ABSTRACT
Introduction
Sacroiliac joint disease is a prominent diagnosis across the world. A novel fixation technique employing a posterior approach, single point, bone allograft transfixation has proven to be helpful anecdotally. The purpose of this is study is to investigate prospectively the safety and efficacy of this approach.
Methods
A multicenter, prospective, single arm study was performed after patient identification and treatment with the novel posterior fusion, single-point transfixation system and followed for 24 months. Target enrollment is 100 patients. Interim results on the first 69 consecutive patients at 6 months is presented. Primary endpoint at 6-month analysis was Pain Intensity reduction by visual analogue scale and functional improvement by Oswestry Disability Index. Adverse events were assessed for safety analysis.
Results
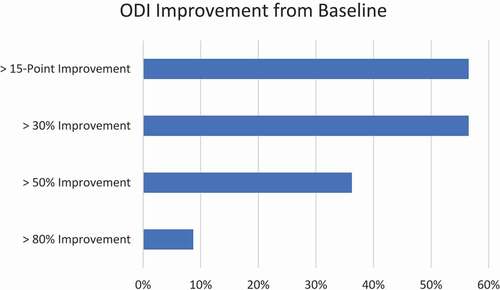
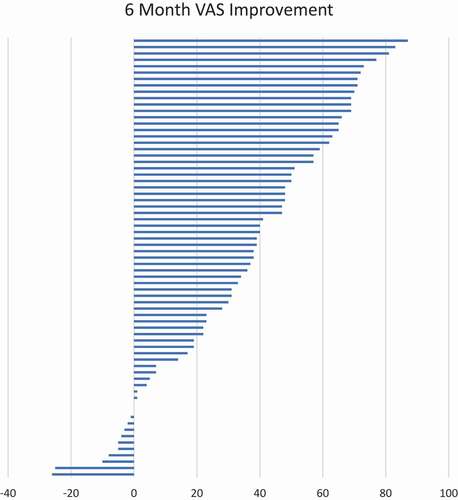
In total, 69 patients were identified for this analysis. At 6 months, a mean improvement of 34.9 was identified by a reduction in VAS and functional improvement was demonstrated by a mean reduction in ODI of 17.7. There were three adverse events, all unrelated to the device.
Conclusion
The posterior single point transfixation is safe and efficacious for the treatment of sacroiliac joint dysfunction with statistical improvements in pain and function.
1. Introduction
Sacroiliac joint dysfunction is a condition affecting the sacroiliac joint (SIJ) resulting in non-radicular low back and buttock pain. Patients with this condition often report referred pain in the posterior thigh, knee, or foot with the posterior thigh constituting up to 50% of patient reported referral patterns [Citation1]. It can be due to primary joint disease from trauma or secondary to rheumatologic, infectious, drug-related, or oncologic diseases [Citation2]. It has a become an increasingly recognized cause of low back pain, affecting 15–30% of people with chronic, non-radicular pain [Citation3].
Conservative treatment for this condition includes physical therapy, chiropractic care, orthotics, and non-steroidal anti-inflammatory drugs. When conservative therapies fail, traditional non-operative interventional therapies include periarticular steroidal injection, intraarticular steroidal injections, radiofrequency ablation (RFA), intraarticular prolotherapy, intraarticular platelet-rich plasma injection, and periarticular botulinum toxin injection [Citation4]. Though effective, these therapies have only been shown to provide short term relief based on limited published studies [Citation5–11]. In situations where conservative and non-operative therapies fail to provide sustained relief, SIJ fusion is often pursued.
Initial open fusion surgeries were associated with long operative times, relatively high estimated blood loss and long postoperative mean length of hospital stay as well as high rates of complications [Citation12]. Subsequently, minimally invasive (MI) SIJ fusion has been pioneered resulting in greater long term pain reduction, shorter operative time, and shorter postoperative mean length of stay [Citation13,Citation14]. Most SIJ fusions are performed in a MI approach with open surgery saved mostly for traumatic fractures [Citation15]. A literature review by Shamrock and et al evaluated the safety profile of the lateral approach MI SIJ fusion of 14 studies revealed a visual analog score (VAS) reduction from 82.42 to 29.03 with a complication rate of 11.11% and a revision rate of 2.56% [Citation16]. Recently, 5 year data was presented for the patients involved in both the open label iMIA and SIFI studies with clinical outcomes as defined by success as Visual Analog Scale (VAS) reduction of at least 20 mm on a 100 mm scale in the absence of a device related adverse event, absence of neurologic worsening, and absence for need of surgical revision, along with a mean reduction from baseline, and further an Oswestry Disability Index (ODI) mean point reduction, along with radiographic analysis [Citation17]. In total, 93 patients were followed for 5 years. In the 5-year period, mean VAS reduction was 54.1 and 82.8% had met the primary endpoint. ODI decreased with a mean improvement of 26.2. In total, 68.8% had an ODI improvement of at least 15 points from baseline [Citation17].
Though minimally invasive, the lateral trans-iliac approach does require gluteus maximus and gluteal fascial dissection as well as drill bone decortication along with a significant postoperative weight bearing restriction [Citation17]. Newer posterior approach techniques have been pioneered, avoiding neurovascular structures allowing for minimally invasive access. Multiple methods currently exist including screw fixation devices and machined allograft placement fixation devices [Citation18].
The need for a minimally invasive, less morbid, non-inferior method to more appropriately care for sacroiliac joint dysfunction is needed.
The posterior approach SIJ fusion with the LinQ implant platform for sacroiliac joint stabilization and arthrodesis was utilized for this study. Formal studies evaluating the safety and efficacy of the allograft placement technique are lacking but review of available literature is promising. Recently Sayed et al published a retrospective review detailing a numeric rating scale (NRS) score reduction from 6.98 to 3.06 in patients with an average follow up of 612.2 days post implant [Citation19]. That said, prospectively acquired data is lacking for the posterior fusion approach, due to the novelty and access to the procedure. Here, we present the first multicenter, single arm, prospective data set, at a planned analysis at 6 months for sacroiliitis treatment utilizing a novel posterior approach, single point, percutaneous machined allograft technique for SIJ fixation. We have chosen to present the first 69 patients consecutively enrolled and implanted in the study, as the precedent to present approximately 50 patients that were followed prospectively following a lateral fusion approach has already been established and published [Citation20].
2. Method
2.1. Design and sites
A multicentered prospective singled armed clinical study was performed across 15 contributing institutions. IRB approval was provided by WCG IRB (Study Number 1275013) for this study to determine the long-term efficacy and safety of the posterior approach, percutaneous allograft placement for sacroiliitis. All subjects provided informed written consent to participate in the study, and all patient data collected were de-identified to provide patient data confidentiality and compliance with the Declaration of Helsinki. The protocol and IRB were each approved by the local governing entities of each involved institution.
2.2. Patient population
Patients between the ages of 21 to 70 years of age with low back pain for greater than 6 months despite conservative care were considered for the study if they had three of four physical exam maneuvers to diagnose sacroiliitis (FABER test, Gaenslen test, Stork/Gillet Test, and Yeoman’s Test). Once diagnosed as having sacroiliitis, patients with an Oswestry Disability Index (ODI) of at least 30% and a VAS of at least 50 mm for low back/buttock were selected. These patients each underwent a local anesthetic only SIJ injection. If patients received an NRS improvement of at least 50% within 30 to 60 min of injection, they were eligible for the study.
Patients with concomitant pain diagnoses such as lumbar disc disease, lumbar disc herniation, lumbar spondylolisthesis, lumbar stenosis, lumbar facet disease, lumbar radiculopathy, or lumbar vertebral body fractures were excluded from the study if these diagnoses were believed to be a major contributor to the symptomatology. Resultingly, this did not exclude subjects with prior lumbar fusion. 11 of the 69 subjects reported on in this interim analysis had a surgical history with lumbar fusion. Additionally, if radiculopathy beyond the buttocks (VAS ≥ 30 mm) was present, these patients were also excluded from the study. Other exclusion factors include a history of steroid SIJ injection within 30 days of the diagnostic injection, complete resolution of pain for 30 days or more after the diagnostic injection, any neuraxial steroid injection within 30 days of the diagnostic injection, having SIJ RFA within the past 6 months of enrollment, history of recent pelvic trauma (< 1 year), history of prior sacral or SIJ surgery, history of endometriosis, history of coccydynia, history of coccygectomy, history of pudendal neuralgia, having an active intrathecal pump therapy for pain, current systemic infection or local infection increasing the risk of surgery, and history of medications decreasing bone quality or soft tissue healing. Principle investigators at each study site were responsible for the diagnosis of sacroiliitis as well as ensuring patients are eligible for the study based on the inclusion and exclusion criteria. Of note, inclusion and exclusion criteria was similar to previously published prospective studies on minimally invasive sacroiliac fusion [Citation20,Citation21].
2.3. Interventions
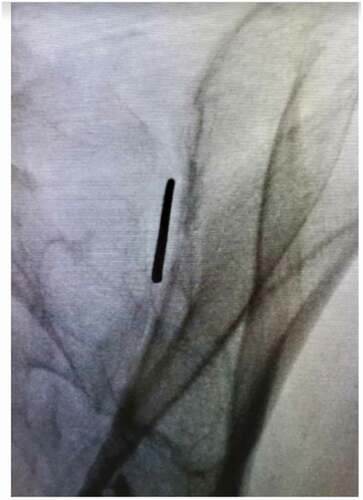
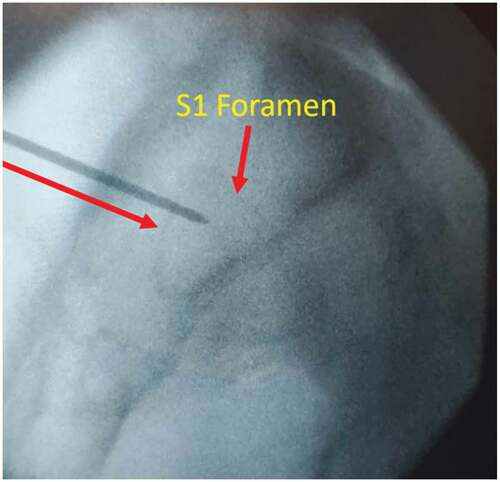
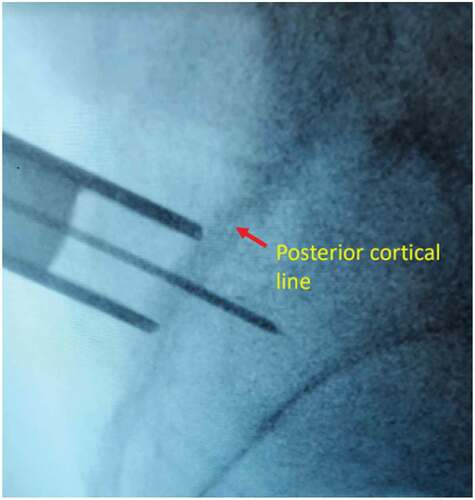
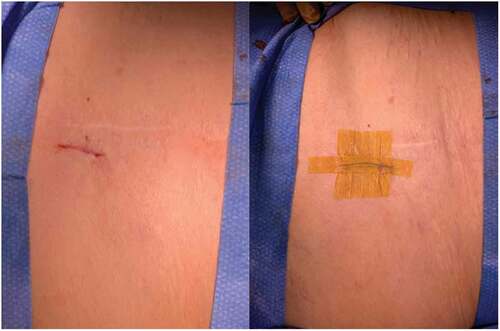
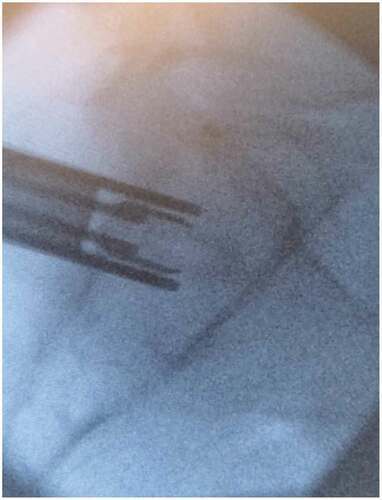
After meeting appropriate inclusion and exclusion criteria, the posterior approach SIJ fusion was performed with the patient in the prone positioning under fluoroscopic guidance as described previously by Sayed et al utilizing the LinQ Fusion Device (PainTeq, Tampa, FL) [Citation18]. Pre-operative surgical planning included review of imaging of the sacroiliac joint and spine to plan for appropriate implantation of the sacroiliac fusion device. Intraoperatively, the medial and lateral borders of the posterior aspect of the SIJ were identified and lined up to allow for a spinal needle to be placed into the joint whereby local anesthetic was used to anesthetize the surgical tract. Once the SIJ was optimally viewed, a 1.5 cm incision was made followed by blunt finger dissection down to the joint. A guide pin was then inserted into the SIJ space and confirmed on lateral views ( and ). Next, a dilator was advanced over the guide pin to the posterior cortical line of the sacrum while in the lateral view (). Once alignment was confirmed, the dilator was gently tapped on the handle with a mallet until the hard stop on the outer dilator met the posterior cortical line of the sacrum. Once the dilator was fully seated on the sacrum, the internal dilator and guide pin were removed. The SIJ was then decorticated with a broaching system. Once in optimal position, the cortical allograft packed with demineralized bone matrix was placed across the dilated joint until fully seated to stabilize and fix the sacroiliac joint. Radiographic confirmation was obtained prior to irrigation, hemostasis, and closure to confirm allograft placement. Surgical instruments were removed at the conclusion of implantation and the incision was irrigated and closed in two stage manner (). All procedures were performed in an outpatient setting with no postoperative weight bearing instructions. Patients received PO analgesics and home dressing changes as needed. Physical therapy was initiated in patients at 6 weeks if deemed appropriate by the treating physician, but not required.
3. Follow-up
Eligible patients are evaluated and identified at enrollment prior to undergoing the diagnostic SIJ injection. If satisfactory results were obtained, they then underwent SIJ fusion after consent, screening and baseline visit. Post procedurally, patients are evaluated at their immediate postoperative visit (7–14 days out for fusion), 1 months, 3 months, 6, months, and 12, 18, and 24 months post treatment. Goal enrollment with follow-up is 100 patients.
4. End points
The primary end points of this study is VAS of at least 20 mm of improvement on 100 mm scale from baseline, ODI improvement of at least 15 points from baseline, PROMIS 29 v2.1, and serious adverse events and mortality related to the device, device related complications, results at 1 month, 3 months, 6 months, 12 months, 18 months, and 24 months post implant, with secondary endpoints to the study include opioid consumption at the follow up visits, satisfactory deployment of the device based on fluoroscopic imaging, procedure time, fluoroscopy time, blood loss, and anesthetic method used. With planned data reporting at 6 months, 12 months, 18 months, and 24 months is underway, this publication represents the interim 6-month data analysis of the first 69 subjects.
The PROMIS-29 v2.1 consists of 7 domains, with evaluation based on the general population, reported as a t score, for measuring pain interference, sleep disturbance, fatigue, anxiety, depression, social participation, and physical function, with instruments of PROMIS SF v1.0 Pain Interference 4a, PROMIS SF v1.0 Sleep Disturbance 4a, PROMIS SF v1.0 Fatigue 4a, PROMIS SF v1.0 Anxiety 4a, PROMIS SF V1.0, Depression 4a, PROMIS SF v2.0, Ability to Participate in Social Roles and Activities 4a, and PROMIS SF v1.0 Physical Function 4a.
4.1. Statistical analysis
For measures including the VAS, ODI, PROMIS 29, and quantitative outcomes, paired t-test was performed. ANOVA regression analysis was utilized to identify and verify the correlations reported. The data analysis was performed utilizing IBM SPSS statistics.
5. Results
After IRB approval, 15 centers participated in this single arm, prospective study, beginning in January 2020, with completed enrollment in March 2022. All actively participating patients met the inclusion and exclusion criteria of the study.
5.1. Patient population
Patient demographics and baseline measures are identified in . The mean age of the patient population served was 60 years old, with 65% female. Baseline VAS on 100 point scale was 75, with baseline mean ODI of 51. Mean BMI was 31 with mean duration of pain of nearly 5 years.
Table 1. Baseline characteristics.
5.2. Subject activity
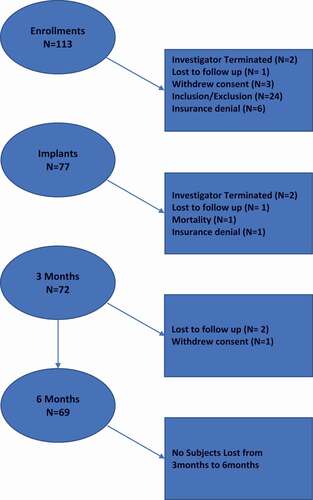
This is an on-going study with an interim analysis done at 6 months for 69 subjects. Study enrollment has ended. This manuscript is an interim analysis focused on the first 69 of 159 subjects reaching 6 months. Future publications will include the rest of the patient cohort. Study and subject activity are highlighted in . : Subject Activity, represents the 69 subjects enrollment and progression through the study for this interim analysis The data presented for analysis, at the time of the data extraction, yielded 69 patients at 6 months. As can be demonstrated, this is a single arm, prospective, multicenter study investigation treatment of symptomatic sacroiliac joint dysfunction, treated by a posteriorly inserted single point allograft within the SIJ joint, offering transfixation with fusion. The total cohort enrolled is 159 patients. We are reporting on the first 69 patients to 6 months. Please see ( and ).
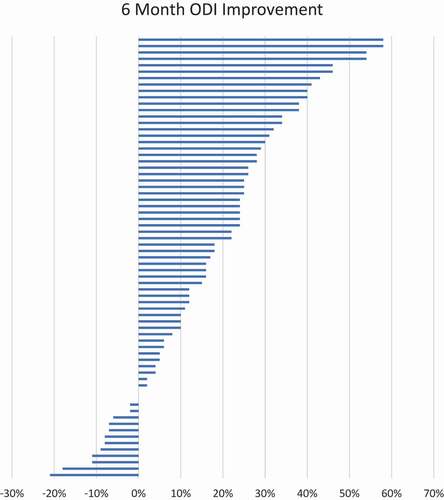
Figure 10. Tornado plot of VAS score reduction from baseline pain at 6 months. Each bar represents one subject’s change in VAS with a positive number signifying a decrease in raw score.
5.3. Surgical characteristics
Surgical characteristics of the study population are identified in . 98.6% of patients were unilateral, with 60.3% of those performed on the RIGHT.
Table 2. Surgical characteristics.
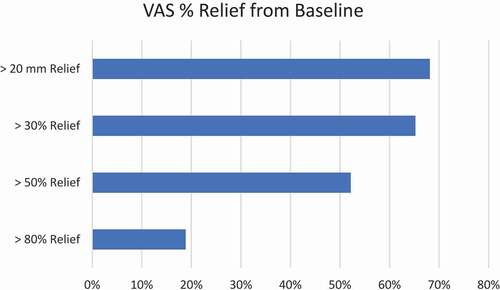
Table 3. For pain intensity assessment and ODI (represented from baseline) and percentage of responders as defined as >20 mm improvement in pain and no adverse events and ODI > 15 point gross improvement at 6 months and p value and SD.
Table 4. Pain intensity improvement by VAS at > 20 mm improvement, 30%, 50%, and 80% of more at 6 months.
Table 5. PROMIS 29v 2.1 table of mean improvement in scores at 6 months from baseline.
Table 6. Adverse events and treatment, ongoing/completed, and x ray times mean (SD).
Table 7. Pain intensity improvement and physical function comparison of the posterior and lateral approaches at 6 months [Citation20].
This comparison can be made confidently for many reasons. First, the inclusion and exclusion criteria for the study were developed to mirror the same criteria for the iMIA study and the INSITE studies, attempting to compare the treatment arms across the prospective data groups. Interestingly, due to commercial coverage, the population served in this study were slightly older than the iMIA cohort ().
Table 8. Comparison of baseline characteristics of patient cohort for SECURE versus iMIA (6 months).
Table 9. Surgical treatment comparison.
Table 10. Adverse event comparison of SECURE 6 month cohort and iMIA 6 month cohort.
5.4. Clinical outcomes
Graph Pain Intensity Responder And Calculation Of Percent Responder At 6 Months
5.5. Safety analysis
6. Discussion
This data represents the first report on any posterior approach for the treatment of sacroiliac joint dysfunction in a prospective multicenter fashion, with an enrollment of > 100 patients. We are reporting on the 6-month interim analysis for nearly 70 patients, as this has been performed for the treatment arm for historical approaches for sacroiliac joint fusion in a prospective manner [Citation20,Citation22], with planned follow-up of the complete cohort at 6, 12, 18 and 24 months [Citation21,Citation23–25]. This represents the first prospective data for posterior sacroiliac joint fusion. We chose to publish the first 69 patients due to the paucity of the data and the previous precedent with the historical standard of lateral fusion approaches.
Based on the above analysis, patients improved from baseline, after treatment, for pain intensity, measured as a VAS, and function, as measured by an ODI, in a statistically significant manner. This alone suggests the importance of the therapy and its efficacy. Furthermore, 100% were performed in the outpatient setting and 83% were done without the need for general anesthesia (MAC). This clearly is different than the predicate lateral fusion approach, with the vast majority from two prospective lateral approach studies requiring general anesthesia and an inpatient hospital stay rate of 60% with average length of stay as 1.89 days [Citation20,Citation21].
The baseline PROMIS 29v2.1 scores of 66.0, 60.3, 60.7, 56.5, 55.5, 38.8, and 34.2 for assessment of Pain Interference, Sleep Disturbance, Fatigue, Anxiety, Depression, Ability To Socially Participate, and Physical Function demonstrated baseline scores similar to those already published for patients entering into pain and spine practices of 64.61, 57.19, 58.50, 53.94, 54.45, 40.06, and 36.23 [Citation26, Citation27,Citation28]. For most PROMIS instruments, a score of 50 is the average for the United States general population, with a standard deviation of 10 16. The average PROMIS-29 instrument T-score is 50 for the US general population, with a standard deviation of 10. A greater T-score signifies a larger measurement on the domain, whereby a greater T-score for negatively worded concepts present worse than average score, and higher T-scores of positively worded attributes represent a better than average score. With sidedness of the change important for interpretation of benefit versus worsening, all patients demonstrated improvement across all instruments, as noted in , which is the first-time improvement for sacroiliac joint dysfunction treatment with fusion has been qualified using a multidimensional tool. This includes improved sleep, which again, has not been demonstrated to date for sacroiliac joint dysfunction treatment.
Regarding safety, there were two adverse events in two patients, one SAE, and death for one patient. All AEs, SAE, and mortality were unrelated to the study or the study device. This suggests that the novel posterior approach with bone allograft placement using a single point fixation is safe and efficacious. Comparably, in the previously published iMIA study, they reported 10 AE’s, 8 SAE’s, and 2 procedural related SAE’s. In the INSITE study, 133 AE’s where reported, 21 SAE’s, in which 17 SAE’s where related to the lateral implant procedure. As can be expected, comparisons to the predicate lateral approach as the gold standard for treatment, is of interest. Comparing the treatment arm of the single arm prospective multicenter data for the posterior approach described here, to the lateral approach described in the iMIA study, at the 6-month outcome endpoint, the pain intensity reduction by the VAS and functional improvement, defined by the ODI, are noninferior (). We acknowledge that a minority of patients in this study did not improve with our intervention. Reasons for non-response include other underlying lumbar disease and nonunion of the implant at 6 months. As sacroiliac pain is a clinical diagnosis based on multiple factors, misdiagnosis is a possibility in a small percentage. Other more rare causes such as device malposition will be further addressed in our 12-month data set with advanced imaging in the study participants.
Further, the novel posterior approach carries less morbidity than the lateral approach. Adverse events, failures at 6 months, and need for revision demonstrate a clear advantage to the posterior approach.
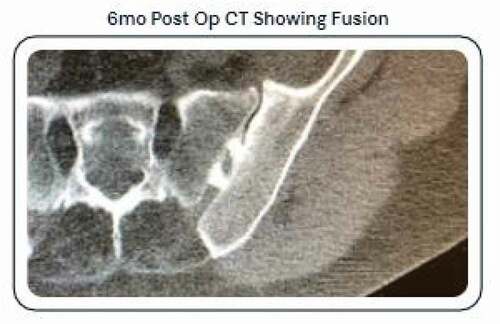
Limitations of the study include this represents a single arm study, as compared to a randomized study with an active conservative treatment arm. The patient cohort represented in the data presented is of the first 69 patients to 6 months, where further study is necessary with a larger number of enrolled patients. As this is an interim manuscript, radiographic post-operative imaging via x-ray or CT was not obtained. We plan to present data at 12 months including post-operative imaging as we feel 6 months is to early a timepoint to assess fusion with a posterior allograft technique based on the authors previous experiences. Other limitations were minimized by strict adherence to the inclusion and exclusion criteria, developed and mirrored from previous SIJ fusion studies and the continued active enrollment of over 100 patients and planned follow-up at 3, 6, 12, 18, and 24 months ().
7. Conclusion
Data is a critical feature to innovation in medical treatments. The data presented is the first prospectively acquired data on any posterior approach for fixation of the sacroiliac joint and represents an important addition in the treatment algorithm, anchored in safety and efficacy. Furthermore, long-term data are required to further qualify this novel posterior approach with a single point fixation strategy for treatment of sacroiliac joint dysfunction.
Declaration of interest
AK Calodney serves as a consultant or receives research support from Medtronic, Nevro, Stryker, Saluda, Nalu, Boston Scientific, Vertos, Painteq, Stimgenics, Spine BioPharma, Saol Therapeutics, Tissuetech, BioRestorative, FUSMobile, APEX Biologix.
JE Pope serves as a consultant for Abbott, Medtronic, Saluda, Flowonix, SpineThera, Vertos, Vertiflex, SPR Therapeutics, Tersera, Aurora, Spark, Ethos, Biotronik, Mainstay, WISE, Boston Scientific, Thermaquil; has received grant and research support from: Abbott, Flowonix, Saluda, Aurora, Painteq, Ethos, Muse, Boston Scientific, SPR Therapeutics, Mainstay, Vertos, AIS, Thermaquil; and is a shareholder of: Vertos, SPR Therapeutics, Painteq Aurora, Spark, Celeri Health, Neural Integrative Solutions, Pacific Research Institute, Thermaquil and Anesthetic Gas Reclamation.
N Azeem serves on the Medical Advisory Board for Painteq.
P Buchanan serves as a consultant and Principal Investigator for Abbott and Painteq.
TR Lubenow serves as a consultant for Abbott, Medtronic, Boston Scientific, Painteq, and Nevro.
S Li is on the scientific advisory boards of: Saluda Medical, Scientific Research: Avanos, Biotronik, Boston Scientific, SGX Medical, Nalu Medical, PainTeq, Saluda Medical, SPR Therapeutics.
Consultant: Abbott, Avanos, Averitas Pharm, Biotronik, Boston Scientific, Nalu Medical, Nevro, PainTeq, Saluda Medical, SPR Therapeutics, Vertos Medical; is on the speaker’s bureau of: Scilex Pharm; and has stock options in: Nalu Medical.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank Shelley Trim, Kamren Murrell, Eric Bruntlett, Nadia Sukhraj-Singh for their support of the study. Painteq has no access to the data or participated in analysis, monitoring, or writing of this manuscript.
Additional information
Funding
References
- Dydyk AM, Forro SD, and Hanna A, et al. Sacroiliac joint injury [Updated 2022 Mar 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Treasure Island (FL); 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557881/
- Buchanan BK, and Varacallo M, et al. Sacroiliitis [Updated 2022 Feb 12]. In: StatPearls. Treasure Island (FL); 2021. PMID: 28846269 Bookshelf ID: NBK448141.
- Cohen SP, Chen Y, Neufeld NJ. Sacroiliac joint pain: a comprehensive review of epidemiology, diagnosis and treatment. Expert Rev Neurother. 2013;13(1):99–116.
- Soto Quijano DA, Otero Loperena E. Sacroiliac joint interventions. Phys Med Rehabil Clin N Am. 2018;29(1):171–183.
- Borowsky CD, Fagen G. Sources of sacroiliac region pain: insights gained from a study comparing standard intra-articular injection with a technique combining intra- and peri-articular injection. Arch Phys Med Rehabil. 2008;89(11):2048–2056.
- Jee H, Lee J-H, Park KD, et al. Ultrasound-guided versus fluoroscopy-guided sacroiliac joint intra-articular injections in the noninflammatory sacroiliac joint dysfunction: a prospective, randomized, single-blinded study. Arch Phys Med Rehabil. 2014;95(2):330–337.
- Soneji N, Bhatia A, Seib R, et al. Comparison of fluoroscopy and ultrasound guidance for sacroiliac joint injection in patients with chronic low back pain. Pain Pract. 2016;16(5):537–544.
- Ferrante FM, et al. Radiofrequency sacroiliac joint denervation for sacroiliac syndrome. Reg Anesth Pain Med. 2001;26(2):137–142.
- Kim WM, Lee HG, Won Jeong C, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16(12):1285–1290.
- Singla V, Batra YK, Bharti N, et al. Steroid vs. platelet-rich plasma in ultrasound-guided sacroiliac joint injection for chronic low back pain. Pain Pract. 2017;17(6):782–791.
- Lee JH, Lee SH, Song SH. Clinical effectiveness of botulinum toxin A compared to a mixture of steroid and local anesthetics as a treatment for sacroiliac joint pain. Pain Med. 2010;11(5):692–700.
- Ou-Yang DC, York PJ, Kleck CJ, et al. Diagnosis and management of sacroiliac joint dysfunction. J Bone Joint Surg Am. 2017;99(23):2027–2036.
- Smith AG, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14.
- Ledonio CG, Polly DW Jr., Swiontkowski MF. Minimally invasive versus open sacroiliac joint fusion: are they similarly safe and effective? Clin Orthop Relat Res. 2014;472(6):1831–1838.
- Ashman B, Norvell DC, Hermsmeyer JT. Chronic sacroiliac joint pain: fusion versus denervation as treatment options. Evid Based Spine Care J. 2010;1(3):35–44.
- Shamrock AG, Patel A, Alam M, et al. The safety profile of percutaneous minimally invasive sacroiliac joint fusion. Global Spine J. 2019;9(8):874–880.
- Whang PG, Darr E, Craig Myer S, et al. Long-term prospective clinical and radiographic outcomes after minimally invasive lateral transiliac sacroiliac joint fusion using triangular titanium implants. Med Devices. 2019;12:411–422.
- Lee DW, Patterson DG, Sayed D. Review of current evidence for minimally invasive posterior sacroiliac joint fusion. Int J Spine Surg. 2021;15(3):514–524.
- Sayed D, Balter K, and Pyles S, et al. A multicenter retrospective analysis of the long-term efficacy and safety of a novel posterior sacroiliac fusion device. J Pain Res. 2021;14:3251–3258.
- Sturesson B, Kools D, Pflugmacher R, et al. iMIA 6 month results. Six month outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion with triangular titanium implants vs. Conservative management. Eur Spine J. 2017;26(3):708–719.
- Whang P, Cher D, Polly D, et al. INSITE 6 month results. Sacroiliac joint fusion using triangular titanium implants vs. Non-surgical management: 6 month outcomes from a prospective randomized controlled trial. Int J Spine Surg. 2015 Jan;9:6.
- Dengler J, Kools D, Pflugmacher R, et al. iMIA 1 year results. 1 year results of a randomized controlled trial of conservative management vs. Minimally invasive surgical treatment for sacroiliac joint pain. Pain Physician. 2017;20:537.
- Dengler J, Kools D, Pflugmacher R, et al. iMIA 2 year results: randomized trial of sacroiliac joint arthrodesis compared with conservative management for chronic low back pain attributed to sacroiliac joint. J Bone Joint Surg Am. 2019;101(5):400–411.
- Polly DW, Cher DJ, Wine KD, et al. INSITE 1-year results.One year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs. non-surgical management for sacroiliac joint dysfunction: 12 month outcomes. Neurosurgery. 2015;77:674–691.
- Polly DW, Swofford J, Whang PG, et al. INSITE 2-year results. Two year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. Non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10:28.
- Dengler J, Kools D, Pflugmacher R, et al. Randomized trial of sacroiliac joint arthrodesis compared with conservative management for chronic low back pain attributed to the sacroiliac joint. J Bone Joint Surg Am. 2019;101:400–411.
- Polly DW, Swofford J, Whang PG. Two-year outcome from an randomized controlled trial of minimally invasive sacroiliac joint fusion vs. Non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016 January;10:28.
- Pope JE, Fishman M, Chakravarthy K, et al. A retrospective, multicenter, quantitative analysis of patients’ baseline pain quality (PROMIS-29) entering into pain and spine practices in the United States (ALIGN). Pain Ther. 2021;10:539–550.