ABSTRACT
Objective
This prospective longitudinal cohort study at six tertiary referral centers in Canada and Denmark describes the clinical efficiency and surgical safety of cochlear implantation with the Oticon Medical Neuro cochlear implant system, including the Neuro Zti implant, the EVO electrode array, and the Neuro One sound processor.
Methods
Patients were adult cochlear implant candidates with bilateral sensorineural hearing loss.
Results
The mean HINT scores in quiet pre-operatively and at 3, 6, and 12 months post-activation were 13%, 58%, 67%, and 72%, respectively, and in noise (+10 dB SNR) 13%, 46%, 53%, and 59%, respectively. The mean improvement from baseline to 6 months post-activation was 54% in quiet and 40% in noise. The surgical major complication incidence rate was 0% and the post-surgical major complication incidence rate (until 12 months post-activation) was 4%. There was no adverse event that was fatal, that required explantation, or that resulted in sound processor nonuse, and no implant failure.
Conclusion
Cochlear implantation with the Oticon Medical Neuro system enables speech identification both in quiet and in noise and audiologic outcomes continue to improve in the year following activation. No substantial adverse events occurred during the surgical implantation procedure and during the 12 months post-activation.
1. Introduction
Cochlear implantation is a well-known treatment option for people with severe to profound sensorineural hearing loss. The Oticon Medical Neuro cochlear implant (CI) system consists of the Neuro Zti implant and the Neuro sound processor (first generation: Neuro One; second generation: Neuro 2). The Neuro Zti implant, available since 2015, has a titanium base and a zirconia casing. The surgery involves a minimally invasive pocket technique and two titanium screws secure the implant to the temporal bone [Citation1]. The Neuro Zti implant is compatible with body and head magnetic resonance imagining (MRI) scans up to 1.5 Tesla with the magnet in place [Citation2]. The electrical stimulation that the Neuro Zti implant delivers uses pulses with an anodic active phase and a capacitive discharge [Citation3]. The pulse amplitude is fixed, and the pulse duration is modulated to code loudness [Citation4]. The typical pulse amplitude is around 0.4 milliampere (mA). The pulse duration can be adjusted in steps of 1 microsecond (μs), from 10 and 120 μs. The typical pulse duration is 25 μs for T levels and 55 μs for C levels. The implant is compatible with two 20-channel electrode arrays, the Classic and the EVO. The Classic electrode array [Citation5], stiffer than the EVO electrode array [Citation6], is better suited for challenging anatomic situations. The EVO electrode array, used in the present study, is better suited for a soft surgery approach [Citation7]. The EVO electrode array has an active length of 24 mm, a proximal diameter of 0.5 mm, and a distal diameter of 0.4 mm. It is flexible and has a smooth surface. It consists of 20 micro-machined titanium-iridium full-band electrodes separated by silicone rings.
The Neuro One sound processor, available since 2015, offers two coding strategies: CRYSTALIS (default) and Main Peak Interleaved Sampling (MPIS). Both CRYSTALIS and MPIS are multiband spectral extraction strategies. Such strategies are also called ‘n-of-m’ as they select ‘n’ frequency channels, out of ‘m’ available, with the highest spectral energy in each stimulation cycle. CRYSTALIS has a high pitch frequency filtering mechanism to provide as much information as possible to the patient. MPIS stimulates a pre-selected maximum number of electrodes per acquisition frame (i.e., an anti-crosstalk function minimizes interaction between electrodes, so two adjacent electrodes are not stimulated at the same time). Both strategies can be combined with either Coordinated Adaptive Processing (CAP; default) or a multi-band compression function (XDP). CAP uses continuous automated environment detection to coordinate the selection of signal processing strategies such as Free Focus, Voice Track, Voice Guard, and Wind Noise. Free Focus applies automatic adaptive directionality with the aim to improve speech intelligibility in noise. Voice Track applies Wiener filter-based multiband single-channel noise reduction to improve speech intelligibility in noise as well as sound quality [Citation8]. Voice Guard applies adaptive back-end dynamic compression in four frequency bands. The amount of compression applied is a function of the electric dynamic range of the CI patient. The input/output function is always compressive, and the amount of compression increases above the knee point. The knee point of each frequency band is constantly adjusted so that 95% of the frequency band’s sound intensity falls under its knee point. Knee points are mapped to 75% of the patient’s electric dynamic range. The wide input dynamic range improves identification of both soft and loud speech compared to logarithmic wide-band compression [Citation9]. Wind Noise applies a dual-microphone wind noise reduction algorithm with the aim to improve listening comfort. DigiMap 4.0 software is used to map the Neuro One.
Two important parameters of CI systems are their clinical efficiency and safety. The clinical efficiency of CIs has been reported extensively. CI benefit can be measured behaviorally in terms of speech perception abilities in quiet and in noise. Several test materials have been devised, including the Hearing in Noise Test (HINT), in which listeners repeat sentences such as the boy fell from the window and the wife helped her husband [Citation10]. HINT performance can be measured in quiet and in noise. As expected, HINT performance typically increases considerably after CI activation; for how long the performance continues to improve post-activation before reaching a plateau is variable but is most likely to be at least 1 year [Citation11,Citation12]. One year after activation, typical scores of adults with postlingual deafness tested in a CI-only condition with English HINT sentences presented at 60 dB SPL range from 69 to 74% in quiet [Citation12,13,Citation13] and from 54 to 61% in 70 dB noise, equivalent to a + 10 dB signal-to-noise ratio (SNR) [Citation11,Citation13]. In the Zwolan et al. study [Citation13], HINT sentences were presented from the front (0-degree azimuth) and speech-shaped noise was presented from the CI side (90-degree or 270-degree azimuth), while the Cusumano et al. study [Citation11] presented both sentences and speech from the front. A recent study reported the monosyllabic word identification scores in quiet and in noise of 44 adult Neuro CI system users [Citation14]; however, the sentence identification scores of Neuro CI system users has yet to be reported.
The perioperative and postoperative safety of CIs has been described at length. Major complications include significant medical problems that are life-threatening, that require hospitalization or surgery with or without explantation or re-implantation, or that result in permanent disability or damage such as tinnitus, facial stimulation, or pain that electrode deactivation cannot alleviate [Citation15]. Minor complications include conditions that resolve spontaneously, without surgical intervention, or with conservative medical management. Cohen and Hoffman [Citation15] reported on CI safety based on a sample of over 1,000 CI recipients in the United States. They classified adverse events into three categories: medical-surgical complications (intra-operative complications), adverse reactions (postoperative complications), and device-related problems (failure of any part of the device). Medical-surgical complications were further defined as major (required surgical intervention or hospitalization) or minor (resolved spontaneously or with noninvasive treatment such as medication). Flap breakdown, facial palsy, and incorrect electrode placement were the most common complications [Citation15]. Subsequent retrospective file reviews have reported on CI surgical safety. In 180 adults implanted with one or two CIs at a Danish center, the overall complication incidence rate was 58.8% and the major complication incidence rate was 1.6% [Citation16]. The most common complications were vertigo/imbalance (25.0%) and wound infection (8.9%). In 168 adults implanted with one CI at a French center, the complication incidence rate was 19.9% and the major complication incidence rate was 5.0% [Citation17]. In 1,017 adults and children implanted with one or two CIs at an Irish center, the major complication incidence rate was 1.7% [Citation18]. The safety of the Neuro Zti has yet to be reported.
The purpose of this study was to prospectively document the clinical efficacy and safety of the Neuro CI system in a sample of adults meeting typical CI candidacy criteria (i.e., bilateral severe to profound sensorineural hearing loss).
2. Methods
This was a multinational, multicenter, prospective, open label, repeated measures clinical study. The clinical trial was conducted in five CI centers in Canada and one CI center in Denmark.
The protocol was registered in the U.S. National Library of Medicine ClinicalTrials.gov database (identifier NCT02941627) and was approved by each of the research ethics committees of the six CI centers. All participants provided written consent.
2.1. Participants
Eligibility criteria were the following: 18 years or older; bilateral severe to profound postlingual sensorineural hearing loss with average of pure-tone air-conduction hearing thresholds ≥ 70 dB HL at 0.5, 1, and 2 kHz, bilaterally; limited benefit from appropriately fitted hearing aid(s), with HINT sentence recognition in quiet ≤ 50% binaurally in the best listening condition; primary implantation; no anatomical contraindications to cochlear implantation (i.e., no potential impediment to full electrode insertion such as otosclerosis, malformation, or ossification as well as absence of central auditory lesions); native in local language (i.e., English, French, or Danish), and; psychologically suitable for cochlear implantation as deemed by the treating CI team.
Six CI centers identified 57 cochlear implant candidates in the 23-month period January 2017 – November 2018 and 53 patients enrolled in the study. One patient passed away in the period from enrollment to surgery. A total of 52 patients underwent surgery and were included in the safety analysis.
The study used the HINT sentence material both for pre-operative candidacy assessment and post-operative clinical efficacy assessment. The HINT English [Citation10], French Canadian [Citation19], and Danish [Citation20] versions were used. The study protocol stated that only the HINT scores of the participants who completed the English-language HINT were to be analyzed (i.e., participants from the four English-speaking Canadian CI centers). This methodological decision was made for two reasons. Firstly, HINT thresholds cannot be directly compared across languages without standardization to a HINT score with a specified mean and standard deviation [Citation21]. Secondly, the two sub-samples of participants that completed the French Canadian and the Danish HINTs were expected to be limited (n < 10 for each). Of the 34 participants from the English-speaking centers, 31 completed the study without protocol deviation and were therefore included in the clinical efficiency analysis.
summarizes the characteristics of the two samples: the 52 participants who underwent surgery (i.e., full sample) and the 31 participants of the full sample who completed the English-language HINT and were included in the clinical efficiency analysis.
Table 1. Patients demographics. Where no missing values are reported, all values were available. ᵞDuration of hearing aid usage is the difference between the age at cochlear implantation and the age at first hearing aid fitting. ᵝDuration of severe to profound hearing loss is the difference between the age at cochlear implantation and the age at onset of severe to profound hearing loss. *CI side is only relevant for implanted patients (i.e., N = 50 for full sample and N = 31 for native English-speaking sample)
2.2. Study procedure
summarizes the study procedure. Screening included a pre-operative evaluation to confirm CI candidacy with tympanometry to rule out middle ear pathology affecting transmission properties and CT or MRI to rule out lesions of the auditory pathways. Participants enrolled in the study 4 weeks before surgery.
Figure 1. Study design. M1 refers to 1-month post-surgery. M3, M6, and M12 refer to 3, 6, and 12 months post-activation, respectively
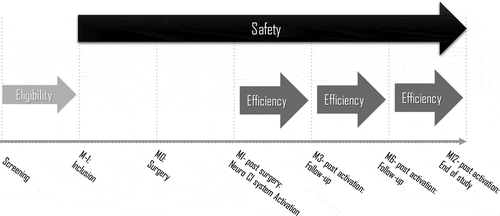
Patients were implanted in the period February 2017 – December 2018. All patients were implanted unilaterally with the Neuro Zti implant and the 20-channel EVO electrode array. CI surgery was performed according to conventional surgical practice, including a transmastoid approach with posterior tympanostomy and insertion of the electrode through the round window [Citation22]. All participating surgeons were experienced in otologic, neurotologic, and CI surgery. Oticon Medical provided training specific to Neuro Zti implantation and EVO electrode array insertion as needed. The duration of each surgery was documented, from its first step, the postauricular incision, to its last step, surgical wound closure and its coverage with a suitable dressing.
The CI centers activated the CI and mapped the Neuro One sound processor 1 month after surgery, following a standardized mapping training provided by Oticon Medical. Participants visited their CI center at 3, 6, and 12 months post-activation for trial measures as well as further mapping. Mapping parameters were not recorded as part of this trial. Electrically evoked Compound Action Potentials (eCAPs) were measured perioperatively. The impedance of the 20 electrodes was measured perioperatively and at every following visit. Furthermore, it was recommended to measure electrically evoked Auditory Brainstem Responses (eABR) and stapedius reflexes perioperatively. Beyond the trial protocol, CI centers performed additional measures and procedures as part of standard care. Unscheduled visits could occur in addition to the planned visits if adverse events occurred or if additional sound processor mapping was needed.
Two main outcomes are reported. Firstly, clinical efficacy is reported on the English-speaking center patients (N = 31). Secondly, safety is reported in terms of surgical safety (intra-operative major complication incidence rate; N = 52) and post-surgical safety (major complication incidence rate, from the surgery to 12 months post-activation, to determine the long-term safety profile of the Neuro CI system; N = 50).
2.3. Outcomes
To assess the clinical efficiency safety of the Neuro CI system, speech perception performance using the HINT sentences was measured both in quiet (HINT-Q) and in noise (HINT-N, at +10 dB SNR) at the inclusion visit and at 3, 6, and 12 months post-activation (baseline, M3, M6, and M12, respectively). Participants were tested in the best-aided condition at baseline and with their CI only at all other visits. The HINT sentence lists were block randomized so that no patient was presented with the same list twice. For all tests, the HINT sentences were presented from one loudspeaker located in front of the participant (0° azimuth). In quiet, the HINT sentences were presented at 60 dB SPL. In noise, the HINT sentences were presented at 65 dB SPL and the speech-shaped noise was presented at 55 dB SPL (+10 dB SNR). Task training was provided with practice sentences. For each test condition, one randomly selected list of 20 sentences was presented. Each HINT sentence includes 4–5 keywords that are scored. The number of words correctly repeated divided by the total number of words for each list is multiplied by 100 to obtain the percentage of correctly repeated words for each list [Citation10]. The clinical trial protocol defined the primary clinical efficiency outcome as the change in HINT-Q scores from baseline to 6 months post-activation.
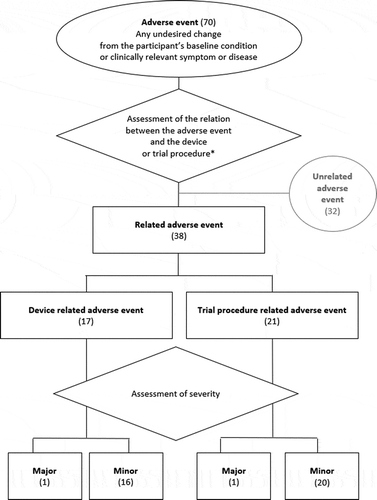
To assess the safety of the Neuro CI system, adverse events were recorded in accordance with Cohen and Hoffman [Citation15]. describes the classification used. An adverse event was defined as any undesired change from the participant’s baseline condition or clinically relevant symptom or disease, regardless of its cause. Adverse events could be related to the device or trial procedure (focus of this study) or unrelated (reported for the sake of completeness). The treating surgeon described the relation between the adverse event and the device and the trial procedure. Furthermore, the severity of the adverse events was classified as major if they were life-threatening, they required hospitalization, surgery with or without explantation or re-implantation, or they resulted in permanent disability or damage such as tinnitus, facial nerve stimulation, or pain that cannot electrode deactivation could not alleviate. Minor adverse events resolved spontaneously, without surgical intervention, or with conservative medical management. The primary safety outcome measure was the incidence rate of major related adverse events a) during surgery and b) in the post-surgical period from activation to 12 months post-activation.
Figure 2. Adverse event classification used following Cohen and Hoffman (1991). The numbers in brackets represent the number of adverse events experienced in the period from surgery to 12 months post-activation. * The treating surgeon also recorded if any of the adverse events was fatal or resulted in explantation or sound processor usage discontinuation. None of these occurrences was reported
2.4. Statistical analyses
Statistical analyses were performed using the software SAS version 9.4 (SAS Institute, Cary NC). Descriptive statistics are used for the safety data. A linear mixed-effects model for repeated measures was fitted to each the HINT-Q and the HINT-N scores with a random intercept for participants and a fixed effect for visits as covariates. To further explore the data, a repeated-measures ANOVA and a post-hoc Tukey test were also conducted for each the HINT-Q and the HINT-N scores to assess mean changes from one time point to another. For all analyses, statistical significance was defined as p ≤ 0.05.
3. Results
3.1. Clinical efficiency
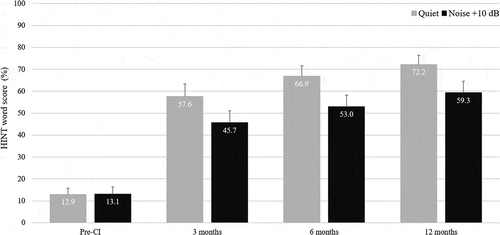
HINT speech performance was measured prior to CI surgery and at 3, 6, and 12 months after CI activation. depicts HINT-Q and HINT-N scores in the 31 native English-speaking participants that completed the study. The mean HINT-Q scores at baseline, M3, M6, and M12 was 12.9% ± 15.7 (CI = [7.2; 18.7]), 57.6% ± 31.6 (CI = [46.0; 69.2]), 66.9% ± 26.5 (CI = [57.1; 76.6]), and 72.2% ± 23.5 (CI = [63.6; 80.8]), respectively. The time course of the HINT-Q improvement was estimated with a linear mixed model for repeated measures using the change from baseline as the response and the participants (random effect) and the visit (fixed effect) as covariates. Mean change from baseline at 3, 6, and 12 months after activation were 44.7% ± 38.5 (CI = [30.6; 58.8]), 53.9% ± 32.6 (CI = [42.0; 65.9]), and 59.3% ± 29.5 (CI = [48.4; 70.1]), respectively. Mean change in performance from M3 to M6, from M3 to M12 and from M6 to M12 were 9.2% ± 14.0 (CI = [4.1; 14.4]), 14.6% ± 17.1 (CI = [8.3; 20.9]), and 5.3% ± 14.7 (CI = [−0.04; 10.7]), respectively.
Figure 3. Mean HINT results in quiet (HINT-Q) and in noise (HINT-N) pre-cochlear implantation and at 3, 6, and 12 months post-activation (N = 31). The error bars represent standard deviations
The linear mixed model revealed that HINT-Q scores increased over the 12 months following activation, with a significant visit effect (F(32) = 26.3, p < 0.001) meaning that overall the HINT-Q scores increased over time. An ANOVA analysis showed that changes in HINT-Q scores between visits were not all similar, with at least one of between-visit changes statistically significantly different from others (F(3) = 18.7, p < 0.001). A post-hoc Tukey test found that the changes in HINT-Q scores from M3 to M6, from M6 to M12, and from M3 to M12 were not significantly different from each other, but the changes from baseline to M3, M6, and M12 were significantly larger.
The same analysis approach was used for HINT-N scores. The mean HINT-N scores at baseline, M3, M6, and M12 were 13.1% ± 17.9 (CI = [6.6; 19.7]), 45.7% ± 30.5 (CI = [34.5; 56.8]), 53.0% ± 31.0 (CI = [41.6; 64.4]), and 59.3% ± 29.7 (CI = [48.4; 70.2]), respectively. Mean change from baseline at 3, 6, and 12 months after activation were 32.5% ± 34.8 (CI = [19.8; 45.3]), 39.9% ± 34.4 (CI = [27.3; 52.5]), and 46.2% ± 34.2 (CI = [33.6; 58.7]), respectively. Mean change in performance from M3 to M6, from M3 to M12 and from M6 to M12 were 7.3% ± 12.8 (CI = [2.6; 12.0]), 13.6% ± 15.8 (CI = [7.9; 19.4]), and 6.3% ± 10.6 (CI = [2.4; 10.2]), respectively.
The linear mixed model revealed that HINT-N scores increased over the 12 months following activation with a significant visit effect (F (32) = 30.14, p < 0.001) meaning that overall, the HINT-N scores increased over time. An ANOVA showed that these changes of HINT-N scores between visits are not all similar, with at least one of the between-visit changes statistically significantly different from others (F (3) = 10.08, p < 0.001). A post-hoc Tukey test found that the changes in HINT-N scores followed the same pattern as HINT-Q scores: changes from M3 to M6, from M6 to M12, and from M3 to M12 were not significantly different from each other, but changes from baseline to M3, M6, and M12 were significantly larger.
3.2. Surgical report
Overall, 58% of participants (n = 29) had a right-ear implantation while 42% (n = 21) had a left-ear implantation. The median surgery duration was 80 minutes and 95% confidence intervals [82.27; 100.31]. The mean surgery duration was 91.29 ± 31.75 minutes, with a range from 47 to 186 minutes. Study surgeons reported that the use of titanium screws was an efficient method of device fixation compared to conventional techniques such a device well or suture fixation. Electrode arrays were fully inserted in 74% of surgeries. In the cases of partial electrode array insertion, an average of 0.82 (± 1.60) extracochlear electrodes was observed.
3.3. Safety
During surgery, no related adverse event (i.e., complication) was recorded (0% surgical complication incidence rate). Of the 52 participants, two could not be implanted. A cholesteatoma undiagnosed during pre-operative imaging prevented cochlear implantation in one patient. Another participant could not be implanted due to electrode handling issues. Post-surgical adverse events were therefore monitored in the 50 implanted participants.
In the period from after surgery to 12 months post-activation, 70 adverse events were recorded in the 50 participants implanted. summarizes the number of adverse events recorded and their types. In total, 32 unrelated adverse events were recorded in 15 of the 50 participants. Examples included influenza, sinusitis, hepatic cancer, renal failure requiring dialysis, pain following teeth extraction, and trauma following physical assault.
Of interest are the 38 complications recorded in 20 of the 50 participants that were either related to the device (17 complications in 12 of the 50 patients) or to the trial procedure (21 complications in 10 of the 50 patients). Those included otalgia, dizziness, and tinnitus. One major device-related complication was recorded: onset of mild tinnitus at surgery, which rose to moderate tinnitus during the study period. One major trial procedure-related complication was recorded: exacerbation of chronic obstructive pulmonary disease requiring hospitalization. The post-surgical major complication incidence rate was therefore 4% (2 complications recorded in 2 of the 50 patients).
During the study, there was no adverse event that was fatal or that led to explantation or to sound processor usage discontinuation. Furthermore, no implant failure was reported. Other than the one case of unresolved tinnitus, there was no report of extracochlear stimulation leading to non-auditory percepts such as facial nerve stimulation. In total, four cases of infections were reported (i.e., 2 chronic bronchitis, 1 influenza, and 1 cystitis). All four were deemed not related to the device. Three cases were deemed not related to the trial procedure, while one case of chronic bronchitis was classified as possibly related to the trial procedure.
4. Discussion
This study reported on the clinical efficacy of the Oticon Medical Neuro CI system and the surgery duration and the safety profile of the Neuro Zti implant, and in particular the incidence of adverse events during surgery and up to 12 months post-activation.
4.1. Clinical efficacy
The mean HINT scores at baseline, M3, M6, and M12 were 13%, 58%, 67%, and 72% in quiet, respectively, and 13%, 46%, 53%, and 59%, respectively, in noise. The mean improvement from baseline to 6 months post activation was 54% in quiet and 40% in noise. These results are comparable to studies that used the same sentence materials and presentation levels in adult CI users [Citation11–13]. Of course, comparisons between studies should be done with caution given the large interindividual variability in CI outcomes as well as methodological differences. In this study, the pre-operative scores were measured in the best-aided condition. For example, pre-operative HINT scores for our sample were similar to those of Massa and Ruckenstein [Citation12], but lower than Lin et al. [Citation23] and Zwolan et al. [Citation13]. The post-operative scores were measured with the CI only and with both the HINT sentences and the noise coming from the same loudspeaker. Two studies report HINT in noise results: in Zwolan et al. [Citation13], the sentences and the noise were presented from different loudspeakers (spatially separated), while the sentences and noise were co-located in Cusumano et al. [Citation11], as in the present study. Factors such as interindividual variability and spatial release from masking are known to affect sentence identification test scores [Citation24].
In the present study, improvements were largest from baseline to 3 months following activation, supporting the large CI treatment effects in adults with postlingual deafness. Speech identification scores continued to improve until 12 months post-activation. Repeated measures of speech perception can lead to procedural and content learning, which can be erroneously described as generalized improvements in speech perception [Citation25]. In this study, HINT lists were block randomized so that no patient was presented with the same list twice to avoid any content learning. However, procedural learning (e.g., increased familiarity with the voice and speech characteristics of the speaker) could have occurred. In young listeners with normal hearing acuity tested with 100 HINT sentences on each of three separate days within a 2-week interval, procedural learning amounted to a 0.4 dB SNR improvement in thresholds, which the researchers described as a small effect [Citation25]. In comparison to [Citation25], participants in the present study had already completed several speech perception tests as part of standard care and were presented with fewer HINT sentences and over a longer period, thereby reducing potential procedural learning. Therefore, although it cannot be ruled out that procedural learning occurred in the present study, its magnitude is likely to be limited.
4.2. Surgical report
The median surgery duration was 80 minutes (range: 47–186 minutes) and the mean was 91 minutes. A retrospective study of 2,025 unilateral cochlear implantations performed in Germany and the U.S. in the period 1997–2007 reported a mean surgery duration of 171 minutes [Citation26]. There is an overall trend for the length of the CI surgical procedure to become shorter [Citation26,Citation27]. Several factors are associated with longer cochlear implantation surgery duration, including less surgeon experience and center experience as well as trainee participation and abnormal anatomy and complications [Citation26–28]. Furthermore, surgery duration is associated with CI manufacturer [Citation26]. Oticon Medical’s screw fixation system requires no bone bed drilling, which could contribute the shorter surgery duration reported in the present study. Study surgeons reported that the use of titanium screws was an efficient method of device fixation compared to conventional techniques such a device well or suture fixation. Flap infection is a common post-surgical complication [Citation15,Citation16]; the present study reported no case of infection at the surgical site. The present study suggests that a shorter CI surgery due to device fixation with screws requires no trade-off with safety nor clinical efficiency and therefore increases CI cost-effectiveness.
4.3. Safety
Using the CI surgical complication classification of Cohen and Hoffman [Citation15], the surgical (i.e., intra-operative) complication incidence rate was 0%. The post-surgical (i.e., in the 12 months following activation) complication incidence rate was 4% for major complications. Using the Clavien-Dindo surgical complication classification [Citation29], the complications reported were very minor (grade I or II out of V). The one major complication recorded, tinnitus, is also mentioned in other reports of CI complications [Citation17,Citation18], but its occurrence is not always systematically recorded in such studies [e.g., 17]. The most common minor complications reported were transient episodes of dizziness and pain – dizziness occurred in 4 of the 50 participants in this study (8%), while it occurred in 25% of 180 patients in Hansen et al. [Citation16] and 4% of 403 patients in Farinetti et al. [Citation17]. In the present study there was no fatal adverse event and no adverse event that led to explantation or to sound processor usage discontinuation. Other than for one case of unresolved tinnitus, no non-auditory percepts, such as facial nerve stimulation, were reported. No device failure was reported, however as device failure is a rare occurrence and this study reported post-surgical adverse events in 50 CI users, the study was not designed to generate reliable device failure incidence rates and these are published elsewhere [Citation30]. Overall, the results show that implantation with the Oticon Medical Neuro Zti is a safe surgical procedure and that few complications occur in the year following activation.
4.4. Strengths and limitations
Strengths of this study include a prospective multicenter design as well as follow-up that spanned to 12-month post-activation. Limitations include variations in rehabilitation procedures, as each center followed their own procedures. Patients were using their CI settings during the HINT test, which also varied from patient to patient. Only one type of behavioral measure for clinical efficacy (i.e., sentence identification in quiet and in noise) was used. Furthermore, only the results of HINT sentence tests performed in English were analyzed. However, as previously discussed it was not felt to be methodologically feasible to combine the results of HINT scores performed in various languages for analysis. Additional auditory perception measures would have been relevant to include, for example according to the Minimum Speech Test Battery for Adult Cochlear Implant Users [Citation31]. This includes speech materials commonly used with CI users such as the CNC words [Citation32,Citation33] and AZBio sentences [Citation12,Citation34]. It could also have been relevant to include multiple noise sources and different noise types. Objective measures of listening effort, ecological momentary assessments, as well as patient-reported outcome measures could have further described the functional benefit that patients derive from the Oticon Medical Neuro CI system. Future studies should explore these other outcome measures.
5. Conclusion
This study shows that cochlear implantation with the Oticon Medical Neuro system enables speech identification both in quiet and in noise and audiologic outcomes continue to improve in the year following activation. Implantation is an efficient and safe procedure, with no substantial adverse events recorded both during surgery and in the period to 12 months post-activation.
Declaration of interest
Oticon Medical covered the trial costs but did not provide honoraria to the investigators and staff of the participating CI centers. M Hoen, C Karoui, A Laplante-Lévesque, and D Gnansia are employees of Oticon Medical. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One peer reviewer was on the advisory committee for Oticon Medical cochlear implant. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Guevara N, Sterkers O, Bébéar JP, et al. Multicenter evaluation of the Digisonic SP cochlear implant fixation system with titanium screws in 156 patients. Ann Otol Rhinol Laryngol. 2010;119(8):501–505.
- Todt I, Rademacher G, Grupe G, et al. Cochlear implants and 1.5 T MRI scans: the effect of diametrically bipolar magnets and screw fixation on pain. J Otolaryngol Head Neck Surg. 2018;47(1):11. .
- Cogan SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng. 2008;10(1):275–309.
- Di Lella FA, Bacciu E, Pasanisi V, et al. Main Peak Interleaved Sampling (MPIS) strategy: effect of stimulation rate variations on speech perception in adult cochlear implant recipients using the Digisonic SP cochlear implant. Acta Otolaryngol. 2010;130(1):102–107. .
- Vashishth A, Fulcheri A, Prasad SC, et al. Cochlear implantation in cochlear ossification: retrospective review of etiologies, surgical considerations, and auditory outcomes. Otol Neurotol. 2018;39(1):17–28.
- Nguyen Y, Miroir M, Kazmitcheff G, et al. Cochlear implant insertion forces in microdissected human cochlea to evaluate a prototype array. Audiol Neurotol. 2012;17(5):290–298.
- Bento RF, Danieli F, de Matos Magalhães AT, et al. Residual hearing preservation with the Evo® cochlear implant electrode array: preliminary results. Int Arch Otorhinolaryngol. 2016;20(4):353–358.
- Guevara N, Bozorg-Grayeli A, Bébéar JP, et al. The Voice Track multiband single-channel modified Wiener-filter noise reduction system for cochlear implants: patients’ outcomes and subjective appraisal. Int J Audiology. 2016;55(8):431–438.
- Bozorg-Grayeli A, Guevara N, Bébéar JP, et al. Clinical evaluation of the xDP output compression strategy for cochlear implants. Eur Arch Otorhinolaryngol. 2016;273(9):2363–2371.
- Nilsson M, Soli SD, Sullivan JA. Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am. 1994;95(2):1085–1099.
- Cusumano C, Friedmann DR, Fang Y, et al. Performance plateau in prelingually and postlingually deafened adult cochlear implant recipients. Otol Neurotol. 2017;38(3):334–338.
- Massa ST, Ruckenstein MJ. Comparing the performance plateau in adult cochlear implant patients using HINT and AzBio. Otol Neurotol. 2014;35(4):598–604.
- Zwolan TA, Henion K, Segel P, et al. The role of age on cochlear implant performance, use, and health utility: A multicenter clinical trial. Otol Neurotol. 2014;35(9):1560–1568.
- Franco-Vidal V, Parietti-Winkler C, Guevara N, et al. The Oticon Medical Neuro Zti cochlear implant and the Neuro 2 sound processor: multicentric evaluation of outcomes in adults and children. Int J Audiol. 2020;59(2):153–160.
- Cohen NL, Hoffman RA, Stroschein M. Medical or surgical complications related to the Nucleus multichannel cochlear implant. Ann Otol Rhinol Laryngol. 1988;97(Suppl 2):8–13.
- Hansen S, Anthonsen K, Stangerup SE, et al. Unexpected findings and surgical complications in 505 consecutive cochlear implantations: A proposal for reporting consensus. Acta Otolaryngol. 2010;130(5):540–549.
- Farinetti A, Gharbia DB, Mancini J, et al. Cochlear implant complications in 403 patients: comparative study of adults and children and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(3):177–182.
- Petersen H, Walshe P, Glynn F, et al. Occurrence of major complications after cochlear implant surgery in Ireland. Cochlear Implants Int. 2018;19(6):297–306.
- Vaillancourt V, Laroche C, Mayer C, et al. Adaptation of the HINT (hearing in noise test) for adult Canadian Francophone populations. Int J Audiol. 2005;44(6):358–361.
- Nielsen JB, Dau T. The Danish hearing in noise test. Int J Audiol. 2011;50(3):202–208.
- Soli SD, Wong LLN. Assessment of speech intelligibility in noise with the Hearing in Noise Test. Int J Audiol. 2008;47(6):356–361.
- House WF. Cochlear implants. Ann Otol Rhinol Laryngol. 1976;85(Suppl 27):1–93.
- Lin FR, Chien WW, Li L, et al. Cochlear implantation in older adults. Med. 2012;91(5):229–241.
- Theunissen M, Swanepoel DW, Hanekom J. Sentence recognition in noise: variables in compilation and interpretation of tests. Int J Audiol. 2009;48(11):743–757.
- Yund EW, Woods DL. Content and procedural learning in repeated sentence tests of speech perception. Ear Hear. 2010;31(6):769–778.
- Majdani O, Schuman TA, Haynes DS, et al. Time of cochlear implant surgery in academic settings. Otolaryngol Head Neck Surg. 2010;142(2):254–259.
- Bennett ML, Sweeney AD, Haynes DS, et al. The utility of a predictive model for cochlear implant operating time. Otol Neurotol. 2016;37(2):e104–9.
- Puram SV, Kozin ED, Sethi RK, et al. Influence of trainee participation on operative times for adult and pediatric cochlear implantation. Cochlear Implants Int. 2015;16(3):175–179.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
- Oticon Medical. 2019 Reliability Report. [cited 2020 Jun 25]. Available from: https://wdh01.azureedge.net/-/media/medical/main/files/for-professionals/ci/reliability-report/212099uk_pbr_reliability-report_2019_version_b_2019-09_low-final_2019-09-16.pdf
- Luxford WM. Minimum speech test battery for postlingually deafened adult cochlear implant patients. Otolaryngol Head Neck Surg. 2001;124(2):125–126.
- Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Dis. 1962;27(1):62–70.
- Bierer JA, Spindler E, Bierer SM, et al. An examination of sources of variability across the consonant-nucleus-consonant test in cochlear implant listeners. Trends Hear. 2016;30(20):2331216516646556.
- Spahr AJ, Dorman MF, Litvak LM, et al. Development and validation of the AzBio sentence lists. Ear Hear. 2012;33(1):112–117.
