ABSTRACT
Tropical pitcher plants (Nepenthes) are carnivorous plants that trap and digest prey using highly modified fluid-filled leaves known as pitchers. Prey are digested by plant-secreted enzymes and pitcher symbionts. Pitchers exert control over abiotic properties of the digestive fluid such as pH levels that can influence its symbionts. Here we examine natural variation in pH and dissolved mineral concentrations in three sympatric Nepenthes species, assessing correlations between fluid properties and pitcher traits. We use addition experiments to investigate differences in protein digestion/absorption rates between species. Fluid pH and dissolved mineral levels both showed distinct patterns corresponding to pitcher developmental stages in N. gracilis and N. rafflesiana, whereas N. ampullaria differs from its congeners in exhibiting far less variation in fluid pH, as well as less clear evidence of protein depletion. This study further elucidates the properties of pitchers as habitats, revealing ways in which the host plant regulates that habitat.
Introduction
Tropical pitcher plants in the genus Nepenthes L. comprise over 160 described species occurring throughout Southeast Asia and a few outlying Paleotropical regions including Madagascar, the Indian subcontinent, New Caledonia, and Australia (McPherson, Robinson, & Fleischmann, Citation2009; Golos et al., Citation2020; Tamizi et al., Citation2020). These plants are characterized by their pitchers, which are modified leaves that evolved for the attraction, capture, and digestion of arthropod prey to fulfill the plants' nutritional requirements in the nitrogen-poor soils where they grow (Juniper, Robins, & Joel, Citation1989). Within the pitcher is a pool of plant-produced fluid and digestive enzymes; in a sense, the pitchers act as the plants’ ‘stomach’. This is a deadly environment for the plants’ prey, which drown and fall victim to the acidic conditions. As in the case of animal guts, however, this digestive pool simultaneously serves as a habitat for specialized organisms that are adapted to withstand these conditions. In nature, pitcher fluid usually harbors living communities of larval dipterans, histiostomatid mites, and microbes including bacteria, fungi, algae, and protists (Dover et al., Citation1928; Bittleston, Citation2018). These miniature aquatic environments are an example of phytotelm systems, communities in plant-held water, such as in tree-holes and bromeliad tanks (Kitching, Citation2001). The arthropod members of the Nepenthes phytotelm community (also known as inquilines) feed on the captured prey and thus may act as kleptoparasites by retaining prey-derived nitrogen in their bodies upon exiting the pitcher (Scharmann et al., Citation2013), or as digestive mutualists by increasing the levels of assimilable nitrogen in the fluid (Lam et al., Citation2017, Citation2018a). These host-symbiont interactions are complex, and the exact balance of costs and benefits is context-dependent (Leong, Lam, & Tan, Citation2018, Citation2019). Thus the plants are likely to benefit from being able to exert control over their resident phytotelm communities.
The community ecology of Nepenthes phytotelmata has been studied for nearly a century (Dover et al., Citation1928; Kitching, Citation1987; Mogi & Chan, Citation1996; Bittleston, Citation2018), and the properties of pitcher fluid as a habitat has been examined as well (Adlassnig, Peroutka, & Lendl, Citation2011). However, many questions remain. Nepenthes pitchers exhibit great morphological diversity (Thorogood, Bauer, & Hiscock, Citation2018), which in some cases has been linked to specialized dietary strategies such as those of the four confirmed coprophagous species (Clarke et al., Citation2009; Chin, Moran, & Clarke, Citation2010; Greenwood et al., Citation2011; Wells et al., Citation2011; Lim et al., Citation2015) as well as the leaf litter-trapping N. ampullaria Jack (Moran, Clarke, & Hawkins, Citation2003), which benefits from absorbing and assimilating the N from the litter it traps (Pavlovič, Slováková, & Šantrůček, Citation2011). Likewise, fluid properties vary across the genus. Some species produce viscoelastic fluid that enhances the capture of flying insects (Gaume & Forterre, Citation2007; Bonhomme et al., Citation2011; Moran et al., Citation2013; Bazile et al., Citation2015). Fluid pH also varies between species, with some species exhibiting moderately acidic levels of pH 5–6 and others reaching exceptionally acidic levels below pH 2 (Kanokratana et al., Citation2016; Saganová et al., Citation2018; Gaume et al., Citation2019; Gilbert et al., Citation2020). Moran et al. (Citation2010) proposed that species maintaining higher pH levels like N. ampullaria may be more reliant on symbiotic insects for digesting prey, compared to more acidic species like N. rafflesiana Jack that rely more on endogenous plant-produced enzymes. Increased acidity aids directly in the killing of insects (Bazile et al., Citation2015) as well as increasing the efficiency of the key enzymes involved in digestion (Saganová et al., Citation2018). Fluid pH is one trait that has a demonstrated strong effect on Nepenthes phytotelm community composition (Kanokratana et al., Citation2016; Gilbert et al., Citation2020). Not only does mean pH vary across species, but species also differ in their range of variance, with some maintaining narrower ranges than others (Bittleston, Citation2018; Gilbert et al., Citation2020).
While the importance of fluid pH to Nepenthes biology is known, questions regarding how this property is regulated in nature remain. How context-dependent is pH regulation? Do sympatric species tend to diverge in pH regulation? These questions require more study (but see (Lam et al., Citation2018b, Citation2019)). Moreover, abiotic fluid properties other than pH are rarely recorded in Nepenthes phytotelm studies, such as the concentrations of dissolved minerals like calcium and magnesium, which are important elements to the nutrition of insects (Clark, Citation1958; Poteat & Buchwalter, Citation2014) and plants alike (White & Broadley, Citation2003). Might pitchers absorb these elements for nutrition, as they do with potassium, phosphorus, and nitrogen (Pavlovič, Masarovičová, & Hudák, Citation2007; Osunkoya, Daud, & Wimmer, Citation2008; Karagatzides & Ellison, Citation2009; Pavlovič et al., Citation2009)?
The concentration of nitrogenous compounds in the fluid, and thus by extension the pitcher's digestive physiology, also has implications for interactions with symbionts. By absorbing nitrogenous waste, pitchers are often considerably less toxic to large metazoan communities than would be similar volumes of standing water in an inert container. Using ion microelectrode measurements in a laboratory context, one study found that two species with relatively long-lived pitchers and low prey-capture rates (N. ampullaria and N. bicalcarata Hook) had lower rates of ammonium uptake compared to N. rafflesiana with its short-lived pitchers and high prey-capture rates (Moran et al., Citation2010). Species may also differ in the enzymatic activity required for breaking down large proteins, prior to N uptake (Saganová et al., Citation2018). Knowledge of how protein digestion and/or absorption rates vary in nature is limited.
Alternative dietary strategies may alter the rates of protein digestion as well as the regulation of other properties of the pitcher fluid environment. In this study, we investigate intra- and interspecific variation in fluid properties in three sympatric Nepenthes species that differ in dietary strategy: the detritivorous N. ampullaria, and two ‘typical’ carnivores, N. gracilis Korth and N. rafflesiana. We measured morphological dimensions of the pitchers, sampling pitchers at each node of each individual plant when possible in order to capture age-related differences. We performed experimental manipulations in the field to assess rates of protein digestion/absorption, and examined natural variation in pH and concentrations of dissolved minerals (containing Ca2+ and/or Mg2+). We discuss the implications of pitcher fluid regulation for interactions with symbionts.
Materials and methods
Study site and sampling scheme
We worked in patches within Kent Ridge Park in Singapore (1°17’13.00"N, 103°47’10.91"E). The site was on the southern side of the ridge, in secondary forest. All measurements were made in June 2019 over a 4-week period during daylight hours (∼07:00-17:00); relative humidity ranged from 73.6% to 99.0% and temperatures ranged from 26.0–30.1°C during the study period. The patches contained wild, syntopic populations of our three focal Nepenthes species: N. ampullaria, N. gracilis, and N. rafflesiana. We collected data from a total of 165 pitchers (from at least 49 individual plants): 38 N. ampullaria pitchers, 44 N. gracilis pitchers (19 plants), and 83 N. rafflesiana pitchers (30 plants). In most cases, we sampled pitchers exhaustively along the length of an individual plant, except for N. ampullaria plants, which had a ‘carpet-like’ growth form (McPherson et al. Citation2009) that was not amenable to distinguishing individuals. We noted the morph of each pitcher (lower or upper, ) and we also categorized pitchers by their developmental stage (age class): ‘unopened’ (the developmental stage just prior to opening, nearly fully inflated, a,f,k), ‘living’ (mature healthy pitchers with no sign of senescence, b,c,g,h,l), ‘senescing’ (upper half of pitcher body undergoing senescence, d,i,m), and ‘dead’ (fully senescent tissues, entire pitcher body and tendril brown and dry, e,j,n). We aimed for equal sampling of the four pitcher age classes per species, but senescing and dead pitchers still capable of retaining fluid were uncommon, as were unopened pitchers mature enough to contain measurable fluid, so these categories were under-sampled relative to living pitchers.
Figure 1. Representative images of Nepenthes pitchers sampled in this study, showing the three species: N. rafflesiana (a-e), N. gracilis (f-j), and N. ampullaria (k-n), as well as the different pitcher age classes and dimorphism: unopened pitchers (a,f,k) (k was manually opened at time of photo), living pitchers, lower morph (b,g,l), living pitchers, upper morph (c,h), senescing pitchers (d,i,m), and dead pitchers (e,j,n). Photos: a-e, g-k, and m-n, T. Goldsborough; f and l K.J. Gilbert.

Pitcher dimensions and fluid properties
We used colorimetric test strips to measure fluid pH (Macherey Nagel pH-Fix 0-14), total hardness (Hach Sofchek), and protein concentration (One + Step 11) in-situ. Total hardness is the overall concentration of dissolved calcium (Ca2+) and magnesium (Mg2+) ions; we generally refer to this metric as the concentration of dissolved minerals. In addition to examining the test strips by eye, we developed a method for getting more precise and replicable values from test strips using observer-independent color data. We photographed the test strip next to the test's reference chromatic scale in front of a white background, using a bright flash. We then analyzed the digital images using a Python script (Goldsborough Citation2020) to estimate the test result by comparing the color of the test strips with its reference chromatic scale. An estimate was calculated by determining the best fit of the red, green, and blue (RGB) values of the test strip with the RGB values of the reference RGB plots. The sum of the differences between each of the RGB values and the reference RGB chromatic scale was used as an estimate for the accuracy of the result. This method yielded reliable results for pH and protein test strips, but the total hardness reference scale was not amenable to this method, so these readings were only done by eye. After observing consistently higher dissolved minerals concentrations in unopened pitchers, we decided to track a single unopened N. rafflesiana pitcher over a period of 9 days in order to probe natural developmental changes in these levels.
We used digital calipers to measure the length from the base of the pitcher to the attachment point of the lid, and the width at the widest section of the pitcher. We measured the pitcher fluid volume by drawing up the fluid into a graduated syringe (Terumo SS-10L); we returned the fluid to the pitcher afterward.
We conducted non-destructive surveys of inquilines and prey during this process. While in the transparent syringe, we visually inspected the fluid for living macroinvertebrate inquilines (total length > 1 mm), which were recorded as either present or absent (binary). The inquilines most commonly encountered in our surveys were the pitcher mosquito larva Tripteroides tenax (Culicidae) and the scuttle fly larva Endonepenthia schuitemakeri (Phoridae). We looked into the pitcher with a flashlight and recorded relative prey volume using a ranked scale of 0-3: 0 for no observed prey, 1 for low amounts of prey, 2 for moderate amounts of prey, and 3 for excessive amounts of prey. This subjective ranking system was calibrated to the norms expected for each particular species, based on how much space was taken up by prey. Excess prey levels were often associated with dark brown fluid and/or putrid scent. In the case of the leaf litter-trapping N. ampullaria, we also recorded the presence and absence of trapped detritus.
All unopened pitchers in this study were sampled by first shining a light through the pitcher wall to confirm the presence of fluid, and then gently popping open the lid along its suture zone. For the single unopened pitcher tracked over time, we observed changes in lid angle and peristome development consistent with natural pitcher development in the ensuing days. In other words, we succeeded in sampling the pitcher quite near the point when it would begin opening naturally.
Protein depletion rates
The initial aim of this study was to examine natural variation in fluid properties, but we found natural protein concentrations were always at undetectable levels, so we decided to conduct manipulative experiments to assess and compare rates of protein digestion/absorption across pitchers. The protein test strips we used depend on a hydrogen ion transfer from an indicator molecule to hydrogen acceptors such as amino group; thus, the test strips are most sensitive to proteins rich in amino groups, such as albumin (Strasinger & Di Lorenzo, Citation2014). Protein depletion rates are likely to be proportional to the combined rates of enzymatic and microbial digestion and absorption, since protein concentrations in pitcher fluids can only be reduced after proteins are both hydrolysed into amino acids or oligo peptides and then removed from fluids by absorption into pitcher wall or microbe cells. We used raw egg white (albumen) from chicken eggs (removing the yolk) to introduce a detectable pulse of amino-rich protein into pitchers that could then be tracked over time. Each pitcher received 1 mL of albumen. We left the pitchers open to the environment (uncovered); while prey capture and other external inputs were possible during the course of the experiment, we found that natural prey capture levels could not result in measurable increases to protein concentration with this volume of albumen. Just as with prey, preliminary trials showed no effect of inquiline presence on rate of protein depletion; inquilines were present in some living pitchers (but no other age classes) in the experiments described here. In one experiment, we examined intraspecific variation by tracking protein levels over a period of 7 days for 9 pitchers of N. rafflesiana: 5 of which were living, 1 unopened, 1 senescing, and 2 dead. We also measured fluid pH for these pitchers prior to albumen addition, and then with each subsequent protein measurement timepoint up until the 48-hour mark. In a second experiment, we examined interspecific variation by tracking protein levels for 5 days in pitchers from all three study species: 5 of N. ampullaria, 5 of N. gracilis, and 4 of N. rafflesiana. Every pitcher came from a separate individual plant and pitchers used in these experiments did not overlap. Final timepoints are missing from some pitchers due to a depleted supply of test strips at the end of the study period.
Statistical analysis
All analyses were performed in R 3.5.0 (RCoreTeam, Citation2013). Five separate analyses were conducted. Where linear mixed-effects models were used, these were performed using the package ‘lme4’ (Bates et al., Citation2014). Generalized additive mixed-effects models (GAMMs) were constructed using the package ‘mgcv’ (Wood, Citation2004, Citation2011). Multimodel selection was performed using the R package ‘MuMIn’ (Barton, Citation2016).
In the experiments comparing age class differences in protein digestion in N. rafflesiana, fluid protein levels were modeled as a smooth spline of time since albumen addition (Time, in days) using GAMMs with the individual pitchers as random intercepts. To model the effect of age class on protein digestion, two additional models containing the variable age class (Age: unopened, living, senescing or dead) and the interaction between age class and time since albumen addition. The three models were compared using Akaike's information criterion with correction for small sample sizes (AICc). The same was done for the experiment comparing interspecific differences in protein digestion, with the variable age class (Age) being replaced by species (Species: N. rafflesiana, N. gracilis or N. ampullaria). Fluid pH was modeled as a smooth spline of time since albumen addition (Time, in days) using GAMMs with individual pitchers as random intercepts. Pitcher age class (Age: unopened, living, senescing or dead) and the interaction between pitcher age class and time were also included as predictors in separate models. The three models were compared using AICc.
In the fluid pH field surveys, fluid pH of individual pitchers were modeled against all possible main effect and one-way interaction combinations of the explanatory variables of pitcher age class (Age: unopened, living, senescing or dead), pitcher morph (Morph: upper or lower pitcher), species (Species: N. rafflesiana, N. gracilis or N. ampullaria), prey volume (Prey: values of 1-3) and pitcher size (Size.PC1: the first principal component of the pitcher dimension variables fluid volume, pitcher width, and pitcher length). Linear mixed-effects models were used to model the data, with site and plant ID included as crossed random intercepts. The fitted models were compared using AICc and inferences were drawn from the top models with ΔAICc < 2 (Anderson & Burnham, Citation2002).
Finally, in the fluid total hardness field surveys, total hardness (in CaCO3 equivalent units) was log-transformed and modeled against all possible main effect and one-way interaction combinations of the explanatory variables of pitcher age class (Age: unopened, living, senescing or dead), pitcher morph (Morph: upper or lower pitcher), species (Species: N. rafflesiana, N. gracilis or N. ampullaria), prey density (Prey: ranks of 1-3) and pitcher size (Size.PC1: the first principal component of the pitcher dimension variables fluid volume, pitcher width, and pitcher length). Linear mixed-effects models were used to model the data, with site and plant ID included as crossed random intercepts. The fitted models were compared using AICc and inferences were drawn from the top models with ΔAICc < 2 (Anderson & Burnham, Citation2002).
To examine correlations between inquiline presence and pitcher properties, we conducted generalized linear mixed models using a logistic regression with plant ID as a random effect and age class, morph, fluid volume, pH, total hardness, and prey density as fixed effects for N. gracilis and N. rafflesiana. In the case of N. ampullaria, morph was not a factor, and presence of trapped detritus was an additional fixed effect.
Results
Protein depletion
In our investigation of intraspecific variation (by age class) in protein depletion rates for N. rafflesiana pitchers, an initial, qualitative inspection of the trends suggested decreasing protein concentration trends through time, with perhaps flatter slopes for the dead/senescing pitchers (a). However, quantitative analysis did not reveal significant differences between age classes. The top ranked model of protein depletion in the intraspecific age class comparisons experiment had a ΔAICc value that was >2.0 units lower than the next best model, and a model weight of 93.4% (a), suggesting that the data strongly supported the model. This model did not contain the term age class, which meant that unopened, living, senescing and dead pitchers did not have different protein depletion responses to the addition of albumen (a).
Figure 2. Results of the manipulative experiment investigating variation in rates of protein digestion within (a) and across (b) Nepenthes species, showing changes in protein concentration in the pitchers through time. The data comparing age classes in panel a are all from N. rafflesiana pitchers. Thin translucent lines represent the trends of individual pitchers, while thick bolded lines represent the top model predictions. In panel a, the shaded region represents the standard error of the prediction.
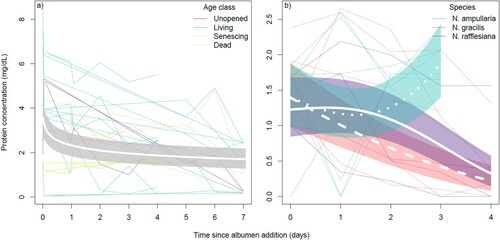
Table 1. Summary of the top models of each subsection of the study. Models are ranked according to the ΔAICc value. All models are presented in subsections involving experiments, while only models with ΔAICc < 2 are presented in subsections involving surveys. df = degrees of freedom.
The top ranked model of protein depletion in the interspecific comparisons experiment had a ΔAICc value that was > 2.0 units lower than the next best model, and a model weight of 69.3% (b), suggesting that the data strongly supported the model. This model contained the term species, and its interaction with the smoothing spline of time (b). Protein concentrations in N. gracilis and N. rafflesiana pitchers decreased gradually with time, but this was not observed in N. ampullaria pitchers (b).
Fluid pH
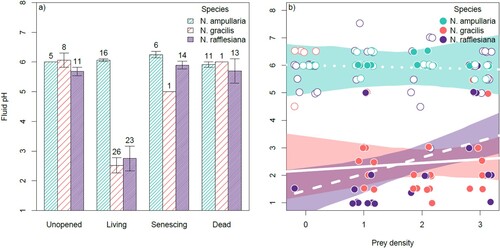
The two top (ΔAICc < 2) models of fluid pH in surveyed pitchers both contained the terms age class, species, prey level, and the interaction between age class and species, while a prey level-species interaction was found in only the model ranked first (c). Differences in fluid pH between species and age classes were found to be very large; in particular, the living pitchers of N. rafflesiana and N. gracilis both had very low pH values of 2-3, while those of N. ampullaria had consistently high pH values of approximately 6 (a). The unopened, senescing, and dead pitchers of all three species also had relatively high pH values of approximately 5–6 (a). Fluid pH also varied with pitcher prey levels, though not in all species. In N. ampullaria, the effect of prey density on fluid pH was negligible (coefficient = −0.05; 95% CI = [−0.48, 0.39]), but this effect was positive in both N. gracilis (coefficient = 0.13; 95% CI = [−0.51, 0.78]) and N. rafflesiana (coefficient = 0.60; 95% CI = [0.06, 1.15]) (b). We note, however, that interspecific differences in the prey density-fluid pH relationship were not strong, since the inclusion of the prey density-species interaction improved model AICc by only 0.60 units (c).
Figure 3. Mean fluid pH (with error bars representing SE) of pitchers of each species and age class (a) and correlation between fluid pH and prey density (b) in the survey. In panel b, living pitchers are represented by filled circles, while all other age classes (i.e. unopened, senescing and dead) are represented by open circles. Lines represent top model predictions of fluid pH in living pitchers, and are colored according to the species they represent. Sample sizes are indicated above each bar.
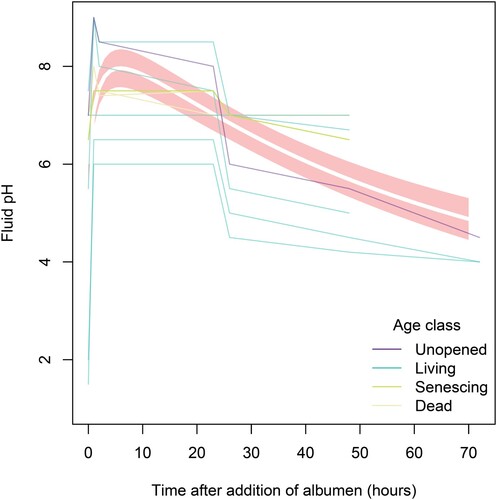
For the experiment examining intraspecific variation in protein depletion for N. rafflesiana pitchers, pH was also observed to decrease through time, following the immediate sharp increase due to albumen addition (). The top ranked model of fluid pH in the albumen addition experiment had a ΔAICc value that was far lower than all other models, and a model weight of 88.2% (d), suggesting that the data were unequivocal in their support of the model. This model did not contain the term age class, which meant that unopened, living, senescing and dead pitchers did not appear to have different fluid pH response profiles to the addition of albumen ().
Figure 4. The response of fluid pH to albumen addition did not differ significantly between pitchers of different age classes in N. rafflesiana. Thin translucent lines represent the trends of individual pitchers, colored by age class, while thick bolded line represents the top model prediction; the shaded region represents the standard error of the prediction.
Dissolved minerals
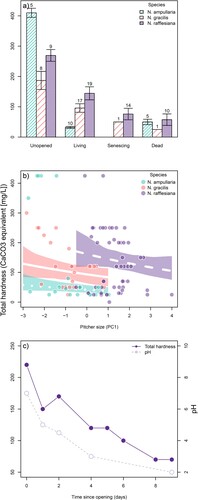
The three top (ΔAICc < 2) models of total hardness in surveyed pitchers all contained the terms age class, species, and the interaction between age class and species, while the term size was found in only the model ranked first, and the term morph, in the model ranked third (d). Differences in total hardness between species and age classes were found to be large, with levels highest in unopened pitchers, and decreasing gradually in living, senescing and dead pitchers (a). The presence of the age class-species interaction term in all top models (d) meant that this decrease with age was not the same between species. In N. rafflesiana and N. gracilis, the decrease in total hardness appeared to be gradual, while in N. ampullaria, the decrease in total hardness was abrupt, with living pitchers having low total hardness, at levels below that of dead pitchers (b). The negative correlation between pitcher size and total hardness in the model ranked first was a weak one (coefficient = −0.11; 95%CI = [−0.23, 0.01]; b).
Figure 5. Total hardness (in CaCO3 equivalents) in surveyed pitchers, as categorized by species and age class (a), or by pitcher size, PC1 = the first principal component of the pitcher dimension variables fluid volume, pitcher width, and pitcher length (b); and in a single N. rafflesiana pitcher which was monitored for 9 days immediately after opening (c). In panel a, bars represent means and error bars represent SEs; in panel b, points represent the observations from each pitcher, and are colored according to the species of that pitcher; lines represent the best model predictions of total hardness in living pitchers in the three species. Sample sizes are indicated above each bar for panel a.
Individual pitcher time series
Tracking total hardness in a single, initially unopened N. rafflesiana pitcher over time also showed that dissolved minerals in the fluid decrease as the pitcher opens and matures (c). The fluid pH also decreased as the pitcher matured. (c)
Inquiline presence
For N. gracilis and N. rafflesiana, inquiline presence was significantly more frequent in living pitchers than other age classes (logistic regression, z value = 2.95, p = 0.003) and less frequent in upper pitchers compared to lower ones (logistic regression, z value = −2.95, p = 0.003); no other factors had a significant effect on inquiline presence (Poisson regression, p > 0.05 for Fluid Volume, pH, Total Hardness, and Prey Density). For N. ampullaria, inquiline presence did not vary with pitcher age class, fluid volume, pH, total hardness, prey density, or presence of trapped detritus (logistic regression, p > 0.05).
Discussion
We sought to document and understand factors underlying the extent of inter- and intraspecific variation in Nepenthes pitcher fluid properties. We found species differences between N. rafflesiana, N. gracilis, and N. ampullaria, as well as some age-related variation within species. Nepenthes ampullaria in particular stood apart from its two congeners in several ways. One difference is that N. ampullaria exhibited a reduced rate of protein depletion. In the 3–4 days for which we tracked protein concentration in the interspecific comparison experiment, pitchers of both N. rafflesiana and N. gracilis showed some indication of active protein digestion/uptake, while those of N. ampullaria showed no decrease in protein concentration with time (). The apparent reduced digestive capacity in N. ampullaria we observed is consistent with SDS-PAGE data showing lower concentrations of the protease nepenthesin in N. ampullaria compared to these same two congeners (Lam & Tan, Citation2020); this may be a consequence of its shift away from insects as the sole nitrogen source (Moran et al., Citation2003). This species’ detritivory and reliance on leaf litter for a considerable portion of its nitrogen may lead to an outsourcing of its digestion to inquilines, which may be more effective at releasing organically-bound nitrogen from plant matter (Moran et al., Citation2003, Citation2010).
Nepenthes ampullaria also demonstrated differences in its pattern of fluid pH regulation that are characteristic of a species that relies little on endogenous enzyme-assisted digestion. For both N. gracilis and N. rafflesiana, the pH of unopened pitchers was relatively high (moderately acidic, pH ∼5-7), living (healthy) pitchers were more acidic (pH 2-3), and senescing and dead pitchers had relatively high pH levels again similar to those of unopened pitchers (). This ontogenetic pattern of post-opening acidification followed by neutralization with age has been previously described in Nepenthes mirabilis (Hua & Li, Citation2005) and provides hydrolytic enzymes like nepenthesin proteases with the low pH required for their function (Athauda et al., Citation2004). In contrast, N. ampullaria was exceptionally invariant in its range of pH levels overall. In fact, all 16 healthy living pitchers of N. ampullaria we sampled had fluid pH values of approximately 6 (a).
Another difference in pH dynamics of N. ampullaria in contrast to those of its congeners is a lack of a response to prey capture. In N. rafflesiana, and to a lesser extent N. gracilis, fluid pH of living pitchers increased with increasing prey density (b). Such an increase may be caused by the failure of fluid regulative mechanisms when pitchers are overloaded with putrefying prey―a phenomenon that often leads to premature pitcher senescence (Clarke & Kitching, Citation1995), and/or the release of large amounts of ammonia by rapidly decomposing prey. It remains unclear why the same positive response of N. ampullaria fluid pH to increasing pitcher prey levels was not observed.
Our experimental albumen additions also suggested links between digestive processes and pH regulation. We tracked pH for the experiment in which we assessed intraspecific variation in protein depletion for N. rafflesiana (). Because albumen is alkaline, its addition caused an immediate increase in pH level, but pH decreased appreciably over time just as protein concentrations did (a), suggesting acidification by the pitcher countering those alkaline inputs. However, we did not find significant differences in the rate of acidification between pitcher age classes (). Just like with pH, pitcher age classes also did not significantly differ in protein depletion in this experiment (a). This was contrary to expectation, as we anticipated that the cessation of physiological activity in dead pitchers would dampen digestion (and acidification) relative to living ones, leading to lower protein depletion. One possibility is that nepenthesins are still present in the dead pitchers, due to the exceptionally long stability of these enzymes: as long as 30 days even when isolated and incubated at 50°C (Athauda et al., Citation2004). It is also possible that microbes still present in the fluid of dead pitchers may contribute to protein digestion, just as they do in the fluid of living pitchers (Lam et al., Citation2017)—inquilines were not present in dead pitchers and may thus be ruled out. Regarding acidification, certain species of soil bacteria (in high population density) are capable of modifying external pH to as extreme of a change as going from neutral to pH 4 in as little as 24 h (Ratzke, Denk, & Gore, Citation2018). We gathered no data on the composition or density of microbes in our study to assess the potential for such a strong microbial effect here. However, Bittleston et al. (Citation2018) demonstrated that these same three species from Singapore differ significantly in their microbial community compositions. Interestingly, Fouilloux et al. (Citation2021) found that various phytotelmata that dry out and subsequently refill with rain would then exhibit the same pre-desiccation pH levels—this held true both for phytotelmata in living as well as dead plant tissues. This suggests that phytotelm container type (size, shape, and location) may select for certain traits such as pH levels that are not dependent upon active metabolism by the hostplant.
Another point to consider regarding protein digestion is that the proteins in albumen are of unknown ecological relevance to Nepenthes. Considering that Nepenthes pitchers produce a variety of different enzymes that may target diverse proteins (Ravee, Salleh, & Goh, Citation2018), different patterns of digestion may occur for different target proteins. On this point, Nepenthes can also digest non-protein nitrogenous compounds, e.g. urea (Yilamujiang et al., Citation2017). In any case, our experiments did at least reveal protein digestion/uptake in our study species, and the absence of conclusive intraspecific variation in the rate of decreasing protein concentration makes the lack of a decrease seen only in N. ampullaria all the more striking. The finding of reduced digestive/absorptive efficiency in N. ampullaria stands in contrast to a recent study showing no reduction in enzymatic efficiency in the coprophagous N. hemsleyana compared to its closest relative, the fully carnivorous N. rafflesiana (Kocáb et al., Citation2021).
As with fluid pH, dissolved mineral concentrations exhibited a pattern of ontogenetic change. In all three species studied, unopened pitchers had the highest levels of dissolved minerals, which then greatly decreased in subsequent pitcher stages (a). However, interspecific differences in dissolved mineral concentrations were not consistent across the pitcher age classes. When considering only unopened pitchers, N. ampullaria consistently had the highest total hardness levels of all, but, the levels in living N. ampullaria pitchers were exceptionally low overall (even compared to dead pitchers) (a). This is interesting, as decomposing leaf litter normally leads to high concentrations of dissolved calcium in tree-hole phytotelmata (Paradise & Dunson, Citation1997; Schmidl, Sulzer, & Kitching, Citation2008; Fouilloux et al., Citation2021). The lower levels of dissolved minerals in the phytotelmata of living Nepenthes pitchers thus more closely resemble those in bromeliads (Richardson, Rogers, & Richardson, Citation2000), which like pitchers, also take up nutrients directly from their tanks.
The decrease in dissolved mineral concentrations after opening may be an indication that pitchers begin absorbing minerals from the fluid once they fully develop. Plants, in general, absorb minerals like calcium from their roots and transport them to the leaves through the vascular system (White & Broadley, Citation2003). Pitcher digestive glands are directly connected to the xylem (Owen & Lennon, Citation1999), so the initial level of dissolved minerals is likely a reflection of what is being secreted into the developing pitchers via this route. Prior to pitcher opening, digestive glands function in fluid secretion and begin functioning for nutrient absorption only after opening (Owen & Lennon, Citation1999). Dissolved minerals would remain low in dead pitchers since vascular transport has ceased. Of note, total hardness did not correlate with pH overall (glm, t value = 0.32, p = 0.75), but when considered only within living pitchers, hardness showed a significant negative correlation (glm, t value = −2.51, p = 0.016). Thus, dissolved minerals do not seem to be responsible for the high pH levels observed in some pitchers. The fact that living pitchers with high dissolved mineral concentrations were actually more acidic could point to a pitcher's ability to counteract fluid pH increases by actively pumping in more protons (Moran et al., Citation2010). Similarly, a study of calcium in the American pitcher plant Sarracenia purpurea L. also found higher calcium levels in more acidic fluid (Meir, Juniper, & Evans, Citation1991). On a different note, we found a slight correlation between total hardness and pitcher size in living pitchers for each species, with bigger pitchers having lower total hardness (b). If the rate of mineral accumulation during development is constant across pitchers, then larger volumes would be more dilute. The regulation of dissolved minerals in Nepenthes merits future study as many unknowns remain.
The differences in the regulation of abiotic fluid properties in N. ampullaria has consequences for their biotic interactions. Nepenthes ampullaria is a species with a reflexed lid, and thus a greater risk of dilution by rain. Despite similarities in pH between the fluids of living pitchers and those of dead ones, N. ampullaria is in fact able to actively regulate its pH levels while alive, preventing the fluid from becoming too acidic, more specifically, buffering against external changes to maintain a narrow pH range (Moran et al., Citation2010). This is hypothesized to create a permissive environment for mutualistic, leaf litter-digesting inquilines (Moran et al., Citation2003, Citation2010; Adlassnig et al., Citation2011). N. ampullaria indeed has richer communities than most Nepenthes species (Gaume et al., Citation2019), and with the exception of dissolved minerals, its fluid environment more closely resembles those of inert tree-holes than do other pitcher phytotelmata. Thus, the evolution of alternative dietary strategies in pitcher plants may result in changes in the physiological regulation of pitcher fluids that further shape the composition of their symbiont communities. The results here have only addressed an assemblage of two fully carnivorous species and one partial detritivore; future work should examine other species with alternative dietary strategies, such as the coprophagous N. lowii, which can also collect leaf litter in its pitchers (Clarke et al., Citation2009). Only a small fraction of Nepenthes species have been the focus of dedicated ecological study (Clarke & Moran, Citation2011), so the full extent of interspecific variation has yet to be revealed. Detailed studies of the regulation of proteins, pH, and dissolved minerals in pitcher fluid throughout the genus will advance our understanding of the ecology and diversification of the group.
Acknowledgements
Singapore NParks enabled this work under permit NP/RP19-056. We thank Hugh Tan, Zhengyang Wang, Chris Baker, William King, and Sylvie Martin-Eberhardt, as well as members of the Pierce Lab at Harvard and the Plant-Arthropod Interactions Group (PLARG) at Michigan State for helpful discussions.
Data availability statement
Data and R scripts will be made available on Harvard Dataverse (https://doi.org/10.7910/DVN/GATZ7D).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Kadeem J. Gilbert
Kadeem J. Gilbert is an Assistant Professor at Michigan State University at the W.K. Kellogg Biological Station. He is interested in symbiotic interactions between plants and insects or microbes, particularly regarding how leaf traits mediate those interactions by altering abiotic properties of their microenvironment. He also studies the ecology and evolutionary biology of carnivorous plants, especially tropical pitcher plants (Nepenthaceae).
Thibaut Goldsborough
Thibaut Goldsborough is a PhD student at the University of Edinburgh. He completed a BSc in Biology and Mathematics at the University of St Andrews and an MSc in Biomedical AI at the University of Edinburgh. He is interested in the use of computational tools for the study of biological systems.
Weng Ngai Lam
Weng Ngai Lam is a postdoctoral research fellow at Nanyang Technological University. He focuses on bottom-up, process-based ecology. He is interested in how plant and invertebrate traits predict ecosystem processes through ecophysiological and demographical processes, and how consumer-resource dynamics determine species interaction outcomes. He works mainly with pitcher plant phytotelmata, leaf litter invertebrate, and forest tree communities.
Felicia Leong
Felicia Leong is a PhD student from the Department of Biological Sciences at the National University of Singapore. She is interested in the structure of plant-plant interaction networks and how they influence tropical forest dynamics.
Naomi E. Pierce
Naomi E. Pierce has been the Hessel Professor of Biology and Curator of Lepidoptera at the Museum of Comparative Zoology at Harvard since 1991. She studies the ecology and evolution of species interactions, and how parasitic and mutualistic life histories can influence the evolutionary trajectories of each partner. Her work has ranged from field studies measuring the costs and benefits of symbioses between ants and other organisms, to genetic analyses of biochemical signaling pathways underlying interactions between plants, pathogens and insects. She is currently interested in whether insects can perceive and use signals in the near infrared.
References
- Adlassnig W, Peroutka M, Lendl T. 2011. Traps of carnivorous pitcher plants as a habitat: composition of the fluid, biodiversity and mutualistic activities. Ann Bot. 107:181–194.
- Anderson DR, Burnham KP. 2002. Avoiding pitfalls when using information-theoretic methods. J Wildl Manage. 66:912–918.
- Athauda SBP, Matsumoto K, Rajapakshe S, Kuribayashi M, Kojima M, Kubomura-Yoshida N, Iwamatsu A, Shibata C, Inoue H, Takahashi K. 2004. Enzymic and structural characterization of nepenthesin, a unique member of a novel subfamily of aspartic proteinases. Biochem J. 381:295–306.
- Barton K. 2016. Package “MuMIn”: Multi-Model Inference. R package, Version 1.15. 6. URL: https://cran.r-project.org/web/packages/MuMIn/index.html [accessed 2016-10-22].
- Bates D, Mächler M, Bolker B, Walker S. 2014. Fitting linear mixed-effects models using lme4. arXiv Preprint ArXiv:1406.5823.
- Bazile V, Le Moguédec G, Marshall DJ, Gaume L. 2015. Fluid physico-chemical properties influence capture and diet in nepenthes pitcher plants. Ann Bot. 115:705–716.
- Bittleston LS. 2018. Commensals of Nepenthes pitchers. In: Aaron M. Ellison, Lubomir Adamec, editors. Carnivorous plants: Physiology, ecology, and evolution. Great Clarendon Street, Oxford, OX2 6DP, UK: Oxford University Press; p. 314–332.
- Bittleston LS, Wolock CJ, Yahya BE, Chan XY, Chan KG, Pierce NE, Pringle A. 2018. Convergence between the microcosms of Southeast Asian and North American pitcher plants. eLife. 7:e36741.
- Bonhomme V, Pelloux-Prayer H, Jousselin E, Forterre Y, Labat JJ, Gaume L. 2011. Slippery or sticky? functional diversity in the trapping strategy of Nepenthes carnivorous plants. New Phytol. 191:545–554.
- Chin L, Moran JA, Clarke C. 2010. Trap geometry in three giant montane pitcher plant species from Borneo is a function of tree shrew body size. New Phytol. 186:461–470.
- Clark EW. 1958. A review of literature on calcium and magnesium in insects. Ann Entomol Soc Am. 51:142–154.
- Clarke C, Kitching R. 1995. Swimming ants and pitcher plants: a unique ant-plant interaction from Borneo. J Trop Ecol. 11:589–602.
- Clarke C, Moran J. 2011. Incorporating ecological context: a revised protocol for the preservation of Nepenthes pitcher plant specimens (Nepenthaceae). Blumea - Biodiversity, Evolution and Biogeography of Plants. 56:225–228.
- Clarke CM, Bauer U, Ch’ien CL, Tuen AA, Rembold K, Moran JA. 2009. Tree shrew lavatories: A novel nitrogen sequestration strategy in a tropical pitcher plant. Biol Lett. 5:632–635.
- Dover C, Fage L, Hirst S, Tams W, Ghosh E. 1928. Notes on the fauna of pitcher-plants from Singapore island. Journal of the Malayan Branch of the Royal Asiatic Society. 6:1–27.
- Fouilloux CA, Serrano Rojas SJ, Carvajal-Castro JD, Valkonen JK, Gaucher P, Fischer MT, Pašukonis A, Rojas B. 2021. Pool choice in a vertical landscape: Tadpole-rearing site flexibility in phytotelm-breeding frogs. Ecol Evol. 11:9021–9038.
- Gaume L, Bazile V, Boussès P, Le Moguédec G, Marshall DJ. 2019. The biotic and abiotic drivers of ‘living’ diversity in the deadly traps of Nepenthes pitcher plants. Biodivers Conserv. 28:345–362.
- Gaume L, Forterre Y. 2007. A viscoelastic deadly fluid in carnivorous pitcher plants. PloS one. 2:e1185.
- Gilbert KJ, Bittleston LS, Tong W, Pierce NE. 2020. Tropical pitcher plants (Nepenthes) act as ecological filters by altering properties of their fluid microenvironments. Sci Rep. 10(1):4431.
- Goldsborough T. 2020. Teststrip reader (Version 2.0). https://github.com/ThibautGoldsborough/teststrip-reader.
- Golos MR, Robinson AS, Barer M, Dančák M, De Witte J, Limberg A, Sapawi NBM, Tjiasmanto W. 2020. Nepenthes fractiflexa (Nepenthaceae), a new Bornean pitcher plant exhibiting concaulescent metatopy and a high degree of axillary bud activation. Phytotaxa. 432:125–143.
- Greenwood M, Clarke C, Lee CC, Gunsalam A, Clarke RH. 2011. A unique resource mutualism between the giant Bornean pitcher plant, Nepenthes rajah, and members of a small mammal community. PloS one. 6:e21114.
- Hua Y, Li H. 2005. Food web and fluid in pitchers of Nepenthes mirabilis in Zhuhai, China. Acta Bot Gall. 152:165–175.
- Juniper BE, Robins RJ, Joel DM. 1989. The carnivorous plants. London, etc.: Academic Press.
- Kanokratana P, Mhuanthong W, Laothanachareon T, Tangphatsornruang S, Eurwilaichitr L, Kruetreepradit T, Mayes S, Champreda V. 2016. Comparative study of bacterial communities in Nepenthes pitchers and their correlation to species and fluid acidity. Microb Ecol. 72:381–393.
- Karagatzides JD, Ellison AM. 2009. Construction costs, payback times, and the leaf economics of carnivorous plants. Am J Bot. 96:1612–1619.
- Kitching R. 1987. A preliminary account of the metazoan food webs in phytotelmata from Sulawesi. Malay. Nat. J. 41:1–12.
- Kitching R. 2001. Food webs in phytotelmata:“bottom-up” and “top-down” explanations for community structure. Annu Rev Entomol. 46:729–760.
- Kocáb O, Bačovčinová M, Bokor B, Šebela M, Lenobel R, Schöner CR, Schöner MG, Pavlovič A. 2021. Enzyme activities in two sister-species of carnivorous pitcher plants (Nepenthes) with contrasting nutrient sequestration strategies. Plant Physiol Biochem. 161:113–121.
- Lam WN, Chong KY, Anand GS, Tan HT. 2019. In situ proteolytic activity in Nepenthes gracilis pitcher plant traps Is affected by both pitcher-extrinsic and pitcher-intrinsic factors. Int J Plant Sci. 180:179–185.
- Lam WN, Chong KY, Anand GS, Tan HTW. 2017. Dipteran larvae and microbes facilitate nutrient sequestration in the Nepenthes gracilis pitcher plant host. Biol Lett. 13:20160928.
- Lam WN, Lai HR, Lee CC, Tan HT. 2018a. Evidence for pitcher trait-mediated coexistence between sympatric Nepenthes pitcher plant species across geographical scales. Plant Ecol Divers. 11(3):283–294.
- Lam WN, Lim RJY, Wong SH, Tan HTW. 2018b. Predatory dipteran larva contributes to nutrient sequestration in a carnivorous pitcher plant. Biol Lett. 14(3):20170716.
- Lam WN, Tan HTW. 2020. Chapter 5: Prey and Carnivory. In: Lam WN, Tan HTW, editor. The pitcher plants (Nepenthes species) of Singapore. Lee Kong Chian Natural History Museum. Singapore: National University of Singapore; p. 75–98.
- Leong FWS, Lam WN, Tan HTW. 2018. A dipteran larva-pitcher plant digestive mutualism is dependent on prey resource digestibility. Oecologia. 188(3):813–820.
- Leong FWS, Lam WN, Tan HTW. 2019. Digestive mutualism in a pitcher plant supports the monotonic rather than hump-shaped stress-gradient hypothesis model. Oecologia. 190:523–534.
- Lim YS, Schöner CR, Schöner MG, Kerth G, Thornham DG, Scharmann M, Grafe TU. 2015. How a pitcher plant facilitates roosting of mutualistic woolly bats. Evol Ecol Res. 16:581–591.
- McPherson S, Robinson A, Fleischmann A. 2009. Pitcher plants of the Old World. Dorset, UK: Redfern Natural History Productions.
- Meir P, Juniper B, Evans D. 1991. Regulation of free calcium concentration in the pitchers of the carnivorous plant Sarracenia purpurea: a model for calcium in the higher plant apoplast? Ann Bot. 68:557–561.
- Mogi M, Chan K. 1996. Predatory habits of dipteran larvae inhabiting Nepenthes pitchers. Raffles Bull Zool. 44:233–245.
- Moran JA, Clarke CM, Hawkins BJ. 2003. From carnivore to detritivore? isotopic evidence for leaf litter utilization by the tropical pitcher plant Nepenthes ampullaria. Int J Plant Sci. 164:635–639.
- Moran JA, Gray LK, Clarke C, Chin L. 2013. Capture mechanism in palaeotropical pitcher plants (Nepenthaceae) is constrained by climate. Ann Bot. 112(7):1279–1291.
- Moran JA, Hawkins BJ, Gowen BE, Robbins SL. 2010. Ion fluxes across the pitcher walls of three Bornean Nepenthes pitcher plant species: flux rates and gland distribution patterns reflect nitrogen sequestration strategies. J Exp Bot. 61(5):1365–1374.
- Osunkoya OO, Daud SD, Wimmer FL. 2008. Longevity, lignin content and construction cost of the assimilatory organs of Nepenthes species. Ann Bot. 102:845–853.
- Owen TP, Lennon KA. 1999. Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae). Am J Bot. 86:1382–1390.
- Paradise CJ, Dunson WA. 1997. Effects of water cations on treehole insect communities. Ann Entomol Soc Am. 90:798–805.
- Pavlovič A, Masarovičová E, Hudák J. 2007. Carnivorous syndrome in Asian pitcher plants of the genus Nepenthes. Ann Bot. 100:527–536.
- Pavlovič A, Singerová L, Demko V, Hudák J. 2009. Feeding enhances photosynthetic efficiency in the carnivorous pitcher plant Nepenthes talangensis. Ann Bot. 104(2):307–314.
- Pavlovič A, Slováková Ľ, Šantrůček J. 2011. Nutritional benefit from leaf litter utilization in the pitcher plant Nepenthes ampullaria. Plant Cell Environ. 34(11):1865–1873.
- Poteat MD, Buchwalter DB. 2014. Calcium uptake in aquatic insects: Influences of phylogeny and metals (Cd and Zn). J Exp Biol. 217:1180–1186.
- Ratzke C, Denk J, Gore J. 2018. Ecological suicide in microbes. Nature Ecology & Evolution. 2:867–872.
- Ravee R, Salleh FM, Goh HH. 2018. Discovery of digestive enzymes in carnivorous plants with focus on proteases. PeerJ. 6:e4914.
- R Core Team. 2013. R: A language and environment for statistical computing. 55:275–286.
- Richardson BA, Rogers C, Richardson M. 2000. Nutrients, diversity, and community structure of two phytotelm systems in a lower montane forest, Puerto Rico. Ecol Entomol. 25:348–356.
- Saganová M, Bokor B, Stolárik T, Pavlovič A. 2018. Regulation of enzyme activities in carnivorous pitcher plants of the genus Nepenthes. Planta. 248(2):451–464.
- Scharmann M, Thornham DG, Grafe TU, Federle W. 2013. A novel type of nutritional ant-plant interaction: Ant partners of carnivorous pitcher plants prevent nutrient export by dipteran pitcher infauna. PloS one. 8:e63556.
- Schmidl J, Sulzer P, Kitching R. 2008. The insect assemblage in water filled tree-holes in a European temperate deciduous forest: Community composition reflects structural, trophic and physicochemical factors. Hydrobiologia. 598:285–303.
- Strasinger SK, Di Lorenzo MS. 2014. Urinalysis and body fluids. FA Davis.
- Tamizi AA, Ghazalli MN, Nikong D, Mat-Esa M, Besi EE, Mohd-Nordin A, Latiff A, Shakri MA. 2020. Nepenthes x setiuensis (Nepenthaceae), a new nothospecies of pitcher plant from montane cloud forest of peninsular Malaysia. Malay Nat J. 72:27–41.
- Thorogood C, Bauer U, Hiscock S. 2018. Convergent and divergent evolution in carnivorous pitcher plant traps. New Phytol. 217(3):1035–1041.
- Wells K, Lakim MB, Schulz S, Ayasse M. 2011. Pitchers of Nepenthes rajah collect faecal droppings from both diurnal and nocturnal small mammals and emit fruity odour. J Trop Ecol. 27:347–353.
- White PJ, Broadley MR. 2003. Calcium in plants. Ann Bot. 92:487–511.
- Wood S. 2011. Gamm4: Generalized additive mixed models using mgcv and lme4 [online]. R Package Version. 0:1–5.
- Wood SN. 2004. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc. 99:673–686.
- Yilamujiang A, Zhu A, Ligabue-Braun R, Bartram S, Witte CP, Hedrich R, Hasabe M, Schöner CR, Schöner MG, Kerth G, Carlini CR. 2017. Coprophagous features in carnivorous Nepenthes plants: a task for ureases. Sci Rep. 7(1):1–9.
