ABSTRACT
Northern corn leaf blight (NCLB) is one of the most important foliar disease in maize, which leads to serious yield losses. It is necessary to identify resistance genes in order to control NCLB. Mitogen-activated protein kinase (MAPK) cascades play important roles in plant defense reactions. Ethylene-response factor (ERF) is involved in plant disease resistance in maize through phosphorylation by MAPK signaling pathway. Here, we found that ZmMPK6-1 positively regulates maize resistance against E. turcicum through enhancing the expression of defense-related genes and enzyme activities. Moreover, ZmMPK6-1 can interact with ZmERF061 which enhanced the transcriptional activation activity of ZmERF061. Taken together, our findings indicated that ZmMPK6-1 would act through improving ZmERF061 transcriptional activation activity to induce defensin gene expression in regulating the maize resistance against E. turcicum. These results revealed the molecular mechanism of ZmMPK6-1-ZmERF061 signaling pathway in response to E. turcicum, which is useful to maize E. turcicum resistance breeding.
Key policy highlights
ZmMPK6-1 positively regulates maize resistance against E. turcicum.
ZmMPK6-1 mutant lines decreased the expression of defense-related genes and enzyme activities.
ZmMPK6-1 can interact with ZmERF061 which enhanced the transcriptional activation activity of ZmERF061.
1. Introduction
Maize (Zea mays. L) is one of the world’s most important food crops, but maize production and quality are often affected by the epidemics of devastating pathogens (Zhou et al. Citation2018; Mueller et al. Citation2020; Mei et al. Citation2023). Northern corn leaf blight (NCLB) caused by Exserohilum turcicum (E. turcicum) is an important foliar disease and leads to serious yield losses of maize worldwide (Mueller et al. Citation2016; Galiano-Carneiro and Miedaner Citation2017). To improve maize’s resistance to E. turcicum infection, it is necessary to identify qualitative and quantitative genes, and elucidate the mechanisms and regulatory networks underlying these gene functions.
During evolution, plants have developed efficient resistant mechanisms to fight off invading pathogens (Boller and He Citation2009; Jones et al. Citation2016; Zhou and Zhang Citation2020). The plant innate immunity system can be classified into two inter-connected components, pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI), and effector-triggered immunity (ETI) (Jones and Dangl Citation2006). Generally, PTI and ETI play crucial roles in plant defense reactions, which are mediated by various signal transduction pathways (Tsuda and Katagiri Citation2010; Cui et al. Citation2015; Yuan et al. Citation2021).
Mitogen-activated protein kinase (MAPK) cascades are vital signaling modules in plant defense reactions. A MAPK cascade is composed of three sequentially phosphorylated protein kinases, including a MAP kinase kinase kinase (MAPKKK), a MAP kinase kinase (MAPKK), and a MAP kinase (MPK). Activated MPKs then positively regulate plant immunity against a series of pathogens via phosphorylation of substrates (Meng and Zhang Citation2013; Xu and Zhang Citation2015; Zhang et al. Citation2018; Sun and Zhang Citation2022; Zhang and Zhang Citation2022). Increasing evidence indicated that MPK3 and MPK6 positively regulate plant defense reactions through defense-related genes activation, camalexin accumulation, ethylene production, and indole glucosinolates biosynthesis (Ren et al. Citation2008; Li et al. Citation2012; Xu and Zhang Citation2014; Xu et al. Citation2016; Yang et al. Citation2020). Arabidopsis thaliana (At) MPK3/AtMPK6 phosphorylated and activated the rate-limiting 1-aminocyclopropane-1-carboxylic acid synthase 2 (ACS2) and ACS6 to regulate pathogen-responsive ethylene biosynthesis (Liu and Zhang Citation2004; Han et al. Citation2010). AtMPK3/AtMPK6-mediated phosphorylation of WRKY33 promoted its transactivation activity and increased camalexin production in response to Botrytis cinerea (B. cinerea) infection (Mao et al. Citation2011; Zhou et al. Citation2020).
Ethylene-response factor (ERF) is the largest family of transcription factors in species, which have been identified in various plants, including Arabidopsis, maize, rice, and soybean (Nakano et al. Citation2006; Zhang et al. Citation2009; Hao et al. Citation2020). ERFs usually positively regulate plant immune response against pathogens through binding to the GCC-box cis-element (GCCGCC), which is present in the promoter regions of defense-related genes (Fujimoto et al. Citation2020). AtERF1 and Octadecanoid-Responsive Arabidopsis 59 (ORA59) have been found to positively regulate the Arabidopsis resistance against B. cinerea through activating the expression of PLANT DEFENSIN 1.2 (PDF1.2) (Berrocal-Lobo et al. Citation2002; Pré et al. Citation2008; Zarei et al. Citation2011). Overexpression of AcERF2, VqERF112, VqERF114, or VqERF072 in Arabidopsis resulted in a significantly enhanced resistance against Pseudomonas syringae pv. tomato (Pst) DC3000 and B. cinerea (Sun et al. Citation2018; Wang et al. Citation2020). Transgenic Arabidopsis overexpression of AtERF11 resulted in enhanced Pst DC3000 resistance (Zheng et al. Citation2019).
Previous studies have shown that several ERF transcription factors play important roles in resistance against pathogens through phosphorylation by AtMPK3/AtMPK6 cascade (Huang et al. Citation2016). AtMPK3/AtMPK6 is involved in regulating Arabidopsis defense against B. cinerea through phosphorylating AtERF6, which enhanced AtERF6 protein stability (Meng et al. Citation2013). Phosphorylation of AtERF72 by AtMPK3/AtMPK6 positively regulated plant resistance to B.cinerea through improving its transactivation activity and activating the transcription of camalexin biosynthesis enzymes (Li et al. Citation2022). MPK3 and MPK6 phosphorylated AtERF1A and promoted its transactivation activity, leading to enhanced resistance to B. cinerea (Wang et al. Citation2022). In soybean, GmMKK4-GmMPK6-mediated phosphorylation of GmERF113 enhanced the protein stability and transcriptional activity of GmERF113, which significantly enhanced soybean resistance to Phytophthora sojae (P.sojae) (Gao et al. Citation2022).
In our previous study, we found that ZmERF061 positively regulates resistance of maize plants against E. turcicum through activating the expression of defense-related genes (Zang et al. Citation2021). Furthermore, we reported that ZmERF061 physically interacts with ZmMPK6-1 using yeast two-hybrid and BiFC assay (Zang et al. Citation2021). However, the function and regulatory mechanism of ZmMPK6-1-ZmERF061 in maize defense reactions are still not well characterized. Here, we further identified that ZmERF061 physically interacts with ZmMPK6-1 using Co-Immunoprecipitation (Co-IP) assay. Meanwhile, ZmMPK6-1 positively regulated maize resistance against E. turcicum. Moreover, ZmMPK6-1 enhanced the transcriptional activation activity of ZmERF061. Taken together, our data in this study revealed that ZmMPK6-1 positively regulates maize resistance to E. turcicum through enhancing ZmERF061 activity. These results revealed the molecular mechanism of ZmMPK6-1-ZmERF061 mediated E. turcicum resistance pathway in maize, which is helpful for enriching the theory of maize E. turcicum resistance.
2. Materials and methods
2.1. Plant materials and treatments
E.turcicum is collected in Jilin province, isolated and stored by Maize Breeding Team in Jilin Agricultural University. The seeds of the maize inbred lines W22 were obtained from Maize Breeding Team in Jilin Agricultural University. ZmMPK6-1 mutants (UFMu-10707, UFMu-07533) were obtained from the Maize Genetics Cooperation Stock Center, which was inserted Mu transposon into W22 genome. The homozygous mutant lines were obtained from self-fertilizing and identified by quantitative real-time polymerase chain reaction (qRT-PCR) and PCR. E. turcicum inoculation was performed as described previously (Zang et al. Citation2021) with minor modification. The maize seeds were grown in a chamber at 25°C with a 16/8-h photoperiod and 70% relative humidity. Three-leaf-stage maize seedlings were inoculated with three drops of conidial suspensions (1 × 105 conidia/ml) and sterile water, respectively. After inoculation 24 h, the leaves were collected and stored at −80°C for qRT-PCR assay.
2.2. Gene expression quantified by qRT-PCR
Total RNAs were extracted using Trizol Reagent (Invitrogen, USA) according to the manufacturer’s instructions. Total RNA (1 µg) was used for cDNA synthesis with the Transcription First Strand cDNA Synthesis kit (TOYOBO, Japan). qRT-PCR was performed using 2 RealStar SYBR Mixture (Genstar, China) on an QuantStudio Q3 system. The experiment was performed with three biological replicates. ZmTub (GRMZM2G066191) was used as the internal control. The relative expression levels were calculated by the 2−ΔΔCt method. The primers are listed in Supplementary Table S1.
2.3. Co-IP Assay
The coding sequence of ZmERF061 was cloned into the Myc tag and ZmMPK6-1 was cloned into the FLAG tag to generate 35S: ZmERF061-Myc and 35S: ZmMPK6-1-FLAG. ZmERF061-Myc and ZmMPK6-1-FLAG were co-transformed into N. benthamiana via Agrobacterium-mediated method. Total proteins were extracted with Co-IP buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM DTT, 1 mM PMSF, 2 mM EDTA, 0.1% Triton X-100, and 1× protease inhibitor cocktail), and then incubated with Anti-FLAG M2 Affinity Gel overnight at 4°C. After washing, separation and blotting, the immunoprecipitated proteins were immunoblotted with anti-Myc antibody (Sigma) or FLAG antibody (Sigma), respectively. The primers are listed in Supplementary Table S1.
2.4. Dual-luciferase transient transcriptional activity assay
Transient dual-luciferase (LUC) assay was performed with N. benthamiana leaves as described previously by Liu et al. (Citation2010). For the reporter, approximately 940 bp promoter region of ZmPR10.2 was cloned into the SalI and HindIII sites of the pGreenII0800 vector to generate pro: ZmPR10.2-LUC. For the effectors, the coding sequence of ZmERF061 and ZmMPK6-1 were fused into pGreenII 62-SK vector driven by the 35S promoter, respectively. The empty vector was used as a negative control. The luciferase activity was detected by a luminometer (Promega Glomax 2020) using Dual-luciferase Reporter Assay System (Promega, USA) with three biological replicates. The primers are listed in Supplementary Table S1.
2.5. Yeast one-hybrid assay
Yeast one-hybrid assay was performed as described previously by Zang et al. (Citation2021). The full-length coding sequence of ZmERF061 and approximately 940 bp promoter region of ZmPR10.2 were cloned into the pGADT7 vector and pAbAi vector, respectively. The pGADT7-ZmERF061 plasmid was co-transformed with pAbAi-pro: ZmPR10.2 plasmid into Y1H Gold yeast strain, which was grown on SD/-Leu/-Ura medium with 200 ng/ml or 300 ng/ml AbA. pGAD-rec-53 + pAbAi-p53 and pGADT7 + pAbAi are positive and negative controls, respectively. The primers are listed in Supplementary Table S1.
2.6. Assessment of plant disease responses
Artificial inoculation procedures were performed as described previously by Zang et al. (Citation2020). The living ear leaves of zmmpk6-1-m1 and zmmpk6-1-m2 mutant lines were inoculated with E. turcicum, and then the inoculated leaves were pictured at 8 days post-inoculation (dpi) with a Nikon D7000 camera. The relative lesion area was measured using the Photoshop CS3 software according to the previously described method by Cui et al. (Citation2009).
2.7. Measurements of SOD and POD activities
To further explore the role of ZmMPK6-1 in response to E. turcicum, we detected the activities of superoxide dismutase (SOD) and peroxide dismutase (POD). For the enzyme activity assays, the fresh leaves (about 0.2 g) were collected at 24 h after infection with E. turcicum conidial suspension and sterile water, respectively. The SOD and POD activities were analyzed as previously described by Li et al. (Citation2015).
2.8. Statistical analysis
The statistical experiments were performed by three biological replicates. All data analyses were performed using the GraphPad Prism 9.0 software. The significant differences between samples were analyzed using Student’s t-tests and two-way ANOVA followed by Tukey’s post hoc test (*P < 0.05, **P < 0.01). Bars indicate standard error of the mean.
3. Results
3.1. ZmERF061 interacts with ZmMPK6-1
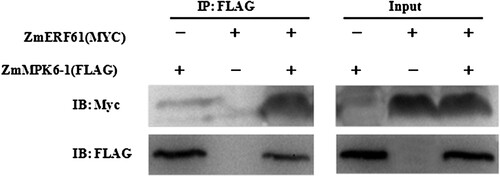
Previous studies demonstrated that ZmERF061 plays a positively role in response to E. turcicum inoculation (Zang et al. Citation2021). Moreover, yeast two-hybrid and bimolecular fluorescence complementation (BiFC) data revealed that ZmERF061 interacts with ZmMPK6-1 (Zang et al. Citation2021). In this study, a Co-IP assay showed that ZmERF061-Myc is successfully detected in the anti-FLAG immunoprecipitates of N. benthamiana leaves co-expressed ZmERF061-Myc and ZmMPK6-1-FLAG (). These results demonstrate that ZmERF061 physically interacts with ZmMPK6-1 in vitro and in vivo.
Figure 1. ZmMPK6-1 can interact with ZmERF061 by Co-IP assay. ZmERF061-Myc was co-expressed with ZmMPK6-1-FLAG in N. benthamiana. The proteins immunoprecipitated from leaf extracts using Anti-FLAG M2 Affinity Gel (IP: FLAG) were analyzed by immunoblotting with anti-Myc (IB: Myc) or anti-FLAG antibody (IB: FLAG). The protein inputs were shown by immunoblotting.
3.2. ZmMPK6-1 positively regulates the resistance to E. turcicum
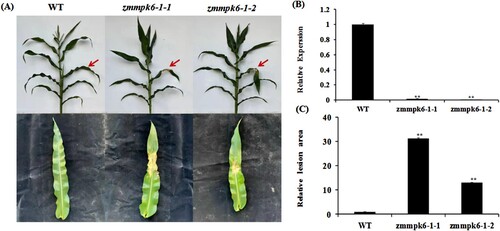
We obtained zmmpk6-1 mutant lines from the Maize Genetics Cooperation Stock Center, named zmmpk6-1-1 and zmmpk6-1-2. PCR and qRT-PCR demonstrated that the expression levels of T4 homozygous zmmpk6-1-1 and zmmpk6-1-2 mutant lines are significantly low in mutant lines ((B) and Figure S1). At 8 days dpi, zmmpk6-1-1 and zmmpk6-1-2 mutant lines exhibited compromised resistance ((A, C)). To further identify the resistance function of ZmMPK6-1, the inoculation identification was performed in the field. As shown in Figure S2, the zmmpk6-1-1 and zmmpk6-1-2 mutant lines decreased the resistance to E. turcicum. These results indicated that ZmMPK6-1 plays a positive role in regulating maize resistance against E. turcicum.
Figure 2. zmmpk6-1 mutant lines exhibited compromised resistance after infection with E. turcicum. (A) Disease symptom was pictured at 8 dpi. (B) Relative expression of ZmMPK6-1 in zmmpk6-1 mutant lines. (C) The relative lesion areas of ZmMPK6-1 mutant lines at 8 dpi. The experiment was performed by three biological replicates and analyzed using Student’s t-tests (**P < 0.01). Bars indicate standard error of the mean.
3.3. ZmMPK6-1 regulates the expression of defense-related genes
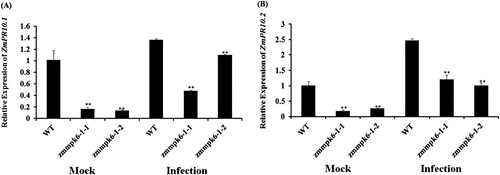
The expression patterns of ZmPR10.1 (GRMZM2G112488) and ZmPR10.2 (GRMZM2G112538) were detected in ZmMPK6-1 mutant lines at 24 h after inoculation with E. turcicum (Infection) and sterile water (Mock). As shown in , the transcripts of ZmPR10.1 and ZmPR10.2 in ZmMPK6-1 mutant lines were remarkably lower than WT plants under mock condition and E. turcicum infection (). These results showed that ZmMPK6-1 participates in the maize resistance against E. turcicum through regulating defense-related genes.
Figure 3. The expression levels of defense genes were decreased in zmmpk6-1 mutant lines after infection with E. turcicum at 24 h. (A) Expression analysis of ZmPR10.1. (B) Expression analysis of ZmPR10.2. The plants were treated with sterile water served as control. The mock-treated WT sample was set to unity. The relative expression levels of genes were analyzed using the 2−ΔΔCT method. The experiment was performed using three biological replicates and analyzed using two-way ANOVA followed by Tukey’s post hoc test (**P < 0.01). Bars indicate standard error of the mean.
3.4. ZmMPK6-1 mutant lines reduce the activities of superoxide dismutase (SOD) and peroxide dismutase (POD) after infection with E. turcicum
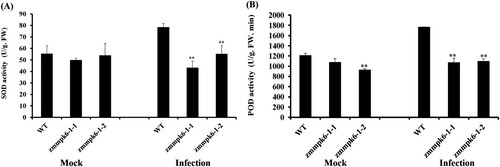
As shown in (A), the activity of SOD in ZmMPK6-1 mutant lines have almost no significant change under mock condition, whereas in ZmMPK6-1 mutant lines the activity of SOD was significantly low after infection with E. turcicum. The activity of POD was significantly low under E. turcicum infection ((B)). These results demonstrated that ZmMPK6-1 is involved in enhancing the activities of SOD and POD in response to E. turcicum infection.
Figure 4. The activities of antioxidant enzymes were decreased in zmmpk6-1 mutant lines after infection with E. turcicum at 24 h. (A) The activities of SOD. (B) The activities of POD. The plants were treated with sterile water served as control. The experiment was performed using three independent biological replicates and analyzed using two-way ANOVA followed by Tukey’s post hoc test (**P < 0.01). Bars indicate standard error of the mean.
3.5. ZmMPK6-1 enhances the transactivation activity of ZmERF061
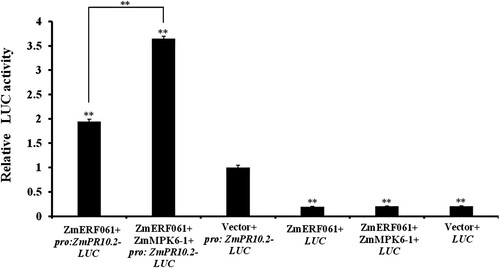
A transient LUC assay in N. benthamiana leaves found that ZmMPK6-1 co-expressed with ZmERF061 shows a significant increase in ZmPR10.2 promoter activity, leading to approximately 3.6-fold increase in the promoter activity of ZmPR10.2 compared to the control (). A yeast one-hybrid assay showed that the yeast cells transfected with pGADT7-ZmERF061 and pAbAi-ZmPR10.2 promoter can grow on SD/-Leu/-Ura medium containing 200 ng/ml or 300 ng/ml of AbA, respectively. It suggested that ZmERF061 specifically binds to the ZmPR10.2 promoter (Figure S3). These results suggest that ZmMPK6-1 co-expressed with ZmERF061 significantly enhanced the transactivation activity of ZmERF061 to ZmPR10.2 promoter.
Figure 5. ZmMPK6-1 enhances the transactivation activity of ZmERF061. The relative LUC activity was normalized to the reference Renilla (REN) luciferase. The empty vector sample was set to unity. The experiment was performed by three biological replicates and analyzed using two-way ANOVA followed by Tukey’s post hoc test (**P < 0.01). Bars indicate standard error of the mean.
4. Discussion
NCBL is one of the most devastating diseases of maize worldwide, which leads to serious losses to corn production (Raymundo and Hooker Citation1981). Breeding resistant varieties are the most effective method to control NCBL. The MAPK cascades as important signal transduction pathways play crucial roles in response to pathogen infection (Zhang and Zhang Citation2022). In this study, we demonstrated that ZmMPK6-1 is involved in mediating maize resistance against E. turcicum by inducing the expression of PR genes and increasing the activities of SOD and POD (). In addition, ZmMPK6-1 can interact with ZmERF061 and enhance ZmERF061 transcriptional activation ( and ). These results are useful for future genetic breeding to improve maize E. turcicum resistance.
Increasing evidence suggested that MPK3 and MPK6 positively participate in regulating plant defense reactions. Silencing of MPK6 in Arabidopsis resulted in reduced disease resistance against Pst DC3000 (Menke et al. Citation2004). MPK3 and MPK6 have been found to positively regulate Arabidopsis resistance against B. cinerea (Han et al. Citation2010). Overexpression of GmMPK6 in soybean led to enhanced resistance to P.sojae (Gao et al. Citation2022). In rice, OsMPK6 has been shown to involve in mediating resistance to X. oryzae pv. Oryzicola (Ma et al. Citation2021) and Magnaporthe oryzae (Ueno et al. Citation2013). In addition, a negative role of MPK6 has been reported in several studies. WIPK and SIPK negatively regulated the plant defense against TMV (Kobayashi et al. Citation2010). Silencing of GmMPK6 in soybean conferred to enhanced resistance against SMV (Liu et al. Citation2014). These results indicated that MPK6 homologs may function differently in response to various pathogens in different plants. Our previous study found that ZmERF061 participates in maize resistance against E. turcicum and can interact with ZmMPK6-1 (Zang et al. Citation2021). However, the function of ZmMPK6-1 in E. turcicum – resistance in maize remains unclear. In this study, we showed that ZmMPK6-1 positively regulates maize resistance to E. turcicum (). These results indicate that ZmMPK6-1 may function as a positive regulator in maize defense reactions.
In the maize–E. turcicum interaction, qualitative resistance usually results in a high level of resistance which is governed by Ht genes. Ht genes display diverse resistance phenotypes based on encoding for different resistance mechanisms (Navarro et al. Citation2020). Some Ht genes can quickly get ineffective when new physiological races appear. Quantitative resistance is an effective method to control NCLB, which can combat multiple races of the pathogen. Thus, it is necessary to identify quantitative genes in order to breed more resistant varieties. In this study, we found that ZmMPK6-1 mutant lines decrease resistance against E. turcicum which is prevalent in Jilin Province (). Considering the yield loss caused by NCLB, the results will be valuable in future resistance breeding programs, however the underlying mechanism of ZmMPK6-1-mediatd quantitative resistance needs to be further investigated. In addition, an increasing number of evidence has showed that ZmMPK6-1 participates in drought-stress and regulating yield-related traits. Wu et al. (Citation2021) found that ZmMPK6-1 may respond to drought-stress through modulating brassinosteroid biosynthesis (Wu et al. Citation2021). Lu et al. (Citation2022) demonstrated that ZmPP2C26 alternative splicing variants negatively regulate drought tolerance in maize through dephosphorylating ZmMAPK3 and ZmMAPK7 (ZmMPK6-1) (Lu et al. Citation2022). Li et al. (Citation2023) showed that ZmMPK6-1 is associated with yield-related traits (Li et al. Citation2023). How ZmMPK6-1 plays different functions in maize needs to further study. Reactive oxygen species (ROS) signaling is an important signaling molecule in plant innate immune (Castro et al. Citation2021). Upon pathogen attack, sustained ROS production can activate the immune system to cope with pathogen invasion. However, overaccumulation of ROS has profound effects on the plant growth and development (Camejo et al. Citation2016). Plant antioxidant system has been developed to maintain the homeostasis of ROS. SOD and POD are the major antioxidant enzymes in plants (Wan et al. Citation2007). Zhao et al. (Citation2023) showed that overexpression of GmWAK1 in soybean enhances the resistance to P. sojae through enhancing the activities of SOD and POD (Zhao et al. Citation2023). ZmERF061 overexpressing transgenic maize plants led to enhanced resistance to E. turcicum via activating SOD and POD (Zang et al. Citation2021). In this study, we found that ZmMPK6-1 mutant lines decreased the activities of SOD and POD after infection with E. turcicum (). Thus, ZmMPK6-1 may positively regulate disease resistance through modulating the activities of SOD and POD. Due to the complicated ROS network, the molecular mechanism by how ZmMPK6-1 regulates antioxidant enzymes activities in response to E. turcicum is required to further investigate.
An increasing number of studies have shown that MPK3/MPK6 cascade positively modulates plant defense against pathogens through phosphorylating various downstream substrates and activating the expression of defense-related genes (Zhang and Zhang Citation2022). MPK3/MPK6-mediated phosphorylation of substrates led to enhanced transactivation activity and protein stability. The transcription factor WRKY33 is phosphorylated by MPK3/MPK6, which enhances its transactivation activity and induces camalexin production through activating the expression of PAD3 and CYP71A13 (Mao et al. Citation2011; Zhou et al. Citation2020). Several ERF transcription factors, such as ERF1, ERF1A, ERF6, ERF72, and ERF104, have been reported to be phosphorylated by MPK3/MPK6 in regulating Arabidopsis immune responses (Bethke et al. Citation2009; Meng et al. Citation2013; Li et al. Citation2022; Wang et al. Citation2022; Zhou et al. Citation2022). Therefore, MPK3/MPK6 may phosphorylate different ERF proteins to precisely regulate a diverse of immune responses. Here, we demonstrated that ZmMPK6-1 is involved in regulating the resistance against E. turcicum through activating the expression of ZmPR10.2 (). Moreover, ZmMPK6-1 can interact with ZmERF061, and ZmMPK6-1 co-expressed with ZmERF061 significantly enhanced the transactivation activity of ZmERF061 to the promoter of ZmPR10.2 ( and , S3). ZmPR10.2 plays a positive role in resistance against pathogen infection. ZmPR10.2 is significantly induced after infection with Fusarium verticillioides Aspergillus flavus (Liu et al. Citation2021; Tran et al. Citation2021). ZmPR10.2 RNAi-silenced maize plants resulted in decreased resistance against Aspergillus flavus (Chen et al. Citation2010). Taken together, our results indicated that ZmMPK6-1 may activate ZmERF061 to induce ZmPR10.2 expression in response to E. turcicum infection. ZmMPK6-1-ZmERF061 signaling pathway may play a crucial role in resistance against E. turcicum, which provide a theoretical basis for breeding disease resistance maize.
5. Conclusion
In this study, we found that ZmMPK6-1 plays a crucial role in resistance against E. turcicum. ZmMPK6-1 mutant lines decreased the expression of defense-related genes and the activities of SOD and POD after infection with E. turcicum. Moreover, ZmMPK6-1 can interact with ZmERF061 and enhanced the transcriptional activation activity of ZmERF061. Taken together, our findings indicated that ZmMPK6-1 would act through improving ZmERF061 transcriptional activation activity to enhance the maize immunity. These results revealed the molecular mechanism of ZmMPK6-1-ZmERF061 mediated defense signaling pathway, which is helpful for maize E. turcicum resistance breeding.
Supplemental Material
Download MS Word (9.5 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Acknowledgments
We thank the Maize Genetics Cooperation Stock Center for the maize mutants. Author contributions: Conceived and designed the experiments: Z. Y. Z. and L. Y. J. Performed the experiments and drafted the manuscript: L. Y. J.; M. R. L.; X. Y. L. Analyzed the data: Z. X. Z. Contributed reagents/materials/analysis tools: Z. Z. Y. and L. Y. J.
Additional information
Funding
Notes on contributors
Liangyu Jiang
Liangyu Jiang, Doctor degree, College of Agriculture, Jilin Agricultural University. His research focused on the maize genetics and breeding.
Mingrui Li
Mingrui Li, Graduate student, College of Agriculture, Jilin Agricultural University. Her research focused on the maize genetics and breeding.
Xiaoyue Liu
Xiaoyue Liu, Graduate student, College of Agriculture, Jilin Agricultural University. Her research focused on the maize genetics and breeding.
Zixin Zhang
Zixin Zhang, Graduate student, College of Agriculture, Jilin Agricultural University. His research focused on the maize genetics and breeding.
Zhenyuan Zang
Zhenyuan Zang, Doctor degree, College of Agriculture, Jilin Agricultural University. Her research focused on the genetics and breeding in maize and soybean.
References
- Berrocal-Lobo M, Molina A, Solano R. 2002. Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J. 29:23–32. doi:10.1046/j.1365-313x.2002.01191.x.
- Bethke G, Unthan T, Uhrig JF, Pöschl Y, Gust AA, Scheel D, Lee J. 2009. Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis thaliana via ethylene signaling. Proc Natl Acad Sci USA. 106:8067–8072. doi:10.1073/pnas.0810206106.
- Boller T, He SY. 2009. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science. 324:742–744. doi:10.1126/science.1171647.
- Camejo D, Guzmán-Cedeño Á, Moreno A. 2016. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol Biochem. 103:10–23. doi:10.1016/j.plaphy.2016.02.035.
- Castro B, Citterico M, Kimura S, Stevens DM, Wrzaczek M, Coaker G. 2021. Stress-induced reactive oxygen species compartmentalization, perception, and signalling. Nat Plants. 7:403–412. doi:10.1038/s41477-021-00887-0.
- Chen ZY, Brown RL, Damann KE, Cleveland TE. 2010. PR10 expression in maize and its effect on host resistance against Aspergillus flavus infection and aflatoxin production. Mol Plant Pathol. 11:69–81. doi:10.1111/j.1364-3703.2009.00574.x.
- Cui H, Tsuda K, Parker JE. 2015. Effector-triggered immunity: from pathogen perception to robust defense. Annu Rev Plant Biol. 66:487–511. doi:10.1146/annurev-arplant-050213-040012.
- Cui HW, Yang YL, Li JT, Luo WF, Miao AM, Hu ZX, Han XN. 2009. A faster method for measuring relative lesion area on leaves based on software photoshop. J Anhui Agric Sci. 37:10760–10762.
- Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M. 2020. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell. 12:393–404.
- Galiano-Carneiro AL, Miedaner T. 2017. Genetics of resistance and pathogenicity in the maize/Setosphaeria turcica Pathosystem and implications for breeding. Front Plant Sci. 8:1490. doi:10.3389/fpls.2017.01490.
- Gao H, Jiang L, Du B, Ning B, Ding X, Zhang C, Song B, Liu S, Zhao M, Zhao Y, et al. 2022. GmMKK4-activated GmMPK6 stimulates GmERF113 to trigger resistance to Phytophthora sojae in soybean. Plant J. 111:473–495. doi:10.1111/tpj.15809.
- Han L, Li GJ, Yang KY, Mao G, Wang R, Liu Y, Zhang S. 2010. Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea-induced ethylene production in Arabidopsis. Plant J. 64:114–127.
- Hao LD, Shi SB, Guo HB, Li M, Hu P, Wei YD, Feng YF. 2020. Genome-wide identification and expression profiles of ERF subfamily transcription factors in Zea mays. Peer J. 8:e9551. doi:10.7717/peerj.9551.
- Huang PY, Catinot J, Zimmerli L. 2016. Ethylene response factors in Arabidopsis immunity. J. Exp. Bot. 67:1231–1241. doi:10.1093/jxb/erv518.
- Jones JD, Dangl JL. 2006. The plant immune system. Nature. 444:323–329. doi:10.1038/nature05286.
- Jones JD, Vance RE, Dangl JL. 2016. Intracellular innate immune surveillance devices in plants and animals. Science. 354:aaf6395. doi:10.1126/science.aaf6395.
- Kobayashi M, Seo S, Hirai K, Yamamoto-Katou A, Katou S, Seto H, Meshi T, Mitsuhara I, Ohashi Y. 2010. Silencing of WIPK and SIPK mitogen-activated protein kinases reduces tobacco mosaic virus accumulation but permits systemic viral movement in tobacco possessing the N resistance gene. Mol Plant Microbe Interact. 23:1032–1041. doi:10.1094/MPMI-23-8-1032.
- Li G, Meng X, Wang R, Mao G, Han L, Liu Y, Zhang S. 2012. Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genet. 8:e1002767. doi:10.1371/journal.pgen.1002767.
- Li Y, Liu K, Tong G, Xi C, Liu J, Zhao H, Wang Y, Ren D, Han S. 2022. MPK3/MPK6-mediated phosphorylation of ERF72 positively regulates resistance to Botrytis cinerea through directly and indirectly activating the transcription of camalexin biosynthesis enzymes. J Exp Bot. 73:413e428.
- Li Z, Li C, Zhang R, Duan M, Tian H, Yi H, Xu L, Wang F, Shi Z, Wang X, et al. 2023. Genomic analysis of a new heterotic maize group reveals key loci for pedigree breeding. Front Plant Sci. 14:12136757.
- Li ZJ, Zhu B, Wang B, Gao JJ, Fu XY, Yao QH. 2015. Stress responses to trichlorophenol in Arabidopsis and integrative analysis of alteration in transcriptional profiling from microarray. Gene. 555:159–168. doi:10.1016/j.gene.2014.10.059.
- Liu H, Wu H, Wang Y, Wang H, Chen S, Yin Z. 2021. Comparative transcriptome profiling and co-expression network analysis uncover the key genes associated with early-stage resistance to Aspergillus flavus in maize. BMC Plant Biol. 21:216. doi:10.1186/s12870-021-02983-x.
- Liu JZ, Braun E, Qiu WL, Shi YF, Marcelino-Guimarães FC, Navarre D, Hill JH, Whitham SA. 2014. Positive and negative roles for soybean MPK6 in regulating defense responses. Mol Plant Microbe Interact. 27:824–834. doi:10.1094/MPMI-11-13-0350-R.
- Liu L, Zhang Y, Tang S, Zhao Q, Zhang Z, Zhang H, Dong L, Guo H, Xie Q. 2010. An efficient system to detect protein ubiquitination by agroinfiltration in Nicotiana benthamiana. Plant J. 61:893–903. doi:10.1111/j.1365-313X.2009.04109.x.
- Liu Y, Zhang S. 2004. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell. 16:3386–3399. doi:10.1105/tpc.104.026609.
- Lu F, Li W, Peng Y, Cao Y, Qu J, Sun F, Yang Q, Lu Y, Zhang X, Zheng L, et al. 2022. ZmPP2C26 alternative splicing variants negatively regulate drought tolerance in maize. Front Plant Sci. 13:851531. doi:10.3389/fpls.2022.851531.
- Ma H, Li J, Ma L, Wang P, Xue Y, Yin P, Xiao J, Wang S. 2021. Pathogen-inducible OsMPKK10.2-OsMPK6 cascade phosphorylates the Raf-like kinase OsEDR1 and inhibits its scaffold function to promote rice disease resistance. Mol Plant. 14:620–632. doi:10.1016/j.molp.2021.01.008.
- Mao G, Meng X, Liu Y, Zheng Z, Chen Z, Zhang S. 2011. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell. 23:1639–1653. doi:10.1105/tpc.111.084996.
- Mei J, Zhou S, Liu W. 2023. Gene-for-gene-mediated resistance to southern corn rust in maize. Trends Plant Sci. 28:255–258. doi:10.1016/j.tplants.2022.12.002.
- Meng X, Zhang S. 2013. MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol. 51:245–266. doi:10.1146/annurev-phyto-082712-102314.
- Meng XZ, Xu J, He YX, Yang KY, Mordorski B, Liu YD, Zhang SQ. 2013. Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell. 25:1126–1142. doi:10.1105/tpc.112.109074.
- Menke FL, van Pelt JA, Pieterse CM, Klessig DF. 2004. Silencing of the mitogen-activated protein kinase MPK6 compromises disease resistance in Arabidopsis. Plant Cell. 16:897–907. doi:10.1105/tpc.015552.
- Mueller DS, Wise A, Sisson AJ, Allen TW, Bergstrom GC, Bissonnette KM, Bradley CA, Byamukama E, Chilvers MI, Collins AA, et al. 2020. Corn yield loss estimates due to diseases in the United States and Ontario, Canada, from 2016 to 2019. Plant Health Prog. 21:238–247. doi:10.1094/PHP-05-20-0038-RS.
- Mueller DS, Wise KA, Sisson AJ, Allen TW, Bergstrom GC, Bosley DB, Bradley CA, Broders KD, Byamukama E, Chilvers MI, et al. 2016. Corn yield loss estimates due to diseases in the United States and Ontario, Canada from 2012 to 2015. Plant Heath Prog. 17:211–222. doi:10.1094/PHP-RS-16-0030.
- Nakano T, Suzuki K, Fujimura T, Shinshi H. 2006. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 140:411–432. doi:10.1104/pp.105.073783.
- Navarro BL, Hanekamp H, Koopmann B, von Tiedemann A. 2020. Diversity of expression types of Ht genes conferring resistance in Maize to Exserohilum turcicum. Front Plant Sci. 11:607850. doi:10.3389/fpls.2020.607850.
- Pré M, Atallah M, Champion A, De Vos M, Pieterse CM, Memelink J. 2008. The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol. 147:1347–1357. doi:10.1104/pp.108.117523.
- Raymundo AD, Hooker AL. 1981. Measuring the relationship between northern corn leaf blight and yield losses. Plant Dis. 65:325–327. doi:10.1094/PD-65-325.
- Ren D, Liu Y, Yang KY, Han L, Mao G, Glazebrook J, Zhang SA. 2008. A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA. 105:5638–5643. doi:10.1073/pnas.0711301105.
- Sun T, Zhang Y. 2022. MAP kinase cascades in plant development and immune signaling. EMBO Rep. 23:e53817. doi:10.15252/embr.202153817.
- Sun XH, Yu G, Li JT, Liu JL, Wang XL, Zhu GL, Zhang XH, Pan HY. 2018. AcERF2, an ethylene-responsive factor of Atriplex canescens, positively modulates osmotic and disease resistance in Arabidopsis thaliana. Plant Sci. 274:32–43. doi:10.1016/j.plantsci.2018.05.004.
- Tran TM, Ameye M, Devlieghere F, De Saeger S, Eeckhout M, Audenaert K. 2021. Streptomyces strains promote plant growth and induce resistance against Fusarium verticillioides via transient regulation of auxin signaling and archetypal defense pathways in maize plants. Front Plant Sci. 12:755733. doi:10.3389/fpls.2021.755733.
- Tsuda K, Katagiri F. 2010. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr Opin Plant Biol. 13:459–465. doi:10.1016/j.pbi.2010.04.006.
- Ueno Y, Yoshida R, Kishi-Kaboshi M, Matsushita A, Jiang CJ, Goto S, Takahashi A, Hirochika H, Takatsuji H. 2013. MAP kinases phosphorylate rice WRKY45. Plant Signal Behav. 8:e24510. doi:10.4161/psb.24510.
- Wan C, Li S, Wen L, Kong J, Wang K, Zhu Y. 2007. Damage of oxidative stress on mitochondria during microspores development in Honglian CMS line of rice. Plant Cell Rep. 26:373–382. doi:10.1007/s00299-006-0234-2.
- Wang L, Liu WD, Wang YJ. 2020. Heterologous expression of Chinese wild grapevine VqERFs in Arabidopsis thaliana enhance resistance to Pseudomonas syringae pv. tomato DC3000 and to Botrytis cinerea. Plant Sci. 293:110421. doi:10.1016/j.plantsci.2020.110421.
- Wang X, Meng H, Tang Y, Zhang Y, He Y, Zhou J, Meng X. 2022. Phosphorylation of an ethylene response factor by MPK3/MPK6 mediates negative feedback regulation of pathogen-induced ethylene biosynthesis in Arabidopsis. J Genet Genomics. 49:810–822. doi:10.1016/j.jgg.2022.04.012.
- Wu X, Feng H, Wu D, Yan S, Zhang P, Wang W, Zhang J, Ye J, Dai G, Fan Y, et al. 2021. Using high-throughput multiple optical phenotyping to decipher the genetic architecture of maize drought tolerance. Genome Biol. 22:185. doi:10.1186/s13059-021-02377-0.
- Xu J, Meng J, Meng X, Zhao Y, Liu J, Sun T, Liu Y, Wang Q, Zhang S. 2016. Pathogen-responsive MPK3 and MPK6 reprogram the biosynthesis of indole glucosinolates and their derivatives in Arabidopsis immunity. Plant Cell. 28:1144–1162. doi:10.1105/tpc.15.00871.
- Xu J, Zhang S. 2014. Regulation of ethylene biosynthesis and signaling by protein kinases and phosphatases. Mol Plant. 7:939e942.
- Xu J, Zhang S. 2015. Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 20:56–64. doi:10.1016/j.tplants.2014.10.001.
- Yang L, Zhang Y, Guan R, Li S, Xu X, Zhang S, Xu J. 2020. Co-regulation of indole glucosinolates and camalexin biosynthesis by CPK5/CPK6 and MPK3/MPK6 signaling pathways. J Integr Plant Biol. 62:1780–1796. doi:10.1111/jipb.12973.
- Yuan M, Ngou BPM, Ding P, Xin XF. 2021. PTI-ETI crosstalk: an integrative view of plant immunity. Curr Opin Plant Biol. 62:102030. doi:10.1016/j.pbi.2021.102030.
- Zang Z, Lv Y, Liu S, Yang W, Ci J, Ren X, Wang Z, Wu H, Ma W, Jiang L, et al. 2020. A novel ERF transcription factor, ZmERF105, positively regulates maize resistance to Exserohilum turcicum. Front Plant Sci. 11:850. doi:10.3389/fpls.2020.00850.
- Zang Z, Wang Z, Zhao F, Yang W, Ci J, Ren X, Jiang L, Yang W. 2021. Maize ethylene response factor ZmERF061 is required for resistance to Exserohilum turcicum. Front Plant Sci. 12:630413. doi:10.3389/fpls.2021.630413.
- Zarei A, Körbes AP, Younessi P, Montiel G, Champion A, Memelink J. 2011. Two GCC boxes and AP2/ERF-domain transcription factor ORA59 in jasmonate/ethylene-mediated activation of the PDF1.2 promoter in Arabidopsis. Plant Mol Biol. 75:321–331. doi:10.1007/s11103-010-9728-y.
- Zhang GY, Chen M, Li LC, Xu ZS, Chen XP, Guo JM, Ma YZ. 2009. Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J Exp Bot. 60:3781–3796. doi:10.1093/jxb/erp214.
- Zhang M, Su J, Zhang Y, Xu J, Zhang S. 2018. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr Opin Plant Biol. 45:1–10. doi:10.1016/j.pbi.2018.04.012.
- Zhang M, Zhang S. 2022. Mitogen-activated protein kinase cascades in plant signaling. J Integr Plant Biol. 64:301–341. doi:10.1111/jipb.13215.
- Zhao M, Li N, Chen S, Wu J, He S, Zhao Y, Wang X, Chen X, Zhang C, Fang X, et al. 2023. GmWAK1, novel wall-associated protein kinase, positively regulates response of Soybean to Phytophthora sojae infection. Int J Mol Sci. 24:798. doi:10.3390/ijms24010798.
- Zheng X, Xing JH, Zhang K, Pang X, Zhao YT, Wang GY, Zang JP, Huang RF, Dong JG. 2019. Ethylene response factor ERF11 activates BT4 transcription to regulate immunity to Pseudomonas syringae. Plant Physiol. 180:1132–1151. doi:10.1104/pp.18.01209.
- Zhou DN, Wang XM, Chen GK, Sun SL, Yang Y, Zhu ZD, Duan CX. 2018. The major Fusarium species causing maize ear and kernel rot and their toxigenicity in Chongqing, China. Toxins. 10:90. doi:10.3390/toxins10020090.
- Zhou J, Mu Q, Wang X, Zhang J, Yu H, Huang T, He Y, Dai S, Meng X, et al. 2022. Multilayered synergistic regulation of phytoalexin biosynthesis by ethylene, jasmonate, and MAPK signaling pathways in Arabidopsis. Plant Cell. 34:3066–3087. doi:10.1093/plcell/koac139.
- Zhou J, Wang X, He Y, Sang T, Wang P, Dai S, Zhang S, Meng X. 2020. Differential phosphorylation of the transcription factor WRKY33 by the protein kinases CPK5/CPK6 and MPK3/MPK6 cooperatively regulates camalexin biosynthesis in Arabidopsis. Plant Cell. 32:2621–2638. doi:10.1105/tpc.19.00971.
- Zhou JM, Zhang Y. 2020. Plant immunity: danger perception and signaling. Cell. 181:978–989. doi:10.1016/j.cell.2020.04.028.
