ABSTRACT
Plant response to individual biotic stresses depends on its physiological state when the challenge is perceived. Optimal conditions for infestation of the spider mite Tetranychus urticae are associated with high temperatures and scarce precipitation. Here, we analyze the impact of previous interactions with the hemibiotrophic bacteria Pseudomonas syringae pv. tomato DC3000 or the necrotroph Dickeya dadantii 3937 on Arabidopsis thaliana plants under mite optimal conditions. Our results showed that both bacterial strains inoculated at adverse low humidity conditions induced the expression of JA-related genes in the plant even when disease symptoms are not observed. This effect was more evident when heat-inactivated bacteria were used, but a significant reduction in mite leaf damage was only detected when plants were previously inoculated with the heat-inactivated hemibiotroph bacteria. These results indicate that bacterial interaction compromises the plant response to subsequent herbivore stress, even under suboptimal conditions for bacterial multiplication.
Introduction
In the field, plant responses are complex processes modulated by the interaction of multiple simultaneous or sequential abiotic and biotic stresses. The response to individual biotic stresses depends on the physiological state at the moment of perception of the challenge. Previous interactions with pathogenic organisms can enhance or inhibit these responses (Lazebnik et al. Citation2014). Regarding herbivore threats, pathogen infections can cause pathogen-induced changes in the plant that may influence the behavior or performance of a subsequent herbivore infestation. Studies have shown diverse results, with both positive and negative impacts of pathogen infection on herbivore behavior. For example, pathogens can repress anti-herbivore defenses such as toxin production or attraction of natural enemies (Hammerbacher et al. Citation2013; Mason et al. Citation2014; Desurmont et al. Citation2016) or enhance the production of volatile compounds to fight against herbivore attacks (Fernandez-Conradi et al. Citation2018; Ederli et al. Citation2021). These canonical plant responses to pathogens and herbivores may also vary depending on how environmental conditions, such as humidity and temperature, deviate from the optimal infection/infestation conditions for each plant-interacting organism (Kim et al. Citation2021).
Defense responses depend on a complex system developed by plants to fight against pathogens and herbivores tightly regulated by hormonal changes. Plants generally defend against biotrophic pathogens that obtain their nutrients from the living host cell, and some phloem-feeding herbivores, by activating defense responses regulated by the salicylic acid (SA) hormone pathway. On the contrary, plants defend against necrotrophic pathogens that benefit from host cell death and chewing herbivores by activating defense responses regulated by jasmonic acid (JA) and ethylene (ET) hormones (Glazebrook Citation2005; Thaler et al. Citation2012). Therefore, pathogen infections can modify later responses by interfering with the activation of defense signaling pathways mediated by hormones and by creating a ‘priming’ effect due to transcriptional reprograming of defense genes prior to the appearance of new threats (De Vos et al. Citation2005; Davila Olivas et al. Citation2016). Despite the relevance of previous infections by pathogens in herbivore performance, most studies used fungi as plant pathogens and scarce information has been provided on the influence of pathogenic bacteria on later herbivore infestation. The most related analysis showed reduced feeding damage caused by the western flower thrip Frankliniella occidentalis in tomato plants previously infiltrated with the pathogenic bacteria Pseudomonas syringae pv. tomato DC3000 (Chen et al. Citation2018). Furthermore, bean plants (Phaseolus vulgaris L.) infected with the phytopathogenic bacterium P. syringae pv. syringae modify the emission of volatile compounds and suffered significantly lower leaf damage caused by the spider mite Tetranychus urticae than uninfected plants (Karamanoli et al. Citation2020).
To gain more insight on this topic, we have chosen a working model composed of Arabidopsis thaliana as plant species, P. syringae pv tomato DC3000 (Pst DC3000) and Dickeya dadantii 3937 (Dd 3937) as pathogenic bacteria with different lifestyles, and T. urticae as herbivore. The spider mite T. urticae is a devastating polyphagous pest that feeds on more than 1,100 different plants (including A. thaliana), 150 of them of agricultural importance (Santamaria et al. Citation2020). T. urticae pierces parenchymatic plant cells using stylets to suck their nutrients, and causes severe chlorosis, leading to a reduction in crop yield (Bensoussan et al. Citation2016). Although JA is the main hormone involved in the establishment of spider mite-induced defense responses, mite feeding also triggers SA signaling pathways (Ament et al. Citation2004; Zhurov et al. Citation2014; Santamaria et al. Citation2017). Pst DC3000 is a hemibiotrophic bacterial pathogen that has been used as a model to study plant-bacteria interactions (Xin and He Citation2013). Pst DC3000 not only infects its natural host, tomato, but also A. thaliana, and carries a large repertoire of potential virulence factors, including a polyketide phytotoxin called coronatine, which structurally mimics the plant hormone jasmonate (JA) (Weiler et al. Citation1994). Dd 3937 is a plant necrotrophic bacterium with a very wide host range, including A. thaliana and important crops; such are tomato and potato (Charkowski et al. Citation2012). A crucial role in the virulence of these two bacterial species has been attributed to chemoreceptor functions driving motility to entry sites like wounds and stomata (Río-Álvarez et al. Citation2015; Santamaría-Hernando et al. Citation2022). In particular, JA released in plant wounds is a strong chemoattractant that drives Dd 3937 entry into the plant apoplast (Antunez-Lamas et al. Citation2009).
High temperatures and scarce precipitation constitute the optimal ecological niche for T. urticae infestation. Thus, spider mite damage will likely intensify with global warming, as predicted by computer models (Migeon and Dorkeld Citation2006–2022). This pest risk due to environmental conditions should be analyzed in the context of the presence of other pathogens. In the case of phytopathogenic bacteria, most of the studies devoted to the analysis of bacterial-plant interactions have been carried out under high humidity conditions in which bacterial infection is optimal. Therefore, changes in plant response due to bacterial presence obtained under these conditions are not translatable to analyze subsequent mite infestations, a plausible scenario in field conditions. Here, we analyze the impact of previous interactions with the hemibiotrophic bacteria Pst DC3000 or the necrotrophic Dd 3937 on the damage caused by the mite T. urticae in A. thaliana under spider mite optimal conditions. Furthermore, we analyze the effects of previous infections on the activation of hormone-associated signaling pathways caused by mites.
Materials and methods
Plant material and growth conditions
A. thaliana Columbia (Col-0) ecotype plants were used. Seeds were surface sterilized with 70% (v/v) ethanol for 2 min, followed by incubation in a solution containing 5% (V/V) sodium hypochlorite and 5% (w/v) SDS for 10 min, and then washed with sterilized distilled H2O. Seeds were planted in a mixture of peat moss and vermiculite (3:2 v/v) and kept for 5 days at 4°C in darkness and then transferred to growth chambers (Sanyo MLR-350-H). Plants were grown at 25°C under a 16/8 h day/night photoperiod for approximately two weeks.
Bacterial strains and inoculation conditions
D. dadantii 3937 was grown in solid King's B (KB) medium (King et al. Citation1954) for 24 h and P. syringae pv. tomato DC3000 in solid KB + 25 μg/ml rifampicin for 48 h. Bacterial cells were collected, washed twice in 10 mM MgCl2, and diluted to 108 CFU/ml for spray inoculations, applied with 5 spray pulses per plant. For Dd 3937 wound inoculation, cells were diluted to 104 CFU/ml and 5 μl of the suspension was placed on wounds performed by piercing three Arabidopsis leaves with a needle. Plants kept at ‘high humidity’ after bacteria inoculation were thoroughly watered to keep the substrate of the pot always humid (75–85% relative humidity). After Dd 3937 inoculation, extra humidity was created by placing a lid on the plant pot trays and temperature was set at 28°C. Bacteria inoculated plants were also kept at ‘low humidity’ conditions (50–60% relative humidity) in growth chambers with scarce watering, keeping dry pot substrate until rosette collection or mite infestation. Bacterial growth in planta was determined 4 days post-inoculation (dpi) by collecting samples from leaves with a 6 mm diameter cork borer. Three leaf disks from the same plant were pooled and homogenized in 10 mM MgCl2 and serial dilutions were dropped onto KB plates for colony-forming unit (CFU) counting. Heat-inactivated bacteria were grown and diluted as indicated above. Then, cells were kept at 70°C for 1 h before spray application. The lack of growth of these cells on solid KB medium confirmed heat inactivation.
Maintenance of spider mites and plant infestation
T. urticae London strain (Acari: Tetranychidae) was provided by Dr. Miodrag Grbic (University of Western Ontario) and reared in bean plants (Phaseolus vulgaris) in growth chambers (Sanyo MLR-350-H) at 25°C ± 1°C and a 16/8 h day/night photoperiod. For mite infestation, 20 T. urticae female adults were carefully placed on the leaf surface of control or bacteria-sprayed Arabidopsis plants using a brush (6 hpi with Dd 3937 and 48 hpi with Pst DC3000). Plants were then incubated in growth chambers at 25°C ± 1°C and a 16/8 h day/night photoperiod in ‘low humidity’ (50–60% relative humidity) conditions.
Determination of gene expression
Arabidopsis rosettes were collected, immediately frozen in liquid nitrogen, and kept at −80°C until use. Plant material of each biological replicate, consisting of pooled 4–6 rosettes, was ground in liquid nitrogen and total RNA was extracted by the phenol/chloroform method, followed by precipitation with 8 M LiCl (Oñate-Sánchez and Vicente-Carbajosa Citation2008). RNA was then quantified spectrophotometrically (ND-1000 spectrophotometer, NanoDrop Technologies, Inc.) and treated with RQ1 DNase (Promega). First-strand cDNA was synthesized from 2 μg RNA in 20 μl volume, using Revert Aid H Minus First Strand cDNA synthesis kit (Thermo Fisher Scientific) following manufacturer’s instructions. Two RT-qPCR technical replicates were performed in a LightCycler 480 System, using 70 ng cDNA from each biological replicate, LightCycler 480 SYBR Green I Master Mix (Roche) and 0.5 μM of specific primers (Supplemental Table 1). The RT-qPCR conditions used were: 40 cycles of 15 s at 95°C, 30 s at 60°C, and 20 s at 72°C. mRNA quantification was expressed as relative expression levels (2−dCt) or fold change (2−ddct) normalized to ubiquitin gene expression.
Plant damage estimation
Bacteria and heat-inactivated bacteria were inoculated by spray and kept at 25°C in low humidity conditions. Mock inoculation with 10 mM MgCl2 was used as control. Mites were applied 6 hpi of Dd 3937 or HI Dd 3937, and 48 hpi of Pst DC3000 or HI Pst DC3000. Plants were kept in the same conditions and four days after mite infestation, leaf damage was evaluated by scanning rosettes (8 plants per treatment) with an HP Scanjet 5590 digital flatbead scanner. Scanning conditions and damaged area were calculated as previously described (Ojeda-Martinez et al. Citation2020).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics software. Gene expression after bacteria inoculation was compared with mock-inoculated samples using independent t-tests. Leaf damage and gene expression after bacteria inoculation + mite infestation were compared with mock-inoculation + mite infestation using one-way ANOVA followed by Tukey’s multiple comparison test.
Results
Effects of optimal and suboptimal conditions for bacterial infection in the response of Arabidopsis to Pst DC3000 and Dd 3937
To analyze the effects of bacterial presence in the plant prior to mite infestation, we carried out bacterial challenge on Arabidopsis plants both in optimal (high humidity and spray for Pst DC3000 and high humidity and wounding for Dd 3937) and suboptimal (low humidity and spray for both bacteria) conditions for infection development. We analyzed symptoms development and transcriptional responses.
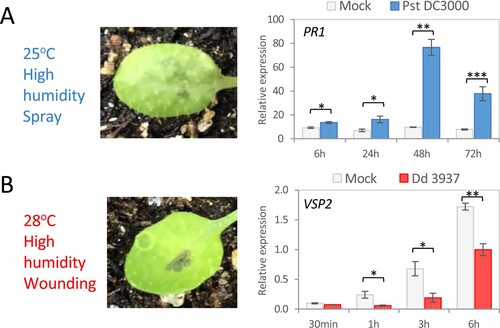
In optimal conditions for bacterial infection, the expression levels of the SA-induced gene PR1 and the JA-induced gene VSP2 were determined for the hemibiotrophic Pst DC3000 and the necrotrophic Dd 3937 inoculations, respectively. As expected, challenge with Pst DC3000 at 25°C and high humidity caused induction of PR1 expression, which peaks at 48 h post-inoculation (hpi) ((A)). In these conditions, water-soaked patches were observed after 48 h ((A)). VSP2 expression at 28°C and high humidity was induced in Dd 3937-inoculated leaves, showing slightly more VSP2 induction the plants mock-inoculated, with a maximum at 6 hpi ((B)). Disease progress was observed in Dd 3937-inoculated plants 6 hpi since maceration symptoms were detected around inoculated wounds ((B)).
Figure 1. Bacterial inoculation of Arabidopsis at optimal conditions. (A) Pst DC3000. Leaf symptoms 48 hpi (left) and relative mRNA expression at different time points of PR1 gene after mock or bacterial inoculation (right). (B) Dd 3937. Leaf symptoms 6 hpi (left) and relative mRNA expression at different time points of VSP2 gene after mock or bacterial inoculation (right). Data are means ± SE of three biological replicates. Asterisks indicate significant differences (t-test; *P < 0.05, **P < 0.01, ***P < 0.001).
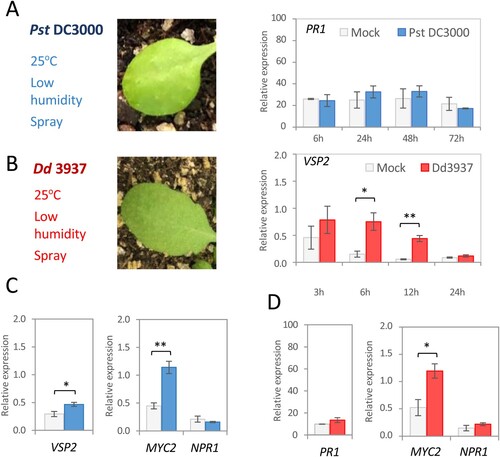
When bacteria were sprayed on leaves at 25°C and low humidity, PR1 induction was not found in response to Pst DC3000 infection, which did not cause any apparent phenotype ((A)). Contrary to wounded plants, mock-sprayed plants did not show induction in the expression of the VSP2 gene. In contrast, the expression of this gene was induced in response to spray-infection with Dd 3937, although no disease symptoms were observed in the leaves ((B)). We also checked whether the optimal conditions of T. urticae affect the expression of other JA- and SA-related genes after bacterial treatment. Expression levels of SA-induced NPR1 and JA-induced MYC2 transcription factors were also determined. Interestingly, while NPR1 was not induced, the expression of the JA-related genes VSP2 and MYC2 was up-regulated 2dpi with Pst DC3000 ((C)). In addition to VSP2, treatment with Dd 3937 also induced the expression of the MYC2 gene at 6 h under low humidity conditions ((D)). Together with phenotypical and transcriptional analysis, samples from plants spray inoculated and kept at 25°C were tested for the multiplication of Dd 3937 and Pst DC3000 in planta at 4 dpi. No colony-forming units (CFU) were observed.
Figure 2. Bacterial inoculation of Arabidopsis at T. urticae optimal conditions. (A) Pst DC3000. Leaf symptoms 48 hpi (left) and relative mRNA expression at different time points of PR1 gene after mock or bacterial inoculation (right). (B) Dd 3937. Leaf symptoms 6 hpi (left) and relative mRNA expression at different time points of VSP2 gene after mock or bacterial inoculation (right). (C) Pst DC3000. Relative mRNA expression at 48h of VSP2, MYC2, and NPR1 genes after mock or bacterial inoculation. (D) Dd 3937. Relative mRNA expression at 6h of PR1, MYC2, and NPR1 genes after mock or bacterial inoculation. Data are means ± SE of three biological replicates. Asterisks indicate significant differences (t-test; *P < 0.05, **P < 0.01).
Transcriptional responses to T. urticae in previous bacterial-inoculated plants
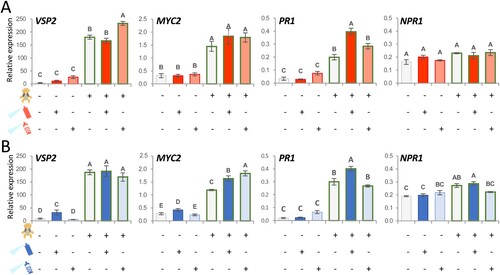
Once established that 48 h of Pst DC3000 and 6 h of Dd 3937 inoculation under suboptimal conditions for bacterial infection alters JA-related plant defense response in Arabidopsis, our aim was to investigate the impact of this gene expression reprograming on the response of the plant to a subsequent T. urticae infestation. JA is the main regulator of induced defenses triggered by spider mite feeding, although induction of the SA pathway has also been reported (Santamaria et al. Citation2020). As expected, induction of genes tested related to the SA and JA pathways was observed after 24 h of spider mite feeding on Arabidopsis leaves (). When the spider mite was applied after Dd 3937 or Pst DC3000 spray-inoculation, the transcription levels of the selected genes were mostly similar to those obtained with the mite stress alone. Differences were found for PR1, which expression was significantly higher in plants previously inoculated with both bacterial strains; and for MYC2, more upregulated when previously inoculated with Pst DC3000. As bacterial infection was not efficiently established under these conditions, we decide to analyze whether the effects on the response to mite infestation were kept when inoculating with heat-inactivated (HI) bacteria. When HI bacteria were applied, significantly higher induction of JA-related genes VSP2 (for Dd 3937) and MYC2 (for Pst DC3000) was observed compared to mite stress alone. On the contrary, PR1 levels increased at levels similar to those observed when only the acarian was applied.
Figure 3. Impact of bacteria inoculation before spider mite infestation on the expression of genes implicated in Arabidopsis defense. The presence of spider mite (first row), bacteria (second row), or heat-inactivated (HI) bacteria (third row) is indicated with (+). Bacteria and HI bacteria were inoculated by spray and kept at 25°C in low humidity conditions. (A) Mites were applied 6 hpi of Dd 3937 or HI Dd 3937. (B) Mites were applied 48 hpi of Pst DC3000 or HI Pst DC3000. In both cases, samples were collected after 24 h of spider mite feeding on the plant. Data are means ± SE of three biological replicates. Different letters indicate significant differences (P < 0.05, One-way ANOVA followed by Tukey HD test).
Effects of prior bacterial inoculation on plant damage caused by T. urticae
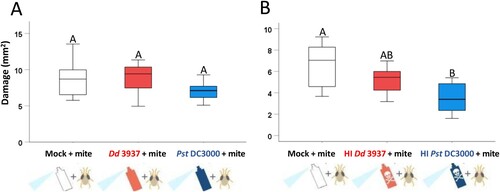
The observed priming effects caused by bacterial inoculations could affect later resistance to T. urticae. To investigate it, mite damage was evaluated 4 days post-infestation in Arabidopsis rosettes previously inoculated with mock, bacteria, or HI bacteria under mite-optimal conditions. When plants were inoculated with full active bacteria, no variations were found in the total area damaged by the mite. However, a significant reduction of mite damage was detected when leaves were previously inoculated with HI Pst DC3000, and a slight non-significant reduction of damage if were inoculated with HI Dd 3937 ().
Figure 4. Mite damage on Arabidopsis plants spray-inoculated with bacteria and HI bacteria. Bacteria (A) and HI bacteria (B) were inoculated by spray and kept at 25°C in low humidity conditions. Mites were applied 6 hpi of Dd 3937 or HI Dd 3937, and 48 hpi of Pst DC3000 or HI Pst DC3000. Plants were kept in the same conditions and damage was quantified 4 days after mite treatment. Data are means ± SE of eight biological replicates. Different letters indicate significant differences (P < 0.05, One-way ANOVA followed by Tukey HD test).
Discussion
The concept of a ‘disease triangle’ states that disease occurs when the plant host is susceptible, the pathogen is virulent, and the environmental conditions are appropriate for infection (Stevens Citation1960). Therefore, suboptimal environmental conditions lead to a reduction in disease severity or even a lack of disease development. However, even if pathogen viability is compromised, plants can detect PAMPs derived from pathogen membrane, cell wall, or motility structures, such as chitin, lipopolysaccharide, oligosaccharides, or flagellin, which act as elicitors of plant defense and can induce resistance to subsequent biotic stresses (Zipfel et al. Citation2004; Zipfel Citation2014; Jia et al. Citation2018; Rebaque et al. Citation2021).
In general, our results support the notion that both bacterial strains inoculated with adverse humidity exert a JA-based priming effect that conditions subsequent infestations with spider mite, even when the multiplication of pathogenic bacteria in the plant is blocked and disease symptoms are not observed. Although Arabidopsis resistance to Pst DC3000 is triggered by exogenous application or endogenous accumulations of SA, Pst DC3000 infection also causes an increase in JA content and up-regulation in the expression of genes related to both SA and JA pathways (Jia et al. Citation2018; Howlader et al. Citation2020). However, while the SA-related pathway response to Pst DC3000 observed under optimal conditions was not found under adverse infection conditions, the JA-related markers VSP2 and MYC2 remain up-regulated. Similarly, whereas a lower induction of wound-associated JA marker genes was observed for Dd 3937 inoculated plants in comparison with mock-inoculated plants, an increase in the expression of the JA-related markers VSP2 and MYC2 in Dd 3937 inoculated plants was found when plants were sprayed at adverse conditions. The JA pathway has been involved in partial resistance of Arabidopsis to Dd 3937 since JA mutants were more susceptible to Dd 3937 infection (Fagard et al. Citation2007). Thus, the increased JA-associated response in adverse conditions would be detrimental to bacterial survival and pathogenesis.
The consequences of modulation of JA-associated responses have previously been reported using virulent bacteria in Arabidopsis. For example, herbivory caused by the insect Trichoplusia ni was affected by prior infection by P. syringae (Groen et al. Citation2013). T. urticae induces mainly the JA pathway in Arabidopsis, but also the SA pathway (Santamaria et al. Citation2020). Therefore, enhanced expression of JA-related markers in response to inoculated bacteria could have a priming effect and would condition subsequent infestation with the spider mite. In fact, the expression of genes associated with the JA and SA pathways after mite infestation varied between bacteria-inoculated and non-inoculated plants. This effect was more evident when heat-inactivated bacteria were used and consisted of increased activation of the JA pathway and a certain inhibition of the SA pathway, regardless of the type of bacteria. The response observed after HI bacterial treatment with respect to that response after living bacteria inoculation could be explained by the lack, in the case of HI treatment, of an active dialog between bacteria and plants. In this dialog, delivering of effector and other virulence factors with plant defense suppression activity is expected. Pst DC3000 secretes coronatine, a mimic of bioactive JA-Ile, that hijacks JA signaling. Coronatine induces JA-related pathways, leading to inhibition of SA accumulation and SA-dependent responses to facilitate bacterial infection in the plant (Zheng et al. Citation2012; Wasternack Citation2017). Therefore, the lower expression of the JA-related genes VSP2 and MYC2 when plants are only infected with HI Pst DC3000 may be associated with the lack of coronatine in these conditions. In fact, the differential regulation of MYC2, PR1, and NPR1 when fully active or HI bacteria were inoculated prior to mite infestation supports the relevance of secretion systems in Pst DC3000. Similarly, differential regulation of VSP2 and PR1 upon mite infestation in plants previously inoculated with fully active or HI Dd 3937 suggests a key role of the secretion systems in Dd 3937. In this way, pectic enzymes released by a type II secretion system (T2SS) of Dd 3937 caused lysis of the plant cell wall in Arabidopsis, activating SA and JA signaling pathways (Fagard et al. Citation2007; Expert et al. Citation2018). Besides, very early activation of T3SS was observed in Dd 3937 at the beginning of plant colonization using transcriptomic analyzes (Chapelle et al. Citation2015). These results confirm that the secretion systems could harbor effectors that affect the activation of the JA pathway. This activation is lost when bacteria are heat-inactivated and lose their capacity to secrete effector molecules.
Pathogen interactions are commonly associated with improved capacity to combat subsequent threats. Thus, the differential transcriptomic responses to mite infestation observed when previous pathogen stress is applied could be translated into reduced plant damage after mite feeding. According to this hypothesis, a significant reduction in damage was only detected when the leaves were previously inoculated with HI Pst DC3000 bacteria. Therefore, particularities in the response to a specific bacteria compromise the plant response to subsequent stress, even under suboptimal conditions. These particularities could be associated with hormonal pathways triggered by the lifestyle of the pathogen, as previously reported. For example, Arabidopsis plants exposed to the fungal necrotroph Botrytis cinerea but not to the biotroph Golovinomyces orontii showed reduced herbivore feeding damage after infestation with the invasive crop pest Eurydema oleracea (Ederli et al. Citation2021). However, our results indicate that the effect on the herbivore is not always a direct consequence of the pathogen lifestyle. In fact, plants were less damaged by mites when previously inoculated with the hemibiotrophic HI Pst DC3000. According to our experiments, the only presence of this heat-inactivated bacteria is able to potentiate the response of the plant to the mite attack.
In conclusion, the effect of a bacterial interaction should not be negligible even when suboptimal conditions for bacterial performance permit the plant to block colonization. The challenge is to predict how the subsequent biotic stress is affected by the transcriptional changes caused by the previous presence of the bacteria. A deeper understanding of the responses of the plants to pathogen threats at different environmental conditions will help design novel strategies to mitigate adverse effects of pests on important agronomic crops.
Supplemental Material
Download MS Word (12.8 KB)Additional information
Funding
Notes on contributors
Estefania Contreras
Estefania Contreras is a postdoctoral fellow at Centro de Biotecnología y Genómica de Plantas (CBGP) in Madrid, where she has been working since 2017 in the research group “Plant-Pest Molecular Interactions”. In the last few years, she has investigated different aspects of the interaction between plants and pests, with a focus on the molecular mechanisms used by plants to combat biotic threats.
Jose J. Rodriguez-Herva
Jose J. Rodriguez-Herva is an associate professor at the Universidad Politécnica de Madrid. His research is conducted in the research group “Phytopathogenic Bacteria” at the Centro de Biotecnología y Genómica de Plantas (CBGP). His research interests are focused on the study of the molecular mechanisms used by phytopathogenic bacteria to infest plants.
Isabel Diaz
Isabel Diaz is a professor at the Universidad Politécnica de Madrid. She leads the research group “Plant-Pest Molecular Interactions” at the Centro de Biotecnología y Genómica de Plantas (CBGP). The final aim of her investigations is to understand plant defence to be applied in biotechnological programs.
Emilia Lopez-Solanilla
Emilia Lopez-Solanilla is a professor at the Universidad Politécnica de Madrid. She leads the research group “Phytopathogenic Bacteria” at the Centro de Biotecnología y Genómica de Plantas (CBGP). Her research is focused on the analysis of the specific mechanisms operating during the first stages of bacterial infection.
Manuel Martinez
Manuel Martinez is a professor at the Universidad Politécnica de Madrid. His research is conducted in the research group “Plant-Pest Molecular Interactions” at the Centro de Biotecnología y Genómica de Plantas (CBGP). His research interests cover different aspects of the molecular response of the plant to biotic stresses, including those related to climate change.
References
- Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC. 2004. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 135:2025–2037.
- Antunez-Lamas M, Cabrera E, Lopez-Solanilla E, Solano R, González-Melendi P, Chico JM, Toth I, Birch P, Pritchard L, Prichard L, et al. 2009. Bacterial chemoattraction towards jasmonate plays a role in the entry of Dickeya dadantii through wounded tissues. Mol Microbiol. 74:662–671.
- Bensoussan N, Santamaria ME, Zhurov V, Diaz I, Grbić M, Grbić V. 2016. Plant-herbivore interaction: dissection of the cellular pattern of Tetranychus urticae feeding on the host plant. Front Plant Sci. 7:1105.
- Chapelle E, Alunni B, Malfatti P, Solier L, Pédron J, Kraepiel Y, Van Gijsegem F. 2015. A straightforward and reliable method for bacterial in planta transcriptomics: application to the Dickeya dadantii/Arabidopsis thaliana pathosystem. Plant J. 82:352–362.
- Charkowski A, Blanco C, Condemine G, Expert D, Franza T, Hayes C, Hugouvieux-Cotte-Pattat N, López Solanilla E, Low D, Moleleki L, et al. 2012. The role of secretion systems and small molecules in soft-Rot enterobacteriaceae pathogenicity. Annu Rev Phytopathol. 50:425–449.
- Chen G, Escobar-Bravo R, Kim HK, Leiss KA, Klinkhamer PGL. 2018. Induced resistance against western flower thrips by the Pseudomonas syringae-derived defense elicitors in tomato. Front Plant Sci. 9:1417.
- Davila Olivas NH, Coolen S, Huang P, Severing E, van Verk MC, Hickman R, Wittenberg AH, de Vos M, Prins M, van Loon JJ, et al. 2016. Effect of prior drought and pathogen stress on Arabidopsis transcriptome changes to caterpillar herbivory. New Phytol. 210:1344–1356.
- Desurmont GA, Xu H, Turlings TC. 2016. Powdery mildew suppresses herbivore-induced plant volatiles and interferes with parasitoid attraction in Brassica rapa. Plant Cell Environ. 39:1920–1927.
- De Vos M, Van Oosten VR, Van Poecke RM, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Métraux JP, Van Loon LC, Dicke M, Pieterse CM. 2005. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant-Microbe Interact. 18:923–937.
- Ederli L, Salerno G, Quaglia M. 2021. In the tripartite combination Botrytis cinerea–Arabidopsis–Eurydema oleracea, the fungal pathogen alters the plant–insect interaction via jasmonic acid signalling activation and inducible plant-emitted volatiles. J Plant Res. 134:523–533.
- Expert D, Patrit O, Shevchik VE, Perino C, Boucher V, Creze C, Wenes E, Fagard M. 2018. Dickeya dadantii pectic enzymes necessary for virulence are also responsible for activation of the Arabidopsis thaliana innate immune system. Mol Plant Pathol. 19:313–327.
- Fagard M, Dellagi A, Roux C, Périno C, Rigault M, Boucher V, Shevchik VE, Expert D. 2007. Arabidopsis thaliana expresses multiple lines of defense to counterattack Erwinia chrysanthemi. Mol Plant-Microbe Interact. 20:794–805.
- Fernandez-Conradi P, Jactel H, Robin C, Tack AJM, Castagneyrol B. 2018. Fungi reduce preference and performance of insect herbivores on challenged plants. Ecology. 99:300–311.
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 43:205–227.
- Groen SC, Whiteman NK, Bahrami AK, Wilczek AM, Cui J, Russell JA, Cibrian-Jaramillo A, Butler IA, Rana JD, Huang GH, et al. 2013. Pathogen-Triggered ethylene signaling mediates systemic-induced susceptibility to herbivory in Arabidopsis. Plant Cell. 25:4755–4766.
- Hammerbacher A, Schmidt A, Wadke N, Wright LP, Schneider B, Bohlmann J, Brand WA, Fenning TM, Gershenzon J, Paetz C. 2013. A common fungal associate of the spruce bark beetle metabolizes the stilbene defenses of Norway spruce. Plant Physiol. 162:1324–1336.
- Howlader P, Bose SK, Jia X, Zhang C, Wang W, Yin H. 2020. Oligogalacturonides induce resistance in Arabidopsis thaliana by triggering salicylic acid and jasmonic acid pathways against Pst DC3000. Int J Biol Macromol. 164:4054–4064.
- Jia X, Zeng H, Wang W, Zhang F, Yin H. 2018. Chitosan oligosaccharide induces resistance to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis thaliana by activating both salicylic acid– and jasmonic acid–mediated pathways. Mol Plant-Microbe Interact. 31:1271–1279.
- Karamanoli K, Kokalas V, Koveos DS, Junker RR, Farré-Armengol G. 2020. Bacteria affect plant-mite interactions Via altered scent emissions. J Chem Ecol. 46:782–792.
- Kim JH, Hilleary R, Seroka A, He SY. 2021. Crops of the future: building a climate-resilient plant immune system. Curr Opin Plant Biol. 60:101997.
- King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 44:301–307.
- Lazebnik J, Frago E, Dicke M, van Loon JJ. 2014. Phytohormone mediation of interactions between herbivores and plant pathogens. J Chem Ecol. 40:730–741.
- Mason CJ, Couture JJ, Raffa KF. 2014. Plant-associated bacteria degrade defense chemicals and reduce their adverse effects on an insect defoliator. Oecologia. 175:901–910.
- Migeon A, Dorkeld F. 2006–2022. Spider mites web: a comprehensive database for the Tetranychidae. Montpellier: Institute for Agronomy Research. The Center for Biology and Management of Populations.
- Ojeda-Martinez D, Martinez M, Diaz I, Santamaria ME. 2020. Saving time maintaining reliability: a new method for quantification of Tetranychus urticae damage in Arabidopsis whole rosettes. BMC Plant Biol. 20:397.
- Oñate-Sánchez L, Vicente-Carbajosa J. 2008. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res Notes. 1:93.
- Rebaque D, Del Hierro I, López G, Bacete L, Vilaplana F, Dallabernardina P, Pfrengle F, Jordá L, Sánchez-Vallet A, Pérez R, et al. 2021. Cell wall-derived mixed-linked β-1,3/1,4-glucans trigger immune responses and disease resistance in plants. Plant J. 106:601–615.
- Río-Álvarez I, Muñoz-Gómez C, Navas-Vásquez M, Martínez-García PM, Antúnez-Lamas M, Rodríguez-Palenzuela P, López-Solanilla E. 2015. Role of Dickeya dadantii 3937 chemoreceptors in the entry to Arabidopsis leaves through wounds. Mol Plant Pathol. 16:685–698.
- Santamaría-Hernando S, López-Maroto Á, Galvez-Roldán C, Munar-Palmer M, Monteagudo-Cascales E, Rodríguez-Herva JJ, Krell T, López-Solanilla E. 2022. Pseudomonas syringae pv. tomato infection of tomato plants is mediated by GABA and l-Pro chemoperception. Mol Plant Pathol. 23:1433–1445.
- Santamaria ME, Arnaiz A, Rosa-Diaz I, González-Melendi P, Romero-Hernandez G, Ojeda-Martinez AD, Garcia A, Contreras E, Martinez M, Diaz I. 2020. Plant defenses against Tetranychus urticae: mind the gaps. Plants 9:464.
- Santamaria ME, Martinez M, Arnaiz A, Ortego F, Grbic V, Diaz I. 2017. MATI, a novel protein involved in the regulation of herbivore-associated signaling pathways. Front Plant Sci. 8:975.
- Stevens R. 1960. Cultural practices in disease control. In: Horsfall J, Dimond A, editors. Plant pathology, an advanced treatise, Vol 3. New York: Academic Press; p. 357–429.
- Thaler JS, Humphrey PT, Whiteman NK. 2012. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 17:260–270.
- Wasternack C. 2017. The Trojan horse coronatine: the COI1-JAZ2-MYC2,3,4-ANAC019,055,072 module in stomata dynamics upon bacterial infection. New Phytol. 213:972–975.
- Weiler EW, Kutchan TM, Gorba T, Brodschelm W, Niesel U, Bublitz F. 1994. The Pseudomonas phytotoxin coronatine mimics octadecanoid signalling molecules of higher plants. FEBS Lett. 345:9–13.
- Xin XF, He SY. 2013. Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu Rev Phytopathol. 51:473–498.
- Zheng XY, Spivey NW, Zeng W, Liu PP, Fu ZQ, Klessig DF, He SY, Dong X. 2012. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe. 11:587–596.
- Zhurov V, Navarro M, Bruinsma KA, Arbona V, Santamaria ME, Cazaux M, Wybouw N, Osborne EJ, Ens C, Rioja C, et al. 2014. Reciprocal responses in the interaction between Arabidopsis and the cell-content-feeding chelicerate herbivore spider mite. Plant Physiol. 164:384–399.
- Zipfel C. 2014. Plant pattern-recognition receptors. Trends Immunol. 35:345–351.
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T. 2004. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 428:764–767.
