Abstract
Nanowires (NWs) have unique electrical and optical properties of value for many applications including lighting, sensing, and energy harnessing. Consumer products containing NWs increase the risk of NWs being released in the environment, especially into aquatic ecosystems through sewage systems. Daphnia magna is a common, cosmopolitan freshwater organism sensitive to toxicity tests and represents a likely entry point for nanoparticles into food webs of aquatic ecosystems. Here we have evaluated the effect of NW diameter on the gut penetrance of NWs in Daphnia magna. The animals were exposed to NWs of two diameters (40 and 80 nm) and similar length (3.6 and 3.8 μm, respectively) suspended in water. In order to locate the NWs in Daphnia, the NWs were designed to comprise one inherently fluorescent segment of gallium indium phosphide (GaInP) flanked by a gallium phosphide (GaP) segment. Daphnia mortality was assessed directly after 24 h of exposure and 7 days after exposure. Translocation of NWs across the intestinal epithelium was investigated using confocal fluorescence microscopy directly after 24 h of exposure and was observed in 89% of Daphnia exposed to 40 nm NWs and in 11% of Daphnia exposed to 80 nm NWs. A high degree of fragmentation was observed for NWs of both diameters after ingestion by the Daphnia, although 40 nm NWs were fragmented to a greater extent, which could possibly facilitate translocation across the intestinal epithelium. Our results show that the feeding behavior of animals may enhance the ability of NWs to penetrate biological barriers and that penetrance is governed by the NW diameter.
Introduction
Fiber-shaped nanomaterials, such as carbon nanotubes (CNTs) and nanowires (NWs), are gaining more and more interest due to their unique electronic, optical, and mechanical properties. These materials are currently investigated for various applications and will most likely be found in consumer products in the near future (Li et al., Citation2006). However, concerns have been raised regarding the potential toxicity of fiber nanomaterials due to their morphological similarities with asbestos fibers (Poland et al., Citation2008; Ryman-Rasmussen et al., Citation2009). It has been shown that the interactions between high aspect ratio nanoparticles and immune cells in vitro can lead to frustrated phagocytosis (Nelson et al., Citation2010; Schinwald et al., Citation2012; Sun et al., Citation2013), a key event in fiber-related pathogenesis. Using in vivo models allows for the assessment of additional important features of fiber-related pathogenesis such as translocation over biological barriers, bio-persistence, and physiological consequences (Adolfsson et al., Citation2013a; Gallentoft et al., Citation2015; Linsmeier et al., Citation2009; Poland et al., Citation2008; Ryman-Rasmussen et al., Citation2009; Silva et al., Citation2014). For instance, Nelson et al. found that silica nanoparticles with an aspect ratio larger than 1 are toxic and induce developmental defects in zebrafish embryos, whereas silica nanomaterials with an aspect ratio of 1 are neither toxic nor teratogenic (Nelson et al., Citation2010). The correlation between nanofiber length and toxicity has been extensively studied in vivo. For instance, the injection of long CNTs into the peritoneal cavity of mice induces an inflammatory response similar to the one induced by long asbestos fibers, whereas the injection of short CNTs had the same effect as the control (Poland et al., Citation2008). CNTs have been extensively studied in the context of fiber uptake and biodistribution (Edgington et al., Citation2014; Parks et al., Citation2013; Poland et al., Citation2008). However, a general problem in CNT studies is the broad size distribution of a given sample, making it difficult to determine the length threshold above which they induce toxicity. Recently, metallic NWs have been used as model particles for high aspect ratio nanoparticles due to their narrow length distribution (Schinwald et al., Citation2012). Using these NWs, the length threshold for mouse pulmonary and pleural pathogenicity has been determined to be 14 μm and 4 μm, respectively. Recently, we have proposed semiconductor NWs as suitable model particles for studying high aspect ratio nanoparticles because of their high compositional and geometrical uniformity (Adolfsson et al., Citation2013a). We have also shown that adding a small amount of indium during the synthesis of gallium phosphide (GaP) NWs can produce inherently fluorescent gallium indium phosphide (GaInP) segments in GaP NWs, which enables the localization of NWs in vivo using confocal fluorescence microscopy (Adolfsson et al., Citation2013b). Moreover, we have shown that hafnium oxide-coated GaP NWs of 5 and 10 μm in length induce a sustained local inflammation when implanted in the rat brain for 12 months, while the brain tissue response to the implantation of 2 μm NWs was similar to the one of controls (Gallentoft et al., Citation2015; Linsmeier et al., Citation2009). Although there is a substantial amount of literature reporting on the effect of diameter for spherical particles (Dobrovolskaia et al., Citation2009; Sanfins et al., Citation2014; Tenzer et al., Citation2011; Walkey et al., Citation2012), there are no data available on the effect of the diameter of high aspect ratio nanoparticles on toxicity and particle distribution in tissue. Here, we take advantage of the high degree of particle geometry-control achievable in semiconductor NW synthesis to investigate the effects of NW diameter on Daphnia magna, a freshwater invertebrate, considered as a likely entry point for nanomaterials into aquatic food webs (Mattsson et al., Citation2015). We study the effect of NWs with similar length and two different diameters on the Daphnia tissue distribution. We investigate (1) whether the NWs are fragmented in the presence of Daphnia and (2) whether there is a diameter-dependent translocation of NWs over the intestinal epithelium in Daphnia magna.
Methods
Nanowires
The NWs were synthesized using Au-particle assisted metal-organic-vapor-phase-epitaxy (MOVPE) and composed of GaP and GaInP, as described earlier (Adolfsson et al., Citation2013b). Briefly, a GaP (111)B substrate decorated with aerosol Au-particles was exposed to precursor gases, phosphine and trimethyl-gallium, at an elevated temperature. The precursors react and crystalize underneath the Au-particle and result in a growing GaP segment with a diameter defined by the Au-particle size, and length determined by the growth time. After a couple of minutes of GaP growth, trimethyl-indium was introduced to produce a fluorescent GaInP segment on top of the grown GaP segment (). A detailed description of the synthesis process and the optical properties of the NWs can be found in our previous work (Adolfsson et al., Citation2013b). After the growth procedure, the NWs were broken off the substrate and suspended in liquid by ultra-sonication for approximately 30 s while immersed in fresh (unmodified) tap water. The NW concentration was determined by counting all NWs on a cover glass containing 0.1 μL of sample solution, using dark field microscopy and the Particle Analyzer plugin in ImageJ (Abramoff et al., Citation2004). The median length (3.6 ± 0.7 μm for 40 nm and 3.8 ± 0.9 μm for 80 nm) and diameter (45 ± 6.1 nm respective 80 ± 10.4 nm) were measured using dark field optical microscopy and scanning electron microscopy (SEM), respectively (Supplementary Table S1, ). The median length of the fluorescent GaInP segment was determined to be 1.2 ± 0.09 μm for 40 nm and 1.1 ± 0.20 μm for 80 nm NWs. Additional information relating to size distributions, exposure metrics, and the tap water used in the experiments can be found in Supplementary Tables S1-S3, respectively.
Figure 1. SEM image of GaP/GaInP NWs. Top, 80 nm in diameter GaP/GaInP nanowire; bottom, 40 nm in diameter GaP/GaInP NW. Scale bar: 1 μm, tilt: 0°.

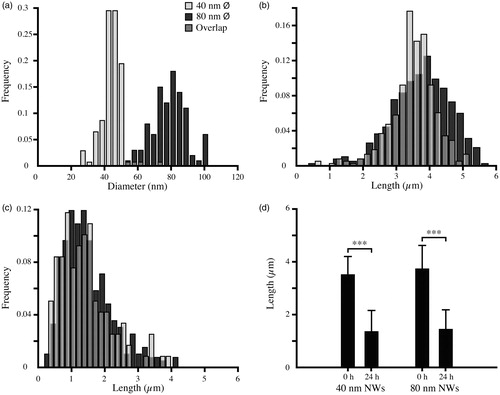
Figure 2. Size distributions of GaP/GaInP NWs. (a) Diameter distribution of NWs before Daphnia filtering measured using SEM. (b) Length distribution before Daphnia filtering measured using dark field optical microscopy. (c) Length distribution after 24 h of Daphnia filtering measured using dark field optical microscopy. (d) Median length and standard deviation for 40 nm and 80 nm NWs before and after Daphnia filtering, measured using dark field optical microscopy (***p < 0.001, Kruskal–Wallis test).
Daphnia magna
We have chosen Daphnia magna as an in vivo model organism to evaluate the consequences of NW exposure. The choice of Daphnia magna as an in vivo model was motivated by the well-established use of Daphnia for eco-toxicity testing and because it is one of the most sensitive organisms for toxicity assessment. Daphnia magna is a freshwater invertebrate commonly found in lakes and ponds worldwide. It is classified as a fine filter-feeder and an adult Daphnia can filtrate 18.5 mL/h (Burns, Citation1969). For uptake of food they use an active selection of particles by direct interception and random internalization of small particles through filtering of the surrounding water (Rosenkranz et al., Citation2009). This feeding behavior allows replacement of up to 3.1% of their body weight each 10 min (Stobbart et al., Citation1977). They can also ingest sediment particles by scraping the bottom or stirring up sediments when the food level is low (Gillis et al., Citation2005). With these mechanisms they can ingest nano- and micro-sized (20 nm to 70 μm) particles from the water (Rosenkranz et al., Citation2009; Zhu et al., Citation2009), and therefore Daphnia magna is a likely entry point for nanomaterials into aquatic food webs (Mattsson et al., Citation2015).
The Daphnia magna used in our study originated from Lake Bysjön, Sweden (55°50′15.3′′ N, 13°17′16.4′′ E) and were kept under controlled laboratory conditions for more than 100 generations at a day–night cycle of 12 h–12 h and at a temperature of 19 °C. The animals were fed twice a week with an algal culture dominated by the green algae, Scenedesmus sp at a concentration of 8.2 × 105 cells/mL.
Exposure
One day after the Daphnia were fed with algae, three Daphnia were transferred to one glass tube with 1.5 mL tap water. The animals were then allowed to empty their gut by swimming in pure water for 4 h, a process that normally takes 2–55 min (Gillis et al., Citation2005). After 4 h, the Daphnia were transferred to either 1.5 mL of tap water containing NWs at a concentration of 6.2 ± 1.6 × 1010 NWs/L (40 nm NWs, n = 13 tubes, and 80 nm NWs, n = 13 tubes) or to 1.5 mL of water without NWs (control, n = 13 tubes). The Daphnia were allowed to filter the medium for 24 h. After exposure, the samples were divided into three groups, one group for localization of ingested NWs, a second group to investigate long-term effects and clearance, and a third group to investigate mortality. In the first group, each Daphnia (n = 9, Daphnia for each treatment) was washed in phosphate-buffered saline (PBS) twice and the arms were cut off before the animals were fixed in formaldehyde (4% v/v) for 6 h. The Daphnia were washed in PBS for 60 min, permeabilized in Triton X-100 (0.1% v/v) overnight, washed twice in PBS, stained with Hoechst 33342 (5 μM) and Alexa Fluor 488 Phalloidin (0.19 μM) for 48 h before being washed three times in PBS. PBS was used as medium throughout the staining protocol in order to preserve tissue structure after permeabilization. After staining, individual Daphnia were mounted in glycerol and arranged in a lateral view. In the second group, Daphnia (n = 9, Daphnia for each treatment) were moved into tubes with 100 mL fresh tap water, to investigate whether the NWs had a long-term toxicity effect. After 24 h of filtering in water, the Daphnia were prepared for confocal imaging using the same procedure as for group one. In the third group, the mortality was investigated directly after exposure (n = 9, Daphnia for each treatment, repeated once), post-exposure after an additional 24 h of filtering fresh tap water (n = 9, Daphnia for each treatment repeated once) and 7 days after exposure (n = 3, Daphnia for each treatment).
Modification of the nanowire suspension due to Daphnia filtering
In order to study the changes in the NW suspension as a result of filtering by Daphnia, we imaged the NW suspensions and measured the NW length before and after filtering using dark field optical microscopy ( and ). The NW suspensions after filtering were studied further using SEM, in order to detect differences between the 40 nm and 80 nm NW lengths with better precision (Supplementary Table S1 and Figures S1 and S2). To exclude the possibility that the changes in the NW suspensions are induced by Daphnia-derived chemical species, NWs were also incubated for 24 h in Daphnia water baths after Daphnia removal from the water. The NW concentration was measured before Daphnia filtering (5.7 ± 1.4 × 1010 NWs/L for 40 nm and 6.3 ± 1.1 × 1010 NWs/L for 80 nm) and after 24 h of NW incubation in Daphnia water (6.0 ± 0.7 × 1010 NWs/L for 40 nm and 6.0 ± 1.7 × 1010 NWs/L for 80 nm) using dark field microscopy.
Analysis of translocation of NWs in Daphnia magna
A defined region of the Daphnia, the posterior head region of the midgut (Supplementary Figure S3), was chosen as sampling area and analyzed using confocal microscopy, where the GaInP fluorescence was used for visualizing the presence of NWs on the basal side of the intestinal epithelium. All samples were analyzed with a Zeiss LSM 510 confocal microscope, using a 63× oil immersion objective (NA = 1.4), and 1AU optical thickness. We used identical gains for control and exposed samples. The laser/filter settings were: 488 nm ex/650LP em for imaging the GaInP fluorescence, 405 nm ex/420-480BP em for Hoechst 33342, 488 nm ex/505-550BP em for Phalloidin-Alexa Fluor 488 and 488 nm ex/505SP detection for 488 nm laser scattering. All images were processed in ImageJ (Abramoff et al., Citation2004) with the FIJI package, using the same brightness/contrast settings.
Results
Mortality
The Daphnia were exposed for 24 h to GaP-GaInP NWs suspended in their surrounding water at a concentration of 6.2 × 1010 NWs/L. The mortality of the Daphnia was assessed immediately after NW exposure (n = 9, Daphnia for each treatment, repeated once), 1 day after the end of the exposure (n = 9, Daphnia for each treatment, repeated once) and 7 days after the exposure (n = 3, Daphnia for each treatment). No mortality could be observed for Daphnia magna exposed to NWs or in the control group (n = 39 for 80 nm, n = 39 for 40 nm, and n = 39 for control).
Nanowire integrity after filtering by Daphnia magna
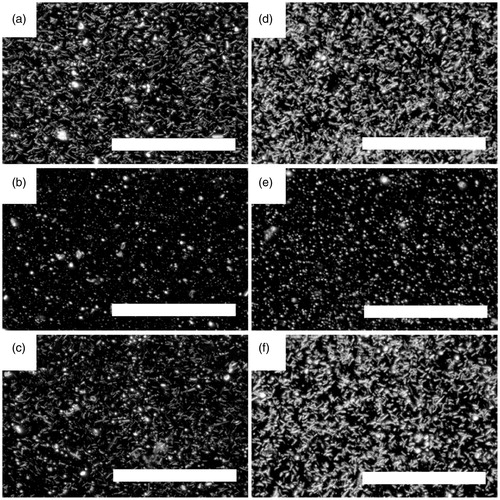
After 24 h of filtering by Daphnia, dark field optical microscopy investigations of the medium indicated that NWs had fractured into smaller fragments (). This was confirmed by the analysis of the length distribution before and after Daphnia filtering using dark field optical microscopy (Supplementary Table S1 and ). In order to compare the fragmented lengths of the two NW populations after filtering with better precision, we also studied the suspensions after filtering using SEM. After 24 h of Daphnia filtering, the median lengths determined using SEM were 402 ± 330 nm and 686 ± 820 nm for 40 and 80 nm diameter NWs, respectively (Supplementary Table S1 and Figures S1 and S2). No fragmentation could be seen for NWs incubated in a water bath in which Daphnia had been allowed to stay for 24 h and been removed before addition of the NWs ().
Figure 3. Dark field optical micrographs of NW suspensions. (a) 40 nm NWs before Daphnia filtering, (b) after 24 h Daphnia filtering of 40 nm NWs, (c) 40 nm NWs after 24 h incubation in Daphnia water, (d) 80 nm NWs before Daphnia filtering, (e) after 24 h Daphnia filtering of 80 nm NWs, (f) 80 nm NWs after 24 h incubation in Daphnia water. Scale bar: 100 μm.
Nanowire localization in the Daphnia tissue
The Daphnia phenotype was studied using confocal microscopy, both immediately after NW exposure and 24 h later. These analyses revealed no phenotypical difference between NW fed and control animals, except in the gut, were red fluorescence was clearly visible in the NW-fed Daphnia (). A large amount of NWs were present in the Daphnia intestine and the majority of the NWs appeared to be confined within the peritrophic membrane (PM) (). However, NWs could also be seen in the intestinal epithelium ( and Supplementary Figure S4). One day after the end of the NW exposure, the intestinal epithelium still contained significant amounts of NWs (Supplementary Figure S5).
Figure 4. Representative confocal images of Daphnia after 24 h of NW exposure. Control Daphnia (a), Daphnia after 24 h of 40 nm NW filtering (b), Daphnia after 24 h of 80 nm NW filtering (c). Stained with Alexa Fluor 488 – Phalloidin (green, actin) and Hoechst 33342 (blue, DNA). The GaInP fluorescence can be seen in red. (See online version of the paper for a color image.)
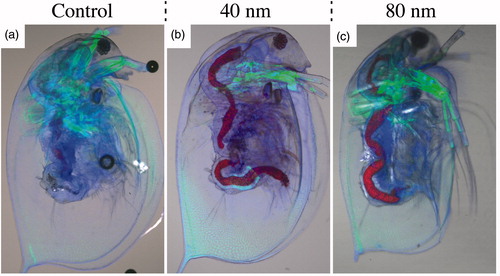
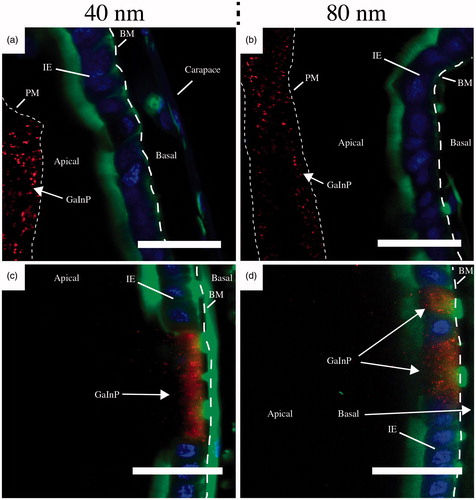
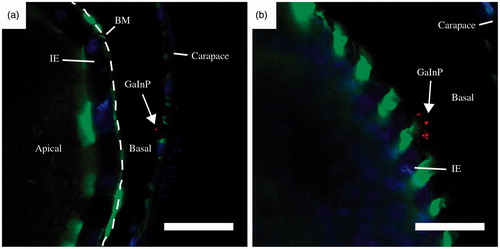
Figure 5. Representative confocal images of Daphnia intestine after 24 h of NW exposure. After 40 nm NW filtering (a) and (c) and after 80 nm NW filtering (b) and (d). Scale bars: 20 μm. Stained with Alexa Fluor 488 – Phalloidin (green, actin) and Hoechst 33342 (blue, DNA). The GaInP fluorescence can be seen in red. BM – basement membrane (dashed line), IE – intestinal epithelium, PM– peritrophic membrane (dotted line). (See online version of the paper for a color image.)
Accumulation of NWs in the intestinal epithelium
For both diameters, NWs were accumulating in the intestinal epithelium. The NWs appear to be either inside cells of the intestinal epithelium (Supplementary Figure S4) or in locations that seem to correlate with one or several cells missing from the epithelium (). Patches of missing cells are also present 24 h after exposure for both diameters (Supplementary Figure S5). Missing cells are visible in every Daphnia in the control group as well (Supplementary Figure S6), but in this group (n = 9) only one cell is missing and not several adjacent ones, as was seen in 17 out of 18 animals in the NW-fed group (n = 18).
Translocation of NWs over the gut epithelium
Translocation of NWs over the gut epithelium into the basal side of the intestinal epithelium was observed in 89% of Daphnia fed with 40 nm NWs (n = 9) and 11% of Daphnia fed with 80 nm NWs (n = 9). Representative images of translocated 40 nm NWs are shown in and a high magnification image of a translocated 40 nm diameter NW is shown in Supplementary Figure S7. The translocated NWs appeared to be shorter than their initial length ( and Supplementary Figure S7).
Figure 6. Representative confocal images of translocated 40 nm NWs after 24 h of NW exposure. Scale bars: 20 μm. Stained with Alexa Fluor 488 – Phalloidin (green, actin) and Hoechst 33342 (blue, DNA). The GaInP fluorescence can be seen in red. BM – basement membrane (dashed line), IE – intestinal epithelium. (See online version of the paper for a color image.)
Discussion
The NWs studied here do not induce any acute toxicity at the concentration tested (6.2 × 1010 NWs/L), which corresponds to 4.6 mg/L for 80 nm and 1.4 mg/L for 40 nm. In contrast, Scanlan et al. classified AgNWs as highly toxic to aquatic organisms based on recorded LC50 values below 1 mg/L after 24 h of exposure, and reported that shorter NWs are more toxic than longer ones (2 μm compared to 20 μm) (Scanlan et al., Citation2013). Artal et al. found toxic effects of AgNWs at 34 μg/L for Daphnia similis after 48 h of exposure (Artal et al., Citation2013; Mwangi et al., Citation2011). It is however possible that the observed toxicity was, at least in part, due to the release of Ag+ ions from the NWs. GaP has been shown to release ionic species in vivo as well. Linsmeier et al. found increased amounts of gallium ions in the brain, liver, and kidneys as a consequence of GaP implantation in the rat abdominal wall. In the mentioned study, it was found that GaP implants resulted in sustained inflammation, whereas the immune response against titanium-based control implants decreased over time (Linsmeier et al., Citation2008). For comparison, a rough estimation of the highest possible concentration of gallium ions generated in the mentioned study can be calculated by assuming that all gallium in the implant dissolved into ions. This results in a gallium ion concentration of 0.8 mM. The same estimation applied to the experiments presented here, results in a gallium ion concentration of 14 μM and 46 μM, respectively, which is negligible compared to the dose used in the earlier study.
Mwangi et al. reported acute toxicity to Hyalella azteca for silicon carbide NWs at 1 g/L after 48 h of exposure (Mwangi et al., Citation2011), which is a higher concentration compared to the acute toxicity concentrations for AgNWs reported above. Although not directly comparable, these studies report markedly different toxicity levels depending on the type of NW studied. This is in agreement with the general conception that many aspects influence the toxic properties of fibers, which complicates generalizations regarding toxicity. It also underlines the importance of isolating and investigating the effect of single parameters. For this reason, the NWs chosen for this work differed mainly with respect to their diameter (median length of 3.6 ± 0.7 μm and 3.8 ± 0.9 μm and diameter of 45 ± 6.1 nm and 80 ± 10.4 nm, respectively).
Our results show that the NWs used in this study were extensively fragmented during the 24 h of exposure. Based on dark field optical microscopy studies of the NW suspensions before and after exposure, the NWs were found to be fragmented from a median length of 3.6 ± 0.7 μm and 3.8 ± 0.9 μm before introducing Daphnia in the medium, to 1.37 ± 0.77 μm and 1.44 ± 0.74 μm for 40 nm and 80 nm NWs, respectively, after 24 h of Daphnia filtering (). Because the lengths of the NW fragments after filtering were comparable to the optical resolution of our dark field setup (NA = 0.4), we reasoned that we would be unable to compare the lengths of the 40 nm and 80 nm NWs using optical microscopy. For this reason, we have used SEM to make this comparison, which resulted in median lengths of 402 ± 330 nm and 686 ± 820 nm for 40 nm and 80 nm NWs, respectively, showing that the 40 nm NWs were broken into smaller fragments than the 80 nm NWs (Supplementary Figure S2). The fragmentation is likely a result of Daphnia filtering and is not due to the presence of substances released by Daphnia in the water. Daphnia filter 18.5 mL per hour and animal (Gillis et al., Citation2005), and consequently the water is filtrated by the Daphnia up to 900 times under the conditions of our experiment. This leads to repeated ingestions of the NWs and most likely fragmentation of the NWs as they are processed through the gut. To our knowledge, there has not been any detailed analysis of NW length after filtering in Daphnia magna. By contrast, Lin et al. found no fragmentation after CeO2 NW exposure to zebrafish larvae. They analyzed pseudofeces using transmission electron microscopy (TEM) and found that the NWs are aggregated but retain their original size and aspect ratio (1 μm long and approximately 10 nm in diameter, Lin et al., Citation2014). Whether these different results reflect a difference in stability between CeO2- and GaP/GaInP NWs or physiological differences between Daphnia and zebrafish larvae is unclear.
The toxicity of highly absorbing substances (such as nanoparticles) can be influenced by the surface to volume ratio of the test vessel since surface absorption will lower the effective concentration (Baumann et al., Citation2014). In our case, there was no significant difference in the number of NWs in test tubes without Daphnia before and after 24 h (see Methods section).
After exposure, NWs were seen to accumulate in the intestinal epithelium for both 40 nm (n = 9) and 80 nm NWs (n = 9) (). The NWs appear to be inside cells of the intestinal lining (Supplementary Figure S4). Whether the cells containing NWs are phagocytic cells, which would explain the mechanism of internalization, is, however, still unknown. Interestingly, we observed high concentrations of NWs in locations where one or several cells of the epithelium were missing (). Missing cells are visible in the control group as well (Supplementary Figure S6), but in this group (n = 9) only one cell is missing and not several adjacent ones, as seen in the NW-fed groups (n = 18). Regions of missing cells may be vacancies after holocrine secretion, which is known to take place in the Daphnia intestine (Heinlaan et al., Citation2011). However, holocrine cells are typically dispersed among other cell types and do not explain the large disruptions of the cellular lining in the Daphnia exposed to NWs. In comparison, Lin et al. showed ultrastructural damage, blunting of microvilli and epithelial atrophy as a consequence of 1 μm long, 10 nm diameter CeO2 NW exposure to zebrafish larvae, but no NWs were taken up into the epithelial cells or gained access to subepitehlial tissue (Lin et al., Citation2014). In the work of Edgington et al., TEM investigations indicated the presence of CNTs in gut epithelial cells of Daphnia magna, but after further analysis using high-resolution TEM, selective area diffraction and X-ray energy-dispersive spectroscopy, this was determined to be an artifact, and not CNTs (Edgington et al., Citation2014). This finding highlights the difficulties in detecting fibers in a complex biological environment without a strong specific signal from the fibers. For this reason, we chose to use fluorescent NWs in this work.
We observed translocation of NWs over the gut epithelium into the basal side of the intestinal epithelium (), and more frequently for the 40 nm diameter NWs compared to the 80 nm NWs (89% for the 40 nm diameter NWs and 11% for the 80 nm diameter NWs). Translocation of NWs into the body cavity of Daphnia has been reported before (Scanlan et al., Citation2013) and although this work involved the use of two populations of NWs with different diameter and length, these two factors were simultaneously changed, making it difficult to isolate the effect of the geometrical aspects.
The translocated NWs appeared to be much shorter than 3.6 μm (Supplementary Figure S7), which was the initial length of the 40 nm NWs in medium before feeding. This indicates that the translocated NWs are fragments of NWs. This finding contrasts with the results of Scanlan et al., who detected intact AgNWs in the hemolymph of Daphnia magna (Scanlan et al., Citation2013). However, in that study, hemolymph was collected by puncture through the carapace, which can lead to NW contamination from the carapace. In our study, confocal microscopy was used to locate the NWs in situ. Note, however, that due to the limited resolution of conventional confocal microscopy we were unable to obtain reliable size information of the translocated NWs.
Finally, it is possible that the diameter also has an indirect effect on translocation across the intestinal epithelium, as the 40 nm NWs fragmented into shorter pieces than the 80 nm NWs during filtering, which could possibly influence their ability to translocate. This leads us to believe that the observed preferential translocation of 40 nm diameter NWs through the gut epithelium is due to the fact that smaller NW diameter facilitates translocation, and/or that the NW fragments were shorter for the 40 nm NWs than for the 80 NWs (Supplementary Figures S1 and S2).
Conclusions
Exposure to NWs for 24 h does not increase the mortality of Daphnia magna neither immediately after NW exposure nor 24 h later. However, the filtering activity of Daphnia magna fragmented the NWs in a diameter-dependent manner. The fragmented 40 nm diameter NWs are shorter compared to 80 nm diameter NWs. Moreover, the 40 nm diameter NW fragments passed through the intestinal epithelium to a higher extent than the 80 nm diameter NWs. This may be a direct effect of the diameter, or due to more extensive fragmentation of the thinner NWs, or a combination of both effects. In a broader context this suggests that biological processes, such as feeding behavior of animals, may facilitate the penetrance of NWs through biological barriers.
Supplementary material available online
Supplementary Tables S1–S3 and Figures S1–S7
Supplementary Materials
Download MS Word (63.8 MB)Acknowledgements
We would like to thank Christian Bergenfeldt, David Göransson, and Masoomeh Ghasemi for contributing to the idea of the project and Henrik Persson for input to this study. The confocal microscopy was performed at the Microscopy Facility at the Department of Biology, Lund University.
Declaration of interest
The authors declare that they have no competing interests.
This study was funded by NanoLund.
References
- Abramoff MDM, Paulo J, Ram SJ. 2004. Image processing with ImageJ. Biophoton Int 11:36–42.
- Adolfsson K, Schneider M, Hammarin G, Hacker U, Prinz CN. 2013a. Ingestion of gallium phosphide nanowires has no adverse effect on Drosophila tissue function. Nanotechnology 24:285101.
- Adolfsson K, Persson H, Wallentin J, Oredsson S, Samuelson L, Tegenfeldt JO, et al. 2013b. Fluorescent nanowire heterostructures as a versatile tool for biology applications. Nano Lett 13:4728–32.
- Artal MC, Holtz RD, Kummrow F, Alves OL, Umbuzeiro Gde A. 2013. The role of silver and vanadium release in the toxicity of silver vanadate nanowires toward Daphnia similis. Environ Toxicol Chem 32:908–12.
- Baumann J, Sakka Y, Bertrand C, Koser J, Filser J. 2014. Adaptation of the Daphnia sp. acute toxicity test: miniaturization and prolongation for the testing of nanomaterials. Environ Sci Pollut Res Int 21:2201–13.
- Burns CW. 1969. Relation between filtering rate, temperature, and body size in 4 species of Daphnia. Limnol Oceanogr 14:693–700.
- Dobrovolskaia MA, Patri AK, Zheng JW, Clogston JD, Ayub N, Aggarwal P, et al. 2009. Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomed Nanotechnol Biol Med 5:106–17.
- Edgington AJ, Petersen EJ, Herzing AA, Podila R, Rao A, Klaine SJ. 2014. Microscopic investigation of single-wall carbon nanotube uptake by Daphnia magna. Nanotoxicology 8:2–10.
- Gallentoft L, Pettersson LM, Danielsen N, Schouenborg J, Prinz CN, Linsmeier CE. 2015. Size-dependent long-term tissue response to biostable nanowires in the brain. Biomaterials 42:172–83.
- Gillis PL, Chow-Fraser P, Ranville JF, Ross PE, Wood CM. 2005. Daphnia need to be gut-cleared too: the effect of exposure to and ingestion of metal-contaminated sediment on the gut-clearance patterns of D. magna. Aquat Toxicol 71:143–54.
- Heinlaan M, Kahru A, Kasemets K, Arbeille B, Prensier G, Dubourguier HC. 2011. Changes in the Daphnia magna midgut upon ingestion of copper oxide nanoparticles: a transmission electron microscopy study. Water Res 45:179–90.
- Li Y, Qian F, Xiang J, Lieber CM. 2006. Nanowire electronic and optoelectronic devices. Mater Today 9:18–27.
- Lin S, Wang X, Ji Z, Chang CH, Dong Y, Meng H, et al. 2014. Aspect ratio plays a role in the hazard potential of CeO2 nanoparticles in mouse lung and zebrafish gastrointestinal tract. ACS Nano 8:4450–64.
- Linsmeier CE, Prinz CN, Pettersson LME, Caroff P, Samuelson L, Schouenborg J, et al. 2009. Nanowire biocompatibility in the brain – looking for a needle in a 3D stack. Nano Lett 9:4184–90.
- Linsmeier CE, Wallman L, Faxius L, Schouenborg J, Bjursten LM, Danielsen N. 2008. Soft tissue reactions evoked by implanted gallium phosphide. Biomaterials 29:4598–604.
- Mattsson K, Hansson LA, Cedervall T. 2015. Nano-plastics in the aquatic environment. Environ Sci Process Impacts 17:1712–21.
- Mwangi JN, Wang N, Ritts A, Kunz JL, Ingersoll CG, Li H, Deng B. 2011. Toxicity of silicon carbide nanowires to sediment-dwelling invertebrates in water or sediment exposures. Environ Toxicol Chem 30:981–7.
- Nelson SM, Mahmoud T, Beaux M 2ND, Shapiro P, McIlroy DN, Stenkamp DL. 2010. Toxic and teratogenic silica nanowires in developing vertebrate embryos. Nanomedicine 6:93–102.
- Parks AN, Portis LM, Schierz PA, Washburn KM, Perron MM, Burgess RM, et al. 2013. Bioaccumulation and toxicity of single-walled carbon nanotubes to benthic organisms at the base of the marine food chain. Environ Toxicol Chem 32:1270–7.
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, et al. 2008. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 3:423–8.
- Rosenkranz P, Chaudhry Q, Stone V, Fernandes TF. 2009. A comparison of nanoparticle and fine particle uptake by Daphnia magna. Environ Toxicol Chem 28:2142–9.
- Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, et al. 2009. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol 4:747–51.
- Sanfins E, Augustsson C, Dahlback B, Linse S, Cedervall T. 2014. Size-dependent effects of nanoparticles on enzymes in the blood coagulation cascade. Nano Lett 14:4736–44.
- Scanlan LD, Reed RB, Loguinov AV, Antczak P, Tagmount A, Aloni S, et al. 2013. Silver nanowire exposure results in internalization and toxicity to Daphnia magna. ACS Nano 7:10681–94.
- Schinwald A, Chernova T, Donaldson K. 2012. Use of silver nanowires to determine thresholds for fibre length-dependent pulmonary inflammation and inhibition of macrophage migration in vitro. Part Fibre Toxicol 9:47.
- Silva RM, Xu J, Saiki C, Anderson DS, Franzi LM, Vulpe CD, et al. 2014. Short versus long silver nanowires: a comparison of in vivo pulmonary effects post instillation. Part Fibre Toxicol 11:52.
- Stobbart RH, Keating J, Earl R. 1977. Study of Sodium Uptake by Water Flea Daphnia magna. Compar Biochem Physiol A 58:299–309.
- Sun B, Wang X, Ji Z, Li R, Xia T. 2013. NLRP3 inflammasome activation induced by engineered nanomaterials. Small 9:1595–607.
- Tenzer S, Docter D, Rosfa S, Wlodarski A, Kuharev J, Rekik A, et al. 2011. Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: a comprehensive quantitative proteomic analysis. ACS Nano 5:7155–67.
- Walkey CD, Olsen JB, Guo H, Emili A, Chan WC. 2012. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc 134:2139–47.
- Zhu XS, Zhu L, Chen YS, Tian SY. 2009. Acute toxicities of six manufactured nanomaterial suspensions to Daphnia magna. J Nanoparticle Res 11:67–75.
