ABSTRACT
Introduction
Surgery and biomedical imaging encompass a big share of the medical-device market. The ever-mounting demand for precision surgery has driven the integration of these two into the field of image-guided surgery. A key-question herein is how imaging modalities can guide the surgical decision-making process. Through performance-based design, chemists, engineers, and doctors need to build a bridge between imaging technologies and surgical challenges.
Areas-covered
This perspective article highlights the complementary nature between the technological design of an image-guidance modality and the type of procedure performed. The specific roles of the involved professionals, imaging technologies, and surgical indications are addressed.
Expert-opinion
Molecular-image-guided surgery has the potential to advance pre-, intra- and post-operative tissue characterization. To achieve this, surgeons need the access to well-designed indication-specific chemical-agents and detection modalities. Hereby, some technologies stimulate exploration (‘go’), while others stimulate caution (‘stop’). However, failing to adequately address the indication-specific needs rises the risk of incorrect tool employment and sub-optimal surgical performance. Therefore, besides the availability of new technologies, market growth is highly dependent on the practical nature and impact on real-life clinical care. While urology currently takes the lead in the widespread implementation of image-guidance technologies, the topic is generic and its popularity spreads rapidly within surgical oncology.
1. Introduction
In surgery, the pre- and intra-operative decisions made by the treating physician, complemented with the surgical dexterity, drive the success of the procedural execution [Citation1]. As such, technologies that can facilitate the decision-making process promise to deliver improved surgical care. Guidance can be achieved by warning the surgeon when to halt, proceed with caution, or move forward with tissue manipulation and/or excision. From an industry perspective, the interest for such technologies has grown substantially over the years. In 2021, the global general surgery devices market was valued at 14.75 billion [Citation2] United-States dollar (USD) and that of medical imaging at USD 38.5 billion [Citation3]. These developments jointly drive the rising popularity of the use of diagnostic biomedical devices in the operating room.
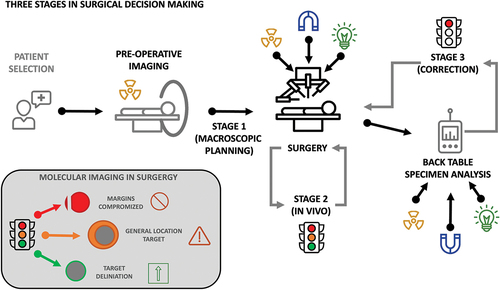
Ideally, biomedical imaging and the accompanying detection devices help provide surgeons with guidance in a traffic-light manner. This concept was previously introduced for surgical excision [Citation4] and adheres to ‘green-flag’ and ‘red-flag’ guidance strategies mentioned in literature [Citation5]. The different colored lights indicate different scenarios: The red-light (stop) indicates that target-margins have been compromised, requiring critical action and reevaluation of the remaining tissue. Orange-light (slow-down) indicates that the target is located nearby and should be approached with caution. Green-light (go) indicates that a clear and precise target delineation is provided, urging the surgeon to proceed with his/her actions. Herein the type and stage of disease, lesion size, and its anatomic localization and relation to the surrounding structures define the form of guidance that is most useful in a specific procedure. This is based on the reasoning that oncological outcome can be improved by the identification of primary tumor margins and the identification of locally confined (lymph node) metastases. In addition, tissue sparing strategies and identification of delicate anatomies may help reduce complications. As such, the technological design of a medical device for image guidance should reflect the type of procedure that is to be performed. Herein, features such as (in-depth) sensitivity, specificity, resolution, measurement time, and ergonomics (e.g. open vs. key-hole surgery), but also the availability of targeted agents can be considered driving factors in technology development and/or selection.
The projected image-guided surgery market is estimated at USD 5.22 billion by 2027 [Citation6], spanning roughly 1/3 of the surgical devices market [Citation7]. To date, traditional intraoperative morphological imaging with radiological modalities, such as C-arm imaging and ultrasound, remains dominant. The most significant area of growth, however, is observed in molecular imaging. Physics dictates that there are substantial differences in the type of information that individual molecular imaging modalities can provide (). This further indicates that the individual modalities have different roles in the surgical decision-making process.
Table 1. Typical characteristics of the most common molecular imaging modalities for surgical guidance.
Most commonly, molecular image-guided surgery comprises radio- and optical-guidance techniques. Radioguidance can ‘visualize’ the location (and state) of a disease both prior to surgery, as well as during surgery, via the use of (portable) gamma cameras and gamma probes. Optical techniques are ideally suitable for superficial visualization of the state of disease during surgery, as well as at pathology [Citation16].
To date, clinical implementation of intraoperative molecular imaging (see ) is dominated by long-known procedures that help ‘illuminate’ anatomical and physiological features [Citation31]. Examples of approaches that take image guidance to the biomarker level (see ) are based on, e.g. the visualization of metabolic function such as protoporphyrin IX (PpIX) [Citation32] or tumor-receptor expression such as folate [Citation33] and the prostate-specific membrane (PSMA) [Citation34]. In these applications not only the physics of the imaging signatures but also the chemicals and their pharmacokinetic behavior dictate utility. In some cases, the availability of (approved) chemicals drives the selection of a device, e.g. indocyanine green and near-infrared fluorescence imaging [Citation35]. In others, the practical value of a device may be directly related to the pharmacokinetics of the chemical used. In the latter sense, it was recently illustrated that the use of a surgical detection modality is directly related to the signal intensity and background staining of specific chemical agents; sentinel node (indocyanine green-99mTc-nanocolloid) versus PSMA-targeted nodal dissections (99mTc-PSMA I&S) [Citation36].
Table 2. Most common surgical applications of molecular imaging, summarizing surgical targets, the anatomies and molecular targets.
From a technology developers’ point of view, the key underlying questions for the selection and utility of technological enablers are: ‘What type of image guidance is required?’ and ‘At which stage of the procedure is guidance needed?” To some these questions may sound rather trivial, but in practice they are not. In fact, differences in surgical targets, anatomies, and surgical approaches (e.g. open, laparoscopic or robot-assisted laparoscopic [Citation37]) all influence the utility of the image guidance technologies. With this special report article, we aim to critically address how typical intraoperative molecular imaging workflows and clinical challenges drive the demand for specific image guidance strategies (). Hereby we also touch upon the role of the involved professionals (e.g. chemists, engineers, and treating physicians) in the development and application of performance-based design of medical devices (e.g. tracers, cameras, and probes).
2. Red-light strategies (target margins have been compromised – stop)
The use of imaging to assess infringement of tumor margins, essentially indicates a ‘red-light’ technology. By that we mean the image-guidance approach has the sole intent to find out if the resection planes indicate that tumor-margins have been compromised. Use of this approach somewhat contradicts the common guideline-based necessity of 5–10 mm safety margins. In vivo investigation (stage 2, ) of resection planes is most common during receptor-targeted optical approaches. Hereby one should realize that optical signals, even those in the near infrared region, suffer from severe tissue induced signal attenuation. Making their identification highly superficial in nature (). That said, radioguidance approaches may also be used to inspect the tumor bed for residual disease. Here, however, deeper lying signals may complicate superficial assessments. Margin infringement can also be defined outside of the patient (ex vivo; stage 3, ). This approach uses so-called ‘back table’ imaging devices to assess resected specimens (e.g. using Cerenkov or PET specimen analyzers; ) [Citation40]. The outcome of which is then communicated with the surgeon for additional resection where needed.
Figure 2. Examples of molecular image guidance technologies at different stages of the procedure. Stage 1, showing PET imaging (top and middle) and SPECT imaging (bottom) for planning of the intervention; Stage 2, showing freehand SPECT navigation (top), DROP-IN gamma probe and gamma camera guidance (middle) and fluorescence flow analysis [Citation38] and optical guidance, for in vivo guidance during the intervention; Stage 3, showing DROP-IN beta probe analysis (top) and PET specimen imager [Citation39] (bottom), for ex vivo back-table analysis during the intervention. Non referenced images originate from authors personal collection. No changes have been made to the referenced images, which are licensed under CC by 4.0 (https://creativecommons.org/licenses/by/4.0/).
![Figure 2. Examples of molecular image guidance technologies at different stages of the procedure. Stage 1, showing PET imaging (top and middle) and SPECT imaging (bottom) for planning of the intervention; Stage 2, showing freehand SPECT navigation (top), DROP-IN gamma probe and gamma camera guidance (middle) and fluorescence flow analysis [Citation38] and optical guidance, for in vivo guidance during the intervention; Stage 3, showing DROP-IN beta probe analysis (top) and PET specimen imager [Citation39] (bottom), for ex vivo back-table analysis during the intervention. Non referenced images originate from authors personal collection. No changes have been made to the referenced images, which are licensed under CC by 4.0 (https://creativecommons.org/licenses/by/4.0/).](/cms/asset/5eaadc4e-9393-4869-8a50-230234c12800/ierd_a_2341102_f0002_oc.jpg)
Independent of the stage in the procedure, when margins are found to be positive, the resection turned out to be irradical (i.e. R1 or R2 resection). Meaning surgeons may need to conduct a second round of resection or adjuvant therapy for local control. While there is no doubt that a secondary resection aimed at residual tumor harvesting could be beneficial, compromising of the margins may mean there is already a chance of tumor spillage (in-transit disease). As complications may be related to the amount of tissue removed, re-excisions also increase the chance of complications, while increasing healthcare costs and possibly decreasing quality-of-life [Citation41].
The limited tissue penetration of, for example, fluorescent, Cerenkov, and beta-particle signals theoretically offers the possibility to minimize the safety margins to a millimeter range and could thus help minimize complications. Unfortunately, the pursuit of safety-margin reduction is inherently linked to a high risk of performing irradical resections. Despite that the typical/theoretical detection depths of optical signals are known (), the actual depth of detection can be very unpredictable in vivo. Here, signal-attenuation and resolution-degration may vary depending on, e.g. tissue type, level of vascularization, and amount of signal accumulated in the tissue target [Citation42]. The lack of effective signal identification may yield false negative results, which in turn result in recurrences. The inherent low signal-intensities of receptor-targeted indications [Citation36] and its accompanying low signal-to-background ratio (SBR), risk to impair the detection sensitivity. When using fluorescence, the lack of sensitivity is often compensated for by increasing the dosing of the tracer used, increasing the dosing to concentration regimes that are typical for drug-based therapy [Citation43]. The downside of dose increases is that they reduce the target-specificity and may enhance the false positive rate [Citation44]. The latter will lead to more, rather than less, invasive resections, and complications. It should hereby be noted that there is a biological limit to the number of receptors that are expressed per targeted cell-type, meaning that there is a limit to the amount of tracer that can selectively bind to the targeted lesion [Citation45].
Devices and technologies that improve the detection sensitivity and in-depth sensitivity of, especially optical, methods are likely to help advance red-light approaches to a next level. Examples of the first are found in fluorescence camera systems that try to maximize sensitivity with improved detectors [Citation46]. Examples of the latter are also found in devices that support life-time imaging [Citation47], spectrally corrected fluorescence [Citation48,Citation49], short-wave infrared (SWIR) fluorescence [Citation50], Cerenkov imaging [Citation51] and optoacoustics [Citation52]. While all these modalities support mm resolution macroscopic imaging, margin assessment could also be supported by microscopic imaging strategies (µm range) such as (fluorescence) confocal imaging [Citation53], optical coherence tomography [Citation54] and Raman scattering detection [Citation55]. Improvements in radioguidance devices tend to be pursued in collimation [Citation56] and/or enlarging the rotational freedom for intraoperative use [Citation57].
3. Orange-light strategies (target nearby – proceed with caution)
We use the term orange-light for strategies that warn the surgeons for the presence of diseased tissue in a certain location but with a limited spatial accuracy (≥5–20 mm). This means that a surgeon can macroscopically assess the target location but cannot define its location down to the µm-mm level. Cornerstones for this type of image-guidance are defined by approaches that rely on preoperative (diagnostic) imaging, e.g. Positron Emission Tomography/X-ray Computed Tomography (PET/CT). Despite the ~3 mm resolution of PET/CT, in the OR, tissue deformations only make such data indicative for the lesion location at the >10 mm scale. Thus, essentially limiting the value of these roadmaps at the surgical stage. Here, it should be noted that preoperative imaging can be used to assess both the sensitivity and specificity of a tracer prior to its use for surgical guidance () [Citation58]. This form of guidance affects surgical decision-making at the planning level (stage 1, ). A successful example of an approach that uses this strategy is sentinel node biopsy. A procedure that relies on the identification of the first tumor draining lymph nodes via lymphoscintigraphy and Single-Photon Emission Computed Tomography/X-ray Computed Tomography (SPECT/CT) via positive contrast prior to being used by surgeons to guide nodal sampling [Citation59]. Similarly, negative contrast at Magnetic Resonance Imaging (MRI) imaging is being used to guide magnetic nodal tracing [Citation60,Citation61]. In these indications, and also in receptor-targeted applications, non-visualizations, or vague imaging outcomes found at preoperative imaging, all have a negative predictive value for the procedural success [Citation36].
Intraoperative orange flag approaches mainly rely on imaging signatures that can effectively penetrate through tissue. This means that there is a preference for gamma-ray-based radioguidance, such as 140 keV 99mTc gamma-rays, to support the highly sensitive detection of targets that are located along the line of detection of a surgical detector. Uniquely, in this form of image-guidance, micro-dosing of tracers (<100 µg/patient) can effectively facilitate sensitive lesion detection, while preserving a high specificity [Citation62]. Given the experimental 10-15 mm tissue penetration limits of far-red and near-infrared-1 (NIR-1) fluorescent emissions [Citation63], this unique type of fluorescence imaging may theoretically provide an indication that a deeper located lesion is being approached [Citation4]. But to be fair, in practice, fluorescence-guidance only delivers on its promise when surgeons have fully exposed its source in tissue. Making it either a red- (see above) or green- (see below) light approach. For fluorescence technologies to become more valuable, enhanced in-depth detection is required. Here NIR-2/SWIR approaches may provide an outcome [Citation50].
From the medical device perspective, refinement of orange-light guidance demands technologies that can connect or even integrate pre- and intraoperative imaging. Herein, the use of preoperative imaging data as roadmap paves the way for the surgical implementation of ‘gps-like’ navigation strategies for, e.g. gamma-probes and fluorescence cameras [Citation64]. The inherent deformations that occur in soft-tissue surgery, demand the development of robust image-to-patient registration-solutions before this can be routinely applied. It also makes the value of navigation strategies dependent on their compatibility with confirmatory red-/green-light strategies. The same is true for the implementation of augmented reality visualizations [Citation65]. Alternatively, advances around intraoperative 3D imaging devices will likely help support the delineation and in-depth assessment of lesions located below the tissue surface. Relevant examples are freehand SPECT [Citation66], robot-assisted SPECT [Citation67], freehand fluorescence tomography [Citation13], and freehand Magnetic Particle Imaging [Citation14].
4. Green-light strategies (clear and precise target delineation – go)
Traditional intraoperative fluorescence image guidance approaches based on Indocyanine Green (ICG) and fluorescein rely on the accumulation of an imaging tracer in a well-defined anatomy, with known location (). With that, the sole function of the image guidance strategy is to highlight boundaries and/or abnormalities therein with a <mm accuracy. This type of (green-light) guidance results in a directional approach that facilitates surgeons to proceed with the procedure (stage 2; ). Well known examples are: 1) angiography (cardiac perfusion, brain perfusion, tissue transplantation, partial nephrectomy, anastomosis, parathyroid glands) [Citation68], 2) cholangiography [Citation69], 3) ureter staining [Citation70], and 4) lymphangiography [Citation71]. All approaches that use well-documented tracer pharmacokinetics to illuminate a target- or healthy-tissue. As such, these approaches suffer less from false positives, e.g. in case of overdosing. That said, ICG has been shown to cause oversampling of lymph nodes (false positives) when this lymphangiographic agent is being misused for sentinel node applications [Citation72]. Again, indications of how pharmacokinetics and dosing can impair the accuracy of an approach.
In green-light applications, anatomies are in direct line-of-sight of the surgeon. As such tissue-induced signal attenuation is minimal. Therefore, this application matches best with the high-spatial resolution and intuitive visual guidance provided by fluorescence imaging (). One should, however, remain aware that fluorescence guidance is not extendable to targets hidden underneath the surface or at unknown locations (see above). When used for such indications, fluorescence guidance therefore remains prone to induce false negative results [Citation43,Citation73]. Perhaps even worse is that active searching for a fluorescent signal could instigate non-directional tissue exploration and manipulation, which may in turn induce complications.
Where the current generation of fluorescence cameras seems to already provide sufficient quality for routine use with ICG (NIR settings) and Fluorescein (photodynamic diagnoses (PDD) settings), further improvements in resolution and, in particular sensitivity, will add to their utility, especially considering the above-mentioned intrinsic low-signal intensities encountered during receptor-targeted imaging. As illustration of further possible improvements: microscopes for neurovascular surgery provide a clear indication for the precision levels that can be achieved [Citation74], hereby image processing at the camera/vision tower level is used to provide an indication for blood flow. Alternative technical advances of camera systems seem to lie in the expansion of the effective spectral window, including the facilitation of multispectral imaging [Citation75]. The latter being a concept that helps identify differently ‘colored’ targets and anatomies simultaneously.
5. Integration of approaches
From a practical perspective, red-, orange-, and green-light read-outs all contribute differently to the procedure. With each individual readout providing a unique and valuable input. At the same time all the individual approaches suffer from intrinsic limitations. Hence, the best way to generate solutions for real-life surgical scenarios is to pursue strategies that combine or even integrate detection technologies. This strategy helps provide a best-of-different-worlds image guidance scenario. Hereby, noninvasive pre-operative imaging seems imperative for accurate patient selection prior to pursuit of intraoperative guidance (stage 1). The latter can be in the form of red-, orange-, and/or green-light guidance (stage 2). Subsequently, the accuracy of the procedure can be validated via post-surgical specimen analysis (stage 3; ). The complementarity in read-out also suggests engineering efforts need to be geared more toward concepts that integrate, e.g. radio-, optical- and/or magnetic-guidance. While hybrid or bi-modal tracers are already clinically available and are becoming increasingly popular, the availability of matching surgical devices seems to be still trailing behind [Citation8,Citation16,Citation76]. However, examples of such surgical devices are found in hybrid radio-/optical-detection probes [Citation77–80], hybrid radio-/optical-cameras [Citation56,Citation81,Citation82], hybrid radio-/ultrasonic-cameras [Citation83,Citation84] and hybrid radio-/optical-tomography [Citation13].
6. Performance-based design of medical devices
Failure to connect technical possibilities with real-life challenges that need to be overcome, for example, the need for a specific type of guidance, may cause a disconcordance between the medical devices created and the clinical needs. If this happens, for example, when a superficial optical imaging technique is used to target small lesions hidden deep in human tissue, widespread adoption will be limited and the risk of late translational failure will be increased. To ensure actual performance enhancement, we need to maintain caution not to have the technological design drive the clinical use, but rather focus on providing enabling technologies.
Currently, a large proportion of the developments in the field of image-guided surgery are driven by subjective (expert) opinions. While this approach can be very effective, the growing complexity of this multidisciplinary field makes it very difficult to assess whose opinion is needed to drive the field forward. For example, you could consider surgeons experts, and some companies have the policy to only pursue a technology when a certain number of surgeons express an interest in a technology. This view is, however, limited by the fact that surgeons tend to specialize in a specific clinical indication and procedure therein, e.g. open nephrectomy versus robot-assisted nephrectomy. Also, few surgeons are experts in chemistry, imaging, and/or engineering. Another group of potential experts are the imaging professionals (nuclear medicine physicians, radiologists, and pathologists). A group that has been specifically trained in the use of advanced imaging technologies in clinical care. Unfortunately, this group nowadays tends to have a relatively low affinity for surgery, chemistry, and/or engineering. Technology oriented chemists and engineers may be able to generate innovative tracers and imaging devices, but in turn tend to have little feeling for the practical constrains encountered during imaging in a surgical theater. Based on the above suggestion to combine the added benefit of different technologies, the most logical path toward establishing experts in device development is to form multidisciplinary teams that are inclusive for experts on different topics.
In addition to forming multidisciplinary expert-teams, there also is a need for more objective benchmarking of the impact that is provided by such developed technologies. Obviously, in surgery, this demands evidence in the form of pathological findings, complications, and long-term outcome measures. But given that image guidance is all about improving the surgical decision-making process, there seems to be an opportunity for technologies that can quantify surgical performance gains. Examples herein are found in the ‘novice versus expert’ analysis conducted for robotic surgery [Citation85] or recording of instrument kinematics and/or operating room logistics during image-guided surgery [Citation36].
7. Conclusions
Development of technologies that do justice to the complexity of (oncological) surgery, with the aim to perform minimal invasive surgery, is a challenging task. This is intensified by the fact that guidance needs to be provided during often hour-long procedures. Moreover, guidance around and toward different targets is required and needs to be applied in different forms (stop, warning, or go). Hence, development of technologies for image-guided surgery not only demands insight in the tracer chemistry, tracer pharmacokinetics, and imaging physics (pros and cons of different modalities), it also demands expert knowledge of the specific aspect of the procedure wherein additional guidance is needed, as well as the procedural constraints (e.g. accessibility). When these aspects are covered, the medical devices have to be able to: 1) identify compromised tumor margins (red-light; example: receptor-targeted fluorescence guided surgery), 2) give a directional indication for possible lesion locations (orange light; example: PSMA-targeted radioguided surgery), and/or 3) visualize the contours of the healthy target tissue (green-light; example: fluorescence angiography). Pursuit of such image-guidance technologies generally relies on the use of complementary procedures. Hybrid strategies, as those that are increasingly being used for sentinel node approaches, seem to provide a logical means to integrate different imaging aspects that are relevant for the surgical setting. This creates opportunities for unique new system engineering possibilities in the form of hybrid devices or development of devices that complement each other during a surgical procedure (e.g. a preoperative SPECT/CT roadmap and the intraoperative robotic use of fluorescence and ‘drop-in’ radioguidance). Key to the translational success of future devices is their ability to enable crucial clinical tasks and the availability of objective performance metrics that can be used to assess to what extend the device impacts surgical care.
8. Expert opinion
Given the complexity, that is to say, different objectives, different anatomies, and approaches pursued surgically, it is to be expected that different surgical procedures demand different types of molecular image-guidance. A mismatch herein can hinder, or delay, routine clinical use. Our intent is to help researchers/engineers in the (molecular) image-guided surgery field to connect their technologies to specific surgical demands. We try to do this by deepening their understanding of the questions: ‘What type of image guidance is required?’ and ‘At which stage of the procedure is guidance needed?.’
Mistakenly introducing an image-guidance technology that does not match the clinical use case brings a great risk of failure and limits widespread adoption. Given the variations of guidance required in the field of oncologic surgery, this makes for a critical aspect. For example, in some cases, the image guidance may be used to stimulate a surgeon to proceed in the pursuit of a target, while in others it may indicate that the surgeon should halt his/her pursuit as there is a risk of tumor spillage or damage to healthy tissues. To classify image guidance, we have suggested a three-way classification, based on the three-color traffic-light principle: red-light (stop), orange-light (caution), and green-light (go). Hereby, classification of an approach in one of these three categories is guided by the physics-based constraints of the underlying detection principles, but also on the tracer availability and their pharmacokinetic behavior. As all three categories provide unique strengths and weaknesses, it is likely that a combination of technologies that facilitate all three categories – similar to an actual traffic light – best serves consecutive stages in the surgical procedure. This is why we expect to see an increase in the use of hybrid (also called bi-modal or multi-modal) modalities, both from a chemical and an engineering perspective.
To provide impact in patient care, all the image-guidance technologies ‘stand’ or ‘fall’ by their compatibility with clinically approved imaging agents. Even if there is a great clinical need and a technology has great potential, when the appropriate tracer is missing, it can simply not be used to guide the surgeons. This is also true the other way around, but, in particular for the sub-discipline of receptor-targeted applications, the demand for tumor-targeted tracers is high. At the moment PSMA-targeting with radioactive tracers is one of the few receptor-targeted image-guidance approaches that is being used in large patient numbers (>500) [Citation62], while other receptor targets are explored at a smaller scale (e.g. folate, vascular endothelial growth factor (VEGF), epidermal growth factor receptors (EGFR), c-mesenchymal-epithelial transition (C-Met), carcinoembryonic antigen (CEA), C-X-C chemokine receptor type 4 (CXCR4), human epidermal growth factor receptor 2 (HER2)) [Citation22,Citation86]. Interestingly, these procedures are enabled by detection technologies that were originally developed for different clinical indications, such as the sentinel node procedure. This indicates that procedural refinements and engineering advances can help to rapidly drive the implementation of new imaging tracers (once approved for clinical use) and new image-guidance indications.
When technologies are implemented correctly, the authors believe the field holds tremendous potential to advance intraoperative tissue characterization. In fact, image-guided surgery technologies are likely to radically change oncological surgical care by supporting precision surgery. With the availability of PSMA-targeting, a widely validated oncological target, we believe the field of urology will remain in the lead in the coming few years. This may, however, change rapidly when more tracers become available for other validated molecular targets and other surgical indications. Efforts that may well be sped-up by the availability of tumor-type independent targets, such as the fibroblast antigen-protein (FAP); nuclear medicine has shown it to provide a valid diagnostic target for >27 different cancer types [Citation87].
Article highlights
Molecular imaging technologies, including tracer agents and detection modalities, have the potential to establish precision surgery for various applications, which we classified in three ‘traffic-light’ categories: red-light (stop), orange-light (caution), and green-light (go).
Red-light technologies image the infringement of critical margins (e.g. tumor margins).
Orange-light technologies warn the surgeon for the nearby presence of (diseased) tissue.
Green-light technologies highlight boundaries and/or abnormalities of clearly defined organs with a sub-mm accuracy.
An integration or combination of technologies that can access all these traffic-light stages during a surgical procedure best serves consecutive stages in the surgical procedure.
Development of novel technologies for image-guided surgery not only demands insight in the tracer chemistry, tracer pharmacokinetics, and imaging physics, it also demands expert knowledge of the specific aspect of the procedure wherein additional guidance is needed, as well as the procedural constraints.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Author contributions
All authors (FvL, TB, DR, and MvO) have substantially contributed to the conception and design of the review article (including search and interpretation of the relevant literature), and all have been involved in the writing and revisions of the review article.
Additional information
Funding
References
- Spencer F. Teaching and measuring surgical techniques: the technical evaluation of competence. Bull Am Coll Surg. 1978;63(3):9–12.
- Research GV. General Surgery Devices Market Size, Share & Trends Analysis Report by Application (Orthopedic, Plastic Surgery, Cardiology, Ophthalmology), by End-Use (Hospitals, ASCs), by Type, by Region, and Segment Forecasts, 2023–2030; 2022. Available from: https://www.grandviewresearch.com/industry-analysis/general-surgery-devices-market
- Insights GM. Medical Imaging Market - by Product (X-Ray Devices, Magnetic Resonance Imaging (MRI), Ultrasound, Computed Tomography, Nuclear Imaging, Mammography), by End-Use (Hospitals, Diagnostic Centers) & Forecast, 2022–2030; 2022. Available from: https://www.gminsights.com/industry-analysis/medical-imaging-market
- Chin PT, Beekman CA, Buckle T, et al. Multispectral visualization of surgical safety-margins using fluorescent marker seeds. Am J Nucl Med Mol Imaging. 2012;2(2):151.
- Hartmans E, Tjalma JJ, Linssen MD, et al. Potential red-flag identification of colorectal adenomas with wide-field fluorescence molecular endoscopy. Theranostics. 2018;8(6):1458. doi: 10.7150/thno.22033
- LLP DBR. The global image-guided surgery devices market to exhibit growth at a CAGR of 7.53% by 2027, assesses DelveInsight 2022. Available from: https://www.globenewswire.com/en/news-release/2022/12/21/2578125/0/en/The-Global-Image-Guided-Surgery-Devices-Market-to-Exhibit-Growth-at-a-CAGR-of-7-53-by-2027-Assesses-DelveInsight.html
- Research P. Surgical Equipment Market (By Product: surgical Sutures & Staplers, Handheld Surgical Device, and Electrosurgical Devices; Application: neurosurgery, Wound Care, Obstetrics & Gynecology, Cardiovascular, Orthopedic, Plastic & Reconstructive Surgery, and Others) - Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2023–2032; 2023. Available from: https://www.precedenceresearch.com/surgical-equipment-market
- Van Oosterom MN, Rietbergen DD, Welling MM, et al. Recent advances in nuclear and hybrid detection modalities for image-guided surgery. Expert review of medical devices. Expert Rev Med Devices. 2019;16(8):711–734. doi: 10.1080/17434440.2019.1642104
- Collamati F, van Oosterom MN, Hadaschik BA, et al. Beta radioguided surgery: towards routine implementation? Q J Nucl Med Mol Imaging. 2021;65(3):229–243. doi: 10.23736/S1824-4785.21.03358-6
- Collamati F, Morganti S, Van Oosterom MN, et al. First-in-human validation of a DROP-IN β-probe for robotic radioguided surgery: defining optimal signal-to-background discrimination algorithm. Eur J Nucl Med Mol Imaging. 2024;1–11. doi: 10.1007/s00259-024-06653-6. Accepted for publication.
- Boykoff N, Grimm J. Current clinical applications of Cerenkov luminescence for intraoperative molecular imaging. Eur J Nucl Med Mol Imaging. 2024;1–10. doi: 10.1007/s00259-024-06602-3
- Erkkilä MT, Reichert D, Hecker-Denschlag N, et al. Surgical microscope with integrated fluorescence lifetime imaging for 5-aminolevulinic acid fluorescence-guided neurosurgery. J Biomed Opt. 2020;25(7):1–71202. doi: 10.1117/1.JBO.25.7.071202
- van Oosterom MN, van der Poel HG, van Leeuwen FWB, et al. Extending the hybrid surgical guidance concept with freehand fluorescence tomography. IEEE Trans Med Imaging. 2020;39(1):226–235. doi: 10.1109/TMI.2019.2924254
- Azargoshasb S, Molenaar L, Rosiello G, et al. Advancing intraoperative magnetic tracing using 3D freehand magnetic particle imaging. Int J CARS. 2022;17(1):211–218. doi: 10.1007/s11548-021-02458-2
- Dadfar SM, Camozzi D, Darguzyte M, et al. Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance. J nanobiotechnol. 2020;18(1):1–13. doi: 10.1186/s12951-020-0580-1
- van Leeuwen FWB, Schottelius M, Brouwer OR, et al. Trending: radioactive and fluorescent bimodal/hybrid tracers as multiplexing solutions for surgical guidance. J Nucl Med. 2020;61(1):13–19. doi: 10.2967/jnumed.119.228684
- Berrens A-C, van Oosterom MN, Slof LJ, et al. Three-way multiplexing in prostate cancer patients—combining a bimodal sentinel node tracer with multicolor fluorescence imaging. Eur J Nucl Med Mol Imaging. 2023;50(4):1262–1263. doi: 10.1007/s00259-022-06034-x
- Cacciamani GE, Shakir A, Tafuri A, et al. Best practices in near-infrared fluorescence imaging with indocyanine green (NIRF/ICG)-guided robotic urologic surgery: a systematic review-based expert consensus. World J Urol. 2020;38(4):883–896. doi: 10.1007/s00345-019-02870-z
- Harke NN, Godes M, Wagner C, et al. Fluorescence-supported lymphography and extended pelvic lymph node dissection in robot-assisted radical prostatectomy: a prospective, randomized trial. World J Urol. 2018;36(11):1817–1823. doi: 10.1007/s00345-018-2330-7
- Jafari MD, Lee KH, Halabi WJ, et al. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc. 2013;27(8):3003–3008. doi: 10.1007/s00464-013-2832-8
- de Vries HMD, Bekers E, van Oosterom MN, et al. C-MET receptor–targeted fluorescence on the road to image-guided surgery in penile squamous cell carcinoma patients. J Nucl Med. 2022;63(1):51–56. doi: 10.2967/jnumed.120.261864
- Van Leeuwen FWB, Van Willigen DM, Buckle T. Clinical application of fluorescent probes. In: Signore A, editor. Nuclear Medicine and Molecular Imaging. Vol. 1. Amsterdam, The Netherlands: Elsevier; 2022. p. 682–695. doi: 10.1016/B978-0-12-822960-6.00104-6
- Lee YJ, Krishnan G, Nishio N, et al. Intraoperative fluorescence‐guided surgery in head and neck squamous cell carcinoma. Laryngoscope. 2021;131(3):529–534. doi: 10.1002/lary.28822
- Okusanya OT, DeJesus EM, Jiang JX, et al. Intraoperative molecular imaging can identify lung adenocarcinomas during pulmonary resection. J Thorac Cardiovasc Surg. 2015;150(1):28–35. e1. doi: 10.1016/j.jtcvs.2015.05.014
- Vonk J, de Wit JG, Voskuil FJ, et al. Epidermal growth factor receptor–targeted fluorescence molecular imaging for postoperative lymph node assessment in patients with oral cancer. J Nucl Med. 2022;63(5):672–678. doi: 10.2967/jnumed.121.262530
- Collarino A, Vidal-Sicart S, Perotti G, et al. The sentinel node approach in gynaecological malignancies. Clin Transl Imaging. 2016;4(5):411–420. doi: 10.1007/s40336-016-0187-6
- de Barros HA, van Oosterom MN, Donswijk ML, et al. Robot-assisted prostate-specific membrane antigen–radioguided salvage surgery in recurrent prostate cancer using a DROP-IN gamma probe: the first prospective feasibility study. Eur Urol. 2022;82(1):97–105. doi: 10.1016/j.eururo.2022.03.002
- Falkenbach F, Knipper S, Koehler D, et al. Safety and efficiency of repeat salvage lymph node dissection for recurrence of prostate cancer using PSMA-radioguided surgery (RGS) after prior salvage lymph node dissection with or without initial RGS support. World J Urol. 2023;41(9):2343–2350. doi: 10.1007/s00345-023-04534-5
- Olmos RAV, Rietbergen DD, Rubello D, et al. Sentinel node imaging and radioguided surgery in the era of SPECT/CT and PET/CT: toward new interventional nuclear medicine strategies. Clin Nucl Med. 2020;45(10):771–777. doi: 10.1097/RLU.0000000000003206
- Nakaseko Y, Ishizawa T, Saiura A. Fluorescence‐guided surgery for liver tumors. J Surg Oncol. 2018;118(2):324–331. doi: 10.1002/jso.25128
- Reinhart MB, Huntington CR, Blair LJ, et al. Indocyanine green: historical context, current applications, and future considerations. Surg Innov. 2016;23(2):166–175. doi: 10.1177/1553350615604053
- Valdes PA, Millesi M, Widhalm G, et al. 5-aminolevulinic acid induced protoporphyrin IX (ALA-PpIX) fluorescence guidance in meningioma surgery. J Neurooncol. 2019;141(3):555–565. doi: 10.1007/s11060-018-03079-7
- Administration USFD FDA approves new imaging drug to help identify ovarian cancer lesions 2021. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-imaging-drug-help-identify-ovarian-cancer-lesions
- Knipper S, Irai MM, Simon R, et al. Cohort study of oligorecurrent prostate cancer patients: oncological outcomes of patients treated with salvage lymph node dissection via prostate-specific membrane antigen–radioguided surgery. Eur Urol. 2023;83(1):62–69. doi: 10.1016/j.eururo.2022.05.031
- Kitai T, Inomoto T, Miwa M, et al. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12(3):211–215. doi: 10.2325/jbcs.12.211
- Azargoshasb S, De Barros HA, Rietbergen DDD, et al. Artificial Intelligence-Supported Video Analysis as a Means to Assess the Impact of DROP-IN Image Guidance on Robotic Surgeons: Radioguided Sentinel Lymph Node versus PSMA-Targeted Prostate Cancer Surgery. Adv Intell Syst. 2023;5(10):2300192. doi: 10.1002/aisy.202300192
- van Oosterom MN, Azargoshasb S, Slof LJ, et al. Robotic radioguided surgery: toward full integration of radio- and hybrid-detection modalities. Clin Transl Imaging. 2023;11(6):533–544. doi: 10.1007/s40336-023-00560-w
- Sun Y, Wang Z, Jiang F, et al. Utility of indocyanine green videoangiography with FLOW 800 analysis in brain tumour resection as a venous protection technique. BMC Surg. 2022;22(1):126. doi: 10.1186/s12893-022-01573-4
- Muraglia L, Mattana F, Travaini LL, et al. First live-experience session with PET/CT specimen imager: a pilot analysis in prostate cancer and neuroendocrine tumor. Biomedicines. 2023;11(2):645. doi: 10.3390/biomedicines11020645
- Costa PF, Püllen L, Kesch C, et al. 18F-PSMA Cerenkov luminescence and flexible autoradiography imaging in a prostate cancer mouse model and first results of a radical prostatectomy feasibility study in men. J Nucl Med. 2023;64(4):598–604. doi: 10.2967/jnumed.122.264670
- Matar-Ujvary R, Haglich K, Flanagan MR, et al. The impact of breast-conserving surgery re-excision on patient-reported outcomes using the BREAST-Q. Ann Surg Oncol. 2023;30(9):5341–5349. doi: 10.1245/s10434-023-13592-3
- Keiser G. Light-Tissue Interactions. Biophotonics: concepts to Applications. Singapore: Springer Singapore; 2016. p. 147–196.
- KleinJan GH, Bunschoten A, van den Berg NS, et al. Fluorescence guided surgery and tracer-dose, fact or fiction? Eur J Nucl Med Mol Imaging. 2016;43(10):1857–1867. doi: 10.1007/s00259-016-3372-y
- Stibbe JA, de Barros HA, Linders DG, et al. First-in-patient study of OTL78 for intraoperative fluorescence imaging of prostate-specific membrane antigen-positive prostate cancer: a single-arm, phase 2a, feasibility trial. Lancet Oncol. 2023;24(5):457–467. doi: 10.1016/S1470-2045(23)00102-X
- Azargoshasb S, Boekestijn I, Roestenberg M, et al. Quantifying the impact of signal-to-background ratios on surgical discrimination of fluorescent lesions. Mol Imaging Biol. 2023;25(1):180–189. doi: 10.1007/s11307-022-01736-y
- Kennedy GT, Azari FS, Bernstein E, et al. First-in-human results of targeted intraoperative molecular imaging for visualization of ground glass opacities during robotic pulmonary resection. Transl Lung Cancer Res. 2022;11(8):1567. doi: 10.21037/tlcr-21-1004
- Alfonso‐Garcia A, Bec J, Weyers B, et al. Mesoscopic fluorescence lifetime imaging: Fundamental principles, clinical applications and future directions. J Biophotonics. 2021;14(6):e202000472. doi: 10.1002/jbio.202000472
- Buckle T, Chin PT, van den Berg NS, et al. Tumor bracketing and safety margin estimation using multimodal marker seeds: a proof of concept. J Biomed Opt. 2010;15(5):056021–8. doi: 10.1117/1.3503955
- Luthman AS, Dumitru S, Quiros‐Gonzalez I, et al. Fluorescence hyperspectral imaging (fHSI) using a spectrally resolved detector array. J Biophotonics. 2017;10(6–7):840–853. doi: 10.1002/jbio.201600304
- Zhu S, Yung BC, Chandra S, et al. Near-infrared-II (NIR-II) bioimaging via off-peak NIR-I fluorescence emission. Theranostics. 2018;8(15):4141. doi: 10.7150/thno.27995
- Olde Heuvel J, de Wit-van der Veen B, van der Poel H, et al. Intraoperative specimen assessment in prostate cancer surgery using Cerenkov luminescence imaging. In: Proc. SPIE 11224, Optics and Ionizing Radiation; 2020; San Francisco. San Francisco: SPIE BiOS; 2020. p. 1122407. doi: 10.1117/12.2542650
- Becker A, Masthoff M, Claussen J, et al. Multispectral optoacoustic tomography of the human breast: characterisation of healthy tissue and malignant lesions using a hybrid ultrasound-optoacoustic approach. Eur Radiol. 2018;28(2):602–609. doi: 10.1007/s00330-017-5002-x
- Ferrer-Roca O. Telepathology and optical biopsy. Int J Telemed Appl. 2009;2009:1–9. doi: 10.1155/2009/740712
- Carrasco-Zevallos OM, Viehland C, Keller B, et al. Review of intraoperative optical coherence tomography: technology and applications. Biomed Opt Express. 2017;8(3):1607–1637. doi: 10.1364/BOE.8.001607
- Orringer DA, Pandian B, Niknafs YS, et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat Biomed Eng. 2017;1(2):0027. doi: 10.1038/s41551-016-0027
- Kang HG, Song SH, Han YB, et al. Proof-of-concept of a multimodal laparoscope for simultaneous NIR/gamma/visible imaging using wavelength division multiplexing. Opt express. 2018;26(7):8325–8339. doi: 10.1364/OE.26.008325
- van Oosterom MN, Simon H, Mengus L, et al. Revolutionizing (robot-assisted) laparoscopic gamma tracing using a drop-in gamma probe technology. Am J Nucl Med Mol Imaging. 2016;6(1):1.
- Berrens A-C, Sorbi MA, Donswijk ML, et al. Strong correlation between SUVmax on PSMA PET/CT and numeric drop-in γ-probe signal for intraoperative identification of prostate cancer lesions. J Nucl Med. 2024;65(4):548–554. accepted for publication. doi: 10.2967/jnumed.123.267075.
- van der Ploeg IM, Valdés Olmos RA, Kroon BB, et al. The yield of SPECT/CT for anatomical lymphatic mapping in patients with melanoma. Ann Surg Oncol. 2009;16(6):1537–1542. doi: 10.1245/s10434-009-0339-2
- Michalik B, Engels S, Otterbach MC, et al. A new bimodal approach for sentinel lymph node imaging in prostate cancer using a magnetic and fluorescent hybrid tracer. Eur J Nucl Med Mol Imaging. 2023;1–7. doi: 10.1007/s00259-023-06522-8
- Winter A, Kowald T, Paulo TS, et al. Magnetic resonance sentinel lymph node imaging and magnetometer-guided intraoperative detection in prostate cancer using superparamagnetic iron oxide nanoparticles. Int j nanomed. 2018;Volume 13:6689–6698. doi: 10.2147/IJN.S173182
- Berrens A-C, Knipper S, Marra G, et al. State of the art in prostate-specific membrane antigen–targeted surgery—a systematic review. Eur Urol Open Sci. 2023;54:43–55. doi: 10.1016/j.euros.2023.05.014
- van Willigen DM, van den Berg NS, Buckle T, et al. Multispectral fluorescence guided surgery; a feasibility study in a phantom using a clinical-grade laparoscopic camera system. Am J Nucl Med Mol Imaging. 2017;7(3):138.
- Boekestijn I, Azargoshasb S, Schilling C, et al. PET-and SPECT-based navigation strategies to advance procedural accuracy in interventional radiology and image-guided surgery. Q J Nucl Med Mol Imaging. 2021;65(3):244–260. doi: 10.23736/S1824-4785.21.03361-6
- Porpiglia F, Checcucci E, Amparore D, et al. Three-dimensional augmented reality robot-assisted partial nephrectomy in case of complex tumours (PADUA ≥ 10): a new intraoperative tool overcoming the ultrasound guidance. Eur Urol. 2020;78(2):229–238. doi: 10.1016/j.eururo.2019.11.024
- Wendler T, Herrmann K, Schnelzer A, et al. First demonstration of 3-D lymphatic mapping in breast cancer using freehand SPECT. Eur J Nucl Med Mol Imaging. 2010;37(8):1452–1461. doi: 10.1007/s00259-010-1430-4
- Azargoshasb S, Berrens AC, Slof LJ, et al. Robot-assisted SPECT - integrating nuclear medicine in robotic urologic surgery. Eur Urol. 2024 Feb 17. doi: 10.1016/j.eururo.2024.01.022. Online ahead of print: S0302-2838(24)00064-2.
- Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. J Biomed Imaging. 2012;2012:1–26. doi: 10.1155/2012/940585
- Serban D, Badiu DC, Davitoiu D, et al. Systematic review of the role of indocyanine green near‑infrared fluorescence in safe laparoscopic cholecystectomy. Exp Ther Med. 2022;23(2):1–10. doi: 10.3892/etm.2021.11110
- Slooter M, Janssen A, Bemelman W, et al. Currently available and experimental dyes for intraoperative near-infrared fluorescence imaging of the ureters: A systematic review. Tech Coloproctol. 2019;23(4):305–313. doi: 10.1007/s10151-019-01973-4
- Suzuki Y, Kajita H, Konishi N, et al. Subcutaneous lymphatic vessels in the lower extremities: comparison between photoacoustic lymphangiography and near-infrared fluorescence lymphangiography. Radiology. 2020;295(2):469–474. doi: 10.1148/radiol.2020191710
- Wit EM, KleinJan GH, Berrens A-C, et al. A hybrid radioactive and fluorescence approach is more than the sum of its parts; outcome of a phase II randomized sentinel node trial in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2023;50(9):2861–2871. doi: 10.1007/s00259-023-06191-7
- Meershoek P, Buckle T, van Oosterom MN, et al. Can intraoperative fluorescence imaging identify all lesions while the road map created by preoperative nuclear imaging is masked? J Nucl Med. 2020;61(6):834–841. doi: 10.2967/jnumed.119.235234
- Miller DR, Ashour R, Sullender CT, et al. Continuous blood flow visualization with laser speckle contrast imaging during neurovascular surgery. Neurophoton. 2022;9(2):021908–021908. doi: 10.1117/1.NPh.9.2.021908
- van Beurden F, van Willigen DM, Vojnovic B, et al. Multi-wavelength fluorescence in image-guided surgery, clinical feasibility and future perspectives. Mol Imaging. 2020;19:1536012120962333. doi: 10.1177/1536012120962333
- Debie P, Devoogdt N, Hernot S. Targeted nanobody-based molecular tracers for nuclear imaging and image-guided surgery. Antibodies. 2019;8(1):12. doi: 10.3390/antib8010012
- Poumellec M, Dejode M, Figl A, et al. Sentinel node detection using optonuclear probe (gamma and fluorescence) after green indocyanine and radio-isotope injections. Gynecol Obstet Fertil. 2016;44(4):207–210. doi: 10.1016/j.gyobfe.2016.02.012
- van den Berg NS, Simon H, Kleinjan GH, et al. First-in-human evaluation of a hybrid modality that allows combined radio-and (near-infrared) fluorescence tracing during surgery. Eur J Nucl Med Mol Imaging. 2015;42(11):1639–1647. doi: 10.1007/s00259-015-3109-3
- Vidal-Sicart S, Seva A, Campos F, et al. Clinical use of an opto-nuclear probe for hybrid sentinel node biopsy guidance: first results. Int J CARS. 2019;14(2):409–416. doi: 10.1007/s11548-018-1816-5
- Yang Y, Biswal NC, Wang T, et al. Potential role of a hybrid intraoperative probe based on OCT and positron detection for ovarian cancer detection and characterization. Biomed Opt Express. 2011;2(7):1918–1930. doi: 10.1364/BOE.2.001918
- KleinJan GH, Hellingman D, van den Berg NS, et al. Hybrid surgical guidance: does hardware integration of γ-and fluorescence imaging modalities make sense? J Nucl Med. 2017;58(4):646–650. doi: 10.2967/jnumed.116.177154
- Lees JE, Bugby SL, Alqahtani MS, et al. A multimodality hybrid gamma-optical camera for intraoperative imaging. Sensors. 2017;17(3):554. doi: 10.3390/s17030554
- Freesmeyer M, Opfermann T, Winkens T. Hybrid integration of real-time US and freehand SPECT: proof of concept in patients with thyroid diseases. Radiology. 2014;271(3):856–861. doi: 10.1148/radiol.14132415
- Pani R, Pellegrini R, Cinti M, et al. Integrated ultrasound and gamma imaging probe for medical diagnosis. J Instrum. 2016;11(3):C03037. doi: 10.1088/1748-0221/11/03/C03037
- Yanik E, Intes X, Kruger U, et al. Deep neural networks for the assessment of surgical skills: A systematic review. J Defense Model Simul. 2022;19(2):159–171. doi: 10.1177/15485129211034586
- Hernot S, van Manen L, Debie P, et al. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019;20(7):e354–e367. doi: 10.1016/S1470-2045(19)30317-1
- Kratochwil C, Flechsig P, Lindner T, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60(6):801–805. doi: 10.2967/jnumed.119.227967