ABSTRACT
Salinity is an important problem for agriculture in the Mediterranean area, and thus, it is essential to develop mitigation strategies to reduce its impact. The main objective of this study was to test the effectiveness of halotolerant plant growth-promoting bacteria (H-PGPB) in improving grapevine salt stress tolerance. Grapevines grafted onto a salt-sensitive rootstock were inoculated with a consortium of H-PGPB. The substrate of half of the plants of each treatment was salinized up to 2 dS m−1. Plants grew for six days under these conditions, and afterward, NaCl was removed to assess plant recovery through growth, physiology, and canopy temperature measurements. Inoculation with H-PGPB had a positive effect on plant physiology, but after salt treatment, grapevines stopped their photosynthetic metabolism, leading to severe defoliation. Remarkably, after salt stress removal, inoculated plants re-sprouted faster, demonstrating that H-PGPB inoculation could be a good practice to increase vineyard resilience to salt stress.
1. Introduction
Grapevine (Vitis vinifera L.) is one of the largest crops worldwide with a total coverage of 7.3 million hectares (ha) (OIV Citation2020). Total wine production, including sparkling and specialty wines, was estimated at 260 million hectoliters (hL) in 2020 (OIV Citation2021). However, grapevine cultivation may be at risk in several regions of the world due to climate change and soil salinization (Cook et al. Citation2014; Fraga et al. Citation2016; Corwin Citation2021; Stavi et al. Citation2021), since more frequent alternating periods of droughts and sudden strong rainfalls usually lead to an accumulation of salts in ground-waters (Tiam-Ren Citation1985; Kalra et al. Citation2002). In semi-arid and hot-summer Mediterranean climates, where the application of irrigation techniques is essential to maintain an optimal level of production and grape quality in vineyards, the use of saline ground-water sources for irrigation can expose grapevine plants to salt stress (Rengasamy Citation2006; Chaves et al. Citation2010; Jamil et al. Citation2011). Since grapevines are classified as a relatively salinity-sensitive species, salt stress is one of the most important abiotic stressors affecting its growth, physiology, yield and quality in hot and dry climate conditions (Shani and Ben-Gal Citation2005).
Salinity induces osmotic stress and ion toxicity, as well as oxidative stress in grapevines (Urdanoz and Aragüés Citation2009). Osmotic stress translates into a reduction in leaf water potential, photosynthesis and transpiration, which ultimately lead to plant growth decrease (Urdanoz and Aragüés Citation2009). On the other hand, ionic toxicity caused by the accumulation of some ions such as Cl− and Na+ can induce toxicity and nutrient imbalances. For example, a decrease in K uptake by root systems commonly occurs under saline conditions (Blumwald Citation2000; Shabala et al. Citation2003; Sairam and Tyagi Citation2004). Ionic cell imbalance due to the accumulation of Na+ is mainly observed in mature leaves, disrupting metabolic processes such as ion transport and respiration (Allen et al. Citation1994), and protein synthesis (Hasegawa et al. Citation2000; Mäser et al. Citation2002; Munns and Tester Citation2008). This leads to leaf marginal necrosis, decreased leaf growth, and ultimately premature senescence (Downton and Millhouse Citation1983). Chloride appears to be the main ion with toxic action for vine growth under saline conditions, and several studies clearly showed the injurious effects of Cl− at concentrations of 300 mmol kg−1 in leaves (Bernstein et al. Citation1969; Maas and Grattan Citation1999; Fisarakis et al. Citation2001; Sinclair and Hoffmann Citation2003).
Grapevines respond to salt stress conditions by activating a series of tolerance mechanisms. The main adaptive stress responses are the increase in osmolyte production (e.g. carbohydrates, amino acids and proteins with osmolyte action), vacuolar sequestration of intracellular ions (Serraj and Sinclair Citation2002; Yokoi et al. Citation2002), as well as the activation of antioxidant machinery for reactive oxygen species (ROS) detoxification (Ozden et al. Citation2009; Subramanyam et al. Citation2011; Sadak Citation2019). Salt exclusion is another tolerance mechanism mediated by plant’s ability to limit Na+ and Cl− uptake, preventing their translocation from roots to shoots (Munns and Gilliham Citation2015), and the undesirable accumulation in berries (Walker et al. Citation2014). The use of salinity tolerant rootstocks can therefore be a useful strategy in vineyards affected by salinity (Walker et al. Citation2019). For instance, Dogridge, SO4, 5C, 420 and Ramsey rootstocks are considered as salt tolerant (Hao et al. Citation2013; Zhou-Tsang et al. Citation2021), as well as 1103 Paulsen, Ramsey and 140 Ruggeri, due to their positive effects in reducing salt accumulation in scion’s leaves and grape bunches (Ollat et al. Citation2016).
Some vineyard management strategies can also help counteract salinity effects in grapevines, such as mulching, that can reduce the evaporation of water from the soil, prolonged irrigation with fresh water to reduce Na+ and Cl− accumulation at the root system level, or rainwater recovery to increase soil drainage (Stevens et al. Citation2011). In addition, some current studies about salinity mitigation strategies in crops are also focused on the use of salinity-tolerant or halophilic microorganisms as inoculants, since they are environmentally friendly and cost-effective solutions (Teo et al. Citation2022). Plant growth promoting bacteria (PGPB) are mutualistic bacteria that promote plant growth and nutrition through direct (e.g. phytohormone production, N fixation, phosphate solubilization, siderophore production) or indirect mechanisms (e.g. protection against plant pathogens, enhancement of abiotic stress tolerance) (Navarro-Torre et al. Citation2020). Among them, halotolerant bacteria are the ones able to survive at high salt concentrations through various mechanisms such as accumulation of osmolytes, production of extracellular proteases, and activation of Na+/H+ antiporters.
Many halotolerant and halophilic bacteria can establish symbiotic associations with halophytes, which are salt tolerant species that can survive at 200 mM NaCl or approximately 20 dS m−1 (Flowers and Colmer Citation2008). Therefore, halophytes, as species from the genus Limonium (Caperta et al. Citation2020) can be an excellent source of halotolerant/halophilic PGPB to potentially improve crop performance under saline stress conditions (Mesa et al. Citation2015a; Citation2015b; Navarro-Torre et al. Citation2016; Citation2017a; Citation2017b; Shurigin et al. Citation2020; Teo et al. Citation2022) (Supplementary Figure S1). However, studies on the use of halotolerant PGPB in grapevines are very scarce (Jiao et al. Citation2016; Ma et al. Citation2017), and are based on single-species inoculation, although several works suggest inoculations with bacterial consortia are more beneficial due to potential complementary effects of different strains in plants under stressful conditions (Glick and Gamalero Citation2021).
The hypothesis of our study was that endophytic bacteria isolated from autochthonous halophyte (salt tolerant) Limonium species (sea-lavender) could promote grapevine tolerance to salt stress, and the goals of this study were to (1) isolate and characterize halophilic/halotolerant bacteria from distinct species of Limonium plants from different saline environments; (2) select the best performing bacteria based on their plant growth promoting traits and NaCl tolerance; and (3) test the selected bacteria in grapevines exposed to saline stress, and assess their potential benefits in improving host’s salt stress tolerance.
2. Materials and methods
2.1. Isolation of culturable bacterial endophytes
Three entire plants of similar age and size of three different Portuguese autochthonous Limonium species (all three considered halophytes) were collected in April 2021. Limonium vulgare Mill. plants were collected in a salt marsh (Sapal das Hortas, Alcochete, Portugal) and L. daveaui Erben in sand dunes (Praia do Samouco, Alcochete, Portugal), both in the Tagus estuary surroundings. Limonium multiflorum Erben in a rocky salt spray environment (Cabo Raso, Cascais, Portugal) (Supplementary Figure S2). Plants were transported to the laboratory in individual bags and stored at 4°C, until utilization.
Root endophytic bacteria were isolated from those plants. Since those plants live in saline soils, their symbiotic microorganisms, including bacteria, are expected to be halotolerant or even halophilic. Roots from each plant were separated from the aerial part and surface-disinfected using 70% ethanol and 6% sodium hypochlorite (Navarro-Torre et al. Citation2016). Then, roots were cut with a sterile scalpel into small pieces (2 cm of length approximately) and crushed in a sterile mortar containing 1 ml of sterile saline solution (0.9% NaCl). The molded paste was diluted to 10−1, 10−2 and 10−3 in saline solution (0.9% NaCl) and plated in three TSA (tryptic soy agar) plates, supplemented or not with 0.3 M NaCl (w/v). All plates were incubated at 28°C for 72 h. In parallel, negative controls were done at each isolation step to ensure that the isolated bacteria were root endophytes and not potential contaminants from the air of the flow chamber, the distilled water, the saline solution, or even epiphytic bacteria remaining in the disinfected roots.
After colony observation, the ones presenting different sizes, colors or morphologies were individually sub-cultured in new TSA plates. A pure culture of each isolate was stored at −80°C in 30% glycerol.
2.2. Taxonomic identification of culturable bacterial endophytes
For the taxonomic identification of the bacterial isolates, each culture was grown in TSB (tryptic soy broth) for 24 h at 28°C at constant shaking (150 rpm). After incubation, cultures were centrifuged at 13,000 rpm for 3 min and the pellets containing the bacteria were used to extract DNA using innuPREP Plant DNA kit (Analytik Jena, Germany), following manufacturer’ instructions.
The amplification of the 16S rRNA gene was done using NZYTaq II DNA polymerase (Nzytech, Portugal) and 16SF27 and 16SR1488 as primers (Navarro-Torre et al. Citation2016) with the following PCR conditions: initial denaturation at 95°C for 3 min, 30 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, extension at 72°C for 30 s, and a final extension step at 72°C for 5 min. The PCR products were purified and sequenced by StabVida company (Portugal).
Each bacterial strain was taxonomically assessed by performing a BLAST analysis of the obtained 16S rRNA gene sequence on the EzBioCloud database using the Ez-Taxon server (Yoon et al. Citation2017). Finally, 16S rRNA gene sequences were deposited in the NCBI GenBank and an accession number was assigned to each sequence ().
Table 1. Identification of culturable bacterial endophytes isolated from roots of Limonium daveaui (LDR), L. vulgare (LVR) and L. multiflorum (LMR) using EzBiocloud database.
2.3. Characterization of bacterial plant growth promoting traits and salt tolerance
To select the halophilic bacterial species or strains with the highest potential to establish mutualistic relationships with plants, their plant growth promoting (PGP) traits were studied. In each bacterial strain PGP traits were studied under control conditions (without NaCl) and in the presence of 0.2 M NaCl (w/v) in the media. The tested PGP properties included indole-3-acetic acid (IAA) and siderophore production, phosphate solubilization, nitrogen fixation, biofilm formation, and the presence of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase gene. Auxins, among which IAA, are plant phytohormones produced also by bacteria, that promote root elongation and ramification (Turan et al. Citation2016), and siderophores are Fe3+ chelators that improve Fe availability for plants (Alori and Babalola Citation2018).
For testing IAA production, bacteria were cultivated in TSB medium supplemented with 100 mg L−1 of L-tryptophan at 28°C for three days at constant shaking. Then, cultures were centrifuged and Salkowski reagent (Gordon and Weber Citation1951) added to the supernatant. The absorbance of the solution was measured at 535 nm and IAA concentration was determined using a IAA pattern curve (Gordon and Weber Citation1951).
For the analysis of siderophore production and phosphate solubilization activity, bacteria were initially cultured on TSB for 24 h at 28°C with constant shaking, and then, 100 μL of each culture was inoculated on TSA plates containing CAS medium (chrome azurol S) (Schwyn and Neilands Citation1987) or on plates containing NBRIP (National Botanical Research Institute’s phosphate growth medium) medium (Nautiyal Citation1999), respectively. Plates were incubated for five days at 28°C and were considered positive when a halo appeared around the bacterial growth, which was orange in the case of the CAS medium and transparent in the case of NBRIP medium, due to the solubilization of Ca3PO4.
To determine if bacteria could fix atmospheric nitrogen, they were incubated at 28°C for five days in NFB (nitrogen free broth) solid medium (Ji et al. Citation2014) and those able to grow in such medium were identified with the capacity to fix nitrogen.
Biofilm formation was also tested in all bacterial strains individually cultured in 24-well-plates filled with TSB medium for four days at 28°C. After this period, biofilm position in the well was determined (surface or bottom) and then, biofilms were stained with 0.01% crystal violet (w/v) (del Castillo et al. Citation2012) to see if the bacterial biomass adhered to the wall of the wells (ring formation).
Finally, the presence of the enzyme ACC deaminase was determined by the amplification of the acdS gene. The ACC deaminase enzyme degrades a precursor on the ethylene formation, contributing to reduce plant stress (Glick Citation2014). The PCR was conducted using NZYTaq II DNA polymerase (Nzytech, Portugal) and the primers acdSf3 and acdSr3, as described in Li et al. (Citation2015). The PCR conditions used were the following: an initial denaturation at 95°C for 3 min, 30 cycles of denaturation at 94°C for 30 s, annealing at 53°C for 30 s, extension at 72°C for 30 s, and final extension at 72°C for 5 min. Electrophoresis was run for 30 min at 120 V in TAE 1X, and then, the gel was stained with EtBr solution and visualized under UV light.
In addition, bacterial tolerance to high NaCl concentrations was evaluated. Each isolate was cultured in TSA plates supplemented with 0, 0.5, 1, 1.5, 2, 2.5, 3, and 3.5 M NaCl (w/v) and incubated for five days at 28°C. Tolerance to NaCl was expressed as the maximum tolerable concentration (MTC) i.e. the highest NaCl concentration at which bacterial growth was observed.
Based on the results obtained, a consortium with the best three bacteria in terms of IAA and siderophore production, phosphate solubilization, N fixation and biofilm production both under saline and non-saline conditions were selected for subsequent analyses: LDR2, LDR25, and LVR13. Their compatibility was tested by culturing them together on the same TSA plate, ensuring contact among their colonies.
2.4. Bacterial transformation with mCherry-containing plasmid
The selected bacterial strains LDR2, LDR25 and LVR13 were transformed with the plasmid pMP7604 by bacterial conjugation. Bacterial strains and plasmids used for this purpose are described in Supplementary Table S1. First, spontaneous antibiotic (ampicillin or rifampicin) resistant mutants were isolated from each strain and incubated overnight at 28°C in TSB under agitation (115 rpm). The next day those cultures were centrifuged at 13,000 rpm for 3 min and the pellets resuspended in 100 μL TSB. Then, each bacterial culture was plated in TSA medium supplemented with the corresponding antibiotic (100 μg mL−1 ampicillin or rifampicin). Plates were incubated for 48 h at 28°C and the newly formed colonies were considered spontaneous mutants to the corresponding antibiotic.
Conjugation was performed by culturing together in TSB the corresponding ampicillin- or rifampicin-resistant bacterial strain (LDR2, LDR25, and LVR13), the helper Escherichia coli DH5α bacteria (containing the plasmid pRK2013), and the donor E. coli DH5α bacteria (containing the pMP7604 plasmid with the tetracyclin resistance gene). The bacterial cultures were incubated for 24 h at 28°C in agitation, and were plated in TSA supplemented with 100 μg mL−1 ampicillin or rifampicin, and tetracycline (40 μg mL−1). Plates were incubated 48 h at 28°C and colonies were selected. The presence of the plasmid was checked by PCR amplification of the mCherry gene using the primers mCherryFw (5′-ATGGTGAGCAAGGGCGAG-3′) and mCherryRv (5′-TTACTTGTACAGCTCGTC-3′).
2.5. Inoculum preparation
Liquid bacterial cultures of LDR2, LDR25 and LVR13 carrying the plasmid with the mCherry gene were used as inoculum for grapevine plants. For this, an individual colony of each bacterium was placed in 50 mL of TSB and was left overnight at 28°C under agitation at 115 rpm. The next day the three bacterial suspensions were processed as in Navarro-Torre et al. (Citation2017a) and were finally mixed in a volume of 800 mL of irrigation water. The optical density (OD600nm) of the final bacterial suspension was 1.374.
2.6. Study of grapevine response to the inoculation with halotolerant bacteria and salt stress
2.6.1. Experimental set up
Thirty-two dormant vines of Aragonez-Tempranillo grafted onto Richter 110 rootstock obtained from Viveiros Vitioeste (Bombarral, Portugal) were used. Richter 110 has excellent adaptability to dry soils but is sensitive/moderately sensitive to salinity (Troncoso de Arce et al. Citation1999; Corso and Bonghi Citation2014; Walker et al. Citation2019; Zhou-Tsang et al. Citation2021). Plants were rooted under greenhouse conditions in rooting beds filled with sterile perlite. When plants presented a well-developed root system, they were transplanted to 3 L square pots filled with a mixture of autoclave-sterilized (1 h at 120°C) peat and perlite (1:2 v/v). Grapevines were kept under greenhouse conditions and watered daily according to their needs. Every week 50 ml of Hoagland’s nutrient solution (Hoaglands and Arnon Citation1972) was applied in each plant.
Three weeks after transplant, half of the plants were inoculated with 50 ml of a bacterial suspension consisting of a consortium of the three previously selected bacterial strains carrying the mCherry gene. This inoculation procedure was repeated every 10 days.
Three months later, half of the plants of each treatment were watered with a saline solution (75 mM) to achieve a final soil electric conductivity (EC) of approximately 2 dS m−1. In non-salinized substrates the EC was 0.3 dS m−1, and was measured with an Orion Star A212 Conductivity Benchtop Meter (Thermofisher Scientific, USA). For six days, grapevines were watered daily to maintain plants under water comfort conditions. After that period, to favor plant recovery, for two days, plants were watered in excess to promote salt leaching and eliminate NaCl present in the substrate, until EC decreased to 0.6 dS m−1 in salinized pots (Supplementary Figure S3). Afterwards, irrigation was re-adjusted to maintain water comfort conditions in grapevines.
To avoid potential differences in soil nutrient content between salt-treated and non-treated plants, non-salinized plants were also watered with the same amounts of water. In the weeks following NaCl elimination, Hoagland’s solution dosage was doubled for treated and untreated plants.
2.6.2. Plant physiology and growth monitorization
Plant performance was monitored by reflectance index assessment (Normalized Difference Vegetation Index-NDVI and Photochemical Reflectance Index-PRI) and by foliar gas exchange parameter measurements. The NDVI and PRI were measured using PlantPen NDVI 300 (PSI, Czech Republic) and PlantPen PRI 200 (PSI, Czech Republic) portable devices, respectively. Photochemical Reflectance Index is indicative of photosynthesis efficiency and plant stress levels (Garbulsky et al. Citation2011) while NDVI reflects plant vigor and, indirectly, chlorophyll status, phosphorus, and nitrogen nutrition (Sembiring et al. Citation1998). Measurements were performed in two mature leaves per plant before salt stress application (i.e. four months after the first bacterial inoculation was done), two days after salt stress application, and during the recovery phase after salt stress elimination (July 28th, August 16th and August 31st) (Supplementary Figure S3). Measurements were performed always at the same time, at 11 am.
Foliar gas exchange parameters (net photosynthesis rate-A, stomatal conductance-gs and transpiration-E) were measured with a CIRAS 3 infrared gas exchange analyzer-IRGA (PP Systems, USA) in two mature leaves per plant. Measurements were performed always at the same time, at 12 am, on the same dates as the reflectance indexes.
Since canopy temperature is commonly used as an indicator of plant stress (Jones Citation2002), thermal images were collected two days after salt stress exposure and during the recovery phase after salt stress elimination. For that purpose, a FLIR A35 thermal camera (Teledyne FLIR®, Germany) equipped with an infrared temperature sensor with manual focus and a spectral range between 7.5 and 14 µm and a 320 × 256 pixel resolution was used. The camera was mounted on a tripod to stably stand approximately 1.5 m above the ground, right in front of the plants. A board was placed behind the grapevines to homogenize the background as much as possible. Data were collected always at 13 pm.
Thermal images were acquired using FLIR Tools® v6.4 and analyzed using ImageJ® software v1.53 (National Institutes of Health, USA). For this, images were converted to 8 bit and calibrated so that the gray scale corresponded to the temperature range recorded by the thermal camera. Then, images of individual plants were manually segmented to ensure accuracy, rather than relying solely on automatic image segmentation methods. To determine the average temperature of the entire plant leaf canopy area, the selected pixels corresponding to the plant canopy were analyzed. Canopy temperature values were not normalized according to dry and wet leaf references (Jones Citation2004; Costa et al. Citation2010) since the aim of the trial was to compare differences between experimental treatments at each timepoint.
Plant growth was monitored by counting the number of green leaves per plant, regardless of their size, and by measuring grapevine shoot length (shoot length was considered 0 when it was dry and with no leaves attached). By the end of the experiment, shoot and root dry biomass were recorded.
2.6.3. Bacterial colonization
Sixteen days after NaCl was eliminated from the substrate, root samples were collected from two non NaCl-treated plants (one inoculated and one non-inoculated), and from one bacteria-inoculated plant previously treated with NaCl.
Roots were fixed using a 4% formaldehyde acetic solution in 70% ethanol. To avoid ethanol-induced tissue dehydration, glacial acetic acid (∼5% of total volume) was added. After 48 h in this fixing solution, roots were washed in a 70% ethanol solution and stored at 4°C until used. Afterwards, samples were impregnated with DP1500 polyethylene glycol (Barbosa et al. Citation2010). Transverse sections of approximately 30 μm thick were prepared with a sliding microtome (Leica SM 2400), were double stained with Safranin/Astrablue (1% aqueous solution) and then rinsed with distilled water. Slides were mounted in a glycerine/water solution and observed on a light microscope (Leitz Dialux 20EB). Images were captured using a digital camera (Leica EC3) and an image software (Leica Application Suite, v.4.0).
For visualizing root bacteria colonization, the slides were observed under a fluorescence microscope (Zeiss Axioskop 2), equipped with a digital camera (Zeiss AxioCam) with a 10× objective. Image acquisition was performed using the Carl Zeiss/AxioVision 4.8 software.
2.6.4. Statistical analysis
Differences in shoot length, number of leaves, NDVI, PRI, A, gs and E before NaCl treatment application were evaluated by a t-student test, after data normality was assessed by a Shapiro–Wilk test.
After salinity stress and during plant recovery phase, different experimental treatments were compared by a one-way ANOVA test followed by a Duncan post hoc test.
At harvest, A, gs, E, NDVI, PRI, canopy temperature, dry shoot and root biomass were analyzed by a two-way ANOVA with interaction where inoculation and salt stress were considered as main factors. Differences among the experimental treatments were further assessed by a Duncan post hoc test. All analyses were performed in SPSS Statistics vs. 23 (IBM) software.
3. Results
3.1. Taxonomic identification of culturable bacterial endophytes isolated from Limonium species
A total of 66 endophytic bacterial strains differing in colony size, color, and morphology were isolated from the three Limonium species growing in different saline environments. Of these, 24 endophytes belonged to L. vulgare roots (LVR strains) being 96% Gram-negative and 4% Gram-positive bacteria. Other 29 bacteria were isolated from L. daveaui roots (LDR strains), of which 90% were Gram-negative and 10% Gram-positive. Finally, from L. multiflorum plants only 13 endophytes (LMR strains) were isolated, of which 85% were Gram-negative and 15% Gram-positive bacteria.
The most represented genera were Pantoea (35%) and Erwinia (27%) (). The Pantoea genus was present in strains isolated from L. vulgare and L. daveaui, being more frequent in L. vulgare (50% of its endophytes) with Pantoea hericii as the most represented species (). On the other hand, the Erwinia genus was present in the three plant species, being especially abundant in L. multiflorum (77% of its endophytes) with Erwinia persicina as the most frequent species (). Other frequent genera such as Pseudomonas, Acinetobacter, Bacillus, Prolinoborus, Staphylococcus, Kluyvera, Cytobacillus, Raoultella, and Weizmannia were also found among the isolated bacterial endophytes ().
Erwinia was the sole genus common to the three Limonium species. By contrast, the genus Kluyvera was exclusive of L. multiflorum, while the genera Cytobacillus, Raoultella, and Weizmannia were only present in L. daveaui, and the genus Staphylococcus was exclusive of the root endosphere of L. vulgare ().
3.2. Characterization of the isolated bacterial endophytes
All endophytes showed at least one of the tested PGP properties and 46% of them showed at least four (). The most common PGP property was biofilm formation, present in 94% of the endophytes. Biofilm formation was observed mainly at the bottom of the wells, although some endophytes were also able to adhere to the wall, showing a characteristic ring. Only 2% of the isolates produced biofilm on the surface of the medium (Supplementary Table S2). The second and third most frequent properties were siderophore and IAA production (present in 83% and 76% of the isolates, respectively). The largest halos, indicative of higher siderophore production, were observed in strains LVR21, LDR21, LVR4, LVR20, LDR11, LVR1, LDR29 and LDR25, in descending order, showing halo diameters from 5 to 6.1 cm (Supplementary Table S2). The best IAA producers were LDR4 and LDR2 with 12.03 and 11.78 mg·L−1 of IAA, respectively (Supplementary Table S2).
Figure 1. Representation of the PGP properties expressed by endophytes isolated from roots of (A) Limonium vulgare (LVR), (B) L. multiflorum (LMR), and (C) L. daveaui (LDR).
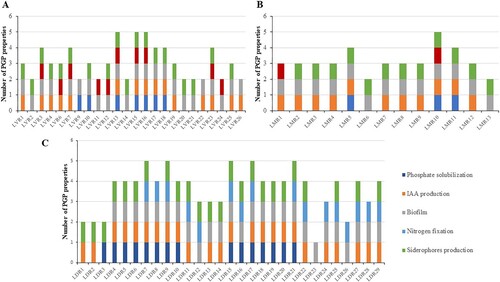
The presence of bacterial growth in a minimal medium without nitrogen source was observed in 42% of the isolates, which suggests that they have the capacity of fixing nitrogen from the air (Supplementary Table S2). Phosphate solubilization (Ca3(PO4)2) was the less frequently observed PGP property across isolates, with strain LVR10 as the best phosphate solubilizer producing a halo of 4.6 cm (Supplementary Table S2). Concerning the ACC deaminase gene acdS, no PCR amplification was observed with the primers used in any endophytic bacteria, and therefore ACC deaminase activity was considered absent in all of them.
All isolated bacteria tolerated up to 0.5 M NaCl (Supplementary Table S2). Fifty percent of the isolated endophytes grew in the presence of 1 M NaCl in the medium while only 12% did it in the presence of 3.5 M NaCl. Endophytes from L. vulgare presented the highest halotolerance, with 29% able to grow at 3.5 M NaCl. Contrastingly, only 15% of bacteria isolated from L. multiflorum tolerated a maximum of 2 M NaCl, being such conditions lethal for all the other bacteria isolated from this plant species.
3.4. The effect of salt on bacterial plant growth promoting traits
In the presence of 0.2 M NaCl, all bacterial strains formed biofilms and the amount of IAA produced also increased in most strains (). The highest increase was found in strain LDR15, since IAA production increased in 57% in the presence of NaCl. Overall, the best IAA producers in the presence of 0.2 M NaCl were LDR25 (22.68 mg L−1), LDR5 (21.87 mg L−1), LDR2 (20.62 mg L−1), and LDR1 (19.81 mg L−1) (Supplementary Table S3). However, decreases in IAA production were also observed in 34% of the bacterial strains. For example, in strain LDR4, IAA production was reduced from 12.03 to 2.93 mg L−1 (Supplementary Table S3).
Figure 2. Percentage of bacterial showing the different plant growth promoting (PGP) properties in absence of NaCl (0 M NaCl) and in presence of 0.2 M NaCl of (A) endophytes from roots of Limonium vulgare (LVR), (B) endophytes from roots of L. daveaui (LDR), and (C) endophytes from roots of L. multiflorum (LMR).
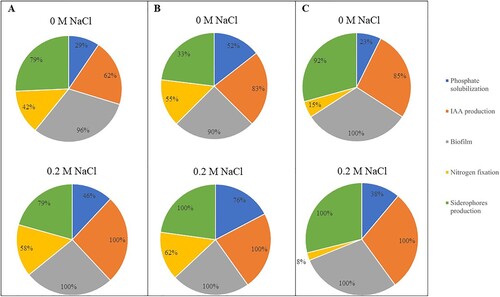
Similar results were observed for phosphate solubilization as the number of phosphate solubilizing bacteria increased in bacteria isolated from L. vulgare (from 29% to 46%), L. daveaui (from 52% to 76%), and L. multiflorum (from 23% to 38%) (). However, the diameter of the halo (representative of solubilized calcium phosphate) decreased in 84% of the strains. The best solubilizers in the presence of NaCl were LVR13 (1.9 cm), LDR13 (1.9 cm), and LDR20 (1.8 cm) (Supplementary Table S3).
The number of siderophore producing bacteria also increased with NaCl, and this trait was expressed by 100% of the endophytes isolated from L. daveaui and L. multiflorum, and by 79% of the bacteria isolated from L. vulgare (). In the presence of NaCl the strains with the highest halo diameters were LDR17 and LDR14 with 5.6 and 5.2 cm, respectively (Supplementary Table S3). Even though the number of bacteria that produced siderophores increased in the presence of NaCl, the diameter of the halo decreased in 56% of the strains. Finally, NaCl also induced an increase in the number of nitrogen fixers in LVR and LDR groups but not in endophytes from L. multiflorum ().
Based on the results obtained, three PGPB strains (Pantoea anthophila LDR2, Pantoea agglomerans LDR25, and Pantoea sp. LVR13) were selected to form a consortium and be further inoculated in grapevines. These strains did not antagonize each other when grown together in TSA medium plates and were all halotolerant. In the presence of 0.2 M NaCl they had the following characteristics: LDR2 showed one of the highest amounts of IAA; LDR25, besides producing high IAA amounts, expressed all the studied PGP properties under saline conditions; and LVR13 expressed all PGP properties and was one of the best phosphate solubilizers in such conditions.
3.5. Study of grapevine response to the inoculation with halotolerant bacteria and to NaCl treatment
3.5.1. Plant performance before and during NaCl treatment
Four months after the first inoculation with the selected PGPB consortium, before NaCl treatment application (Supplementary Figure S3), inoculated grapevines showed significantly higher A, gs, E, NDVI and PRI values than non-inoculated plants (). However, no differences were found between inoculated and non-inoculated plants in the number of leaves (10.6 ± 0.06 and 10.1 ± 0.06, respectively) and shoot length (13.2 ± 3.41 and 16.2 ± 5.41, respectively).
Figure 3. Grapevine leaf gas exchange parameters. (A) Net photosynthesis rate, A; (B) stomatal conductance, gs; (C) transpiration rate, E; (D) normalized difference vegetation index, NDVI; and (E) photochemical reflectance index, PRI measured at different time points in Aragonez cv. grapevines grafted onto Richter 110 rootstocks inoculated or not with a bacterial consortium and treated or not with a saline (NaCl) solution. Bars indicate the mean value per treatment ± standard error. Different letters at each time point indicate significant differences according to the Duncan multiple comparison test, and ‘ns’ indicates non-significant differences in a particular measurement time. The dashes on July 29 and August 18 indicate that there were not enough plants with mature leaves in the corresponding treatments for performing the analysis.
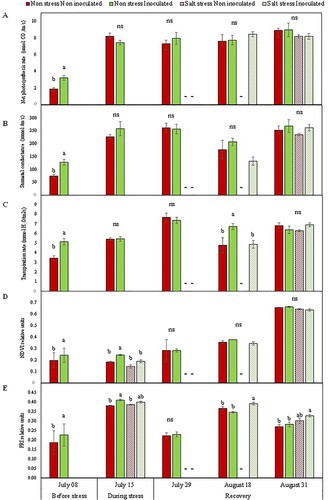
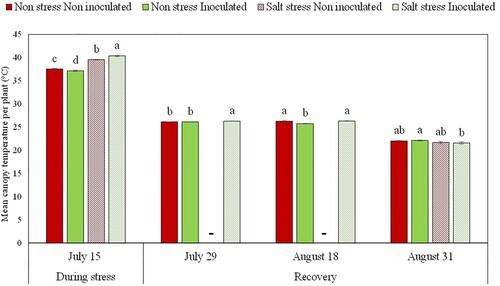
When reflectance indexes were measured one day after NaCl treatment application, before any visual symptom was observed in grapevine plants, a decrease was found in NDVI both, in inoculated and non-inoculated plants exposed to salt stress (21.4% and 22.2% decrease, respectively), which had closed stomata and zero photosynthesis rate (). Furthermore, canopy temperature assessed by thermography images (Supplementary Figure S4) showed that salt stress induced an increase of 3.2°C and 2°C in inoculated and non-inoculated plants, respectively (). At this same time point, in plants not exposed to salt stress we observed that the inoculated ones presented 33% and 8% higher NDVI and PRI, and 0.4°C lower canopy temperature ((D,E); ), although no differences were found in A, gs and E ().
Figure 4. Grapevine canopy temperature obtained from infra-red images of Aragonez cv. grapevines grafted onto Richter 110 rootstocks inoculated or not with a bacterial consortium and treated or not with a saline (NaCl) solution. Bars indicate the mean value per treatment ± standard error. Different letters at each time point indicate significant differences according to the Duncan multiple comparison test, and ‘ns’ indicates non-significant differences at that particular measurement time. The dashes on July 29 and August 18 indicate that there were not enough plants with mature leaves in the non-inoculated and salt stressed treatment for performing the analysis.
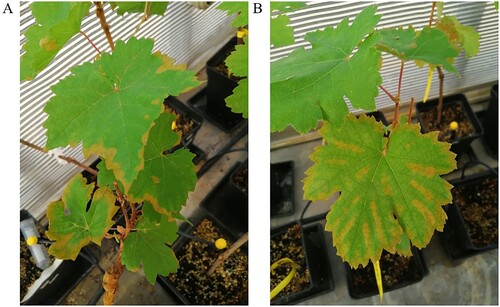
Two days after saline solution application, the first salt stress symptoms were observed (). Symptomatic leaves of inoculated and non-inoculated plants showed small marginal and inter-nerval leaf burnt. At the third day after the saline treatment, plants started defoliating and by the sixth day, all leaves were senescent, and a high degree of defoliation was observed.
3.5.2. Plant recovery
Ten days after salinity stress elimination, on July 28th, 62.5% of the bacteria-inoculated plants started to show new buds and leaves, while in the group of non-inoculated grapevines only 40% showed such signals of recovery. In both cases, news leaves were too small for gas exchange measurements (Supplementary Figure S5), and therefore, these parameters were only measured in inoculated and non-inoculated plants not-previously treated with salt. In these cases, no significant differences were found between the two experimental groups for A, gs, E, NDVI or PRI parameters (). On the other hand, it was possible to perform infrared image analysis on PGPB-inoculated plants that had been exposed to salt stress, because they had already a small canopy with small leaves (Supplementary Figure S4). In those plants, canopy temperature was significantly higher than in plants non-exposed to salt stress ().
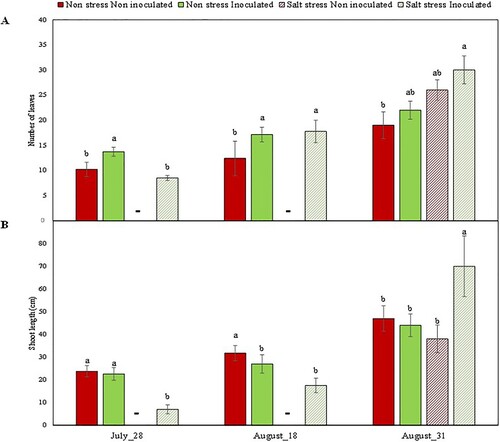
One month after NaCl elimination, on August 18th, in grapevines that had not been exposed to salt stress, the number of leaves was significantly higher in inoculated plants than in the non-inoculated ones (). On the other hand, in plants that had been exposed to salt stress, 75% of the PGPB-inoculated plants showed new leaves and buds, while only 60% of the non-inoculated grapevines did. Besides, inoculated plants produced a high number of new leaves, similar to the one found in inoculated plants not subjected to salt stress, although with much smaller dimensions and not all mature (). At that time point, four of these plants had sufficiently developed leaves for PRI, NDVI, gas exchange measurements and canopy temperature analysis, while only one non-inoculated plant from the salt stress treatment had mature leaves. Therefore, this experimental treatment could not be included in the subsequent statistical analyses.
Figure 6. Number of green leaves (A) and green shoot length (B) in Aragonez cv. grapevines grafted onto Richter 110 rootstock, inoculated or not with bacteria and exposed or not the NaCl stress. Bars indicate the mean value per treatment ± standard error. Different letters indicate significant differences according to Duncan test.
When physiological parameters were analyzed, no significant differences were observed in A and gs among the experimental groups, inoculated or not, exposed to salt stress or not ((A,B)) presenting mean values (± standard error) of 7.93 ± 0.55 µmol CO2/m2s and 171.96 ± 22.24 mmol/m2s, respectively. However, PGPB-inoculated plants that had not been subjected to salt stress had the highest transpiration rates compared to the other experiment treatments ((C)). These plants were also the ones showing the lowest canopy temperature (). Concerning PRI, the highest values were found in PGPB-inoculated plants that were recovering after salt stress ((D,E)) but no differences were found in NDVI values among the experimental treatments.
On August 31st, one month after salt removal, the new leaves in stress-recovered plants were larger in size and measurements could be performed in both treatments (inoculated and non-inoculated). The length of the new green shoots was highest in inoculated plants recovering from the salt stress (70 cm ± 13.4), and no significant differences were found in that parameter among the remaining experimental treatments (). The number of leaves was also the highest in inoculated plants recovering from the salt stress, although many of these leaves were still small in size. Non-inoculated plants non-exposed to salt stress were the ones with the lowest number of leaves ().
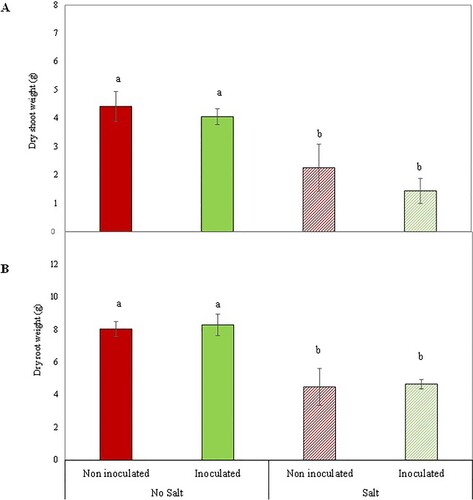
At that same time point, the two-way ANOVA showed a significant negative effect of salt stress factor in NDVI, canopy temperature, shoot and root dry biomass, while PGPB-inoculation factor did not show a significant effect (Supplementary Table S4; and ; ). However, the multiple mean comparison test did not show significant differences across the experimental treatments for NDVI nor any of the gas exchange parameters (). Contrastingly, salt stress seemed to have a positive effect on PRI values (Supplementary Table S4; (E)).
Figure 7. Dry shoot biomass (g) (A) and dry root biomass (g) (B) of Aragonez variety grapevines grafted onto Richter 110 rootstocks inoculated or not with a bacterial consortium and treated or not with a saline (NaCl) solution. Bars indicate the mean value per treatment ± standard error. Different letters indicate significant differences according to the Duncan. In the right part of each chart, the results of the two-way ANOVA test for the factors Inoculation and Stress are presented. The asterisk indicates significant differences at P = 0.05, while ‘***’ highly significant differences at P < 0.01 and the ‘ns’ indicates non-significant differences.
3.6. Bacterial colonization in grapevine roots
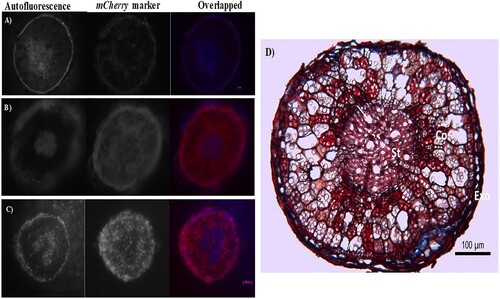
Fifteen days after salt removal, when root samples were collected to evaluate bacterial colonization, the red fluorescence from the mCherry protein was recorded in inoculated plants within epidermal, cortical parenchyma, and phloem cells, indicating that the bacteria were present in such sites (). No red fluorescence was detected in the roots of the non-inoculated plant. In both, inoculated and non-inoculated plants, autoflourescence due to compounds like lignin and suberin usually deposited in the xylem tissue was also observed ().
Figure 8. Root transverse sections of grapevines non-inoculated or inoculated with plant growth promoting bacteria (PGPB). Richter rootstock roots from (A) non-inoculated plants (control); (B) plants inoculated with a consortium of three selected PGPB strains; (C) plants inoculated with a consortium of three PGPB strains and exposed to saline stress. The root sections were observed by fluorescence microscope (Zeiss Axioskop 2), equipped with a digital camera (Zeiss AxioCam) with a 10× objective. (A–C) Intense blue lignin autofluorescence (arrowed) is visualized in the exodermis and stelle while the red fluorescence (mCherry marker) is mainly distributed in the cortex and phloem living tissues. Note absence of red labeling in control root sections. (D) Transverse sectional of grapevine root during maturation. Cortex (Co), stele (St), exodermis (Exo), vessel (arrow) and starch grains (*). Cell walls unlignified appear blue (stained with astra blue) while those lignified will appear red (stained with safranin).
4. Discussion
4.1. Differences in bacterial salt tolerance according to their original environment and host plant
There is an urgent need to find strategies that allow crops to grow under adverse conditions such as soil salinization, which is becoming in an increasing threat for agriculture. One of these strategies is the inoculation with halophilic/halotolerant PGPB, due to their demonstrated capacity to promote plant growth and physiology under saline conditions (Yasin et al. Citation2018; Teo et al. Citation2022). In our study, three species of halophytes of the genus Limonium were used to isolate endophytic bacteria, and all of them were found to be halotolerant and showed at least one of the tested PGP properties, making them good candidates for inoculation in salinity-affected agricultural soils (Yasin et al. Citation2018; Kumar S et al. Citation2021; Teo et al. Citation2022).
The root endosphere of the studied Limonium species was mainly formed by Gram negative bacteria, being the most representative genera Pantoea and Erwinia. Other studies of Limonium plants’ microbiome (i.e. L. vulgare plants growing in a marsh in the Netherlands), revealed that Pseudomonas was the most abundant genus, although species from other genera were similar to those obtained in our work, such as E. persicina (Wang et al. Citation2017). In Limonium sinense roots the most frequently isolated bacterial genus was Glutamicibacter, but Bacillus and Pseudomonas species were also found, as in our study (Qin et al. Citation2018). These results indicate that the root endosphere microbiome composition depends on the plant species as well as on the environment (Mora-Ruíz et al. Citation2016; Navarro-Torre et al. Citation2016; Citation2017b; Furtado et al. Citation2019).
The most salt tolerant bacteria were those isolated from L. vulgare, while strains isolated from L. multiflorum were the least tolerant. These results could be explained by the ecological conditions in which each Limonium species thrives. Limonium vulgare is exposed to more challenging stressful conditions, such as inundation with brackish water during the high tides two times a day (Costa et al. Citation2014), whereas L. daveaui thrives on sandy-brackish soils only occasionally touched by brackish water during equinoctial tides, and occasionally flooded by fresh water during winter or springtime (Costa et al. Citation2014; Caperta and Carapeto Citation2020). In contrast, L. multiflorum inhabits rocky areas exposed to salt spray but only very seldom inundated by sea water (Caperta et al. Citation2014).
Although a general increase in bacterial IAA production and a decrease in the production of siderophores and phosphate solubilization were found at 0.2 M of NaCl, this depended largely on the bacterial strain (), as also reported by other authors, who found that the differential expression of bacterial PGP properties in presence of NaCl depended on both the salt concentration and the bacterial genotype (Ali et al. Citation2022; Kapadia et al. Citation2022).
4.2. Benefits of halotolerant bacteria inoculation in grapevine plants
In this work, under non-saline conditions, a beneficial effect of the PGPB inoculation was observed in grapevine plants three months after the first bacterial inoculation. The presence of the inoculated bacteria was detected in exodermal cells, in cortical parenchyma, and in phloem tissues from grapevine root cross-sections, indicating a successful colonization of halotolerant bacteria in these plants (). A previous study on plants inoculated with two bacterial strains labeled with fluorescent proteins (green fluorescent protein-gfp and red fluorescent protein-dsRED) showed fluorescent marks either along the root surface or along root hairs (Rolli et al. Citation2017). Contrastingly, our study provides evidence of tissue specific localization of the PGPB in the grapevine root endosphere, which was restricted to living cells and absent in the xylem vessels.
The inoculated grapevines presented higher values of A, gs, E, NDVI and PRI (). Other studies also found that inoculation with a PGPB consortium can improve the physiology of their hosts (Glick Citation2005; Ahmad et al. Citation2013; Castillo-Aguilar et al. Citation2017) as well as their nutritional status and growth (Dodd and Pérez-Alfocea Citation2012; Santi et al. Citation2013; Paul and Lade Citation2014). In the particular case of grapevines, Salomon et al. (Citation2016), observed an improved Malbec vine physiology when inoculated with a consortium of three PGPB (Microbacterium imperiale, Kocuria erythromyxa and Terribacillus saccharophilus). This consortium was able to produce IAA, biofilms and siderophores, to fix nitrogen and solubilize phosphate, which could have contributed to the observed positive effect on plant physiology (Salomon et al. Citation2016). In the case of Rolli et al. (Citation2017), from the 15 strains tested, only three promoted plant growth under field conditions, which was attributed to an improvement in plant auxin balance or even to an improvement in microbial interactions in the root microbiome (Rolli et al. Citation2017).
The halotolerant PGPB consortium used for grapevine inoculation in our study was able to produce auxins and siderophores, fix nitrogen, and solubilize phosphates (), which could have contributed to the observed beneficial effect in our grapevines.
4.3. Halotolerant bacteria promoted a faster grapevine recovery after salt stress exposure
Two days after NaCl treatment application, grapevines showed marginal and internerval necrosis, especially in mature leaves (). Salt damage in the oldest leaves could be due to excessive amount of salt entering the plant, ultimately leading to ion toxicity and causing premature senescence, which may also be mediated by increased production of abscisic acid and ethylene (Kefu et al. Citation1991; Baneh et al. Citation2014). The accumulation of Na+ in mature leaves accelerates their death, and the loss of these leaves decreases the supply of carbohydrates and/or hormones to the meristematic regions, thus inhibiting plant growth (Belew et al. Citation2010). Indeed, in this study, by the sixth day after NaCl application, all leaves of salt-stressed plants were senescent, and a high degree of defoliation could be observed.
The severity of leaf necrosis and the degree of growth decline in grapevines exposed to salinity stress depends on the susceptibility of the grapevine variety and the rootstock, as well as on the applied salt type and dose (Gomez-del-Campo et al. Citation2002; Owais Citation2015). Cabernet Sauvignon variety grapevines grafted onto Ruggeri rootstock at three different salinity levels (1.8, 3.3 and 4.8 dS m−1) showed that dry matter production at the medium and high salinity levels was 18% and 25% lower than in the lowest salinity treatment (Ben-Asher et al. Citation2006). In our case, the EC of the substrate of salt-stressed plants was 2 dS m−1, but grapevines presented more severe stress symptoms in leaves than the ones shown in Ben-Asher et al. (Citation2006). These findings confirm that Aragonez variety grapevines grafted onto Richter 110 are sensitive to salt stress. Moreover, salt tolerant cultivars can normally sustain their transpiration rates and stomatal conductances under saline conditions (Baneh et al. Citation2014), which was not observed in the present study. In fact, some studies show that the Richter 110 rootstock has medium salt tolerance in the open field but can show medium to high salt sensitivity under greenhouse conditions (Troncoso de Arce et al. Citation1999; Corso and Bonghi Citation2014; Zhou-Tsang et al. Citation2021). Similarly, Tempranillo/Aragonez variety is classified as a moderately salinity-sensitive variety, and can show an important growth decrease in saline soils, mainly due to an osmotic effect rather than ionic toxicity (Urdanoz and Aragüés Citation2009).
After NaCl application, a strong decrease in reflectance indexes and in plant physiology parameters was observed (), which is in agreement with previous works demonstrating that salinity stress can lead to foliar damage, limited photosynthesis capacity, decreased chlorophyll a and b contents and ultimately a reduction in shoot and root growth (Hu et al. Citation2006; Shibli et al. Citation2007; Walker et al. Citation2010; Fozouni et al. Citation2012; Baneh et al. Citation2014; Kim et al. Citation2017). In fact, the first response of grapevines to salinity stress is stomatal closure (Flexas et al. Citation2000; Baneh et al. Citation2014), which leads to a strong decrease in net photosynthetic rate and to reduced photo-assimilation (Tee et al. Citation2004; Parida and Das Citation2005; Li et al. Citation2010; Torabi et al. Citation2014; Li and Jiang Citation2017). This disturbance in plant physiology can explain the observed decrease in shoot and root biomass in salt-stressed grapevines (). Moreover, stomatal closure can lead to an increase in plant temperature since no evaporative cooling can happen. This was also observed in our study, where two days after NaCl treatment application, salt-stressed grapevines had significantly higher temperatures in the canopies than the non-treated ones ().
Although A, gs and E were zero two days after NaCl application in both inoculated and non-inoculated plants, canopy temperature was found to be higher in inoculated plants under salt stress (), revealing that canopy temperature assessment by infrared imaging can be an efficient method to detect physiological changes due to bacteria inoculation under severe salinity stress conditions, therefore being useful for early stress phenotyping.
As demonstrated in several previous works, leaf temperature can be used for early prediction of several environmental constraints on plants (Gómez-Bellot et al. Citation2015; Acosta-Motos et al. Citation2016; Umair et al. Citation2018), including salinity (Esmaeili et al. Citation2017). This parameter is correlated with stomatal conductance (Zhou-Tsang et al. Citation2021) and photosynthesis rates (Greer Citation2012; Evans Citation2013). At high temperatures, between 30 and 45°C, there is a negative correlation between temperature and photosynthesis, and therefore, high canopy temperature is indicative of stress (Greer Citation2012). Since at the time of salt stress application the highest leaf temperatures were measured in grapevines exposed to salinity, being 39.6°C and 40.4°C for non-inoculated and inoculated plants, respectively, this may indicate that these plants were the ones that suffered the most stress (). At highly stressful conditions, due to the reduced plant C assimilation and the high C costs that endophytic microorganisms impose to their hosts, mutualistic symbioses may turn into commensalistic or even parasitic symbioses (Drew et al. Citation2021). This could explain the higher stress sensed by the plant. On the contrary, at temperatures between 15 and 25°C there is a positive linear correlation between temperature and photosynthesis (Greer Citation2012). For this reason, at harvest time, since leaf temperatures were near 22°C, the highest canopy temperature measured in inoculated plants not exposed to salt stress may be an indicator of a better plant performance (), while the decrease in canopy temperature in the plants that were recovering from salt stress may be indicating the opposite (Supplementary Table S4).
The use of halotolerant PGPB isolated from saline soils to improve crop tolerance to salt stress has already been studied in previous works, including in grapevines (Jiao et al. Citation2016; Ma et al. Citation2017). These bacteria can lead to an improvement in growth parameters as well as in photosynthesis performance and chlorophyll pigments (chlorophyll a, chlorophyll b and chlorophyll ab) under saline conditions (Han et al. Citation2011; Ma et al. Citation2017; Yasin et al. Citation2018; Ansari et al. Citation2019). In our work, regardless of the negative effect of salt stress on both inoculated and non-inoculated plants, after NaCl was eliminated from the substrate, PGPB-inoculated plants were able to recover faster than the non-inoculated ones. This indicates a positive effect of the inoculation in grapevines, even if these plants were not their original host (Limonium species). Furthermore, by the end of the experiment, inoculated plants that had been exposed to salt stress had the highest number of leaves and shoot length, revealing a fast stimulation for regrowth after salt stress. However, since those leaves were still small and new shoots were very young and green, this was not translated yet into a higher shoot biomass (). Similarly, no differences were observed in root biomass among inoculated and non-inoculated plants that had been exposed to salt stress, probably because the new leaves were too young and may not be fixing sufficient C yet to simultaneously promote shoot and root growth.
During the recovery phase, grapevine plants that had been exposed to salt stress showed similar A and gs values than the ones that had not been exposed to the stress, and by the end of the experiment no significant differences were found among inoculated and non-inoculated plants that had been or not exposed to salt stress ((A,B)). This indicates that the C fixation metabolism was fully operative again in the new leaves of salt-exposed plants. Contrary to what was observed at the beginning of the experiment, the effect of PGPB was no longer significant.
Overall, in our study, the most important benefit of the halotolerant PGPB inoculation in grapevine plants was at plant re-growth, as the recovery rate after strong salt stress exposure was much faster than in non-inoculated plants. Several mechanisms could have been responsible for these findings, like, the synthesis of phytohormones (Zhang et al. Citation2010; Gopalakrishnan et al. Citation2015; Gupta et al. Citation2015; Qu et al. Citation2016; Egamberdieva et al. Citation2017; Ma et al. Citation2017), or an stimulation of nutrient uptake in plants (e.g. P, K, N) (Glick et al. Citation2007; Babalola Citation2010; Gupta et al. Citation2015; Xiao et al. Citation2018; Liu et al. Citation2019; Trdan et al. Citation2019). Since the PGPB consortium used in this study was not able to produce ACC deaminase, it is not expected that the reduced ethylene levels could be behind the faster leaf sprouting in salt-stressed plants. In addition, the faster recovery in those plants could be due to an activation of antioxidant systems (Ma et al. Citation2017). Several authors associate the reduction of the stress level in plants with the increase of the antioxidant enzyme activities like catalase, superoxide dismutase, ascorbate peroxidase or guaiacol peroxidase (Flores-Duarte et al. Citation2022a; Citation2022b; Citation2022c; Khan et al. Citation2023) and with a decrease of malondialdehyde (MDA) levels (Khan et al. Citation2023). Based on previous studies, the activation of antioxidant mechanisms in plants by PGPB could reduce ROS levels faster in inoculated plants than in non-inoculated ones, thereby contributing to a faster recovery after NaCl treatment.
5. Conclusions
In this work, we demonstrated that the use of halotolerant/halophilic bacteria isolated from halophytes can be a sustainable strategy to improve plant resilience to salt stress in other hosts, like grapevines, since they promoted a faster recovery after salt stress-induced defoliation. Our results also revealed that thermography was the most effective method for early plant stress monitoring, suggesting that it has a large potential for mapping salt stress in vineyards. However, further field studies are necessary to evaluate if these halotolerant PGPB are competitive under vineyard conditions and if they establish mutualistic symbioses with other rootstocks in the presence of the soil/root native microbiota. If proven, they could represent a suitable method to sustain grapevine growth and production in vineyards with salinity problems in a context of climate change.
Supplemental Material
Download PDF (577.9 KB)Acknowledgements
SN-T thanks Federation of European Microbiological Societies (FEMS) (FEMS-GO-2020-203) and the University of Sevilla (Plan Propio de Investigación y Tranferencia, Ayudas A1-I.3A1 (2021) and III.2B (2022)) for the grants obtained to conduct scientific stays at ISA-ULisboa. AN thanks the FCT for the contract CEECIND/01769/2017. VS thanks the FCT for the contract (DL57/2016/CP1382/CT0004). Authors are grateful to Carla Silva and Luisa Brito, from the Microbiology Department of ISA-ULisboa, for their help in bacterial isolation, to J. Miguel Costa, for his help in thermography image analysis and to Teresa Quilhó from CEF/ISA/ULisboa for the advice on root anatomy. Authors thank Ignacio D. Rodriguez-Llorente for the critical review of the manuscript and the useful comments made on it, and Emilia Sorci, for the proofreading of the article.
Disclosure statement
No potential conflict of interest was reported by the author(s ).
Additional information
Funding
Notes on contributors
Salvadora Navarro-Torre
Salvadora Navarro-Torre obtained her PhD in Biology in 2017 at the University of Seville (Spain). Her research line is focused on the use of the plant-microbiome interaction as a sustainable tool to recover degraded soils and/or to improve the growth of crops under different stressful conditions. She works with plant growth promoting bacteria (PGPB) and, in the beginning, with halophytes plants, although at the moment she is focused on the study of the rhizobia-legume relationship to improve the development of legumes in agricultural soils affected by climate change. Currently, she works at the University of Seville as a substitute professor combined with research periods. During her postdoctoral career, she visited different foreign laboratories, including the Instituto Superior de Agronomia in Lisbon (Portugal).
Sara Ferrario
Sara Ferrario graduated as Agro-environmental technician at the Gregorio Mendel Higher Technical Institute for Agriculture (Italy), and obtained her bachelor’s degree in Agricultural and Forestry Sciences and Technologies at the Università Cattolica del Sacro Cuore (Italy). She has a Double Master’s degree in Viticulture and Oenology Engineering and she conducted her thesis at the Instituto Superior de Agronomia-Universidade de Lisboa on the use of plant-growth promoting bacteria for improving grapevine tolerance to salt stress.
Ana D. Caperta
Ana D. Caperta is Assistant Professor at the Instituto Superior de Agronomia-Universidade de Lisboa (ISA-UL) and researcher at Linking Landscape, Environment, Agriculture and Food Research Center (LEAF) of the same university. Her main area of scientific activity is plant reproduction and conservation. She leads a working group that uses halophytes (salt tolerant plants) with sexual and apomixis (asexual seed production) reproduction. Her research aims are at resolving taxonomy and evolutionary questions associated with reproductive modes in species of Limonium Mill. These species are widespread in coastal environments all over the world, and have a huge environmental importance and conservation value. Other research goals are oriented to find sustainable solutions for cultivation of valued halophytes (species of genus Arthrocnemum, Limonium, Suaeda) and reintroduction of such species into natural habitats. She established, and currently curates, a Limonium ex situ collection at ISA.
Gonçalo Victorino
Gonçalo Victorino is a PhD in Agronomic Engineering (Instituto Superior de Agronomia, University of Lisbon) specialized on the use of image analysis to estimate vineyard yield, within the scope of precision viticulture. During his PhD, he developed skills regarding the use of various imaging devices linked to robotic platforms for data collection in field conditions. Since his PhD he has been continuing his research at the Linking Landscape, Environment, Agriculture and Food Research Center of the Instituto Superior de Agronomia-Universidade de Lisboa, where he is now working on the study of the effect of agrivoltaic systems in vineyard performance. Throughout his career, he benefited from research grants from Portugal (Fundação para a Ciência e a Tecnologia PhD grant FRH/BD/132305/2017) and the European Commision (Rise vWise project, grant agreement ID: 872394, sponsored by the European Community’s Horizon 2020 Program) where he had the opportunity to carry out scientific work at Stellenbosch University, South Africa over a period of three months.
Marion Bailly
Marion Bailly graduated in 2022 as an Agronomic Engineer from the Institut Agro Dijon (formerly AgroSup Dijon). During her studies, she did several internships: a research internship at Linking Landscape, Environment, Agriculture and Food Research Center of the Instituto Superior de Agronomia de Lisboa-Universidade de Lisboa, where she isolated and characterized halophillic plant growth promoting bacteria, another one in a French farm, and another one as a junior consultant for her final graduation thesis. Through these different experiences, she was able to discover many fields and missions that led her to specialize on strategies and organizations implemented by companies and agricultural and agri-food sectors (SOFEAA). After presenting her final year project and obtaining her diploma, she is now working since January 2023 at Mars Wrigley Confectionary as a Network Production Planner, where she is currently discovering the functioning of the supply chain on an international scale.
Vicelina Sousa
Vicelina Sousa holds a PhD in Forestry Engineering and Natural Resources (Instituto Superior de Agronomia-Universidade de Lisboa, ISA-ULisboa). She is currently a researcher at the Forest Research Centre (CEF, research group ForTec: Forest Products and Biorefineries), and at the Associate Laboratory TERRA – Laboratory for Sustainability of Land Use and Ecosystem Services of ISA-ULisboa. Her research focuses are on wood science and technology, namely wood properties, wood formation, wood anatomy and wood identification. Other research interests are on non-wood forest products topics, namely on bark anatomy and properties, and on tree and plant responses to different environmental conditions.
Wanda Viegas
Wanda Viegas is senior researcher at Linking Landscape, Environment, Agriculture and Food Research Center (LEAF). She has renowned experience in genetics and epigenetics in grapevines as well as in other crops, as demonstrated by the more than 200 publications that she has on those topics.
Amaia Nogales
Amaia Nogales holds a PhD in Biology (University of Barcelona in 2009), and she is dedicated to the study of the microbiome of agricultural species, specially grapevines, being a specialist in arbuscular mycorrhizal fungi, pathogenic fungi and plant-growth promoting bacteria. Since 2016, she is an Assistant Researcher at the Linking Landscape, Environment, Agriculture and Food Research Center (LEAF) of the Instituto Superior de Agronomia-Universidade de Lisboa, having been responsible for creating a new research area focused on Agricultural Microbiology and Soil Health, which aims to increase crop productivity and adaptation to new/adverse soil and climate conditions.
References
- Acosta-Motos JR, Ortuño MF, Álvarez S, López-Climent MF, Gómez-Cadenas A, Sánchez-Blanco MJ. 2016. Changes in growth, physiological parameters and the hormonal status of Myrtus communis L. plants irrigated with water with different chemical compositions. J Plant Physiol. 191:12–21. doi:10.1016/j.jplph.2015.11.010.
- Ahmad A, Azooz MM, Prasad MNV. 2013. Salt stress in plant. New York: Springer. doi:10.1007/978-1-4614-6108-1
- Ali B, Wang X, Saleem MH, Sumaira HA, Afridi MS, Khan S, Zaib-Un-Nisa UI, Amaral Júnior ATD, Alatawi A, Ali S. 2022. PGPR-mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants. 11(3):345. doi:10.3390/plants11030345.
- Allen R, Smith M, Perrier A, Pereira L. 1994. An update for the definition of reference evapotranspiration. ICID Bulletin. 43:1–35.
- Alori ET, Babalola OO. 2018. Microbial inoculants for improving crop quality and human health in Africa. Front Microbiol. 9:1–12. doi:10.3389/fmicb.2018.02213.
- Ansari FA, Ahmad I, Pichtel J. 2019. Growth stimulation and alleviation of salinity stress to wheat by the biofilm forming Bacillus pumilus strain FAB10. Appl Soil Ecol. 143:45–54. doi:10.1016/j.apsoil.2019.05.023.
- Babalola OO. 2010. Beneficial bacteria of agricultural importance. Biotechnol Lett. 32(11):1559–1570. doi:10.1007/s10529-010-0347-0.
- Baneh HD, Attari H, Hassani A, Abdollahi R, Taheri M, Shayesteh FG. 2014. Genotypic variation in plant growth and physiological response to salt stress in grapevine. Philipp Agric Sci. 97(2):113–121.
- Barbosa ACF, Pace MR, Witovisk L, Angyalossy V. 2010. A new method to obtain good anatomical slides of heterogeneous plant parts. IAWA J. 31(4):373–383. doi:10.1163/22941932-90000030.
- Belew D, Astatkie T, Mokashi MN, Getachew Y, Patil CP. 2010. Effects of salinity and mycorrhizal inoculation (Glomus fasciculatum) on growth responses of grape rootstocks (Vitis spp). SAJEV. 31(2):82–88. doi:10.21548/31-2-1404.
- Ben-Asher J, Tsuyuki I, Bravdo BA, Sagih M. 2006. Irrigation of grapevines with saline water: I. Leaf area index, stomatal conductance, transpiration and photosynthesis. Agric Water Manag. 83(1-2):13–21. doi:10.1016/j.agwat.2006.01.002.
- Bernstein L, Ehlig CF, Clark RA. 1969. Effect of grape rootstocks on chloride accumulation in leaves. J Am Soc Hortic Sci. 7(5):195–219. doi:10.1007/s11104-004-2695-9.
- Blumwald E. 2000. Sodium transport and salt tolerance in plants. Curr Opin Cell Biol. 12(4):431–434. doi:10.1016/s0955-0674(00)00112-5.
- Caperta A, Carapeto A. 2020. Limonium daveaui: ficha de avaliação do risco de extinção. In: Lista Vermelha da Flora Vascular de Portugal Continental. Sociedade Portuguesa de Botânica Associação Portuguesa de Ciência da Vegetação, Lisbon.
- Caperta AD, Espírito-Santo MD, Silva V, Ferreira A, Paes AP, Róis AS, Costa JC, Arsénio P. 2014. Habitat specificity of a threatened and endemic, cliff-dwelling halophyte. AoB Plants. 6:plu032. doi:10.1093/aobpla/plu032.
- Caperta AD, Róis AS, Teixeira G, Garcia-Caparros P, Flowers TJ. 2020. Secretory structures in plants: lessons from the Plumbaginaceae on their origin, evolution and roles in stress tolerance. Plant Cell Environ. 43(12):2912–2931. doi:10.1111/pce.13825
- Castillo-Aguilar CC, Zúñiga-Aguilar JJ, Guzmán-Antonio AA, Garruña R. 2017. PGPR inoculation improves growth, nutrient uptake and physiological parameters of Capsicum chinense plants. Phyton-Int J Exp Bot. 86:199–204.
- Chaves MM, Zarrouk O, Francisco R, Costa JM, Santos T, Regalado AP, Rodrigues ML, Lopes CM. 2010. Grapevine under deficit irrigation: hints from physiological and molecular data. Ann Bot. 105(5):661–676. doi:10.1093/aob/mcq030.
- Cook BI, Smerdon JE, Seager R, Coats S. 2014. Global warming and 21st century drying. Clim Dyn. 43:2607–2627. doi:10.1007/s00382-014-2075-y.
- Corso M, Bonghi C. 2014. Grapevine rootstock effects on abiotic stress tolerance. Plant Sci Today. 1:108–113. doi:10.14719/pst.2014.1.3.64.
- Corwin DL. 2021. Climate change impacts on soil salinity in agricultural areas. Eur J Soil Sci. 72:842–862. doi:10.1111/ejss.13010.
- Costa JC, Neto C, Monteiro-Henriques T, Arsénio P, Portela-Pereira E, Caperta A, Izco J. 2014. Coastal halophilous limonium communities from west Iberian Peninsula. Documents Phytosociologiques. 3(1):214–227.
- Costa JM, Grant O, Chaves M. 2010. Use of thermal imaging in viticulture: current application and future prospects. In: Delrot S, Medrano H, Or E, Bavaresco L, Grando S, editor. Methodologies and results in grapevine research. Dordrecht: Springer; p. 135–150. doi:10.1007/978-90-481-9283-0_10.
- del Castillo I, Hernández P, Lafuente A, Rodríguez-Llorente ID, Caviedes MA, Pajuelo E. 2012. Self-bioremediation of cork-processing wastewaters by (chloro)phenol-degrading bacteria immobilised onto residual cork particles. Water Res. 46(6):1723–1734. doi:10.1016/J.WATRES.2011.12.038.
- Dodd IC, Pérez-Alfocea F. 2012. Microbial amelioration of crop salinity stress. J Exp Bot. 63(9):3415–3428. doi:10.1093/jxb/ers033.
- Downton WJS, Millhouse J. 1983. Turgor maintenance during salt stress prevents loss of variable fluorescence in grapevine leaves. Plant Sci Lett. 31(1):1–7. doi:10.1016/0304-4211(83)90124-4.
- Drew GC, Stevens EJ, King KC. 2021. Microbial evolution and transitions along the parasite–mutualist continuum. Nat Rev Microbiol. 19:623–638. doi:10.1038/s41579-021-00550-7.
- Egamberdieva D, Wirth S, Jabborova D, Räsänen LA, Liao H. 2017. Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. J Plant Interac. 12(1):100–107. doi:10.1080/17429145.2017.1294212.
- Esmaeili A, Poustini K, Ahmadi H, Abbasi A. 2017. Use of IR thermography in screening wheat (Triticum aestivum L. cultivars for Salt Tolerance. Arch Agron Soil Sci. 63(2):161–170. doi:10.1080/03650340.2016.1204541.
- Evans JR. 2013. Improving photosynthesis. Plant Physiol. 162(4):1780–1793. doi:10.1104/pp.113.219006.
- Fisarakis I, Chartzoulakis K, Stavrakas D. 2001. Response of Sultana vines (V. vinifera L.) on six rootstocks to NaCl salinity exposure and recovery. Agric Water Manag. 51(1):13–27. doi:10.1016/S0378-3774(01)00115-9.
- Flexas J, Briantais J-M, Cerovic Z, Medrano H, Moya I. 2000. Steady-state and maximum chlorophyll fluorescence responses to water stress in grapevine leaves: a new remote sensing system. Remote Sens Environ. 73(3):283–297. doi:10.1016/S0034-4257(00)00104-8.
- Flores-Duarte NJ, Caballero-Delgado S, Pajuelo E, Mateos-Naranjo E, Redondo-Gómez S, Navarro-Torre S, Rodríguez-Llorente ID. 2022c. Enhanced legume growth and adaptation to degraded estuarine soils using Pseudomonas sp. nodule endophytes. Front Microbiol. 13:1005458. doi:10.3389/fmicb.2022.1005458.
- Flores-Duarte NJ, Mateos-Naranjo E, Redondo-Gómez S, Pajuelo E, Rodriguez-Llorente ID, Navarro-Torre S. 2022b. Role of nodulation-enhancing rhizobacteria in the promotion of Medicago sativa development in nutrient-poor soils. Plants. 11(9):1164. doi:10.3390/plants11091164.
- Flores-Duarte NJ, Pérez-Pérez J, Navarro-Torre S, Mateos-Naranjo E, Redondo-Gómez S, Pajuelo E, Rodríguez-Llorente ID. 2022a. Improved Medicago sativa nodulation under stress assisted by Variovorax sp. endophytes. Plants. 11(8):1091. doi:10.3390/plants11081091.
- Flowers TJ, Colmer TD. 2008. Salinity tolerance in halophytes. New Phytol. 179:945–963. doi:10.1111/j.1469-8137.2008.02531.x.
- Fozouni M, Abbaspour N, Doulati Baneh H. 2012. Leaf water potential, photosynthetic pigments and compatible solutes alterations in four grape cultivars under salinity. Vitis-J Grapevine Res. 51(4):147–152. doi:10.5073/vitis.2012.51.147-152.
- Fraga H, Santos JA, Malheiro AC, Oliveira AA, Mountinho-Pereira GVJ. 2016. Climatic suitability of Portuguese grapevine varieties and climate change adaptation. Int J Climatol. 36:1–12. doi:10.1002/joc.4325.
- Furtado BU, Golebiewski M, Skorupa M, Hulisz P, Hrynkiewicz K. 2019. Bacterial and fungal endophytic microbiomes of Salicornia europaea. Ap Environ Microbiol. 85:e00305–19. doi:10.1128/AEM.00305-19.
- Garbulsky MF, Peñuelas J, Gamon J, Inoue Y, Filella I. 2011. The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies. A review and meta-analysis. Remote Sens Environ. 115(2):281–297. doi:10.1016/j.rse.2010.08.023.
- Glick BR. 2005. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol Lett. 251(1):1–7. doi:10.1016/j.femsle.2005.07.030.
- Glick BR. 2014. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 169:30–39. doi:10.1016/j.micres.2013.09.009.
- Glick BR, Cheng Z, Czarny J, Duan J. 2007. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol. 119(3):329–339. doi:10.1007/s10658-007-9162-4.
- Glick BR, Gamalero E. 2021. Recent developments in the study of plant microbiomes. Microorganisms. 9(7):1533. doi:10.3390/microorganisms9071533.
- Gómez-Bellot MJ, Nortes PA, Sánchez-Blanco MJ, Ortuño MF. 2015. Sensitivity of thermal imaging and infrared thermometry to detect water status changes in Euonymus japonica plants irrigated with saline reclaimed water. Biosyst Eng. 133:21–32. doi:10.1016/j.biosystemseng.2015.02.014.
- Gomez-del-Campo M, Ruiz C, Lissarrague JR. 2002. Effect of water stress on leaf area development, photosynthesis, and productivity in Chardonnay and Airén grapevines. AJEV. 53(2):138–143.
- Gopalakrishnan S, Sathya A, Vijayabharathi R, Varshney RK, Gowda CLL, Krishnamurthy L. 2015. Plant growth promoting rhizobia: challenges and opportunities. Biotech. 5(4):355–377. doi:10.1007/s13205-014-0241-x.
- Gordon SA, Weber RP. 1951. Colorimetric estimation of indoleacetic acid. Plant Physiol. 26(1):192–195. doi:10.1104/pp.26.1.192.
- Greer DH. 2012. Modelling leaf photosynthetic and transpiration temperature-dependent responses in Vitis vinifera cv. semillon grapevines growing in hot, irrigated vineyard conditions. AoB Plants. 2012:pls009. doi:10.1093/aobpla/pls009.
- Gupta G, Parihar S, Ahirwar N, Snehi DSK, Singh V. 2015. Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. JMBT. 7(2):96–102. doi:10.4172/1948-5948.1000188.
- Han J, Song Y, Liu Z, Hu Y. 2011. Culturable bacterial community analysis in the root domains of two varieties of tree peony (Paeonia ostii). FEMS Microbiol Lett. 322(1):15–24. doi:10.1111/j.1574-6968.2011.02319.x.
- Hao Y, Zhang K, Yang R, Wang YA. 2013. Effect of different rootstock varieties on photosynthesis and fluorescence characteristics of riesling grape. Acta Agric Bor Sin. 6(23):113–117.
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. 2000. Plant cellular and molecular resoponses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 51:463–499. doi:10.1146/annurev.arplant.51.1.463.
- Hoagland D, Arnon DI. 1972. The water culture method for growing plants without soil. Berkeley, CA: California Agricultural Experiment Station.
- Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L. 2006. Overexpressing a NAM. ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. PNAS. 103(35):12987–12992. doi:10.1073/pnas.0604882103.
- Jamil A, Riaz S, Ashraf M, Foolad MR. 2011. Gene wxpression profiling of plants under salt stress. Crit Revi Plant Sci. 30(5):435–458. doi:10.1080/07352689.2011.605739.
- Ji SH, Gururani MA, Chun SC. 2014. Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol Res. 169(1):83–98. doi:10.1016/j.micres.2013.06.003.
- Jiao J, Ma Y, Chen S, Liu C, Song Y, Qin Y, Yuan C, Liu Y. 2016. Melatonin-producing endophytic bacteria from grapevine roots promote the abiotic stress-induced production of endogenous melatonin in their hosts. Front Plant Sci. 7:1387. doi:10.3389/fpls.2016.01387.
- Jones HG. 2002. Use of the thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. Plant Cell Environ. 22(9):1043–1055. doi:10.1046/j.1365-3040.1999.00468.x.
- Jones HG. 2004. Application of thermal imaging and infrared sensing in plant physiology and ecophysiology. Adv Bot Res. 41:107–163. doi:10.1016/S0065-2296(04)41003-9.
- Kalra N, Chander S, Pathak H, Aggarwal PK, Gupta NC, Sehgal M, Debashis C. 2002. Impacts of climate change. Outlook Agric. 36(2):109–118.
- Kapadia C, Patel N, Rana A, Vaidya H, Alfarraj S, Ansari MJ, Gafur A, Poczai P, Sayyed RZ. 2022. Evaluation of plant growth-promoting and salinity ameliorating potential of halophilic bacteria isolated from saline soil. Front Plant Sci. 15(13):946217. doi:10.3389/fpls.2022.946217.
- Kefu Z, Munns R, King RW. 1991. Abscisic acid levels in NaCl-treated barley, cotton and saltbush. Aus J Plant Physiol. 18:17–24. doi:10.1071/PP9910017.
- Khan WU, Yasin NA, Ahmad SR, Nazir A, Naeem K, Nadeem QUA, Nawaz S, Ijaz M, Tahir A. 2023. Burkholderia cepacia CS8 improves phytoremediation potential of Calendula officinalis for tannery solid waste polluted soil. Int J Phytoremediation. 28:1–13. doi:10.1080/15226514.2023.2183717.
- Kim MJ, Kim HJ, Pak JH, Cho HS, Choi HK, Jung HW, Lee DH, Chung YS. 2017. Overexpression of AtSZF2 from Arabidopsis showed enhanced tolerance to salt stress in soybean. Plant Breed Biotechnol. 5(1):1–15. doi:10.9787/PBB.2017.5.1.1.
- Kumar S D, Sindhu SS, Kumar R. 2021. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr Res Microb Sci. 3:100094. doi:10.1016/j.crmicr.2021.100094.
- Li HQ, Jiang XW. 2017. Inoculation with plant growth-promoting bacteria (PGPB) improves salt tolerance of maize seedling. Russ J Plant Physiol. 64(2):235–241. doi:10.1134/S1021443717020078.
- Li R, Shi F, Fukuda K, Yang Y. 2010. Effects of salt and alkali stresses on germination, growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L. Soil Sci Plant Nutr. 56(5):725–733. doi:10.1111/j.1747-0765.2010.00506.x.
- Li Z, Chang S, Ye S, Chen M, Lin L, Li Y, Li S, An Q. 2015. Differentiation of 1-aminocyclopropane-1-carboxylate (ACC) deaminase from its homologs is the key for identifying bacteria containing ACC deaminase. FEMS Microbiol Ecol. 91(10):1–11. doi:10.1093/femsec/fiv112.
- Liu J, Tang L, Gao H, Zhang M, Guo C. 2019. Enhancement of alfalfa yield and quality by plant growth-promoting rhizobacteria under saline-alkali conditions. J Sci Food Agric. 99(1):281–289. doi:10.1002/jsfa.9185.
- Ma Y, Jiao J, Fan X, Sun H, Zhang Y, Jiang J, Liu C. 2017. Endophytic bacterium pseudomonas fluorescens RG11 may transform tryptophan to melatonin and promote endogenous melatonin levels in the roots of four grape cultivars. Front Plant Sci. 7:2068. doi:10.3389/fpls.2016.02068.
- Maas EV, Grattan SR. 1999. Crop growths as affected by salinity. In: Skaggs RW, van Schilfgaarde J, editor. Agricultural drainage vol 38. Agronomy. Wisconsin: American Society of Agronomy Madison; p. 55–110.
- Mäser P, Hosoo Y, Goshima S, Horie T, Eckelman B, Yamada K, Yoshida K, Bakker EP, Shinmyo A, Oiki S, et al. 2002. Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. PNAS. 99(9):6428–6433. doi:10.1073/pnas.082123799.
- Mesa J, Mateos-Naranjo E, Caviedes MA, Redondo-Gómez S, Pajuelo E, Rodríguez-Llorente ID. 2015a. Scouting contaminated estuaries: heavy metal resistant and plant growth promoting rhizobacteria in the native metal rhizoaccumulator Spartina maritima. Mar Pollut Bull. 90:150–159. doi:10.1016/j.marpolbul.2014.11.002.
- Mesa J, Mateos-Naranjo E, Caviedes MA, Redondo-Gómez S, Pajuelo E, Rodríguez-Llorente ID. 2015b. Endophytic cultivable bacteria of the metal bioaccumulator Spartina maritima improve plant growth but not metal uptake in polluted marshes soils. Front Microbiol. 6:1450. doi:10.3389/fmicb.2015.01450.
- Mora-Ruíz MR, Font-Verdera F, Orfila A, Rita J, Roseselló-Móra R. 2016. Endophytic microbial diversity of the halophyte Arthrocnemum macrostachyum across plant compartments. FEMS Microbiol Ecol. 92:fiw145. doi:10.1093/femsec/fiw145.
- Munns R, Gilliham M. 2015. Salinity tolerance of crops – What is the cost? New Phytol. 208(3):668–673. doi:10.1111/nph.13519.
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 59(1):651–681. doi:10.1146/annurev.arplant.59.032607.092911.
- Nautiyal CS. 1999. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 170(1):265–270. doi:10.1111/j.1574-6968.1999.tb13383.x.
- Navarro-Torre S, Barcia-Piedras JM, Caviedes MA, Pajuelo E, Redondo-Gómez S, Rodríguez-Llorente ID, Mateos-Naranjo E. 2017a. Bioaugmentation with bacteria selected from the microbiome enhances Arthrocnemum macrostachyum metal accumulation and tolerance. Mar Pollut Bull. 117:340–347. doi:10.1016/j.marpolbul.2017.02.008.
- Navarro-Torre S, Barcia-Piedras JM, Mateos-Naranjo E, Redondo-Gómez S, Camacho M, Caviedes MA, Pajuelo E, Rodriguez-Llorente ID. 2017b. Assessing the role of endophytic bacteria in the halophyte Arthrocnemum macrostachyum salt tolerance. Plant Biol. 19(2):249–256. doi:10.1111/plb.12521.
- Navarro-Torre S, Bessadok K, Flores-Duarte NJ, Rodríguez-Llorente ID, Caviedes MA, Pajuelo E. 2020. Helping legumes under stress situations: inoculation with beneficial microorganisms. In: Hasanuzzaman H, editor. Legume crops-prospects, production and uses. London: IntechOpen; p. 115–135. doi:10.5772/INTECHOPEN.91857
- Navarro-Torre S, Mateos-Naranjo E, Caviedes MA, Pajuelo E, Rodríguez-Llorente ID. 2016. Isolation of plant-growth promoting and metal resistant cultivable bacteria from Arthrocnemum macrostachyum in the Odiel marshes with potential use in phytoremediation. Mar Pollut Bull. 110:133–142. doi:10.1016/j.marpolbul.2016.06.070.
- OIV. 2020. Statistical report on world vitiviniculture. Paris: International Organisation of Vine and Wine.
- OIV. 2021. State of the vitivinicultural world in 2020. Paris: International Organisation of Vine and Wine.
- Ollat N, Peccoux A, Papura D, Esmenjaud D, Marguerit E, Tandonnet JP, Bordenave L, Cookson SJ, Barrieu F, Rossdeutsch L, et al. 2016. Rootstocks as a component of adaptation to environment. In: Gerós H, Chaves M.M., Gil H.M., Delrot S., editor. Grapevine in a changing environment: A molecular and ecophysiological perspective. Chichester: John Wiley; p. 68–108. doi:10.1002/9781118735985.ch4
- Owais S. 2015. Morphological and physiological responses of six grape genotypes to NaCl salt stress. Pak J Biol Sci. 18:240–246. doi:10.3923/pjbs.2015.240.246.
- Ozden M, Demirel U, Kahraman A. 2009. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci Hortic. 119(2):163–168. doi:10.1016/j.scienta.2008.07.031.
- Parida AK, Das AB. 2005. Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf. 60(3):324–349. doi:10.1016/j.ecoenv.2004.06.010.
- Paul D, Lade H. 2014. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron Sustain Dev. 34(4):737–752. doi:10.1007/s13593-014-0233-6.
- Qin S, Feng WW, Zhang YJ, Wang TT, Xiong YW, Xing K. 2018. Diversity of bacterial microbiota of coastal halophyte Limonium sinense and amelioration of salinity stress damage by symbiotic plant growth-promoting actinobacterium Glutamicibacter halophytocola KLBMP 5180. Appl Environ Microbiol. 19:e01533–18. doi:10.1128/AEM.01533-18.
- Qu L, Huang Y, Zhu C, Zeng H, Shen C, Liu C, Zhao Y, Pi E. 2016. Rhizobia-inoculation enhances the soybean’s tolerance to salt stress. Plant Soil. 400(1):209–222. doi:10.1007/s11104-015-2728-6.
- Rengasamy P. 2006. World salinization with emphasis on Australia. J Exp Bot. 57(5):1017–1023. doi:10.1093/jxb/erj108.
- Rolli E, Marasco R, Saderi S, Corretto E, Mapelli F, Cherif A, Borin S, Valenti L, Sorlini C, Daffonchio D. 2017. Root-associated bacteria promote grapevine growth: from the laboratory to the field. Plant Soil. 410(1-2):369–382. doi:10.1007/s11104-016-3019-6.
- Sadak MS. 2019. Physiological role of trehalose on enhancing salinity tolerance of wheat plant. Bull Natl Res Cent. 43(1):53. doi:10.1186/s42269-019-0098-6.
- Sairam RK, Tyagi A. 2004. Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci. 86(3):407–421. doi:10.1007/1-4020-4225-6.
- Salomon MV, Purpora R, Bottini R, Piccoli P. 2016. Rhizosphere associated bacteria trigger accumulation of terpenes in leaves of Vitis vinifera L. cv. Malbec that protect cells against reactive oxygen species. Plant Physiol Biochem. 106:295–304. doi:10.1016/j.plaphy.2016.05.007.
- Santi C, Bogusz D, Franche C. 2013. Biological nitrogen fixation in non-legume plants. Ann Bot. 111(5):743–767. doi:10.1093/aob/mct048.
- Schwyn B, Neilands JB. 1987. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 160(1):47–56. doi:10.1016/0003-2697(87)90612-9.
- Sembiring H, Raun WR, Johnson GV, Stone ML, Solie JB, Phillips SB. 1998. Detection of nitrogen and phosphorus nutrient status in bermudagrass using spectral radiance. J Plant Nutr. 21(6):1189–1206. doi:10.1080/01904169809365477.
- Serraj R, Sinclair TR. 2002. Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Enviro. 25(2):333–341. doi:10.1046/j.1365-3040.2002.00754.x.
- Shabala S, Shabala L, van Volkenburgh E. 2003. Effect of calcium on root development and root ion fluxes in salinised barley seedlings. Funct Plant Biol. 30(5):507–514. doi:10.1071/FP03016.
- Shani U, Ben-Gal A. 2005. Long-term response of grapevines to salinity: osmotic effects and ion toxicity. Am J Enol Vitic. 56(2):148–154.
- Shibli RA, Kushad M, Yousef GG, Lila MA. 2007. Physiological and biochemical responses of tomato microshoots to induced salinity stress with associated ethylene accumulation. Plant Growth Regul. 51(2):159–169. doi:10.1007/s10725-006-9158-7.
- Shurigin V, Egamberdieva D, Li L, Davranov K, Panosyan H, Birkeland N K, Wirth S, Bellingrath-Kimura SD. 2020. Endophytic bacteria associated with halophyte Seidlitzia rosmarinus Ehrenb. ex Boiss. from saline soil of Uzbekistan and their plant beneficial traits. J Arid Land. 12:730–740. doi:10.1007/s40333-020-0019-4.
- Sinclair C, Hoffmann AA. 2003. Monitoring salt stress in grapevines: are measures of plant trait variability useful? J Appl Ecol. 40(5):928–937. doi:10.1046/j.1365-2664.2003.00843.x.
- Stavi I, Thevs N, Priori S. 2021. Soil salinity and sodicity in drylands: A review of causes, effects, monitoring, and restoration measures. Front Environ Sci. 9:330. doi:10.3389/fenvs.2021.712831.
- Stevens RM, Harvey G, Norton S, Frahn W. 2011. Over-canopy saline sprinkler irrigation of grapevines during different growth stages. Agric Water Manag. 101(1):62–70. doi:10.1016/j.agwat.2011.09.003.
- Subramanyam K, Sailaja KV, Subramanyam K, Muralidhara Rao D, Lakshmidevi K. 2011. Ectopic expression of an osmotin gene leads to enhanced salt tolerance in transgenic chilli pepper (Capsicum annum L. Plant Cell, Tissue Organ Cult. 105(2):181–192. doi:10.1007/s11240-010-9850-1.
- Tee E, Burrows DM, Boland A-M, Putland S. 2004. Best irrigation: management practices for viticulture in the Murray darling basin. Knoxfield: Department of Primary Industries.
- Teo HM, Aziz A, Wahizatul AA, Bhubalan K, Siti Nordahliawate MS, Muhamad Syazlie CI, Ng LC. 2022. Setting a plausible route for saline soil-based crop cultivations by application of beneficial halophyte-associated bacteria: A review. Microorganisms. 10:657. doi:10.3390/microorganisms10030657.
- Tiam-Ren Y. 1985. Physical chemistry of paddy soils. Beijing: Springer Nature.
- Torabi M, Halim RA, Choukan R. 2014. Physiological adaptation of Alfalfa genotypes to salt stress (one of deleterious impacts of llimate change). In: Behnassi M, Shahid SA, Mintz-Habib N, editor. Science, policy and politics of modern agricultural system: global context to local dynamics of sustainable agriculture. Dordrecht: Springer Netherlands; p. 181–194. doi:10.1007/978-94-007-7957-0_12
- Trdan S, Vučajnk F, Bohinc T, Vidrih M. 2019. The effect of a mixture of two plant growth-promoting bacteria from Argentina on the yield of potato, and occurrence of primary potato diseases and pest–short communication. Acta Agric Scan B Soil Plant Sci. 69(1):89–94. doi:10.1080/09064710.2018.1492628.
- Troncoso de Arce A, Matte C, Cantos M, Lavee S. 1999. Evaluation of salt tolerance of in vitro-grown grapevine rootstock varieties. Vitis. 38(2):55–60.
- Turan M, Kıtır N, Alkaya U, Gunes A, Tufenkci Ş, Yıldırım E, Nikerel E. 2016. Making soil more accessible to plants: The case of plant growth promoting rhizobacteria. In: Rogovelo EC, editor. Plant growth. London: IntechOpen; p. 61–69. doi:10.5772/64826.
- Umair M, Inam-ul-Haq M, Saeed M, Altaf A, Azam F, Hayat S. 2018. A brief review on plant growth promoting rhizobacteria (PGPR): A key role in plant growth promotion. Plant Protection. 2(2):77–82.
- Urdanoz V, Aragüés R. 2009. Three-year field response of drip-irrigated grapevine (Vitis vinifera L., cv. tempranillo) to soil salinity. Plant Soil. 324(1):219–230. doi:10.1007/s11104-009-9948-6.
- Walker RR, Blackmore DH, Clingeleffer PR. 2010. Impact of rootstock on yield and ion concentrations in petioles, juice and wine of Shiraz and Chardonnay in different viticultural environments with different irrigation water salinity. Aust J Grape Wine Res. 16(1):243–257. doi:10.1111/j.1755-0238.2009.00081.x.
- Walker RR, Blackmore DH, Clingeleffer PR, Emanuelli D. 2014. Rootstock type determines tolerance of Chardonnay and Shiraz to long-term saline irrigation. Aust J Grape Wine Res. 20(3):496–506. doi:10.1111/ajgw.12094.
- Walker RR, Blackmore DH, Clingeleffer PR, Holt H, Pearson W, Francis IL. 2019. Efect of rootstock on yield, grape composition and wine sensory attributes of Shiraz grown in a moderately saline environment. Aust J Grape Wine Res. 25(4):414–429. doi:10.1111/ajgw.12409.
- Wang M, Li E, Liu C, Jousset A, Salles JF. 2017. Functionality of root-associated bacteria along a salt marsh primary succession. Front Microbiol. 8:2102. doi:10.3389/fmicb.2017.02102.
- Xiao X, Fan M, Wang E, Chen W, Wei G. 2018. Interactions of plant growth-promoting rhizobacteria and soil factors in two leguminous plants. App Microbiol Biotechnol. 101(23-24):8485–8497. doi:10.1007/s00253-017-8550-8.
- Yasin NA, Khan WU, Ahmad SR, Ali A, Ahmad A, Akram W. 2018. Imperative roles of halotolerant plant growth-promoting rhizobacteria and kinetin in improving salt tolerance and growth of black gram (Phaseolus mungo). Environ Sci Pollut Res. 25:4491–4505. doi:10.1007/s11356-017-0761-0.
- Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA, Hasegawa PM, Pardo JM. 2002. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 30(5):529–539. doi:10.1046/j.1365-313X.2002.01309.x.
- Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J. 2017. 16S, introducing EzBioCloud: a taxonomically united database of assemblies. rRNA gene sequences and whole-genome. IJSEM. 67:1613–1617. doi:10.1099/ijsem.0.001755.
- Zhang H, Murzello C, Sun Y, Kim M-S, Xie X, Jeter RM, Zak JC, Dowd SE, Paré PW. 2010. Choline and osmotic-stress tolerance induced in Arabidopsis by the soil microbe Bacillus subtilis (GB03). Mol Plant Microbe Interact. 23(8):1097–1104. doi:10.1094/mpmi-23-8-1097.
- Zhou-Tsang A, Wu Y, Henderson SW, Walker AR, Borneman AR, Walker RR, Gilliham M. 2021. Grapevine salt tolerance. Aust J Grape Wine Res. 27(2):149–168. doi:10.1111/ajgw.12487.