 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In recent years, anthropogenic activities and climate change have significantly increased exposure of plants to environmental stresses (single or multiple) and pollutants, which negatively affect plant growth, survival, and productivity. Plants may activate an armament of defenses against such environmental stresses. Isoprene, the most abundant biogenic volatile organic compound emitted by plants, is supposed to induce stress tolerance directly, by quenching reactive oxygen species, or indirectly by strengthening photosynthetic membranes and reprogramming expression of genes that are involved in antioxidant defense mechanisms. On the other hand, isoprene is also involved in tropospheric chemistry that contributes to the production of air pollutants when mixing with anthropogenic gases. In this review, we summarized current knowledge about the impact of air and soil pollutants on isoprene emission from plants, focusing on possible feedback and feedforward mechanisms that may affect whole ecosystem functioning and evolution of plant species. Despite limited available information, especially about long-term effects of soil pollutants, it may be speculated that isoprene generally improves fitness of plants challenged by air and soil pollutants, and their interaction with other organisms.
Introduction
Plants are sessile but not passive components of the ecosystems, and they interact with the environment in several ways. Biogenic volatile organic compounds (BVOCs) are gases that are emitted by organisms in all terrestrial and marine ecosystems (Loreto et al. Citation2014). Plants emit worldwide more than 1 Pg C per year as BVOCs (Guenther et al. Citation1995, Citation2012), about half of which is isoprene (Guenther et al. Citation2006). Leaf BVOCs may be constitutively emitted (generally leaf life-long) or induced by abiotic and biotic stresses (Loreto and Schnitzler Citation2010). Some constitutive BVOCs may also be induced by stresses (Harrison et al. Citation2013). More than 1700 BVOCs have been identified, which are emitted by 90 different plant families belonging to both angiosperms and gymnosperms (Knudsen et al. Citation2006), as well as ferns and mosses (Hanson et al. Citation1999). As the detection systems get more accurate and high-throughput, the idea that all plants emit BVOCs is becoming realistic. Several roles have been suggested in the protection of plant tissues/cellular integrity, in the interaction between plants and other organisms, both attractants and repellents, as well as in the allelopathic interaction (Puig et al. Citation2018; Lazazzara et al. Citation2022).
Synthesis of most significant BVOCs occurs through three pathways: the lipoxygenase (LOX), the shikimic acid, and the terpenoid pathways (Pichersky and Gershenzon Citation2002). Terpenoids or isoprenoids are the largest group of specialized plant metabolites and derive from two precursors: isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) (McGarvey and Croteau Citation1995). Among terpenoids, isoprene (C5H8) is the simplest and most volatile BVOC. Isoprene is formed by the chloroplastic methyl erythritol 4-phosphate (MEP) pathway through isoprene synthase (IspS), which catalyzes the removal of pyrophosphate (PPi) from DMAPP. Constitutive, light-dependent emissions of volatile isoprenoids are generally limited to large amounts of isoprene, especially from fast-growing plants (Loreto and Fineschi Citation2015), and of monoterpenes from some families of trees and bushes (Loreto and Schnitzler Citation2010). Emission of isoprene is a metabolic cost for plants, but benefits may outweigh the cost, especially under high temperature (Jardine et al. Citation2012) and oxidative stress (Vickers et al. Citation2009). Isoprene was thought to quench reactive oxidative species (ROS) such as hydrogen peroxide (H2O2) (Loreto and Velikova Citation2001), singlet oxygen (1O2) (Affek and Yakir Citation2002), or reactive nitrogen species (RNS) (Velikova et al. Citation2005a), and to stabilize chloroplast membranes (Velikova et al. Citation2011a) facilitating photosynthetic electron transport rate (Pollastri et al. Citation2019). The antioxidant role of isoprene is now revised, as isoprene induces a significant shift in the transcriptome and metabolome (Monson et al. Citation2021; Dani and Loreto Citation2022) that improves the ability of plants to tolerate various stresses. The general role of isoprene as a stress protective agent remains unrivaled. Other, stress-induced, BVOCs provide a clearer action as stress relievers (Paré and Tumlinson Citation1999; Mithöfer and Boland Citation2012), improving protection against biotic (e.g. herbivores or pathogens attacks) (Dicke and Baldwin Citation2010), or abiotic stresses (e.g. drought, high temperatures, or oxidative pollutants) (Loreto and Schnitzler Citation2010).
Isoprene plays several roles in atmosphere chemistry, all of which are due to its oxidation (Heald et al. Citation2009; Archibald et al. Citation2010). When anthropogenic volatile pollutants such as nitrogen oxides (NOx) are absent, isoprene further cleanses the atmosphere of ozone. In the presence of NOx, however, isoprene participates in reactions leading to increased ozone formation (Fehsenfeld et al. Citation1992) under a well-established stoichiometry (Kanakidou et al. Citation2005). As the emission of isoprene to the atmosphere is so prevalent, the impact of environmental factors such as light intensity, atmospheric CO2 concentration, temperature, relative humidity, and nutrient status on isoprene emission has attracted great attention (Loreto and Schnitzler Citation2010; Harrison et al. Citation2013). Climate change impact on isoprene emission has been mainly attributed to positive long-term (enzyme-driven) and short-term (substrate-driven) feedback of rising temperature (Lehning et al. Citation2001; Rennenberg et al. Citation2006), implying that future emissions of isoprene will also increase (Arneth et al. Citation2008). This may be counteracted by an often large (and largely unexplained) inhibition of isoprene in rising CO2 (Rosenstiel et al. Citation2003; Guidolotti et al. Citation2011). However, the inhibitory impact of rising CO2 seems to be lost as the temperature gets higher, and the overall impact of climate change is therefore expected to lead to a heavier load of isoprene in the atmosphere (Sharkey and Monson Citation2017). We summarized studies on the effects of air and soil pollutants ( and ) on isoprene emission, and possible induction or repression factors.
Table 1. Air pollutant feedback on isoprene emission and possible induction or repression factors.
Table 2. Feedback of soil pollutants on isoprene emission and possible repression and induction factors.
It may be also hypothesized that, in response to increasing environmental stresses and global warming, a shift of native plants toward species and genotypes able to emit isoprene constitutively or an induced manner will occur (Lerdau Citation2007), and that pioneer species invading new environments largely benefit from emitting more complex volatile isoprenoids (Llusià et al. Citation2010). Here, we focus on reviewing anthropogenic atmospheric and soil pollutants and climate change that could also affect isoprene emission by natural vegetation and thus alter further the load of isoprene in the atmosphere.
Focus on isoprene and air pollution
Isoprene and the chemistry of the troposphere
Most of the plant BVOCs have relatively short lifetimes in the atmosphere ranging from less than a minute to few hours depending on the atmospheric conditions (Blande et al. Citation2014). In the case of isoprene, rapid reaction with reactive oxygen and nitrogen species ubiquitous in the atmosphere leads not only to ozone production, but also to the appearance of secondary products of isoprene oxidation. For example, methylvinyl-ketone (MVK), methacrolein (MACR), and 2-methyltetrols like 2-methylthreitol and 2-methylerythritol have been found in the natural aerosol of Amazonia forests characterized by high isoprene emissions (Claeys et al. Citation2004).
Formaldehyde is also produced by isoprene oxidation and, despite the low yield (<10%), this BVOC has been used as an important proxy of isoprene natural planetary sources by satellite inspection (Palmer et al. Citation2006). MVK and MACR are markers of isoprene oxidation also in planta and therefore it is possible for these secondary BVOCs to be directly emitted by plants and not only formed by isoprene reactions in the atmosphere (Jardine et al. Citation2012). Recent results, however, suggest that MVK and MACR might be produced in planta by pathways other than isoprene oxidation (Kai et al. Citation2012), and that MVK may even be further oxidized to methyl ethyl ketone (MEK), making the pattern of interactions between plant BVOCs and atmospheric chemistry even more complex (Cappellin et al. Citation2019).
Indeed, ozonolysis (Pinto-Zevallos et al. Citation2010) results in the formation of many secondary organic aerosols (SOAs) (Seinfeld and Pandis Citation2006; Laothawornkitkul et al. Citation2009) with relevant climatic impacts (Claeys et al. Citation2004; Paulot et al. Citation2009). Isoprene, monoterpenes, and other terpenoids characterized by high emission rates and high reactivity with the atmospheric oxidants that are present in polluted and urban areas (, ozone, hydroxyl radical (OH·)), are major contributors of SOA burden (Kanakidou et al. Citation2005; Goldstein and Galbally Citation2007). Field studies have shown that under conditions characterized by moderate to high BVOC levels,
predominantly reacts with BVOCs (Brown and Stutz Citation2012) to produce multifunctional compounds such as organic nitrates (ONs) (Nah et al. Citation2016; Faxon et al. Citation2018).
Impact of atmospheric determinants of climate change on isoprene
The two main atmospheric constituents affecting isoprene emission are carbon dioxide (CO2) and ozone (O3). Anthropogenic CO2 emission is the most important forcing variable affecting changes in climate since the beginning of the industrial era. Over time, CO2 concentrations have continued to steadily increase in the atmosphere, reaching 424 ppm in May of 2023 (https://gml.noaa.gov/ccgg/trends/weekly.html). A recent meta-analysis (Feng et al. Citation2019) summarized decades of experimental data (e.g. Rosenstiel et al. Citation2003; Possell et al. Citation2005) showing a largely negative impact of rising CO2 on isoprene emission, while emission of monoterpenes is substantially unaffected by CO2. The negative impact of rising CO2 on isoprene has surprised scientists, as isoprene is almost totally made by photosynthetic carbon (Delwiche and Sharkey Citation1993; Sharkey et al. Citation2020), and photosynthesis is stimulated by CO2 (Long et al. Citation2004). It has been suggested that the decrease of isoprene emission when CO2 increases is related: (i) to photorespiration stimulation, and to the consequent reduction of pyruvate available to the MEP pathway (Rosenstiel et al. Citation2003); or (ii) to competition for phosphoenolpyruvate (PEP) a cytosolic substrate that may support chloroplastic demand (Loreto and Fares Citation2007); or (iii) to an inhibitory effect on IspS activity (Scholefield et al. Citation2004). A hypothesis that the CO2 inhibition is related to a triose phosphate utlilization limitation of photosynthesis was recently ruled out (Lantz et al. Citation2019). Guidolotti et al. (Citation2011) found an inverse relationship between isoprene and intercellular CO2 concentration (Ci), which holds even at currently ambient CO2 concentration (>400 ppm). This supports the notion that the CO2-dependent reduction of isoprene reflects fine biochemical adjustments. The most recent data indicates that CO2 inhibits the MEP pathway enzyme 4-hydroxy-3-methylbut-2-en-1-yl diphosphate reductase (Sahu et al. Citation2023). An increase in CO2 may also indirectly stimulate isoprene emission at whole canopy and ecosystem level, because of higher photosynthesis, growth rate, and biomass accumulation (Arneth et al. Citation2007). However, several lines of evidence indicate that photosynthesis and plant growth/productivity do not always increase linearly with rising in CO2 (Ainsworth and Long Citation2005).
Moreover, a significant interspecific variability in CO2-responsiveness of isoprene emission was observed and is unexplained. Such variability in the reduction of isoprene emission could be caused by a significant variation in the size and composition of the precursor pools responsible for isoprene emissions (Niinemets et al. Citation2021). Squire et al. (Citation2014) found that climate change, which includes both rising temperature and CO2, increased isoprene emissions by natural vegetation, and the effect is expected to continue as long as CO2 overfertilizes plants (Squire et al. Citation2014). Future increase of isoprene emission by natural vegetation is expected when accounting for rising temperature only (Sanderson et al. Citation2003; Lathière et al. Citation2005; Wu et al. Citation2012). By modeling temperature and CO2 interaction (which includes direct and indirect CO2 effects) indeed it is confirmed that isoprene emissions will be stimulated over the twenty-first century (Arneth et al. Citation2007; Heald et al. Citation2009). A framework modeling study based on a scenario where the effect of climate and natural vegetation changes (driven by the rising of temperature and by the expansion of broadleaf forests respectively) co-occur, suggests an increase of isoprene emission by ∼42% by 2050, which drops to ∼4% if CO2 inhibition of isoprene emission is also included (Tai et al. Citation2013). However, the CO2 suppression is temperature-dependent and almost non-existent at 35°C (Sahu et al. Citation2023). shows the contrasting effects of CO2 on isoprene, indicating that further studies are needed to determine the potential effect of high CO2 levels in the long term.
The other gas that has received large attention for its feedback on isoprene is ozone. While generally, CO2 improves plant growth (Long et al. Citation2004), ozone is a serious environmental stress that causes heavy damage to photosynthesis. Indeed, when ozone enters the leaf, it is degraded to other ROS, which can cause oxidative stress and damage to the lipo-protein bilayer of the photosynthetic membranes, with consequent rapid chloroplast degradation (Loreto and Velikova Citation2001).
High doses of ozone could cause an initial stimulation of isoprene emission (Velikova et al. Citation2005a) due to higher expression of IspS and to enhanced enzyme activity (Fares et al. Citation2006). It seems that this upregulation is more evident in leaves developing under enriched O3 atmosphere which build up a better resistance to the pollutant (Fares et al. Citation2006). However, plants adapted to high O3 are not able to further stimulate isoprene emission when exposed to a following stimulus (Calfapietra et al. Citation2008). If O3 does not damage photosynthesis, isoprene emission is maintained and, as already mentioned, often stimulated. Under these conditions, isoprene may scavenge ROS made by O3 (Velikova et al. Citation2004), may make the photosynthetic membranes more resistant to ROS-dependent oxidation (Velikova et al. Citation2011a; Pollastri et al. Citation2019), or may simply prime a general activation of the antioxidant capacity, as well as other metabolic changes (Monson et al. Citation2021).
As isoprene is also stimulated by high temperatures, when high O3 episodes are more frequent, the antioxidant role of isoprene may be more relevant when associated with heat waves (Jardine et al. Citation2012) and with acute O3 exposures damaging photosynthesis. A first line of defense against O3 is stomatal closure. Loreto and Fares (Citation2007) showed that leaf damage is associated with O3 concentration inside leaves rather than the atmospheric O3. However, under prolonged or chronic exposure to O3 that overcomes the epidermal barrier, photosynthesis is severely impaired, and consequently isoprene emission is also restrained. Indeed, O3 often irreversibly damages plant tissues leading to reduced crop yields and forest growth (Mills et al. Citation2011). The uptake of O3 inside mesophyll causes oxidation of cell wall components, damages photosynthetic apparatus with detrimental effects on growth rate and biomass production, and accelerates leaf senescence (Ashmore Citation2005; Fares et al. Citation2006; Wittig et al. Citation2009). Meta-analysis data analysis shows that isoprene and photosynthesis are reduced to similar extent (10%) by high O3 exposure (Feng et al. Citation2019). However, isoprene emission is significantly increased by exposure of leaves to high UV-b (Harley et al. Citation1996; Tiiva et al. Citation2007a) and UV-a (Pallozzi et al. Citation2013b) radiation, which is a requisite for O3 formation in the atmosphere. Thus, the overall impact of air pollution on isoprene emission needs additional field testing where all factors dynamically interact together.
We speculate that both effects of climate change and environmental stress could lead to an increase of isoprene-emitting species in polluted environments in response to the negative effects (e.g. oxidative stress) resulting from increased air and soil contaminants.
summarizes the interaction between plant isoprene emissions and atmospheric pollutants in cities and industrial areas, which may have two effects: on one hand this interaction may increase the O3 load and high O3 episodes may exacerbate environmental stresses; on the other hand, this same interaction may favor evolution of a vegetation that is resistant to O3 pollution and associated oxidative stresses. As isoprene is involved in generic antioxidant protection (Loreto and Schnitzler Citation2010) this may lead to higher isoprene emission by both native and alien (invasive) species (Lerdau Citation2007). The two effects may feedback on each other, and the loop may cause unpredictable consequences. Llusià et al. (Citation2010) suggested that protection against multiple environmental stress conferred by high capacity to emit terpenoids accounted for the success of invasive plant species in Hawaii. Similarly, establishment and proliferation of Artemisia vulgaris in a new habitat seems to be related to its capacity to emit BVOCs (Barney et al. Citation2005). On the other hand, it is also conceivable that human-driven land use change, by replacing natural vegetation with agricultural crops, has selected against high isoprene emitters (Loreto and Fineschi Citation2015). Changes in natural vegetation (reduction of isoprene-emitting species) could affect air quality (Tai et al. Citation2013; Hantson et al. Citation2017). Clearly, understanding the future effects of climate change on isoprene emission is a very complex task, because of the wide range of multiple and concomitant environmental factors that could have synergistic or antagonistic effects.
Figure 1. Dynamic of plant isoprene emission and settlement of alien isoprene-emitting species induced by anthropogenic processes determining environmental stresses or by climate change. Positive or negative feedback (+/−) refers to the direct effects of pollutants on the isoprene emission capacity. ↑ represents the direct effects of anthropogenic processes and climate change on the increasing of air and soil determinants and settlement of new isoprene emitter species. Figure was created with BioRender.com.
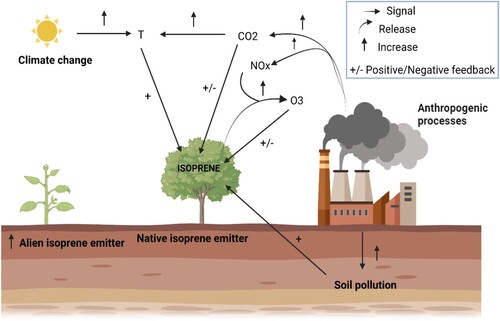
The future rising of environmental stresses other than O3 pollution but still related to anthropogenic processes and known to trigger oxidative stresses may also lead to a positive feedback for isoprene (both constitutive and induced, ) and other BVOCs biosynthesis and emission, acting as a plant-defense-system in response to climate change and warming (Peñuelas and Llusià Citation2003). For example, field measurements showed that white oak tree canopies have higher isoprene emission rates when exposed to more sunlight, reduced water availability, and high temperature (Sharkey et al. Citation1996). Interestingly, these plants did not show any anomalies in their growth and an increased thermotolerance (Singsaas et al. Citation1997) and it is suggested that the quenching of ROS by isoprene could be an effective way to reduce the negative effects of oxidative stress compounds (Velikova et al. Citation2005a).
Besides CO2 and O3, aerosols may also affect isoprene emission. Indeed, varying in size and composition (McMurry Citation2000) natural and anthropogenic aerosols are of particular interest as they act as condensation nuclei of particles (Novakov and Penner Citation1993) and absorb solar radiation (Andreae and Crutzen Citation1997). We have already considered that isoprene and more complex volatile terpenoids play a direct or indirect role in the formation of ozone and SOA (see above). While monoterpenes are well-known aerosol precursors, directly, or after ozonolysis (Joutsensaari et al. Citation2005), the impact of isoprene on aerosol has been more controversial. Early studies showing that isoprene may contribute to SOA formation (Claeys et al. Citation2004) have received experimental validation (Claeys and Maenhaut Citation2021), but large emissions by isoprene in forests have been also shown to inhibit particle formation (Kiendler-Scharr et al. Citation2009), and to suppress the formation of anthropogenic SOA (Li et al. Citation2022).
Aerosols may also feedback on isoprene emission, for example by dimming light available to photosynthesis. An earth system model study showed that the global land isoprene emission is weakly sensitive (−1% to 2%) to aerosol pollution (Strada and Unger Citation2016). However, at the regional scale, anthropogenic aerosol pollution led to a reduction (−2 to −12%) in annual average isoprene emission over Europe and China (Strada and Unger Citation2016). Thus, the impact of aerosol on isoprene emission seems to be variable and needs to be studied further and on different ecosystems.
Focus on isoprene and soil pollution
Soils may also contribute to the exchange of BVOCs, as sinks or sources, depending on the very diverse soil composition in terms of microorganisms, flora, and fauna, thus expanding functional considerations on trophic interactions between aboveground and belowground plant compartments (Peñuelas et al. Citation2014). While the complex relationships between isoprene and atmospheric pollution have been largely investigated, much less is known about the impact of soil pollution on isoprene. It could be speculated that isoprene antioxidant action also improves resistance to soil pollution.
‘Soil pollution’ refers to the presence of a chemical or substance out of place and/or present at a higher-than-normal concentration that has adverse effects on any non-targeted organism (FAO and ITPS Citation2015). Soil pollution acts as an abiotic stress on plants. It triggers ROS production, leading to adaptive plant responses including the improvement of the primary antioxidant redox system and the increase of the biosynthesis of secondary metabolites (Ferrer et al. Citation2018). Except natural areas with specific geological conditions, the major soil pollutants are related to anthropogenic (industrial) activities that release different kinds of pollutants, from complex hydrocarbons released by oil industries to very simple chemical elements such as heavy metals released as byproducts of several processes or nutrient elements such as nitrates from excess fertilization or phosphates from commercial cleaning industries. shows studies focused on the feedback of soil pollutants on isoprene emission and the potential suppression and induction factors.
Heavy metal soil pollution is a problem of major importance for plant productivity and survival (Foy et al. Citation1978; Salt et al. Citation1998; Fargašová and Molnárová Citation2010). There are several cases in which the effect of heavy metal pollution on BVOC emissions has been investigated. Velikova et al. (Citation2011b) suggested that heavy metal (Ni) pollution increases both constitutive (isoprene) and induced (monoterpenes and sesquiterpenes) isoprenoid emissions. Indeed, other reports indicate that high doses of copper could induce emission of BVOCs (Obara et al. Citation2002; Mithöfer et al. Citation2004) some of which characterize the interplay between plants and herbivores (Winter et al. Citation2012). Soil cadmium stress seems to increase total leaf VOC emission (Lin et al. Citation2022), and induces an upregulation of IspS over time (Li et al. Citation2017).
Moreover, in unstressed leaves, it was found that isoprene enhanced the expression of defense related genes that may be involved in resistance against heavy metal stress (Zuo et al. Citation2019). For example, in unstressed transgenic models (Arabidopsis and tobacco) and in non-emitting lines fumigated with isoprene, tolerance genes for heavy metal detoxification (MRPs and HIPP32) were upregulated, together with tolerance genes for other soil stresses like salinity (like CIPK20, NCED3) and drought (NCED5, ATAF1) (Zuo et al. Citation2019). This transcriptome upregulation of stress-tolerance genes could lead to increased resistance of emitting species when interacting with challenging environments.
The impact of nutrients on isoprene emission has received even wider coverage. Nutrient excess is often a consequence of pollution and over-fertilization, and finally eutrophication (Shortall Citation2013). Nitrates seem to generally elicit production and emission of isoprene, possibly making more N available for isoprene synthase biosynthesis (Litvak et al. Citation1996; Fernández-Martínez et al. Citation2018). On the other hand, excess of phosphorus in soils has a clear inhibitory effect on isoprene emission, assessed in different experiments (Fares et al. Citation2008; Cocozza et al. Citation2019; Cocozza et al. Citation2020) but never explained physiologically. Intuitively, high phosphorous should be beneficial for the synthesis of a molecule that requires large inputs of phosphorylated substrates of isoprene, like adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH) (Sharkey and Yeh Citation2001). High phosphorus also stimulates photosynthesis which supplies carbon for isoprene synthesis. However, uncoupling of isoprene synthesis and photosynthesis is often observed, for example under elevated CO2 (cfr. see above), or under water and salt stress (Loreto and Schnitzler Citation2010). It was proposed that competition with mitochondrial respiration for pyruvate or phosphoenolpyruvate is responsible for the inhibition of isoprene emission under high phosphorous nutrition (Fares et al. Citation2008), similar to what may occur under elevated CO2 (Rosenstiel et al. Citation2003), although we think that all pyruvate for isoprene synthesis comes from the Calvin-Benson cycle (Sharkey et al. Citation2020).
While different nutrients may have opposite effects on isoprene emission, a reduction of the intensity of emitted BVOCs in plants cultivated under high level of fertilization seems to be a convergent result (Fernández-Martínez et al. Citation2018), which may also explain why storage of BVOCs into reservoirs is a lost trait in recently evolved angiosperm crops. Evolution against emission of BVOCs may have an important trade-off in terms of improved plant productivity in the absence of stress, but losing the capacity to synthesize and emit BVOCs may not pay off when plants must defend themselves from abiotic stresses or must communicate with other organisms. We hypothesize that native and pioneer plants of polluted areas emit more isoprene (constitutive and induced) and speculate that the capacity to produce large amounts of isoprene may confer an adaptive advantage in a rapidly changing climate characterized by more frequent extreme events and pollution episodes.
Water and salt are important components of soils. Isoprene synthesis and emission continues even under drought or high salinity, despite concurrent photosynthesis inhibition (Brilli et al. Citation2007). The massive literature covering the impact of these stresses on isoprene has been reviewed (e.g. Loreto and Schnitzler Citation2010; Loreto et al. Citation2014; Monson et al. Citation2021) and the topic is beyond the scope of this work aiming at reviewing only impacts of soil pollutants. However, we redirect the readers to cited references for a more complete understanding of the impact of water and salt on BVOCs, and particularly on isoprene.
Soil structure and composition influence the development and morphology of the root system. The root is the anchorage system of plants and is critical for the uptake of nutrients required for plant growth and physiology, including the isoprene pathway. If for the aboveground part of plants (leaves) there is much scientific evidence on the effects and activities of in situ emission of isoprene, less well studied is whether belowground heterotrophic tissues (roots) can also emit isoprene, and if roots are also influenced when plants acquire or enhance their capacity to emit isoprene. Although isoprene is mainly emitted from leaves, there is evidence that the root systems of poplar (Ghirardo et al. Citation2011) and transgenic Arabidopsis (Loivamäki et al. Citation2007; Miloradovic van Doorn et al. Citation2020) emit a small amount of isoprene. It was shown that the constitutive promoter of isoprene synthase (PcIspS) is present and active in specific regions of roots (Cinege et al. Citation2009; Miloradovic van Doorn et al. Citation2020).
There are reports of isoprene affecting root development. Miloradovic van Doorn et al. (Citation2020) recently proposed a ROS-related role of isoprene in roots, showing an altered lateral root development and differences in ROS accumulation in roots. ROS are known to be involved in many pathways, especially under a challenging environment, being signals able to activate defenses responses also coordinating the developmental processes with environmental conditions (Locato et al. Citation2018). An interplay between ROS and hormones, in particular auxin, ethylene, and abscisic acid has also been reported (Xia et al. Citation2015). Isoprene also seems to interfere with many hormones, especially those sharing the same MEP pathway (cytokinins and abscisic acid, Barta and Loreto Citation2006; Dani et al. Citation2022) but possibly also with auxins (Dani and Loreto Citation2022). The effect of these interactions on roots is unknown, but a possible scheme of signaling function of internal isoprene in roots in relation with ROS was proposed (Miloradovic van Doorn et al. Citation2020). ROS signaling affects hormonal networks and signaling processes that regulate response to environmental drivers (Mittler Citation2017). It was proposed that isoprene could adjust ROS by quenching (direct) or changing gene expression (indirect) and so regulate all ROS-related pathways, including those involving phyto-hormones. Even if the molecular mechanism of this interplay is still relatively unknown, it could influence the growth of the root system, in particular the development of lateral roots (Miloradovic van Doorn et al. Citation2020). Isoprene also affects the root proteome and many of the proteins affected are involved in redox and stress responses (Miloradovic van Doorn et al. Citation2020). Soil pollutants directly affect root development and the determinants of root system architecture (Lombardi et al. Citation2021) and this might also contribute to change BVOC emission by aboveground and belowground plant organs ( and ), in turn altering plant capacity to cope with pollution and environmental constraints (e.g. drought stress).
Finally, root volatiles often are key elements of plant–plant communication and of interactions of plants with soil microbiome (), with positive consequences on priming defensive responses, facilitating root nutrient uptake, or counteracting the negative effects of pollutants. For example, BVOCs emitted from roots may facilitate interactions with arbuscular mycorrhizal fungi, expanding their beneficial functions, from improving resistance to soil stresses to enhancing nutrient availability (Asensio et al. Citation2012). However, whether these same functions may be attributed to isoprene is unclear. We hypothesize that this beneficial interaction (BVOCs-soil microbiome) could lead to better plant tolerance to soil stress and have positive feedback on plant biomass (). In leaves, isoprene does not seem to be a messenger able to be captured by receptors and induce priming in receiving plants (Giordano et al. Citation2021), but it seems to influence insect feeding (Laothawornkitkul et al. Citation2008; Loivamäki et al. Citation2008). This may very well be the case in roots as well, where the emission of isoprene by plants is also elusive, and possibly tiny.
Figure 2. Soil pollutants effects on root ROS generation and root-related BVOCs and the communication plant-plant and plant-microbiome through the soil. + or – refers to the direct effect of isoprene or BVOCs while ↑ to the direct effects of soil pollution. Only for this purpose we refer to BVOCs only for unclear evidence of isoprene belowground communication. Figure was created with BioRender.com.
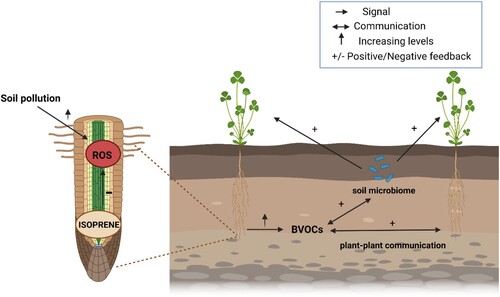
Soil constraints increase ROS formation, and it was shown that ROS, and ROS scavenging enzymes, play crucial roles in early-stage root-mycorrhiza interaction (Baptista et al. Citation2007; Nanda et al. Citation2010; Ditengou et al. Citation2015). Several studies have shown that the intensity of ROS burst is important for root-microbes (mutualistic or pathogen) interaction and contact (Baptista et al. Citation2007; Nanda et al. Citation2010), and plant redox balance could be fundamental to differentiate between the various microbes. ROS adjustment by isoprene (direct or indirect) could be crucial for regulating root redox balance, and root isoprene emission could facilitate the interaction and communication with soil microbiome (). It is known that volatiles are a signal for aboveground plant communication (Ninkovic et al. Citation2021). Whereas the role of isoprene as a message allowing such communication, both above and below ground, is still unclear, other root BVOCs have been extensively studied and are known to effectively elicit belowground communication (Schenkel et al. Citation2015; Abbas et al. Citation2022). If BVOCs are stimulated by soil pollution this may favor belowground plant–plant communication, perhaps priming the defensive system of plants and improving plant resistance to soil pollution and other soil-borne stresses ().
Conclusion and future directions
Over the past years, isoprene emission has been well studied for its effects on atmospheric pollution and on plant defense, but the reciprocal impact of isoprene, soil, and atmospheric pollutants is more elusive and complicated, especially when considering that direct measurements of isoprene emission are missing (in soils) and that long-term responses at whole plant and community level have not been extensively investigated yet (both in soils and air).
Future studies should evaluate the long-term effect of pollutants, including evolutionary impacts on the composition of natural and semi-natural forests around cities and industrial areas where anthropogenic pollution may be persistent over time. This would allow a better evaluation on how policies of re-forestation and afforestation of these areas may impact on air quality, also considering climate change pressures, which may lead to regional expansion of broadleaf forests, the main emitters of isoprene and in boreal areas (Wu et al. Citation2012). and highlight the contrasting effects of air and soil pollutants on isoprene and show how many studies focus on isoprene emission at foliar level. Future studies are needed at whole plant level especially for the potential long-term effect. Soil pollution impacts on isoprene are largely uninvestigated, and the impact of soil microorganisms on isoprene emission by plants is also largely unknown, although preliminary experiments indicate that beneficial microorganisms such as mycorrhiza (Pollastri et al. Citation2018) and plant growth-promoting rhizobacteria (Brunetti et al. Citation2021) may stimulate isoprene emission.
Acknowledgements
M.B is grateful to ‘The Company of Biologists’ for providing the traveling fellowship award (www.biologists.com) and BioRender.com for the creation and editing of the images. Author contribution: Conceptualization: Manuel Bellucci and Francesco Loreto; Writing original-draft preparation: Manuel Bellucci and Francesco Loreto; Writing review and editing: Manuel Bellucci, Laura De Gara, Vittoria Locato, Francesco Loreto, and Thomas D. Sharkey Funding acquisition: Laura De Gara and Francesco Loreto Supervision: Laura De Gara, Vittoria Locato, Francesco Loreto, and Thomas D. Sharkey. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Manuel Bellucci
Manuel Bellucci is a PhD student of ‘Science and engineering for the Human and the environment’ at Department of Science and Technology for Sustainable Development and One Health of the Campus Bio-Medico University of Rome, in collaboration with the Department of Biology of the National Research Council (CNR) of Italy and Edmund Mach Foundation. His main activities cover: (i) stress resistance and response of plants against abiotic stresses and (ii) biosynthesis, emission, and activities of isoprenoids in plants.
Vittoria Locato
Vittoria Locato is Associate Professor of Plant Physiology at Campus Bio-Medico (CBM) University of Rome from 2018. She has obtained a Master Degree in Biological Sciences cum laude (2003) and a Doctorate in Physiology and Cell Biotechnology (2008) from University of Bari. Her main research interests cover: redox signaling in plant responses to environmental stress; identification of tolerance traits in crop; plant biotechnology applied to the production of ‘bioactive’ molecules derived from plant cells.
Thomas D. Sharkey
Thomas D. (Tom) Sharkey is a University Distinguished Professor of Biochemistry and Molecular Biology and is a member of the Michigan State University/US Department of Energy Plant Research Laboratory. The two main interests in his lab are carbon metabolism of photosynthesis and isoprene emission and signaling in plants. A major aspect of his research is studying how these processes will be affected by climate change, both the temperature changes and carbon dioxide changes that are occurring.
Laura De Gara
Laura De Gara is Full Professor of Plant Physiology at the Department of Science and Technology for Sustainability and One Health of the Università Campus Bio-Medico di Roma. Her main scientific interests cover: (i) stress physiology, with particular attention to climate change-related stress and to redox homeostasis and ROS-dependent signaling and (ii) metabolism of bioactive molecules in plants.
Francesco Loreto
Francesco Loreto is Full Professor of Plant Physiology at the Department of Biology of the University of Naples Federico II and a Research Associate at the National Research Council of Italy (CNR), Institute for Sustainable Plant Protection (IPSP). Prof. Loreto main interests cover: (i) stress physiology, especially the study of the effect of biotic and abiotic stresses on photosynthesis limitations and plant productivity. (ii) Biosynthesis, emission, and functional roles of biogenic volatile organic compounds.
References
- Abbas F, O’Neill Rothenberg D, Zhou Y, Ke Y, Wang H-C. 2022. Volatile organic compounds as mediators of plant communication and adaptation to climate change. Physiol Plant. 174(6):e13840. doi: 10.1111/ppl.13840.
- Affek H, Yakir D. 2002. Protection by isoprene against singlet oxygen in leaves. Plant Physiol. 129:269–277. doi: 10.1104/pp.010909.
- Ainsworth EA, Long SP. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165(2):351–372. doi: 10.1111/j.1469-8137.2004.01224.x.
- Andreae MO, Crutzen PJ. 1997. Atmospheric aerosols: biogeochemical sources and role in atmospheric chemistry. Science. 276(5315):1052–1058. doi: 10.1126/science.276.5315.1052.
- Archibald A, Cooke M, Utembe S, Shallcross D, Derwent R, Jenkin M. 2010. Impacts of mechanistic changes on HOx formation and recycling in the oxidation of isoprene. Atmos Chem Phys. 10:8097–8118. doi: 10.5194/acp-10-8097-2010.
- Arneth A, Miller P, Scholze M, Hickler T, Schurgers G, Smith B, Prentice I. 2007. CO2 inhibition of global terrestrial isoprene emissions: potential implications for atmospheric chemistry. Geophys Res Lett. 34. doi: 10.1029/2007GL030615.
- Arneth A, Schurgers G, Hickler T, Miller P. 2008. Effects of species composition, land surface cover, CO2 concentration and climate on isoprene emissions from European forests. Plant Biol. 10:150–162. doi: 10.1055/s-2007-965247.
- Asensio D, Rapparini F, Penuelas J. 2012. AM fungi root colonization increases the production of essential isoprenoids vs. nonessential isoprenoids especially under drought stress conditions or after jasmonic acid application. Phytochemistry. 77:149–161. doi: 10.1016/j.phytochem.2011.12.012.
- Ashmore M. 2005. Assessing the future global impacts of ozone on vegetation. Plant Cell Environ. 28:949–964. doi: 10.1111/j.1365-3040.2005.01341.x.
- Baptista P, Martins A, Pais MS, Tavares RM, Lino-Neto T. 2007. Involvement of reactive oxygen species during early stages of ectomycorrhiza establishment between Castanea sativa and Pisolithus tinctorius. Mycorrhiza. 17(3):185–193. doi: 10.1007/s00572-006-0091-4.
- Barney J, Hay A, Weston L. 2005. Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J Chem Ecol. 31:247–265. doi: 10.1007/s10886-005-1339-8.
- Barta C, Loreto F. 2006. The relationship between the methyl-erythritol phosphate pathway leading to emission of volatile isoprenoids and abscisic acid content in leaves. Plant Physiol. 141(4):1676–1683. doi: 10.1104/pp.106.083063.
- Behnke K, Ghirardo A, Janz D, Kanawati B, Esperschütz J, Zimmer I, Schmitt-Kopplin P, Niinemets Ü, Polle A, Schnitzler JP, Rosenkranz M. 2013. Isoprene function in two contrasting poplars under salt and sunflecks. Tree Physiol. 33(6):562–578. doi: 10.1093/treephys/tpt018.
- Bibbiani S, Colzi I, Taiti C, Guidi Nissim W, Papini A, Mancuso S, Gonnelli C. 2018. Smelling the metal: volatile organic compound emission under Zn excess in the mint Tetradenia riparia. Plant Sci. 271:1–8. doi: 10.1016/j.plantsci.2018.03.006.
- Blande J, Holopainen J, Niinemets Ü. 2014. Plant volatiles in polluted atmospheres: stress responses and signal degradation. Plant Cell Environ. 37:1892–1904. doi: 10.1111/pce.12352.
- Blande JD, Tiiva P, Oksanen E, Holopainen JK. 2007. Emission of herbivore-induced volatile terpenoids from two hybrid aspen (Populus tremula×tremuloides) clones under ambient and elevated ozone concentrations in the field. Glob Chang Biol. 13(12):2538–2550. doi: 10.1111/j.1365-2486.2007.01453.x.
- Brilli F, Barta C, Fortunati A, Lerdau M, Loreto F, Centritto M. 2007. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol. 175(2):244–254. doi: 10.1111/j.1469-8137.2007.02094.x.
- Brown S, Stutz J. 2012. Nighttime radical observations and chemistry. Chem Soc Rev. 41:6405–6447. doi: 10.1039/c2cs35181a.
- Brunetti C, Saleem AR, Della Rocca G, Emiliani G, De Carlo A, Balestrini R, Khalid A, Mahmood T, Centritto M. 2021. Effects of plant growth-promoting rhizobacteria strains producing ACC deaminase on photosynthesis, isoprene emission, ethylene formation and growth of Mucuna pruriens (L.) DC. in response to water deficit. J Biotechnol. 331:53–62. doi: 10.1016/j.jbiotec.2021.03.008.
- Calfapietra C, Scarascia Mugnozza G, Karnosky DF, Loreto F, Sharkey TD. 2008. Isoprene emission rates under elevated CO2 and O3 in two field-grown aspen clones differing in their sensitivity to O3. New Phytol. 179(1):55–61. doi: 10.1111/j.1469-8137.2008.02493.x.
- Calfapietra C, Wiberley AE, Falbel TG, Linskey AR, Mugnozza GS, Karnosky DF, Loreto F, Shareky TD. 2007. Isoprene synthase expression and protein levels are reduced under elevated O3 but not under elevated CO2 (FACE) in field-grown aspen trees. Plant Cell Environ. 30(5):654–661. doi: 10.1111/j.1365-3040.2007.01646.x.
- Cappellin L, Loreto F, Biasioli F, Pastore P, McKinney K. 2019. A mechanism for biogenic production and emission of MEK from MVK decoupled from isoprene biosynthesis. Atmos Chem Phys. 19:3125–3135. doi: 10.5194/acp-19-3125-2019.
- Centritto M, Haworth M, Marino G, Pallozzi E, Tsonev T, Velikova V, Nogues I, Loreto F. 2014. Isoprene emission aids recovery of photosynthetic performance in transgenic Nicotiana tabacum following high intensity acute UV-B exposure. Plant Sci. 226:82–91. doi: 10.1016/j.plantsci.2014.06.004.
- Cinege G, Louis S, Hänsch R, Schnitzler J-P. 2009. Regulation of isoprene synthase promoter by environmental and internal factors. Plant Mol Biol. 69(5):593–604. doi: 10.1007/s11103-008-9441-2.
- Claeys M, Graham B, Vas G, Wang W, Vermeylen R, Pashynska V, Cafmeyer J, Guyon P, Andreae M, Artaxo P, Maenhaut W. 2004. Formation of secondary organic aerosols through photooxidation of isoprene. Science. 303:1173–1176. doi: 10.1126/science.1092805.
- Claeys M, Maenhaut W. 2021. Secondary organic aerosol formation from isoprene: selected research, historic account and state of the art. Atmosphere. 12(6):728. doi: 10.3390/atmos12060728.
- Cocozza C, Brilli F, Miozzi L, Pignattelli S, Rotunno S, Brunetti C, Giordano C, Pollastri S, Centritto M, Accotto GP, et al. 2019. Impact of high or low levels of phosphorus and high sodium in soils on productivity and stress tolerance of Arundo donax plants. Plant Sci. 289:110260. doi: 10.1016/j.plantsci.2019.110260.
- Cocozza C, Brilli F, Pignattelli S, Pollastri S, Brunetti C, Gonnelli C, Tognetti R, Centritto M, Loreto F. 2020. The excess of phosphorus in soil reduces physiological performances over time but enhances prompt recovery of salt-stressed Arundo donax plants. Plant Physiol Biochem. 151:556–565. doi: 10.1016/j.plaphy.2020.04.011.
- Dani KGS, Loreto F. 2022. Plant volatiles as regulators of hormone homeostasis. New Phytol. 234(3):804–812. doi: 10.1111/nph.18035.
- Dani KGS, Pollastri S, Pinosio S, Reichelt M, Sharkey TD, Schnitzler J-P, Loreto F. 2022. Isoprene enhances leaf cytokinin metabolism and induces early senescence. New Phytol. 234(3):961–974. doi: 10.1111/nph.17833.
- Delwiche C, Sharkey T. 1993. Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ. 16:587–591. doi: 10.1111/j.1365-3040.1993.tb00907.x.
- Dicke M, Baldwin IT. 2010. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci. 15(3):167–175. doi: 10.1016/j.tplants.2009.12.002.
- Ditengou FA, Müller A, Rosenkranz M, Felten J, Lasok H, van Doorn MM, Legué V, Palme K, Schnitzler J-P, Polle A. 2015. Volatile signalling by sesquiterpenes from ectomycorrhizal fungi reprogrammes root architecture. Nat Commun. 6(1):6279. doi: 10.1038/ncomms7279.
- FAO, ITPS. 2015. Status of the World’s Soil Resources (SWSR) - main report. Rome, Italy. http://www.fao.org/3/a-i5199e.pdf.
- Fares S, Barta C, Brilli F, Centritto M, Ederli L, Ferranti F, Pasqualini S, Reale L, Tricoli D, Loreto F. 2006. Impact of high ozone on isoprene emission, photosynthesis and histology of developing Populus alba leaves directly or indirectly exposed to the pollutant. Physiol Plant. 128:456–465. doi: 10.1111/j.1399-3054.2006.00750.x.
- Fares S, Brilli F, Noguès I, Velikova V, Tsonev T, Dagli S, Loreto F. 2008. Isoprene emission and primary metabolism in Phragmites australis grown under different phosphorus levels. Plant Biol. 10:38–43. doi: 10.1055/s-2007-965429.
- Fares S, Oksanen E, Lännenpää M, Julkunen-Tiitto R, Loreto F. 2010. Volatile emissions and phenolic compound concentrations along a vertical profile of Populus nigra leaves exposed to realistic ozone concentrations. Photosynth Res. 104(1):61–74. doi: 10.1007/s11120-010-9549-5.
- Fargašová A, Molnárová M. 2010. Assessment of Cr and Ni phytotoxicity from cutlery-washing waste-waters using biomass and chlorophyll production tests on mustard Sinapis alba L. seedlings. Environ Sci Pollut Res. 17(1):187–194. doi: 10.1007/s11356-009-0136-2.
- Faubert P, Tiiva P, Rinnan Å, Räsänen J, Holopainen JK, Holopainen T, Kyrö E, Rinnan R. 2010. Non-methane biogenic volatile organic compound emissions from a subarctic peatland under enhanced UV-B radiation. Ecosystems. 13(6):860–873. doi: 10.1007/s10021-010-9362-1.
- Faxon C, Hammes J, Le Breton M, Pathak RK, Hallquist M. 2018. Characterization of organic nitrate constituents of secondary organic aerosol (SOA) from nitrate-radical-initiated oxidation of limonene using high-resolution chemical ionization mass spectrometry. Atmos Chem Phys. 18:5467–5481. doi: 10.5194/acp-18-5467-2018.
- Fehsenfeld F, Calvert J, Fall R, Goldan P, Guenther A, Hewitt CN, Lamb B, Liu S, Trainer M, Westberg H, Zimmerman P. 1992. Emissions of volatile organic compounds from vegetation and the implications for atmospheric chemistry. Global Biogeochem Cycles. 6:389–430. doi: 10.1029/92GB02125.
- Feng Z, Yuan X, Fares S, Loreto F, Li P, Hoshika Y, Paoletti E. 2019. Isoprene is more affected by climate drivers than monoterpenes: a meta-analytic review on plant isoprenoid emissions. Plant Cell Environ. 42(6):1939–1949. doi: 10.1111/pce.13535.
- Fernández-Martínez M, Llusia J, Filella I, Niinemets Ü, Arneth A, Wright I, Loreto F, Penuelas J. 2018. Nutrient-rich plants emit a less intense blend of volatile isoprenoids. New Phytol. 220:773–784. doi: 10.1111/nph.14889.
- Ferrer MA, Cimini S, López-Orenes A, Calderón AA, De Gara L. 2018. Differential Pb tolerance in metallicolous and non-metallicolous Zygophyllum fabago populations involves the strengthening of the antioxidative pathways. Environ Exp Bot. 150:141–151. doi:10.1016/j.envexpbot.2018.03.010.
- Foy CD, Chaney R, White M. 1978. The physiology of metal toxicity in plants. Annu Rev Plant Physiol. 29:511–566. doi: 10.1146/annurev.pp.29.060178.002455.
- Ghirardo A, Gutknecht J, Zimmer I, Brüggemann N, Schnitzler J-P. 2011. Biogenic volatile organic compound and respiratory CO2 emissions after 13C-labeling: online tracing of C translocation dynamics in poplar plants. PLoS One. 6(2):e17393. doi: 10.1371/journal.pone.0017393.
- Giordano D, Facchiano A, D’Auria S, Loreto F. 2021. A hypothesis on the capacity of plant odorant-binding proteins to bind volatile isoprenoids based on in silico evidences. Elife. 10:e66741. doi: 10.7554/eLife.66741.
- Goldstein AH, Galbally IE. 2007. Known and unexplored organic constituents in the earth's atmosphere. Environ Sci Technol. 41(5):1514–1521. doi: 10.1021/es072476p.
- Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, Mckay WA, et al. 1995. A global model of natural volatile organic compound emissions. J Geophys Res. 100:8873–8892. doi: 10.1029/94JD02950.
- Guenther A, Jiang X, Heald C, Sakulyanontvittaya T, Duhl T, Emmons L, Wang X. 2012. The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci Model Dev Discuss. 5. doi: 10.5194/gmdd-5-1503-2012.
- Guenther A, Karl T, Harley P, Wiedinmyer C, Palmer PI, Geron C. 2006. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos Chem Phys. 6(11):3181–3210. doi: 10.5194/acp-6-3181-2006.
- Guidolotti G, Calfapietra C, Loreto F. 2011. The relationship between isoprene emission, CO2 assimilation and water use efficiency across a range of poplar genotypes. Physiol Plant. 142:297–304. doi: 10.1111/j.1399-3054.2011.01463.x.
- Hanson DT, Swanson S, Graham LE, Sharkey TD. 1999. Evolutionary significance of isopreneemission from mosses. Am J Bot. 86(5):634–639. doi: 10.2307/2656571.
- Hantson S, Knorr W, Schurgers G, Pugh TAM, Arneth A. 2017. Global isoprene and monoterpene emissions under changing climate, vegetation, CO2 and land use. Atmos Environ. 155:35–45. doi: 10.1016/j.atmosenv.2017.02.010.
- Harley P, Deem G, Flint S, Caldwell M. 1996. Effects of growth under elevated UV-B on photosynthesis and isoprene emission in Quercus gambelii and Mucuna pruriens. Glob Chang Biol. 2(2):149–154. doi: 10.1111/j.1365-2486.1996.tb00060.x.
- Harrison SP, Morfopoulos C, Dani KGS, Prentice IC, Arneth A, Atwell BJ, Barkley MP, Leishman MR, Loreto F, Medlyn BE, et al. 2013. Volatile isoprenoid emissions from plastid to planet. New Phytol. 197(1):49–57. doi: 10.1111/nph.12021.
- Heald C, Wilkinson M, Monson RK, Alo C, Wang G, Guenther A. 2009. Response of isoprene emission to ambient CO2 changes and implications for global budgets. Glob Chang Biol. 15:1127–1140. doi: 10.1111/j.1365-2486.2008.01802.x.
- Jardine K, Monson R, Abrell L, Saleska S, Arneth A, Jardine AB, Ishida Y, Yañez-Serrano A, Artaxo P, Karl T, et al. 2012. Within-plant isoprene oxidation confirmed by direct emissions of oxidation products methyl vinyl ketone and methacrolein. Glob Chang Biol. 18:973–984. doi: 10.1111/j.1365-2486.2011.02610.x.
- Joutsensaari J, Loivamäki M, Vuorinen T, Miettinen P, Nerg A-M, Holopainen JK, Laaksonen A. 2005. Nanoparticle formation by ozonolysis of inducible plant volatiles. Atmos Chem Phys. 5(6):1489–1495. doi: 10.5194/acp-5-1489-2005.
- Kai H, Hirashima K, Matsuda O, Ikegami H, Winkelmann T, Nakahara T, Iba K. 2012. Thermotolerant cyclamen with reduced acrolein and methyl vinyl ketone. J Exp Bot. 63(11):4143–4150. doi: 10.1093/jxb/ers110.
- Kaling M, Kanawati B, Ghirardo A, Albert A, Winkler JB, Heller W, Barta C, Loreto F, Schmitt-Kopplin PE, Schnitzler J-P. 2015. UV-B mediated metabolic rearrangements in poplar revealed by non-targeted metabolomics. Plant Cell Environ. 38(5):892–904. doi: 10.1111/pce.12348.
- Kanakidou M, Seinfeld J, Pandis S, Barnes I, Dentener F, Facchini M, Van Dingenen R, Ervens B, Nenes A, Nielsen C, et al. 2005. Organic aerosol and global climate modelling: a review. Atmos Chem Phys. 5:1053–1123. doi: 10.5194/acp-5-1053-2005.
- Kiendler-Scharr A, Wildt J, Dal Maso M, Hohaus T, Kleist E, Mentel TF, Tillmann R, Uerlings R, Schurr U, Wahner A. 2009. New particle formation in forests inhibited by isoprene emissions. Nature. 461(7262):381–384. doi: 10.1038/nature08292.
- Knudsen J, Eriksson R, Gershenzon J, Ståhl B. 2006. Diversity and distribution of floral scent. Bot Rev. 72:1–120. doi: 10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2.
- Lantz AT, Solomon C, Gog L, McClain AM, Weraduwage SM, Cruz JA, Sharkey TD. 2019. Isoprene suppression by CO2 is not due to triose phosphate utilization (TPU) limitation. Front For Glob Chang. 2. doi: 10.3389/ffgc.2019.00008.
- Laothawornkitkul J, Paul ND, Vickers CE, Possell M, Taylor JE, Mullineaux PM, Hewitt CN. 2008. Isoprene emissions influence herbivore feeding decisions. Plant Cell Environ. 31(10):1410–1415. doi: 10.1111/j.1365-3040.2008.01849.x.
- Laothawornkitkul J, Taylor JE, Paul ND, Hewitt CN. 2009. Biogenic volatile organic compounds in the earth system. New Phytol. 183(1):27–51. doi: 10.1111/j.1469-8137.2009.02859.x.
- Lathière J, Hauglustaine DA, de Noblet N, Krinner G, Folberth G. 2005. Past and future changes in biogenic volatile organic compound emissions simulated with a global dynamic vegetation model. Geophys Res Lett. 32. doi: 10.1029/2005GL024164.
- Lazazzara V, Avesani S, Robatscher P, Oberhuber M, Pertot I, Schuhmacher R, Perazzolli M. 2022. Biogenic volatile organic compounds in the grapevine response to pathogens, beneficial microorganisms, resistance inducers, and abiotic factors. J Exp Bot. 73(2):529–554. doi: 10.1093/jxb/erab367.
- Lehning A, Zimmer W, Zimmer I, Schnitzler J-P. 2001. Modeling of annual variations of oak (Quercus robur L.) isoprene synthase activity to predict isoprene emission rates. J Geophys Res. 106:3157–3166. doi: 10.1029/2000JD900631.
- Lerdau M. 2007. ECOLOGY: a positive feedback with negative consequences. Science. 316:212–213. doi: 10.1126/science.1141486.
- Li D, Chen Y, Shi Y, He X, Chen X. 2009. Impact of elevated CO2 and O3 concentrations on biogenic volatile organic compounds emissions from Ginkgo biloba. Bull Environ Contam Toxicol. 82(4):473–477. doi: 10.1007/s00128-008-9590-7.
- Li K, Zhang X, Zhao B, Bloss WJ, Lin C, White S, Yu H, Chen L, Geng C, Yang W, et al. 2022. Suppression of anthropogenic secondary organic aerosol formation by isoprene. npj Clim Atmos Sci. 5(1):12. doi: 10.1038/s41612-022-00233-x.
- Li M, Xu J, Algarra Alarcon A, Carlin S, Barbaro E, Cappellin L, Velikova V, Vrhovsek U, Loreto F, Varotto C. 2017. In planta recapitulation of isoprene synthase evolution from ocimene synthases. Mol Biol Evol. 34(10):2583–2599. doi: 10.1093/molbev/msx178.
- Li S, Agathokleous E, Li S, Yuan X, Du Y, Feng Z. 2023. Sensitivity of isoprene emission rate to ozone in greening trees is concurrently determined by isoprene synthesis capacity and stomatal conductance. Sci Total Environ. 891:164325. doi: 10.1016/j.scitotenv.2023.164325.
- Lin T, Zhu G, He W, Xie J, Li S, Han S, Li S, Yang C, Liu Y, Zhu T. 2022. Soil cadmium stress reduced host plant odor selection and oviposition preference of leaf herbivores through the changes in leaf volatile emissions. Sci Total Environ. 814:152728. doi: 10.1016/j.scitotenv.2021.152728.
- Litvak M, Loreto F, Harley P, Sharkey T, Monson R. 1996. The response of isoprene emission rate and photosynthetic rate to photon flux and nitrogen supply in aspen and white oak trees. Plant Cell Environ. 19:549–559. doi: 10.1111/j.1365-3040.1996.tb00388.x.
- Llusià J, Peñuelas J, Sardans J, Owen S, Niinemets Ü. 2010. Measurement of volatile terpene emissions in 70 dominant vascular plant species in Hawaii: aliens emit more than natives. Glob Ecol Biogeogr. 19:863–874. doi: 10.1111/j.1466-8238.2010.00557.x.
- Locato V, Cimini S, De Gara L. 2018. ROS and redox balance as multifaceted players of cross-tolerance: epigenetic and retrograde control of gene expression. J Exp Bot. 69(14):3373–3391. doi: 10.1093/jxb/ery168.
- Loivamäki M, Gilmer F, Fischbach RJ, Sörgel C, Bachl A, Walter A, Schnitzler J-P. 2007. Arabidopsis, a model to study biological functions of isoprene emission? Plant Physiol. 144(2):1066–1078. doi: 10.1104/pp.107.098509.
- Loivamäki M, Mumm R, Dicke M, Schnitzler J-P. 2008. Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proc Natl Acad Sci USA. 105(45):17430–17435. doi: 10.1073/pnas.0804488105.
- Lombardi M, De Gara L, Loreto F. 2021. Determinants of root system architecture for future-ready, stress-resilient crops. Physiol Plant. 172(4):2090–2097. doi: 10.1111/ppl.13439.
- Long SP, Ainsworth EA, Rogers A, Ort DR. 2004. Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol. 55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610.
- Loreto F, Centritto M, Barta C, Calfapietra C, Fares S, Monson RK. 2007. The relationship between isoprene emission rate and dark respiration rate in white poplar (Populus alba L.) leaves. Plant Cell Environ. 30(5):662–669. doi: 10.1111/j.1365-3040.2007.01648.x.
- Loreto F, Delfine S. 2000. Emission of isoprene from salt-stressed Eucalyptus globulus leaves. Plant Physiol. 123(4):1605–1610. doi: 10.1104/pp.123.4.1605.
- Loreto F, Dicke M, Schnitzler J-P, Turlings TCJ. 2014. Plant volatiles and the environment. Plant Cell Environ. 37(8):1905–1908. doi: 10.1111/pce.12369.
- Loreto F, Fares S. 2007. Is ozone flux inside leaves only a damage indicator? Clues from volatile isoprenoid studies. Plant Physiol. 143:1096–1100. doi: 10.1104/pp.106.091892.
- Loreto F, Fineschi S. 2015. Reconciling functions and evolution of isoprene emission in higher plants. New Phytol. 206(2):578–582. doi: 10.1111/nph.13242.
- Loreto F, Schnitzler J-P. 2010. Abiotic stresses and induced BVOCs. Trends Plant Sci. 15:154–166. doi: 10.1016/j.tplants.2009.12.006.
- Loreto F, Sharkey TD. 1990. A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta. 182(4):523–531. doi: 10.1007/BF02341027.
- Loreto F, Velikova V. 2001. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 127(4):1781–1787. doi: 10.1104/pp.010497.
- McGarvey DJ, Croteau R. 1995. Terpenoid metabolism. Plant Cell. 7(7):1015–1026. doi: 10.1105/tpc.7.7.1015.
- McMurry PH. 2000. A review of atmospheric aerosol measurements. Atmos Environ. 34(12):1959–1999. doi: 10.1016/S1352-2310(99)00455-0.
- Mills G, Hayes F, Simpson D, Emberson L, Norris D, Harmens H, Büker P. 2011. Evidence of widespread effects of ozone on crops and (semi-)natural vegetation in Europe (1990–2006) in relation to AOT40- and flux-based risk maps. Glob Chang Biol. 17:592–613. doi: 10.1111/j.1365-2486.2010.02217.x.
- Miloradovic van Doorn M, Merl-Pham J, Ghirardo A, Fink S, Polle A, Schnitzler J-P, Rosenkranz M. 2020. Root isoprene formation alters lateral root development. Plant Cell Environ. 43(9):2207–2223. doi: 10.1111/pce.13814.
- Mithöfer A, Boland W. 2012. Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol. 63:431–450. doi: 10.1146/annurev-arplant-042110-103854.
- Mithöfer A, Schulze B, Boland W. 2004. Biotic and heavy metal stress response in plants: evidence for common signals. FEBS Lett. 566(1–3):1–5. doi: 10.1016/j.febslet.2004.04.011.
- Mittler R. 2017. ROS are good. Trends Plant Sci. 22(1):11–19. doi: 10.1016/j.tplants.2016.08.002.
- Monson RK, Fall R. 1989. Isoprene emission from aspen leaves 1: influence of environment and relation to photosynthesis and photorespiration. Plant Physiol. 90(1):267–274. doi: 10.1104/pp.90.1.267.
- Monson RK, Weraduwage SM, Rosenkranz M, Schnitzler J-P, Sharkey TD. 2021. Leaf isoprene emission as a trait that mediates the growth-defense tradeoff in the face of climate stress. Oecologia. 197(4):885–902. doi: 10.1007/s00442-020-04813-7.
- Nah T, Sanchez J, Boyd C, Ng N. 2016. Photochemical aging of α-pinene and β-pinene secondary organic aerosol formed from nitrate radical oxidation. Environ Sci Technol. 50:222–231. doi: 10.1021/acs.est.5b04594.
- Nanda AK, Andrio E, Marino D, Pauly N, Dunand C. 2010. Reactive oxygen species during plant-microorganism early interactions. J Integr Plant Biol. 52(2):195–204. doi: 10.1111/j.1744-7909.2010.00933.x.
- Niinemets Ü, Rasulov B, Talts E. 2021. CO2-responsiveness of leaf isoprene emission: why do species differ? Plant Cell Environ. 44(9):3049–3063. doi: 10.1111/pce.14131.
- Ninkovic V, Markovic D, Rensing M. 2021. Plant volatiles as cues and signals in plant communication. Plant Cell Environ. 44(4):1030–1043. doi: 10.1111/pce.13910.
- Novakov T, Penner JE. 1993. Large contribution of organic aerosols to cloud-condensation-nuclei concentrations. Nature. 365(6449):823–826. doi: 10.1038/365823a0.
- Obara N, Hasegawa M, Kodama O. 2002. Induced volatiles in elicitor-treated and rice blast fungus-inoculated rice leaves. Biosci Biotechnol Biochem. 66:2549–2559. doi: 10.1271/bbb.66.2549.
- Pallozzi E, Fortunati A, Marino G, Loreto F, Agati G, Centritto M. 2013a. BVOC emission from Populus × canadensis saplings in response to acute UV-A radiation. Physiol Plant. 148(1):51–61. doi: 10.1111/j.1399-3054.2012.01687.x.
- Pallozzi E, Marino G, Fortunati A, Loreto F, Centritto M. 2013b. Effect of exposure to UVA radiation on photosynthesis and isoprene emission in populus × euroamericana. In: Kuang T, Lu C, Lixin Z, editors. Photosynthesis research for food, fuel and the future. Berlin: Springer; p. 763–767.
- Palmer P, Abbot D, Fu T-M, Jacob D, Chance K, Kurosu T, Guenther A, Wiedinmyer C, Young J, Pilling M, et al. 2006. Quantifying the seasonal and interannual variability of North American isoprene emissions using satellite observations of the formaldehyde column. J Geophys Res. 111. doi: 10.1029/2005JD006689.
- Paré PW, Tumlinson JH. 1999. Plant volatiles as a defense against insect herbivores. Plant Physiol. 121(2):325–332. doi: 10.1104/pp.121.2.325.
- Paulot F, Crounse JD, Kjaergaard HG, Kürten A, St Clair JM, Seinfeld JH, Wennberg PO. 2009. Unexpected epoxide formation in the gas-phase photooxidation of isoprene. Science. 325(5941):730–733. doi: 10.1126/science.1172910.
- Pegoraro E, Abrell L, Van Haren J, Barron-Gafford G, Grieve KA, Malhi Y, Murthy R, Lin G. 2005. The effect of elevated atmospheric CO2 and drought on sources and sinks of isoprene in a temperate and tropical rainforest mesocosm. Glob Chang Biol. 11(8):1234–1246. doi: 10.1111/j.1365-2486.2005.00986.x.
- Peñuelas J, Asensio D, Tholl D, Wenke K, Rosenkranz M, Piechulla B, Schnitzler J-P. 2014. Biogenic volatile emissions from the soil. Plant Cell Environ. 37(8):1866–1891. doi: 10.1111/pce.12340.
- Peñuelas J, Llusià J. 2003. BVOCs: plant defense against climate warming? Trends Plant Sci. 8(3):105–109. doi: 10.1016/S1360-1385(03)00008-6.
- Pichersky E, Gershenzon J. 2002. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol. 5(3):237–243. doi: 10.1016/S1369-5266(02)00251-0.
- Pinto-Zevallos D, Blande J, Souza S, Nerg A-M, Holopainen J. 2010. Plant volatile organic compounds (VOCs) in ozone (O3) polluted atmospheres: the ecological effects. J Chem Ecol. 36:22–34. doi: 10.1007/s10886-009-9732-3.
- Pollastri S, Jorba I, Hawkins TJ, Llusià J, Michelozzi M, Navajas D, Peñuelas J, Hussey PJ, Knight MR, Loreto F. 2019. Leaves of isoprene-emitting tobacco plants maintain PSII stability at high temperatures. New Phytol. 223(3):1307–1318. doi: 10.1111/nph.15847.
- Pollastri S, Savvides A, Pesando M, Lumini E, Volpe MG, Ozudogru EA, Faccio A, De Cunzo F, Michelozzi M, Lambardi M, et al. 2018. Impact of two arbuscular mycorrhizal fungi on Arundo donax L. response to salt stress. Planta. 247(3):573–585. doi: 10.1007/s00425-017-2808-3.
- Possell M, Hewitt CN. 2011. Isoprene emissions from plants are mediated by atmospheric CO2 concentrations. Glob Chang Biol. 17(4):1595–1610. doi: 10.1111/j.1365-2486.2010.02306.x.
- Possell M, Hewitt CN, Beerling D. 2005. The effects of glacial atmospheric CO2 concentrations and climate on isoprene emissions by vascular plants. Glob Chang Biol. 11:60–69. doi: 10.1111/j.1365-2486.2004.00889.x.
- Puig CG, Gonçalves RF, Valentão P, Andrade PB, Reigosa MJ, Pedrol N. 2018. The consistency between phytotoxic effects and the dynamics of allelochemicals release from eucalyptus globulus leaves used as bioherbicide green manure. J Chem Ecol. 44(7):658–670. doi: 10.1007/s10886-018-0983-8.
- Rennenberg H, Loreto F, Polle A, Brilli F, Fares S, Beniwal RS, Gessler A. 2006. Physiological responses of forest trees to heat and drought. Plant Biol. 8(5):556–571. doi: 10.1055/s-2006-924084.
- Rosenstiel TN, Potosnak MJ, Griffin KL, Fall R, Monson RK. 2003. Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature. 421(6920):256–259. doi: 10.1038/nature01312.
- Sahu A, Mostofa MG, Weraduwage SM, Sharkey TD. 2023. Hydroxymethylbutenyl diphosphate accumulation reveals MEP pathway regulation for high CO2-induced suppression of isoprene emission. Proc Natl Acad Sci USA. 120(41):e2309536120. doi: 10.1073/pnas.2309536120.
- Salt DE, Smith RD, Raskin I. 1998. Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol. 49(1):643–668. doi: 10.1146/annurev.arplant.49.1.643.
- Sanderson M, Jones CD, Collins W, Johnson CE, Derwent R. 2003. Effect of climate change on isoprene emissions and surface ozone levels. Geophys Res Lett. 30:1936. doi: 10.1029/2003GL017642.
- Schenkel D, Lemfack MC, Piechulla B, Splivallo R. 2015. A meta-analysis approach for assessing the diversity and specificity of belowground root and microbial volatiles. Front Plant Sci. 6:707. doi: 10.3389/fpls.2015.00707.
- Scholefield P, Doick K, Herbert B, Hewitt CN, Schnitzler J-P, Pinelli P, Loreto F. 2004. Impact of rising CO2 on emissions of volatile organic compounds: isoprene emission from Phragmites australis growing at elevated CO2 in a natural carbon dioxide spring. Plant Cell Environ. 27:393–401. doi: 10.1111/j.1365-3040.2003.01155.x.
- Seinfeld JH, Pandis S. 2006. Atmospheric chemistry and physics: from air pollution to climate change. 2nd ed. New York: Wiley-Interscience.
- Sharkey TD, Loreto F, Delwiche CF. 1991. High carbon dioxide and sun/shade effects on isoprene emission from oak and aspen tree leaves. Plant Cell Environ. 14(3):333–338. doi: 10.1111/j.1365-3040.1991.tb01509.x.
- Sharkey TD, Monson RK. 2017. Isoprene research – 60 years later, the biology is still enigmatic. Plant Cell Environ. 40(9):1671–1678. doi: 10.1111/pce.12930.
- Sharkey TD, Preiser AL, Weraduwage SM, Gog L. 2020. Source of 12C in Calvin-Benson cycle intermediates and isoprene emitted from plant leaves fed with 13CO2. Biochem J. 477(17):3237–3252. doi: 10.1042/BCJ20200480.
- Sharkey TD, Singsaas EL, Vanderveer PJ, Geron C. 1996. Field measurements of isoprene emission from trees in response to temperature and light. Tree Physiol. 16(7):649–654. doi: 10.1093/treephys/16.7.649.
- Sharkey TD, Yeh S. 2001. Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol. 52:407–436. doi: 10.1146/annurev.arplant.52.1.407.
- Shortall OK. 2013. “Marginal land” for energy crops: exploring definitions and embedded assumptions. Energy Policy. 62:19–27. doi: 10.1016/j.enpol.2013.07.048.
- Singsaas EL, Lerdau M, Winter K, Sharkey TD. 1997. Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol. 115(4):1413–1420. doi: 10.1104/pp.115.4.1413.
- Squire OJ, Archibald AT, Abraham NL, Beerling DJ, Hewitt CN, Lathière J, Pike RC, Telford PJ, Pyle JA. 2014. Influence of future climate and cropland expansion on isoprene emissions and tropospheric ozone. Atmos Chem Phys. 14(2):1011–1024. doi: 10.5194/acp-14-1011-2014.
- Strada S, Unger N. 2016. Potential sensitivity of photosynthesis and isoprene emission to direct radiative effects of atmospheric aerosol pollution. Atmos Chem Phys. 16(7):4213–4234. doi: 10.5194/acp-16-4213-2016.
- Sun Z, Hüve K, Vislap V, Niinemets Ü. 2013. Elevated [CO2] magnifies isoprene emissions under heat and improves thermal resistance in hybrid aspen. J Exp Bot. 64(18):5509–5523. doi: 10.1093/jxb/ert318.
- Sun Z, Niinemets Ü, Hüve K, Noe SM, Rasulov B, Copolovici L, Vislap V. 2012. Enhanced isoprene emission capacity and altered light responsiveness in aspen grown under elevated atmospheric CO2 concentration. Glob Chang Biol. 18(11):3423–3440. doi: 10.1111/j.1365-2486.2012.02789.x.
- Tai A, Mickley L, Heald C, Wu S. 2013. Effect of CO2 inhibition on biogenic isoprene emission: implications for air quality under 2000 to 2050 changes in climate, vegetation, and land use. Geophys Res Lett. 40:3479–3483. doi: 10.1002/grl.50650.
- Teuber M, Zimmer I, Kreuzwieser J, Ache P, Polle A, Rennenberg H, Schnitzler J-P. 2008. VOC emissions of Grey poplar leaves as affected by salt stress and different N sources. Plant Biol. 10(1):86–96. doi: 10.1111/j.1438-8677.2007.00015.x.
- Tiiva P, Rinnan R, Faubert P, Räsänen J, Holopainen T, Kyrö E, Holopainen JK. 2007a. Isoprene emission from a subarctic peatland under enhanced UV-B radiation. New Phytol. 176(2):346–355. doi: 10.1111/j.1469-8137.2007.02164.x.
- Tiiva P, Rinnan R, Holopainen T, Mörsky SK, Holopainen JK. 2007b. Isoprene emissions from boreal peatland microcosms; effects of elevated ozone concentration in an open field experiment. Atmos Environ. 41(18):3819–3828. doi: 10.1016/j.atmosenv.2007.01.005.
- Tognetti R, Johnson JD, Michelozzi M, Raschi A. 1998. Response of foliar metabolism in mature trees of Quercus pubescens and Quercus ilex to long-term elevated CO2. Environ Exp Bot. 39(3):233–245. doi: 10.1016/S0098-8472(98)00013-6.
- Trowbridge AM, Asensio D, Eller ASD, Way DA, Wilkinson MJ, Schnitzler J-P, Jackson RB, Monson RK. 2012. Contribution of various carbon sources toward isoprene biosynthesis in poplar leaves mediated by altered atmospheric CO2 concentrations. PLoS One. 7(2):e32387. doi: 10.1371/journal.pone.0032387.
- Velikova V, Edreva A, Loreto F. 2004. Endogenous isoprene protects Phragmites australis leaves against singlet oxygen. Physiol Plant. 122:219–225. doi: 10.1111/j.0031-9317.2004.00392.x.
- Velikova V, Pinelli P, Pasqualini S, Reale L, Ferranti F, Loreto F. 2005a. Isoprene decreases the concentration of nitric oxide in leaves exposed to elevated ozone. New Phytol. 166(2):419–426. doi: 10.1111/j.1469-8137.2005.01409.x.
- Velikova V, Tsonev T, Pinelli P, Alessio GA, Loreto F. 2005b. Localized ozone fumigation system for studying ozone effects on photosynthesis, respiration, electron transport rate and isoprene emission in field-grown Mediterranean oak species. Tree Physiol. 25(12):1523–1532. doi: 10.1093/treephys/25.12.1523.
- Velikova V, Várkonyi Z, Szabó M, Maslenkova L, Nogues I, Kovács L, Peeva V, Busheva M, Garab G, Sharkey TD, Loreto F. 2011a. Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiol. 157(2):905–916. doi: 10.1104/pp.111.182519.
- Velikova V, Tsonev T, Loreto F, Centritto M. 2011b. Changes in photosynthesis, mesophyll conductance to CO2, and isoprenoid emissions in Populus nigra plants exposed to excess nickel. Environ Pollut. 159(5):1058–1066. doi: 10.1016/j.envpol.2010.10.032.
- Vickers C, Possell M, Cojocariu C, Velikova V, Laothawornkitkul J, Ryan A, Mullineaux P, Hewitt CN. 2009. Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant Cell Environ. 32:520–531. doi: 10.1111/j.1365-3040.2009.01946.x.
- Wilkinson MJ, Monson RK, Trahan N, Lee S, Brown E, Jackson RB, Polley HW, Fay PA, Fall R. 2009. Leaf isoprene emission rate as a function of atmospheric CO2 concentration. Glob Chang Biol. 15(5):1189–1200. doi: 10.1111/j.1365-2486.2008.01803.x.
- Winter TR, Borkowski L, Zeier J, Rostás M. 2012. Heavy metal stress can prime for herbivore-induced plant volatile emission. Plant Cell Environ. 35(7):1287–1298. doi: 10.1111/j.1365-3040.2012.02489.x.
- Wittig V, Ainsworth E, Naidu S, Karnosky D, Long S. 2009. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: a quantitative meta-analysis. Glob Chang Biol. 15:396–424. doi: 10.1111/j.1365-2486.2008.01774.x.
- Wu S, Mickley L, Kaplan J, Jacob D. 2012. Impacts of changes in land use and land cover on atmospheric chemistry and air quality over the 21st century. Atmos Chem Phys. 12:1597–1609. doi: 10.5194/acp-12-1597-2012.
- Xia X-J, Zhou Y-H, Shi K, Zhou J, Foyer CH, Yu J-Q. 2015. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot. 66(10):2839–2856. doi: 10.1093/jxb/erv089.
- Yu H, Blande JD. 2021. Diurnal variation in BVOC emission and CO2 gas exchange from above- and belowground parts of two coniferous species and their responses to elevated O3. Environ Pollut. 278:116830. doi: 10.1016/j.envpol.2021.116830.
- Yu H, Blande JD. 2022. A potential ozone defense in intercellular air space: clues from intercellular BVOC concentrations and stomatal conductance. Sci Total Environ. 852:158456. doi: 10.1016/j.scitotenv.2022.158456.
- Yuan X, Calatayud V, Gao F, Fares S, Paoletti E, Tian Y, Feng Z. 2016. Interaction of drought and ozone exposure on isoprene emission from extensively cultivated poplar. Plant Cell Environ. 39(10):2276–2287. doi: 10.1111/pce.12798.
- Yuan X, Feng Z, Liu S, Shang B, Li P, Xu Y, Paoletti E. 2017a. Concentration- and flux-based dose–responses of isoprene emission from poplar leaves and plants exposed to an ozone concentration gradient. Plant Cell Environ. 40(9):1960–1971. doi: 10.1111/pce.13007.
- Yuan X, Shang B, Xu Y, Xin Y, Tian Y, Feng Z, Paoletti E. 2017b. No significant interactions between nitrogen stimulation and ozone inhibition of isoprene emission in Cathay poplar. Sci Total Environ. 601-602:222–229. doi: 10.1016/j.scitotenv.2017.05.138.
- Zuo Z, Weraduwage SM, Lantz AT, Sanchez LM, Weise SE, Wang J, Childs KL, Sharkey TD. 2019. Isoprene acts as a signaling molecule in gene networks important for stress responses and plant growth. Plant Physiol. 180(1):124–152. doi: 10.1104/pp.18.01391.
