous studies on histochemical methods have indicated that wheat stripe rust (Puccinia striiformis f. sp. tritici) can induce the generation of H2O2 and
at the seedling or adult plant stages (Wang et al. Citation2007; Zhang, Wang, et al. Citation2012). Plants also respond to stress by activating various antioxidant enzymes (Singh et al. Citation2016; Zhou et al. Citation2017). For example, Chen et al. (Citation2015) found that the activities of peroxidase (POD), catalase (CAT) and glutathione peroxidase (GPx) in wheat increased significantly after Pst infection.
Wood vinegar (WV) is a reddish-brown liquid obtained by the condensation and separation of gases from agricultural and forestry wastes after the process of dry distillation pyrolysis, and has a distinctive smoke smell and is acidic (Liu et al. Citation2016). It contains more than 200 components, including organic compounds, such as acids, phenols, aldehydes, ketones, alcohols and esters (Yang and Xue Citation2014), as well as trace elements, such as K, Ca, Mg, Zn, Mn and Fe (Wu et al. Citation2015). WV is inexpensive to produce and will not pollute the environment when reasonably applied. It is gradually being applied in various fields of agricultural production (Yang et al. Citation2007). WV can not only be used as a new type of liquid organic fertilizer but can also act as a plant growth regulator, which enables it to regulate plant growth and improve crop quality (Mungkunkamchao et al. Citation2013). In addition, wood vinegar has a significant impact on soil microbial diversity and can improve the quality of soil (Loo et al. Citation2008). The application of WV can accelerate the growth of leaves and prevent diseases and insect pests (Cao et al. Citation2017).
Tobacco is an important cash crop, and wildfire disease is one of the primary leaf diseases of tobacco, which is mainly caused by Pseudomonas syringae pv. tabaci (Pst) (Wang et al. Citation2016). This pathogen occurs all over the world and can be transmitted through air and rain (Barreneche et al. Citation1998). For a long time, chemical pesticides have been widely used to prevent and control tobacco diseases. There are problems, such as pathogen resistance, pesticide residues and environmental pollution. Therefore, it is imperative to control the application of chemical pesticides. To produce tobacco with high quality, yield and safety, and fully utilize the characteristics of WV to control tobacco diseases and pests, this study used this type of vinegar to alleviate the infection of Pst on tobacco leaves. By analyzing the relevant parameters, such as photosynthetic carbon assimilation, photochemical reactions and the metabolism of ROS, this study reveals the mechanism of WV in alleviating the photosynthetic inhibition and oxidative damage in tobacco leaves infected with Pst and provides new insights for its potential application on crops to enhance their resistance to biotic stress.
2. Materials and methods
2.1. Experimental materials and treatment
Tobacco seedlings (Nicotiana tabacum L. cv ‘Longjiang 911) were used as experimental materials, and the seeds were procured from the Heilongjiang Tobacco Science Research Institute. In early March 2021, tobacco seeds were sown in a matrix formed by fully mixed peat and vermiculite (1:1, [v/v]), cultured in an incubator with temperatures of 25°C/23°C (light/dark), light intensity of 400 µmol·m−2·s−1, light cycle of 12 h/12 h (light/dark) and relative humidity of 65%. The seeds were watered regularly and managed at the seedling stage. When the seedlings grew to two leaves with a terminal bud, the tobacco seedlings with the same growth trend were selected and transplanted into flowerpots that were 15 cm in diameter and 12 cm high. One plant was grown in each pot, and the cultivation medium was 260 g of sterilized vermiculite per pot. One-half Hoagland nutrient solution was added regularly (Zhang, Li, et al. Citation2018). When the seedlings grew to six leaves with a terminal bud, plants with the same growth were randomly divided into four groups, which were labeled as the control group (CK), WV group, Pseudomonas syringae pv. tabaci group (Pst) and wood vinegar+ Pseudomonas syringae pv. tabaci group (Pst + WV). The seedlings of WV and Pst + WV treatment groups were sprayed with 1% wood vinegar, and 100 ml was sprayed evenly on the front and back of each leaf. In order to eliminate the factors of WV absorption by soil and plant roots, the soil surface was covered with a layer of plastic film before spraying. The CK and Pst treatment groups were sprayed with deionized water. Wood vinegar was prepared by Mudanjiang Tobacco Science Research Institute by high-temperature carbonization of walnut shell. It mainly includes organic acids (26.64%), phenols (25.80%), aldehydes (20.35%), ketones (11.31%), nitrogen-containing organic compounds (5.44%) and alcohols (5.02%).
The stock solution of wildfire pathogen was provided by Heilongjiang Tobacco Science Research Institute. A volume of 200 µL of stock solution was pipetted into 100 mL of liquid LB medium and incubated at room temperature with shaking at 110 rpm. After shaking for 24 h, 1.5 mL of bacterial solution was mixed with 0.5 mL of glycerol and was stored at −80°C. When infecting leaves, the preserved bacterial solution was poured into LB liquid media and cultured for 3 days, and the bacterial solution OD600 = 0.1 (Li Citation2021). Inoculate with acupuncture method, and quantitatively inoculate 10 µL of wildfire pathogen solution on the same part of tobacco seedlings with the same leaf age. Each tobacco plant was inoculated with two leaves, and each leaf has six inoculation points.
2.2. Measurements and methods
2.2.1. Determination of chlorophyll content
The acetone-ethanol solvent extraction method was used to determine the chlorophyll content. Fresh leaves (about 0.5 g) without main vein from each group were immersed in 2 mL of the mixture of acetone and ethanol (V:V = 1:1) and oscillated in the dark until the green completely faded. Absorbance values at 665 and 649 nm were measured using a spectrophotometer (Agilent Technologies, China). The contents of chlorophyll a (Chl a), chlorophyll b (Chl b), chlorophyll a/b (Chl b), chlorophyll a + b (Chl a + b), carotenoid (Car) content and carotenoid/chlorophyll a + b (Car/Chl a + b) were measured as described by Porra (Citation2002).
2.2.2 Determination of chlorophyll fluorescence kinetic curve (OJIP) and 820 nm light absorption curve (MR820)
The chlorophyll fluorescence kinetic curve (OJIP curve) and the 820 nm light absorption curve (MR820) of the second fully expanded leaf of tobacco seedlings after 0.5 h dark adaptation were measured by a M-PEA plant efficiency instrument (Hansatech, King’s Lynn, UK). The induced light intensity was 3000 μmol·m−2·s−1 pulsed red light during the determination of OJIP curve. The measurements started from 10 μs to record the relative fluorescence intensity (Fv) and ended at 2 s where the points O, J, I and P on the curve represent the corresponding points on the curve when the time was 0, 2, 30 and 1000 ms, respectively. Point L represents the corresponding point on the curve at 0.15 ms, and point K represents the corresponding point on the curve at 0.3 ms. O-P, O-J and O-K were standardized on the OJIP curve. This involved setting the relative fluorescence intensity (Fo) of the O point to 0, and the relative fluorescence intensity (Fp) of the P, J and K points to 1. The standardized formula was as follows: VO-P = (Ft − Fo)/(Fp − Fo), VO-J = (Ft − Fo)/(FJ − Fo) and VO-K = (Ft − Fo)/(FK − Fo), where Ft represents the relative fluorescence intensity of each time point. The relative variable fluorescence intensities of the L, K and J points on the standardization curve were expressed as VL,VK and VJ, VL = (FL − Fo)/(FK − Fo), VK = (FK − Fo)/(FJ − Fo) and VJ = (FJ − Fo)/(FP − Fo). A JIP test analysis was performed using the calculations described by Strasser and Strasser (Citation1995) as follows: PSII maximum photochemical efficiency (Fv/Fm), photosynthetic performance index based on light absorption (PIABS), energy absorbed by the unit reaction center (ABS/RC), energy captured by the unit reaction center for reducing QA (TRo/RC), energy captured by the unit reaction center for electron transfer (ERo/RC), energy dissipated per unit reaction center (DIo/RC), light energy absorbed per unit area (ABS/CSm), light energy captured per unit area (TRo/CSm), electron transfer yield per unit area (ETo/CSm) and heat dissipation per unit area (DIo/CSm). The activity of PS I reaction center is expressed by the relative value of the difference between the maximum value (Io) and the minimum value (Im) of 820 nm light absorption, i.e. △I/Io = (Io − Im)/Io (Oukarroum et al. Citation2013; Zhang, Xu, et al., Citation2018).
2.2.3. Determination of active oxygen content and antioxidant enzyme activities
Hydrogen peroxide (H2O2) was determined using the method described by Alexieva et al. (Citation2001), and slightly changed (Yang et al. Citation2021). The rate of production of superoxide anions () was determined by hydroxylamine oxidation method (Elstner and Heupel Citation1976). The activity of superoxide dismutase (SOD) was assayed by the nitrogen blue tetrazole method as previously described by Li (Citation2000). Catalase (CAT) activity was determined by monitoring the decrease in absorbance at 240 nm caused by the decomposition of H2O2 (Chance and Maehly Citation1955). Peroxidase activity (POD) was assayed at 470 nm using hydrogen peroxide and guaiacol as the reaction substrates (Cakmak and Marschner Citation1992). The activity of ascorbate peroxidase (APX) was determined as described by Nakano and Asada (Citation1982). Glutathione peroxidase (GPx) activity was assayed using a plant glutathione peroxidase kit (GSH-PX; Grice, China).
2.2.4. RT–PCR analysis
Tobacco leaves (0.1 g) were ground in liquid nitrogen to extract the total RNA using an OMEGA Plant RNA Kit (Omega Bio-tek, Norcross, GA, USA), and a PrimeScript RT Reagent Kit (TaKaRa Bio, Inc., Kusatsu, Japan) was used to synthesize the single-strand cDNA according to the manufacturer's instructions. Quantitative RT–PCR was performed using a Lightcycler 96 fluorescence real-time PCR system (qTOWER3 qPCR Thermal Cycler, Analytik Jena, Jena, Germany). The PCR cycling protocol consisted of an initial denaturation at 94°C for 10 min, followed by 40 cycles of 94°C for 20 s and 60°C for 20 s. After the final cycle, a melting curve analysis was performed over a temperature range of 60–95°C in increments of 1°C to verify the reaction specificity. The actin gene was used as a constitutive reference, and the relative expression was measured by the 2−ΔΔCt method (Livak and Schmittgen Citation2001). The primer used in this study are given in Additional file 1.
2.3 Statistical analysis
Microsoft Excel 2016 (Redmond, WA, USA) and SPSS 22.0 (IBM, Inc., Armonk, NY, USA) were used to analyze the data measured. All the data were expressed as the mean ± SE (standard error) of three biological replicates. The measured differences between the treatment groups were compared by a one-way analysis of variance (ANOVA) test and Tukey–Kramer’ method, with the level of significance established at P < 0.05 for all the tests.
3. Results and analysis
3.1. Plant phenotype and chlorophyll content
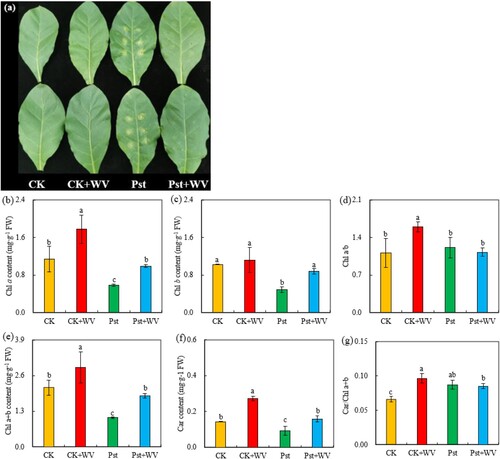
As shown in , compared with the injection of distilled water (CK), the leaves of WV sprayed treatment become greener, but the leaves wilted and had lesions after infection by Pst. However, pretreatment with WV alleviated the damage of Pst infection to leaves and reduced the lesion areas. Compared with the CK, the WV treatment increased the contents of Chl a, Chl b, Chl a + b and Car, while the parameters of these four pigments in the Pst-infected leaves decreased significantly. However, after WV pretreatment, the parameters of the four groups increased by 70%, 80.45%, 74.86% and 72.83% (all P< 0.05), respectively. Compared with the CK, treatment with WV increased the parameters of Chl a/b and Car/Chl a + b, while the WV pretreatment had no significant effect on the parameters of Chl a/b and Car/Chl a + b in the leaves infected with Pst.
Figure 1. Effects of WV on phenotype (a), Chl a content (b), Chl b content (c), Chl a/b (d), Chl a + b content (e), Car content (f) and Car/Chl a + b (g) of tobacco leaves infected by Pst. Note: The data in the figure are from three biological repeats (n = 3), and represent means ± standard error (SE). Significant differences were expressed by different letters (P < 0.05).
3.2. PSII and PSI photochemical activities
As shown in (a), compared with the CK, the WV treatment reduced the values of Fo, FJ, FI and FP on an OJIP curve in tobacco leaves, while Fo increased significantly, and FJ, FI and FP decreased significantly in the leaves infected with Pst. Compared with the leaves directly infected by Pst, pretreatment with WV decreased the Fo and FJ but increased the FI and FP. After Pst infection, the 820 nm optical signal drop of leaves decreased significantly, while pretreatment with WV increased the optical signal drop of the Pst-infected leaves ((b)). Compared with the CK, the WV treatment had no significant effect on the Fv/Fm, PIABS and ΔI/Io. The Fv/Fm, PIABS and ΔI/Io of the Pst-infected leaves decreased by 32.48%, 89.62%, and 46.27%, respectively (all P < 0.05). Pretreatment with WV alleviated its downward trend. Compared with the leaves directly infected by Pst, Fv/Fm, PIABS and ΔI/Io significantly increased by 38.60%, 4.94-fold and 72.22% following pretreatment with WV, respectively ().
Figure 2. Effects of WV on the OJIP curve (a), MR820 curve (b), Fv/Fm (c), PIABS (d), and ΔI/Io (e) of tobacco leaves infected by Pst. Note: The data in the figure are from three biological repeats (n = 3), and represent means ± standard error (SE). Significant differences were expressed by different letters (P < 0.05).
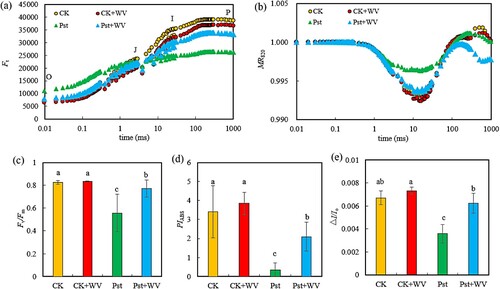
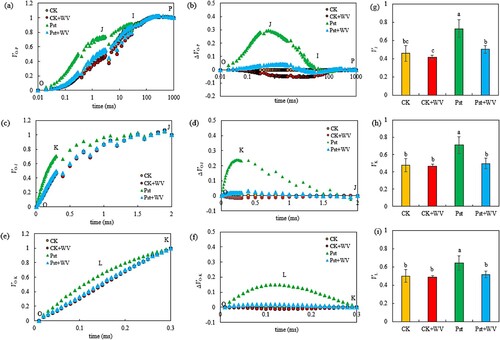
Figure 3. Effects of WV on the VO-P (a), ΔVO-P (b), VO-J (c), ΔVO-J (d), VO-K (e), ΔVO-K (f), VJ (g), VK (h) and VL (i) of tobacco leaves infected by Pst. Note: The data in the figure are from three biological repeats (n = 3), and represent means ± standard error (SE). Significant differences were expressed by different letters (P < 0.05).
3.3 Standardized OJIP curve and PSII electron transfer capacity on the donor and receptor sides
The OJIP curve of each treatment group was standardized according to the O-P, and the change in the relative fluorescence intensity (VJ) of point J was the most clearly affected. The O-J and O-K curves were standardized. The difference between the relative fluorescence intensity (VK) of point K at 0.3 ms on the O-J curve and the relative fluorescence intensity (VL) of point L at 0.15 ms on the O-K curve were found to be the most obvious changes. Therefore, VJ, VK and VL were analyzed quantitatively. The results showed that compared with the CK, the WV treatment had no significant effect on the leaf VJ, VK and VL but significantly increased these three parameters in Pst-infected leaves. Compared with the leaves directly infected with Pst, VJ, VK and VL pretreated with WV decreased by 30.82%, 29.86% and 19.80%, respectively (all P< 0.05).
3.4 Light energy absorption and utilization parameters
As shown in , compared with the CK, the WV treatment increased the ETo/RC of leaves. Infection with Pst significantly reduced its value, but pretreatment with WV increased it. ABS/CSm, TRo/CSm and ETo/CSm decreased significantly, but DIo/CSm increased significantly. Following pretreatment with WV, ABS/CSm, TRo/CSm and ETo/CSm in the Pst-infected leaves increased, and DIo/CSm decreased ().
Figure 4. Effects of WV on the light energy absorption and utilization parameters of tobacco leaves infected by Pst. Note: The data in the figure are from three biological repeats (n = 3), and represent means ± standard error (SE). Significant differences were expressed by different letters (P < 0.05).
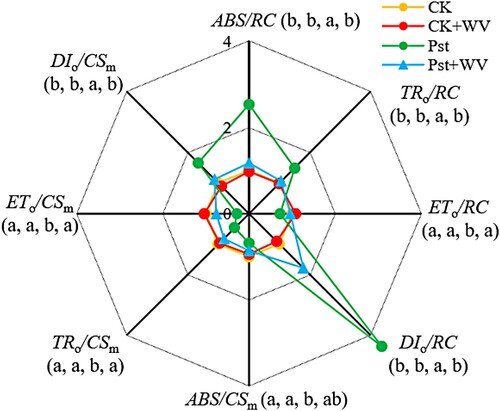
Figure 5. Effects of WV on the generation rate of (a), H2O2 content (b), CAT activities (c), SOD activities (d), POD activities (e), APX activities (f) and GPX activities (g) of tobacco leaves infected by Pst. Note: The data in the figure are from three biological repeats (n = 3), and represent means ± standard error (SE). Significant differences were expressed by different letters (P < 0.05).
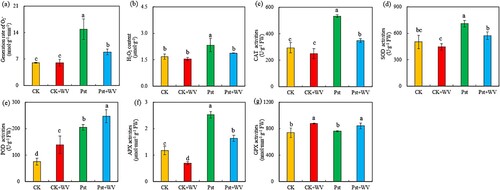
3.5 ROS content and ROS scavenging enzyme activities
Compared with the CK, the WV treatment had little effect on the rate of production of , H2O2 content, and the activities of CAT and SOD, but this treatment significantly improved the activities of POD and GPx. In Pst-infected leaves, the rate of production of
and the content of H2O2 increased by 1.47-fold (P < 0.05) and 39.01% (P < 0.05), and the activities of CAT, SOD, POD, APX and GPx increased by 81.39% (P < 0.05), 41.79% (P < 0.05), 1.71-fold (P < 0.05), 1.15-fold (P < 0.05) and 3.02% (P > 0.05), respectively. Pretreatment with WV significantly reduced the rate of production of
, H2O2 content and the activities of CAT, SOD and APX in Pst-infected leaves, but the activities of POD and GPX increased by 21.02% and 10.63% (both P < 0.05), respectively.
3.6 qRT-PCR analysis of the key gene expression of photosynthetic and antioxidant enzymes
In , according to the qRT-PCR determination of a single reference gene, we know that compared with the CK, the WV treatment significantly decreased the levels of expression of PsbB, but the expression of other genes did not change significantly in PSII reaction center. But Pst infection significantly down regulated the expression levels of PsbA, PsbB, PsbC and PsbD genes and the expression levels of OEC-related genes PsbO and PsbP. However, WV pretreatment significantly increased the expression of the above genes. In the PSI reaction center, compared with direct Pst infection, WV pretreatment significantly increased the levels of expression of the key genes PsaA, PsaB, PsaC and PsaF. In photosynthetic electron transport and ATPase, compared with CK, there was no significant change in gene expression in WV treatment. However, WV pretreatment increased the expression levels of PetE, ATPa and ATPc in Pst-infected leaves.
Figure 6. Effects of WV on the key gene expression of photosynthetic and antioxidant enzymes of tobacco leaves infected by Pst. Note: The data in the figure are from three biological repeats (n = 3), and represent means ± standard error (SE). Significant differences were expressed by different letters (P < 0.05).
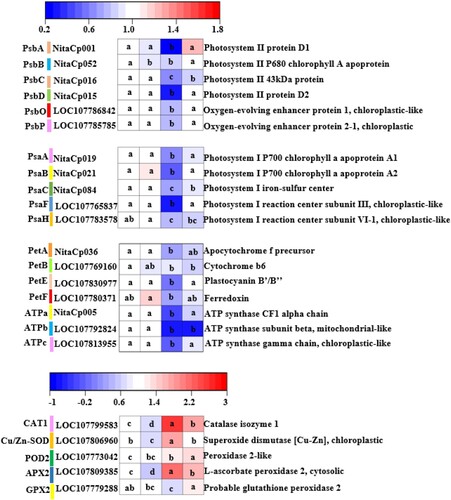
Compared with the CK, the WV treatment significantly reduced the levels of expression of CAT1, Cu/Zn-SOD and APX2 in leaves. The levels of expression of CAT1, Cu/Zn-SOD, POD2, APX2 and GPX2 were significantly upregulated in Pst-infected leaves. Pretreatment with WV decreased the levels of expression of CAT1, Cu/Zn-SOD and APX2 and increased the levels of expression of POD2 and GPX2 in Pst-infected leaves.
4. Discussion
Photosynthesis is not only an important basic physiological activity of plants but also a process sensitive to environmental factors, including biological stress, such as pathogen infection (Kottapalli et al. Citation2009). Chlorophyll is an important component of photosynthesis, and its content reflects the strength of this process (Fromme et al. Citation2003; Wang et al. Citation2022). In this experiment, Pst infection significantly reduced the contents of Chl a, Chl b and Chl a + b in tobacco leaves, indicating that Pst infection inhibited the capture and utilization of light energy. Pradhan et al. (Pradhan et al. Citation2015) found that the infection with wheat stripe mosaic virus (WSMV) significantly reduced the yield of wheat, which was primarily owing to the decrease in chlorophyll content in wheat leaves caused by infection with this pathogen. In a study by Bertamini et al. (Citation2002) a phytoplasma infection (Stolbur subgroup [Bois noir BN]) inhibited the contents of photosynthetic pigments and the carbon assimilation process of grape (Vitis vinifera L. cv. Chardonnay). Pretreatment with WV increased the chlorophyll content of Pst-infected leaves. This may be because wood vinegar contains magnesium, which is a critical component of chloroplast molecules and an activator of enzymes involved in photosynthetic metabolism (Wang, Li, et al., Citation2019). Wang, Chen, et al. (Citation2019) found that spraying an appropriate concentration of WV effectively alleviated the effect of low temperature on chlorophyll synthesis in rice (Oryza sativa L.). Carotenoid (car) serves as an antenna pigment in photosynthesis to transmit the captured light energy to chlorophyll (Polívka et al. Citation2004) and can scavenge free radicals (Dall’Osto et al. Citation2007; Polívka and Frank Citation2010). The decrease of Car increases the sensitivity of plant photosynthesis to stress (Cazzaniga et al. Citation2012). In this experiment, Pst infection reduced the content of Car in tobacco leaves, but pretreatment with WV alleviated the effect of Pst infection on Car in tobacco leaves.
Chlorophyll fluorescence is closely related to photosynthesis, and the effect of any environmental factors on plant photosynthesis can be reflected by the chlorophyll fluorescence of leaves, particularly the function of PSII (Maxwell and Johnson Citation2000; Kalaji et al. Citation2016; Citation2017; Zhang et al. Citation2019). Among them, the decrease in photosynthetic rate will inevitably affect the absorption, transmission and transformation of light energy by plants, and the most important manifestation is a decrease in photochemical activity (Wang et al. Citation2020). In this study, infection with Pst significantly reduced Fv/Fm, PIABS and ΔI/Io of tobacco leaves and inhibited their normal photosynthesis. Photosynthetic inhibition is one of the main hazards of pathogen infection (Kottapalli et al. Citation2009), and it is also an important reason why pathogen infections inhibit plant growth and reduce crop yields (Roloff et al. Citation2004; Ficke et al. Citation2018).Saeed et al.(Citation2007) found that infection with the fungus Verticillium dahlia and the nematode Pratylenchus penetrans reduced the efficiency of potato (Solanum tuberosum L.) leaves to utilize light energy under field conditions. However, the Fv/Fm, PIABS and ΔI/Io of the Pst-infected leaves increased following pretreatment with WV. In addition, the levels of expression of PsbA, PsbB, PsbC and PsbD that encode the core protein of PSII reaction center and those of PsaA, PsaB, PsaC and PsaF that encode the core protein of PSI reaction center were upregulated. WV was found to improve the photosynthetic capacity of crops, which was primarily attributed to the increase in chlorophyll content by the phenolics, organic acids and trace elements in wood vinegar (Fromme et al. Citation2003; Li et al. Citation2015; Wang et al. Citation2022). The change in the content of chlorophyll in this experiment also proved this point of view.
To further analyze the alleviating effects of infection with Pst on the damaged parts of photosynthetic apparatus and WV in tobacco leaves, the original OJIP curves of each treatment were standardized. Compared with the CK, infection with Pst significantly increased VJ, VK and VL in tobacco leaves. The increase of VJ reflects the inhibition of electron transfer from QA to QB on the PSII receptor side, resulting in the increase in accumulation (Zhang, Xu, et al. Citation2020). Therefore, this shows that the electron transfer block of Pst-infected tobacco leaves primarily occurs from QA to QB on the receptor side of PSII. The increase in VK was considered to be a specific marker of the impaired OEC activity of the PSII donor side oxygen release complex (Zhang, Li, et al. Citation2012). The decrease in OEC activity on the PSII electron donor side will lead to the incomplete breakdown of water and the production of H2O2, while the obstruction of electron transfer on the PSII receptor side will lead to the leakage of excess electrons, which will attack the free O2 in the cell and generate
(Wang et al. Citation2021). The increase in VL was a specific marker of thylakoid dissociation (Essemine et al. Citation2012). The thylakoid membrane contains photosynthetic pigments and electron transport chain components on which the light reaction takes place. Therefore, Pst infection changes the fluidity of thylakoid membranes, and the integrity of thylakoid function and structure was destroyed (Zhang et al. 2018). Pretreatment with VW reduced the VJ, VK and VL of Pst-infected leaves. Therefore, pretreatment with WV can alleviate the obstruction of electron transfer on the PSII receptor side, the damage caused by OEC on the PSII donor side and the damage to thylakoid membrane caused by Pst infection. The levels of expression of the OEC-related genes PsbO and PsbP were significantly upregulated in leaves infected by Pst following pretreatment with WV. In addition to PSII and PSI, photosynthetic electron transport also involves the Cytochrome b6/f (Cytb6/f) complex, plastocyanin (PC), ferredoxin (Fd) and ATP synthase subunit alpha subunit (ATPase) (Brestic et al. Citation2015). In this study, pretreatment with WV increased the levels of expression of PetE, ATPa and ATPc in Pst-infected leaves. The change in specific activity parameters of the unit reaction center can not only analyze the absorption and utilization of light energy but also reflect the activity of the reaction center (Strasserf and Srivastava Citation1994). The results showed that infection with Pst reduced the ABS/CSm, TRo/CSm and ETo/CSm but increased DIo/CSm. The leaves were protected against the damage of photoinhibition by increasing heat dissipation. The heat dissipation per unit area of the leaves pretreated with WV decreased, and the absorbed light energy was used more effectively in photosynthetic carbon assimilation to improve the photosynthetic efficiency of leaves.
One of the most rapid defense responses against pathogen attack is the oxidative burst, which constitutes the production of ROS, primarily and H2O2 at the invasion site (Apel and Hirt Citation2004). This response involves pathogenic and symbiotic interactions (Doke Citation1983). In this experiment, the rate of production of
and the content of H2O2 of Pst-infected leaves increased significantly. The increase in these products may significantly degrade unsaturated fatty acids in the cell membranes and cause lipid peroxidation (Apel and Hirt Citation2004). Zhang, Zhang, et al. (Citation2020) also found that the contents of
and H2O2 increased significantly when the fungal pathogen Lasiodiplodia theobromae infected peach (Prunus persica L.), causing lipid peroxidation of the host cell membrane. In addition, a large amount of H2O2 accumulated during the infection of wheat by the fungus wheat needle spore leaf blight (Septoria tritici), which promoted the occurrence of the disease (Shetty et al. Citation2007). In this experiment, pretreatment with WV reduced the content of ROS in Pst-infected leaves. Under different environmental stresses, plants can reduce or eliminate oxidative damage through complex antioxidant defense systems (Singh et al. Citation2016; Zhou et al. Citation2017). SOD, CAT and POD are important enzymes that plants utilize to adapt to a variety of stresses, and they are also important components of the plant antioxidant system. APX is one of the important protective enzymes in the plant membrane lipid peroxidase defense system. GPX is also a type of enzyme that is found widely in organisms that catalyze the degradation of H2O2. Its activity can be used as an important indicator to measure changes in the antioxidant system. The activities of CAT, SOD, POD and APX in Pst-infected leaves increased, indicating that when infected with Pst, tobacco leaves can eliminate oxygen free radicals by inducing antioxidant enzymes to cooperate with each other and accumulate antioxidant enzymes to reduce the peroxidation of membrane systems. In addition, the levels of expression of CAT1, Cu/Zn-SOD, POD2 and APX2 were upregulated in the Pst-infected leaves. When gray mold (Botrytis cinerea) infects its host tomato (Solanum lycopersicon), the antioxidant enzymes, such as SOD and CAT enzymes in tomato leaves, were destroyed, and the antioxidant capacity of the host decreased significantly (Kuniak and Skodowska Citation2005). However, the activities of SOD and CAT enzymes in leaves increased rapidly during the infection of wheat by the fungus Pyricularia oryzae (Debona et al. Citation2012). In this experiment, pretreatment with WV decreased the activities of CAT, SOD and APX in Pst-infected leaves but increased the activities of POD and GPX, indicating that under Pst stress, treatment with WV eliminated excessive H2O2 free radicals and reduced the toxicity of stress by enhancing the activities of POD and GPX in leaves. Moreover, the upregulation of the expression of POD2 and GPX2 in Pst-infected leaves pretreated by WV also proved this hypothesis.
5. Conclusion
The chlorophyll content of Pst-infected tobacco leaves decreased, which inhibited the capture and utilization of light energy. However, pretreatment with WV alleviated the degradation of chlorophyll, and the chlorophyll content of leaves increased. Pst infection significantly reduced the Fv/Fm, PIABS and ΔI/Io of leaves and inhibited their normal photosynthesis. Pretreatment with WV can not only improve the activity of reaction center of Pst-infected leaves, but the levels of expression of PsbA, PsbB, PsbC and PsbD, which encode the core protein of PSII reaction center, and PsaA, PsaB, PsaC and PsaF, which encode the core protein of PSI reaction center were upregulated. Pretreatment with WV can also alleviate the obstruction of electron transfer on the PSII receptor side caused by Pst infection, the damage caused by OEC on the PSII donor side and damage to the thylakoid membrane. The levels of expression of PetE, ATPa and ATPc in Pst-infected leaves were significantly upregulated when pretreated with WV. The heat dissipation per unit area of the leaves pretreated with WV decreased, and the absorbed light energy was more effectively used to assimilate carbon photosynthetically to improve the photosynthetic efficiency of leaves. Pst infection increased the content of ROS in leaves, but WV reduced the toxicity of Pst infection by increasing the activities of POD and GPX and upregulating the expression of POD2 and GPX2 genes.
Supplemental Material
Download MS Word (15.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Yuan Ye
Yuan Ye is an Associate Researcher in Mudanjiang Tobacco Science Research Institute. His research interests lie in the area of Plant physiology and crop cultivation.
Sun Hongwei
Sun Hongwei is an Associate Researcher in Mudanjiang Tobacco Science Research Institute. His research interests lie in the area of Plant physiology and crop cultivation.
Wang Yue
Wang Yue is a Doctoral Student in Northeast Forestry University. Her research interests lie in the area of Plant physiology and molecular biology.
Xu Zisong
Xu Zisong is a Postgraduate Student in Northeast Agricultural University. His research interests lie in the area of Plant physiology.
Han Shixin
Han Shixin is an engineer in Mudanjiang Tobacco Company. His research interests lie in the area of Plant physiology and crop cultivation.
He Guoqiang
He Guoqiang is an Associate Researcher in Mudanjiang Tobacco Science Research Institute. His research interests lie in the area of Plant physiology and crop cultivation.
Yin Kuide
Yin Kuide is a Professor in Heilongjiang Bayi Agricultural University. His research interests lie in the area of Plant physiology.
Huihui Zhang
Huihui Zhang is an Associate Professor in Northeast Forestry University. His research interests lie in the area of Plant physiology and molecular biology.
References
- Alexieva V, Sergiev I, Mapelli S, Karanov E. 2001. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 24(12):1337–1344.
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 55(1):373–399.
- Barreneche T, Bodenes C, Lexer C, Trontin JF, Fluch S, Streiff R, Plomion C, Roussel G, Steinkellner H, Burg K, et al. 1998. A genetic linkage map of Quercus robur L. (Pedunculate oak) based on RAPD, SCAR, microsatellite, minisatellite, isozyme and 5S rDNA markers. Theor Appl Genet. 97(7):1090–1103.
- Berger S, Sinha AK, Roitsch T. 2007. Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen interactions. J Exp Bot. 58(15–16):4019–4026.
- Bertamini M, Nedunchezhian N, Tomasi F, Grando MS. 2002. Phytoplasma [stolbur-subgroup (bois noir-BN)] infection inhibits photosynthetic pigments, ribulose-1,5-bisphosphate carboxylase and photosynthetic activities in field grown grapevine (Vitis vinifera L. cv. chardonnay) leaves. Physiol Mol Plant Pathol. 61(6):357–366.
- Brestic M, Zivcak M, Kunderlikova K, Sytar O, Shao HB, Kalaji HM, Allakhverdiev S. 2015. Low PSI content limits the photoprotection of PSI and PSII in early growth stages of chlorophyll b-deficient wheat mutant lines. Photosynth Res. 125(1–2):151–166.
- Cakmak I, Marschner H. 1992. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 98(4):1222–1227.
- Camejo D, Cedeno AG, Herrera AM. 2016. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol Biochem. 103:10–23.
- Cao Y, Zhang HN, Meng J, Yang QG, Zhang XY, Kang ZK, Zhou GC. 2017. Effects of wood vinegar and sodium naphthalene acetate on photosynthesis and yield of peanut. Agric Res Arid Areas. 35(1):185–191.
- Cazzaniga S, Li ZR, Niyogi KK, Bassi R, Dall'Osto L. 2012. The Arabidopsis szl1 mutant reveals a critical role of beta-carotene in photosystem I photoprotection. Plant Physiol. 159(4):1745–1758.
- Chance B, Maehly AC. 1955. Assay of catalases and peroxidases. Methods Enzymol. 2:764–817.
- Chaves-Gómez JL, Chaez-Arias CC, Caro SG, Cotes AM, Restrepo-Diaz H. 2019. Physiological response of cape gooseberry seedlings to three biological control agents under Fusarium oxysporum f. sp. physali infection. Plant Dis. 104(2):388–397.
- Chen Y, Mao H, Wu N, Ma J, Zhang H. 2019. Effects of stripe rust infection on the levels of redox balance and photosynthetic capacities in wheat. Int J Mol Sci. 21(1):268.
- Chen YE, Cui JM, Su YQ, Yuan S, Yuan M, Zhang HY. 2015. Influence of stripe rust infection on the photosynthetic characteristics and antioxidant system of susceptible and resistant wheat cultivars at the adult plant stage. Front Plant Sci. 6:779.
- Cheng DD. 2017. Responses of photosynthesis and respiration to Pseudomonas syringae pv. tabaci infection in tobacco leaves. Harbin: Northeast Forestry University.
- Cheng DD, Liu MJ, Sun XB, Zhao M, Chow WS, Sun GY, Zhang ZS, Hu YB. 2016. Light suppresses bacterial population through the accumulation of hydrogen peroxide in tobacco leaves infected with Pseudomonas syringae pv. tabaci. Front Plant Sci. 7:512.
- Dall’Osto L, Cazzaniga S, North H, Marion-Poll A, Bassi R, Notes A. 2007. The Arabidopsis aba4-1 mutant reveals a specific function for neoxanthin in protection against photooxidative stress. Plant Cell. 19(3):1048–1064.
- Debona D, Rodrigues FA, Rios JA, Nascimento KJT. 2012. Biochemical changes in the leaves of wheat plants infected by Pyricularia oryzae. Phytopathology. 102(12):1121–1129.
- Doke N. 1983. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol. 23(3):345–357.
- Elstner EF, Heupel A. 1976. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem. 70(2):616–620.
- Essemine J, Govindachary S, Ammar S, Bouzid S, Carpentier R. 2012. Enhanced sensitivity of the photosynthetic apparatus to heat stress in digalactosyl-diacylglycerol deficient Arabidopsis. Environ Exp Bot. 80:16–26.
- Ficke A, Cowger C, Bergstrom G, Brodal G. 2018. Understanding yield loss and pathogen biology to improve disease management: Septoria 1 Nodorum blotch – a case study in wheat. Plant Dis. 102(4):696–707.
- Fromme P, Melkozernov A, Jordan P, Krauss N. 2003. Structure and function of photosystem I: interaction with its soluble electron carriers and external antenna systems. FEBS Lett. 555(1):40–44.
- Garry G, Jeuffroy HM, Ney B, Tivoli B. 2007. Effects of ascochyta blight (Mycosphaerella pinodes) on the photosynthesizing leaf area and the photosynthetic efficiency of the green leaf area of dried-pea (Pisum sativum). Plant Pathol. 47(4):473–479.
- Ito M, Yamamoto Y, Kim CS, Ohnishi K, Hikichi YS, Kiba A. 2014. Heat shock protein 70 is required for tabtoxinine-β-lactam-induced cell death in Nicotiana benthamiana. J Plant Physiol. 171(2):173–178.
- Jia XC, Zeng HH, Bose SK, Wang WX, Yin H. 2020. Chitosan oligosaccharide induces resistance to Pst DC3000 in Arabidopsis via a non-canonical N-glycosylation regulation pattern. Carbohydr Polym. 250:116939.
- Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA, Cetner MD, Lukasik I, Goltsev V, Ladle RJ. 2016. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant. 38(4):102.
- Kalaji HM, Schansker G, Brestic M, Bussotti F, Calatayud A, Ferroni L, Goltsev V, Guidi L, Jajoo A, Li PM, et al. 2017. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth Res. 132(1):13–66.
- Kim YM, Bouras N, Kay NNV, Strelkov SE. 2010. Inhibition of photosynthesis and modification of the wheat leaf proteome by Ptr ToxB: a host-specific toxin from the fungal pathogen Pyrenophora tritici-repentis. Proteomics. 10(16):2911–2926.
- Kocal N, Sonnewald U. 2008. Cell wall-bound invertase limits sucrose export and is involved in symptom development and inhibition of photosynthesis during compatible interaction between tomato and Xanthomonas campestris pv vesicatoria. Plant Physiol. 148(3):1523–1536.
- Kottapalli KR, Rakwal R, Shibato J, Burow G, Tissue D, Burke J, Puppala N, Burow M, Payton P. 2009. Physiology and proteomics of the water-deficit stress response in three contrasting peanut genotypes. Plant Cell Environ. 32(4):380–407.
- Kuniak E, Skodowska M. 2005. Fungal pathogen-induced changes in the antioxidant systems of leaf peroxisomes from infected tomato plants. Planta. 222(1):192–200.
- Kunkel BN, Brooks DM. 2002. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol. 5(4):325–331.
- Lee S, Yang DS, Uppalapati SR, Summer LW, Mysore KS. 2013. Suppression of plant defense responses by extracellular metabolites from Pseudomonas syringae pv. tabaci in Nicotiana benthamiana. BMC Plant Biol. 13(1):65.
- Li H. 2021. Study on the mechanism of exogenous ammonium ions affecting the propagation, motility and pathogenicity and gene expression of Pseudomonas syringae pv. tabaci. Henan: Henan Agricultural University.
- Li HS. 2000. Principles and techniques of plant physiological and biochemical experiments. Wuhan: Higher Education Press.
- Li X, Liu TG, Chen WQ, Zhong SF, Zhang HY, Tang ZX, Chang ZJ, Wang L, Zhang M, Li LQ, et al. 2015. Wheat WCBP1 encodes a putative copper-binding protein involved in stripe rust resistance and inhibition of leaf senescence. BMC Plant Biol. 15:239.
- Liu CF, Li M, Gao PY, Yan XL, Li XY. 2016. Research progress of source, chemical compositions and application of wood vinegar. Chinese Agric Sci Bull. 32(1):28–32.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 25(4):402–408.
- Loo AY, Jain K, Darah I. 2008. Antioxidant activity of compounds isolated from the pyroligneous acid, Rhizophora apiculata. Food Chem. 107(3):1151–1160.
- Maxwell K, Johnson GN. 2000. Chlorophyll fluorescence-a practical guide. J Exp Bot. 51(345):659–668.
- Miguel AT, Jonathan DGJ, Dangl JL. 2006. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 141(2):373–378.
- Mittler R. 2016. ROS are good. Trends Plant Sci. 22(1):11–19.
- Mungkunkamchao T, Kesmala T, Pimratch S, Toomsan B, Jothityangkoon D. 2013. Wood vinegar and fermented bioextracts: natural products to enhance growth and yield of tomato (Solanum lycopersicum L.). Sci Hortic. 154:66–72.
- Nakano Y, Asada K. 1982. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiology. 22(5):867–880.
- Oukarroum A, Goltsev V, Strasser RJ. 2013. Temperature effects on pea plants probed by simultaneous measurements of the kinetics of prompt fluorescence, delayed fluorescence and modulated 820nm reflection. Plos One. 8(3):e59433.
- Polívka T, Frank HA. 2010. Molecular factors controlling photosynthetic light harvesting by carotenoids. Acc Chem Res. 43(8):1125–1134.
- Polívka T, Pullerits T, Frank HA, Cogdell RJ, SundstrÖm V. 2004. Ultrafast formation of a carotenoid radical in LH2 antenna complexes of purple bacteria. J Phys Chem B. 108(39):15398–15407.
- Porra RJ. 2002. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res. 73(1-3):149–156.
- Pradhan GP, Xue Q, Jessup KE, Hao B, Rush CM. 2015. Physiological responses of hard red winter wheat to infection by wheat streak mosaic virus. Phytopathology. 105(5):621–627.
- Roloff I, Scherm H, Van-Iersel MW. 2004. Photosynthesis of blueberry leaves as affected by septoria leaf spot and abiotic leaf damage. Plant Dis. 88(4):397–401.
- Saeed IAM, Macguidwin AE, Rouse DI, Malek C. 2007. A field study on the influence of Verticillium dahliae and Pratylenchus penetrans on gas exchange of potato. Plant Dis. 91(12):1531–1535.
- Shetty NP, Mehrabi R, Lutken H, Haldrup A, Kema GHJ, Collinge DB, Jorgensen HJL. 2007. Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat. New Phytol. 174(3):637–647.
- Singh R, Singh S, Parihar P, Mishra RK, Tripathi DK, Singh VP, Chauhan DK, Prasad SM. 2016. Reactive oxygen species (ROS): beneficial companions of plants? Developmental processes. Front Plant Sci. 7:1299.
- Steffens B, Sauter M. 2009. Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. Plant Cell. 21(1):184–196.
- Strasser BJ, Strasser RJ. 1995. Measuring fast fluorescence transients to address environmental questions: The JIP-test.
- Strasserf RJ, Srivastava A. 1994. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol. 61(1):32–42.
- Turner JG, Taha RR. 1984. Contribution of tabtoxin to the pathogenicity of Pseudomonas syringae pv. tabaci. Physiol Plant Pathol. 25(1):55–69.
- Wang CF, Huang LL, Buchenauer H, Han QM, Zhang HC, Kang ZS. 2007. Histochemical studies on the accumulation of reactive oxygen species (O2- and H2O2) in the incompatible and compatible interaction of wheat – Puccinia striiformis f. sp. tritici. Physiol Mol Plant Pathol. 71(4–6):230–239.
- Wang HY, Chen L, Ma XW, Liu XB, Zhang YP, Quan J, Shan JR, Zhao W. 2019. Effect of wood vinegar on cold resistance of rice seedlings under low temperature stress. J Northeast Agric Univ. 50(2):1–8.
- Wang S, Li QP, Wang JF, Yan Y, Zhang GL, Yan Y, Zhang HF, Wu JJ, Chen F, Wang XJ, et al. 2019. YR36/WKS1-mediated phosphorylation of PsbO, an extrinsic member of photosystem II, inhibits photosynthesis and confers stripe rust resistance in wheat. Mol Plant. 12(12):1639–1650.
- Wang TL, Li J, Yang YW, Zhao YC. 2016. Analysis of coding region for proteins containing signal peptides of Pseudomonas syringae pv. tabaci yuexi-1 strain. Acta Tabacaria Sinica. 22(1):92–100.
- Wang Y, Guo DD, Wang JC, Tian B, Li YY, Sun GY, Zhang HH. 2022. Exogenous melatonin alleviates NO2 damage in tobacco leaves by promoting antioxidant defense, modulating redox homeostasis, and signal transduction. J Hazard Mater. 424:127265.
- Wang Y, Wang JC, Guo DD, Zhang HB, Che YH, Li YY, Tian B, Wang ZH, Gun GY, Zhang HH. 2021. Physiological and comparative transcriptome analysis of leaf response and physiological adaption to saline alkali stress across pH values in alfalfa (Medicago sativa). Plant Physiol Biochem. 167:140–152.
- Wang Y, Zhang HH, Wang JC, Zhao HC, He GQ, Huang D, Yang FW, Zhao MC, Che YH, Teng ZY, et al. 2020. Elevated NO2 damages the photosynthetic apparatus by inducing the accumulation of superoxide anions and peroxynitrite in tobacco seedling leaves. Ecotoxicol Environ Saf. 196:110534.
- Wang ZG. 2012. Studies on key-factor analysis of tobacco wildfire diseases’s occurrence and its control techniques. Xian: Southwest University.
- Wu QM, Zhang SY, Hou BX, Zheng HJ, Deng WX, Liu DH, Tang WJ. 2015. Study on the preparation of wood vinegar from biomass residues by carbonization process. Bioresour Technol. 179:98–103.
- Xu Q, Tang CL, Wang XD, Sun ST, Zhao JR, Kang ZS, Wang XJ. 2019. An effector protein of the wheat stripe rust fungus targets chloroplasts and suppresses chloroplast function. Nat Commun. 10(1):5571.
- Xue CL, Liu ZG, Dai L, Bu JD, Jiang ZH, Gao WL, Zhao J. 2018. Changing host photosynthetic, carbohydrate, and energy metabolisms play important roles in phytoplasma infection. Phytopathology. 108(9):1067–1077.
- Yang FW, Zhang HB, Wang Y, He GQ, Wang JC, Guo GG, Li T, Sun GY, Zhang HH. 2021. The role of antioxidant mechanism in photosynthesis under heavy metals Cd or Zn exposure in tobacco leaves. J Plant Interact. 16(1):354–366.
- Yang XM, Xue SP. 2014. Study on the comprehensive application of wood vinegar in agricultural production. Sci Technol Vision. 12:306.
- Yang YC, Huang ZM, Zhang JD. 2007. Determination of trace elements in wood vinegar by ICP-MS. Studies Trace Elements Health. 24(6):45–46.
- Zhang H, Zhang DM, Wang F, Hsiang T, Liu JW, Li GH. 2020. Lasiodiplodia theobromae-induced alteration in ROS metabolism and its relation to gummosis development in Prunus persica. Plant Physiol Biochem. 154:43–53.
- Zhang HC, Wang CF, Cheng YL, Chen XM, Han QM, Huang LL, Wei GR, Kang ZS. 2012. Histological and cytological characterization of adult plant resistance to wheat stripe rust. Plant Cell Rep. 31(12):2121–2137.
- Zhang HH, Li X, Zhang SB, Yin ZP, Zhu WX, Li JB, Meng L, Zhong HX, Xu N, Wu YN, Sun GY. 2018. Rootstock alleviates salt stress in grafted mulberry seedlings: physiological and PSII function responses. Front Plant Sci. 9:1806.
- Zhang HH, Shi GL, Shao JY, Li X, Li MB, Liang M, Xu N, Sun GY. 2019. Photochemistry and proteomics of mulberry (Morus alba L.) seedlings under NaCl and NaHCO3 stress. Ecotoxicol Environ Saf. 184:109624.
- Zhang HH, Xu N, Wu XY, Wang JR, Ma SL, Li X, Sun GY. 2018. Effects of four types of sodium salt stress on plant growth and photosynthetic apparatus in sorghum leaves. J Plant Interact. 13(1):506–513.
- Zhang HH, Xu ZS, Guo KW, Huo YZ, He GQ, Sun HW, Guan YP, Xu N, Yang W, Sun GY. 2020. Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicol Environ Saf. 202:110856.
- Zhang ZS, Li G, Gao HY, Zhang LT, Yang C, Liu P, Meng QW. 2012. Characterization of photosynthetic performance during senescence in stay-green and quick-leaf-senescence Zea mays L. inbred lines. Plos One. 7(8):e42936.
- Zhou X, Zhu T, Zhu LS, Luo SS, Deng XG, Lin HH, Xi DH. 2017. The role of photoreceptors in response to cucumber mosaic virus in Arabidopsis thaliana. J Plant Growth Regul. 36(2):257–270.
- Zurbriggen MD, Carrillo N, Tognetti VB, Melzer M, Peisker M, Hause B, Hajirezaei MR. 2009. Chloroplast-generated reactive oxygen species play a major role in localized cell death during the non-host interaction between tobacco and Xanthomonas campestris pv. vesicatoria. Plant J. 60(6):962–973.
