Abstract
Despite the great potential of using positively charged gold nanoparticles (AuNPs) in nanomedicine, no systematic studies have been reported on their synthesis optimization or colloidal stability under physiological conditions until a group at the National Institute of Standards and Technology recently succeeded in producing remarkably stable polyethyleneimine (PEI)-coated AuNPs (Au-PEI). This improved version of Au-PEI (Au-PEI25kB) has increased the demand for toxicity and teratogenicity information for applications in nanomedicine and nanotoxicology. In vitro assays for Au-PEI25kB in various cell lines showed substantial active cytotoxicity. For advanced toxicity research, the frog embryo teratogenesis assay–Xenopus (FETAX) method was employed in this study. We observed that positively-charged Au-PEI25kB exhibited significant toxicity and teratogenicity, whereas polyethylene glycol conjugated AuNPs (Au-PEG) used as comparable negative controls did not. There is a characteristic avidity of Au-PEI25kB for the jelly coat, the chorionic envelope (also known as vitelline membrane) and the cytoplasmic membrane, as well as a barrier effect of the chorionic envelope observed with Au-PEG. To circumvent these characteristics, an injection-mediated FETAX approach was utilized. Like treatment with the FETAX method, the injection of Au-PEI25kB severely impaired embryo development. Notably, the survival/concentration curve that was steep when the standard FETAX approach was employed became gradual in the injection-mediated FETAX. These results suggest that Au-PEI25kB may be a good candidate as a nanoscale positive control material for nanoparticle analysis in toxicology and teratology.
Introduction
From conceptual design to materialization in the laboratory, decades of research on nanoparticle (NP) based bio-conjugates have led to a new paradigm called nanomedicine (Niemeyer Citation2001; Hamley Citation2003; Katz and Willner Citation2004; Rosi and Mirkin Citation2005; Whitesides Citation2005; De, Ghosh, and Rotello Citation2008). NP-platformed drug delivery systems can play an important role in the field of nanomedicine by improving the efficacy and efficiency of high drug doses via targeted delivery while reducing the toxic adverse effects (Cho et al. Citation2008; Hu and Zhang Citation2009), and recently by inducing selective toxicity against cancer cells (Ahamed et al. Citation2021). Due to the enhanced permeation and retention (EPR) effect (Maeda Citation2001; Kratz et al. Citation2008), the use of nano-scale vehicles for delivery could increase drug moiety accumulation in cancer cells, thereby minimizing either kidney excretion or cellular uptake (by healthy cells). Among the various NPs applied, gold NPs (AuNPs) have shown broad potential for use in various applications (Bose et al. Citation2014; Singh et al. Citation2018; Sztandera, Gorzkiewicz, and Klajnert-Maculewicz Citation2019).
The attraction to AuNPs originates largely from their chemical stability, biocompatibility (although much smaller gold nanoclusters do not necessarily exhibit this property (Pan et al. Citation2009)), commercial availability, and easily modifiable surface functionalities (Daniel and Astruc Citation2004). In particular, positively-charged AuNPs have shown great potential for use in biomedical applications because of their cationic surface characteristics that can induce electrostatic approach/interaction to the anionic surfaces of bio-entities, resulting in cellular uptake (Cho et al. Citation2009; Arvizo et al. Citation2010; Taylor et al. Citation2010), and/or gene transfection (Thomas and Klibanov Citation2003; Song et al. Citation2010; Lee et al. Citation2011a; Elbakry et al. Citation2012; Chen et al. Citation2014; Ding et al. Citation2014).
Polyethyleneimine (PEI) is the most widely-used polycation for the development of positively charged AuNPs (hereafter referred to as Au-PEIs) due to its characteristic properties. These include roles as both reducing and stabilizing agents during Au-PEI formation (Sun, Dong, and Wang Citation2005; Note, Kosmella, and Koetz Citation2006; Kim et al. Citation2008), water solubility, proton scavenging efficacy (pH buffering) (Boussif et al. Citation1995; Thomas and Klibanov Citation2003), interactions with polyanions (Kramer et al. Citation1998) and biomaterials (Boussif et al. Citation1995), low-cost, and commercial availability. Due to these attractive features, Au-PEIs have been prepared by several research groups—as cited by Cho et al. (Citation2015, Citation2019)—and used for further investigation, including layer-by-layer assembly and subsequent gene transfection studies. However, they also have disadvantages, such as the known cytotoxicity (Moghimi et al. Citation2005; Hunter Citation2006), organo-toxicity in aquatic vertebrates (Hu et al. Citation2017; Zhang et al Citation2020) of PEIs and perhaps of Au-PEIs. The interaction between Au-PEIs and the cell membrane surface indicates that Au-PEIs could induce cellular uptake, which can be advantageous for specific nanomedical applications. However, the active interaction between Au-PEIs and bio-entities may induce cytotoxicity as an undesirable side-effect. Interestingly, if one shifts to the opposing perspective, this seemingly negative interaction between Au-PEIs and cellular biomolecules may also have beneficial attributes. Selected types of Au-PEI interactions in vitro and in vivo may allow these NPs to serve as active nanoscale positive control materials in nanotoxicology. For the conventional toxicology study, control materials are fundamental for assay reproducibility and repeatability. Likewise, nanotoxicology assays require nanoscale control materials other than soluble chemical toxins. The expressed demands of such materials have emerged from global standards-related agencies; ISO TC229, OECD Testing Programme of Manufactured Nanomaterials, and International Alliance for NanoEHS Harmonization (IANH) (Lynch et al. Citation2009; Roebben et al. Citation2011). Our group at the National Institute of Standards and Technology (NIST) has been addressing these needs since the early stage of nanotoxicology. AuNP reference materials (RMs 8011, 8012, and 8013, NIST, Gaithersburg MD) have tentatively served as potential nanoscale negative controls in nanotoxicological analysis (Nelson et al. Citation2013); meanwhile, to date, validated nanoscale positive controls for establishing standardized protocols of nanotoxicology assays remain scarce (Kim et al. Citation2013a). The main hypothesis of this study, as mentioned above, is whether the inherent toxicity of the PEI chain itself will be retained following conjugation to the gold core. In addition, if structural and morphological differences between polymeric chains (PEI) and colloids (Au-PEI) can modulate toxicity mechanisms depending on the type of material, it will open further avenues for nanomaterial toxicology research.
In general, NPs must exhibit several critical characteristics for their successful use in either nanomedical or nanotoxicological applications. These characteristics include monodisperse size distributions, long-term chemical and physical integrity, hydrophilicity, and colloidal stability in physiologically relevant conditions such as isotonicity, pH, and temperature (Cho et al. Citation2014). Previously, several different groups of researchers have used their own various conditions for Au-PEI preparation, and therefore, it is unclear which specific features of Au-PEIs can be associated with the colloidal behavior observed in the biological environment. Besides the features mentioned above, additional properties, including the toxicity of Au-PEIs attributable to variables such as molar mass and/or PEI structure, Au-PEI size, cell type, assay medium, and morphological transformation (colloidal stability) should also be considered (Sullivan et al. Citation2003; Hu and Zhang Citation2009; Hu et al. Citation2010; Song et al. Citation2010; Cebrián et al. Citation2011; Kim et al. Citation2011; Lee et al. Citation2011b; Elbakry et al. Citation2012; Tao et al. Citation2013; Chen et al. Citation2014; Pyshnaya et al. Citation2014). Therefore, it is essential to conduct careful systematic studies on all designed/prepared nanomaterials (as preliminary studies before proceeding with biological activity tests) and to assess their potential utility as bioactive candidates for nanomedicine and avoid unexpected/unintentional false results or artifacts. Recently, Cho et al. (Citation2019) reported the development of a robust, reproducible method for the preparation of Au-PEIs, which enables the analysis of the systemic structure–function relationship, as well as the link between the synthesis method and product properties. In that study authors performed a multi-parameter analysis involving 96 reaction combinations and identified an optimal set of synthetic conditions and parameters that consistently yield a superior product with the required physical and stability properties necessary for nanomedicine applications (Cho et al. Citation2020).
Along with in vitro cytotoxicity analysis of AuNPs, evaluation of the hazardous effects of Au-NPs in vivo is important in practical toxicology research. Moreover, performing these studies during embryogenesis, a highly sensitive life stage, will further provide basic information relevant for nanotoxicology. To date, most embryo toxicity studies on engineered NPs, including AuNPs, have been conducted using zebrafish (Danio rerio) (Haque and Ward Citation2018), an established in vivo testing model, to identify mechanisms underlying the induced toxicity (Hill et al. Citation2005), whereas no reports exist for AuNPs tested using traditional amphibian systems. The Frog Embryo Teratogenesis Assay–Xenopus (FETAX) (Mouche, Malesic, and Gillardeaux Citation2017), which was originally standardized and improved in the early 1990s (ASTM International E1439) has been broadly used to examine the teratogenic potency of environmental pollutants, test drugs (Fort and Paul Citation2002; Bonfanti et al. Citation2004; Chae et al. Citation2015; Williams et al. Citation2015), and metal oxide nanomaterials (Nations et al. Citation2011; Bacchetta et al. Citation2012), but not AuNPs. In most studies on AuNPs with zebrafish (Browning et al. Citation2009; Asharani et al. Citation2011; Geffroy et al. Citation2012; Truong et al. Citation2012; Kim et al. Citation2013b; Dedeh et al. Citation2015; Dayal et al. Citation2016), the tested materials were either negatively charged or neutral AuNPs, but positively-charged (cationic) AuNPs have rarely been utilized. The only examples of cationic AuNPs were reported by Truong et al. (Citation2012, Citation2013, Citation2017) and Kim et al. (Citation2013b) who used N, N, N-trimethylammonium ethanethiol-stabilized AuNPs (Au-TMATs). To the best of our knowledge, no reports exist on the use of Au-PEIs. Although Au-TMATs exhibit toxic developmental effects during embryogenesis, it remains unclear whether these will be useful in nanomedicine as drug carriers or nanoscale positive controls due to their size. According to the definition of engineered NPs by the National Institute of Occupational Safety and Health (https://www.cdc.gov/niosh/docs/2009-116/), these include particles ‘with at least one dimension of approximately between 1 and 100 nanometers’, and Au-TMATs (which are mainly ≈ 1.5 nm in diameter) lie at the extreme low end of this range. Additionally, their preparation process is more complicated than for Au-PEIs.
In this study, we employed the Au-PEI synthetic route (Au-PEI25kB) developed by Cho et al. (Citation2019, Citation2020), which show outstanding colloidal stability and have well-characterized physicochemical properties. In addition to Au-PEI, we employed pegylated AuNPs (Au-PEG) as a counter-part control material, a nanoscale negative control. Since Au-PEG has been used extensively in nanomedicine (Reznickova et al. Citation2019; Wang et al. Citation2020), we expect Au-PEG to show only minimal cytotoxicity to cells. If negligible-toxicity is confirmed in this study system, Au-PEG NPs could be a replacement for citrate-stabilized AuNPs as negative controls−which currently are the most often used AuNPs−due to their instability in the physiological media (Cho and Hackley Citation2018). Thus, we used the standardized FETAX method to evaluate the potential developmental toxicity of the positively-charged PEI, PEI-coated AuNP (Au-PEI25kB), and further to identify any differences from that of the neutral polymer, polyethylene glycol (PEG)-coated AuNPs (Au-PEG5k-OMe). Furthermore, we assessed the potential cytotoxic effects of these AuNPs on various endothelial cells, since they line the route for intravenous (IV)-introduction of drugs with the expectation of an EPR effect in the mammalian system. The results of this study provide information regarding the biological activity required to assess the use of Au-PEI25kB and Au-PEG5k-OMe as candidate carriers for a drug delivery system or as nanoscale control materials for nanotoxicology.
Materials and methodsFootnote1
Measurement methods and instrumentation for physicochemical properties such as UV-Vis, dynamic light scattering (DLS), zeta potential (z-p), thermogravimetric analysis (TGA), stability tests, cell viability assay, and cell count assay are described in Supplemental online material(SM).
Materials
Gold(III) chloride hydrate (HAuCl4•3H2O, ACS reagent), and branched 25 kDa PEI (PEI25kB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methoxy polyethylene glycol thiol (OMe-PEG-SH; 5 kDa) was obtained from Laysan Bio Inc. (Arab, AL, USA). All chemicals were used without further purification. Deionized (DI) water (18.2 MΩ·cm) was produced by an Aqua Solutions (Jasper, GA) Type I biological grade water purification system.
AuNP preparation
Au-PEI25kB
To 10 mL of aqueous HAuCl4 (2.5 mmol/L) solution, 1 mL of stock solution of aqueous branched PEI (25 kDa (PEI25kB), 10% of the mass fraction) was added in a borosilicate glass vial (EPA vials, Thermo Scientific, Waltham, MA) at room temperature (r.t.) and heated to 80 °C on a magnetic hot plate with stirring (≈ 12 Hz or 700 rpm). The temperature was increased from r.t. at a rate of approximately 5 °C/min. After reaching 80 °C, the reaction mixture was stirred for 1.5 h, removed from the hot plate and allowed to cool to ambient temperature. The product was purified using a centrifugal filter (Amicon Ultra15, 100 kDa molecular weight cut off, filtered and refilled with deionized (DI) water to 10 mL, the process was repeated three times), and adjusted to a final volume of 10 mL with DI water yielding an Au concentration of ≈ 2.5 mmol/L, which was similar to the starting concentration.
Au-PEG5k-OMe
For comparison, PEG-stabilized AuNPs (Au-PEG5k-OMe) were prepared as follows: 100 mL of aqueous HAuCl4 (0.25 mmol/L) was heated to reflux. To this reflux, 1 mL of aqueous sodium citrate (120 mmol/L) was added and stirred for 30 min. The red suspension was cooled to r.t., and 1 mL of aqueous methoxy PEG thiol (5 mmol/L; molar mass 5 kDa) was added and stirred for another 3 h at room temperature. The entire reaction mixture was purified by centrifugal filtration (Amicon Ultra15, MWCO 100 kDa); Four filters were used at a time for this process. First 15 mL of the reaction mixture was placed in each filter and centrifugal filtered, then the next 15 mL of the reaction mixture underwent the same centrifugation and was washed with water twice (15 mL at each). The final concentration of purified Au-PEG5k-OMe was adjusted to that of Au-PEI25kB in a total 10 mL (≈ 2.5 mL of residual Au-PEG5k-OMe per filter). The demand for higher concentrations of AuNP samples for specific tests was adjusted by further centrifugal filtration; Note: This particular PEG was chosen by consideration of linear PEG structure, unlike branched PEI. Surface thicknesses between Au-PEI and Au-PEG could be significantly different if 25 kDa PEG used.
FETAX and attachment/penetration assay
For treatment-mediated FETAX (Tr-FETAX), the original FETAX protocol was followed (Mouche, Malesic, and Gillardeaux Citation2017). The FETAX range determination experiment used concentrations of [2500 µmol/L (stock) to 0.00017 µmol/L (Au-PEI25kB)], [2500 µmol/L (stock) to 2.6 µmol/L (Au-PEG5k-OMe)], [238 000 µmol/L (1% stock, mass fraction) to 0.004915 µmol/L (unit concentration; unit molar mass = 42; PEI25kB alone)] and [227 300 µmol/L (1% stock, mass fraction) to 73.6 µmol/L (unit concentration; unit molar mass = 44; PEG alone)], the series of five-fold dilutions from the stocks. For microinjection-mediated FETAX (Inj-FETAX), the AuNP stock was concentrated six-fold via Amicon centrifugal filtration. For an examination on attachment/penetration of AuNPs on/through the jelly coat, we omitted the de-jellying step as following the original FETAX protocol (Mouche, Malesic, and Gillardeaux Citation2017) for dimethyl sulfoxide (DMSO)-dissolvable, permeable compounds. Other range-finding and definitive FETAX procedures included de-jellying with L-cysteine solution before the seeding step. After in vitro fertilization, embryos that did not show the perfect cleavage feature at the 32-cell stage were removed to obtain a zero base spontaneous variation rate in the control group and increase the data credibility. After incubation at 23 °C for an additional 2 h, from stage 8–45 [5–96 h post-fertilization (hpf)], the embryos were incubated in chemical-containing FETAX media. At stage 45 (96 hpf), the anesthetized embryos (0.01% MS-222) were examined for hemorrhaging and then subsequently euthanized by the addition of MS-222 (0.1% final concentration) for 30 min. Finally, the embryos were fixed with 10% formaldehyde to count other aspects of malformation. All FETAX experiments were repeated three times using the same number of embryos.
For Inj-FETAX, the FETAX medium was replaced daily, but no additional injections were performed other than the first injection at the one-cell stage. The AuNPs were diluted in the FETAX medium. For the control, 10 nL of the FETAX medium was injected.
Transmission electron microscopy (TEM) studies
The vitelline membrane (chorionic membrane) was removed at the eight-cell stage using forceps, and the embryos were immersed in 6× concentrated stock (15 000 µmol/L) of AuNPs in a 96-well plate, with one in each well, and the plates were incubated at 23 °C for 4 h. Next, the embryos were washed in FETAX medium three times, followed by 5 min fixation using 2% glutaraldehyde (Sigma-Aldrich, St. Louis, MO) + 4% formaldehyde (Sigma-Aldrich) in FETAX medium. The embryos were then fixed in 2% glutaraldehyde + 4% formaldehyde in 0.1 mol/L sodium cacodylate (Trihydrate, Sigma-Aldrich) buffer overnight at 4 °C. The next day, the embryos were washed three times with 0.1 mol/L sodium cacodylate buffer and stained with 1% osmium tetroxide and 0.5% uranyl acetate for 1 h each. Subsequently, ethanol solutions (35%, 50%, 70%, 95%, and 100%) were used for sample dehydration. Next, the samples were immersed in Embed 812 resin (Fisher Scientific, Hampton, NH) overnight to enable resin infiltration and incubated in an oven (55 °C) for 48 h to complete the resin-curing process. Thin sections from the sample blocks were prepared using a Leica UC7 microtome and the sections were stained with uranyl acetate and lead citrate for 2 min each. The images were obtained using a Hitachi H7600 TEM.
For each sample, both low magnification images (0.00318 µm/pixel, HV = 80.0 kV, 5000×) that provided an overview of the target area to search for regions containing gold, and high magnification images (0.795 nm/pixel, HV = 80.0 kV, 20 000×) to search for and verify gold particles were obtained.
Statistics
LC50 [median lethal concentration] and EC50 [median effective (teratogenic) concentration] were calculated using Prism version 8 for Mac (GraphPad LLC, San Diego, CA, USA) and performing nonlinear regression (curve fit) analysis with the equation for the four-parameter logistic curve (Sigmoidal 4PL). Curve charts for LC50 and EC50 with Au-PEI25kB and PEI25kB were obtained from the sigmoidal 4PL analysis.
The survival and malformation rates from multiple comparisons among various concentrations of Au-PEI25kB were evaluated using a one-way analysis of variance (ANOVA; nonparametric Kruskal–Wallis test) using the same software. The survival and malformation rates between the Au-PEG5k-OMe treatment and the control groups did not show significant differences using the Kolmogorov–Smirnov test. The Chi-square test was used to evaluate malformation features relative to the control using the Prism 8 software. For TEM data analysis, arbitrary values for the linear or spatial AuNP counts were evaluated using the nonparametric t-test (Kolmogorov–Smirnov D test) to compare the Au-PEI25kB and Au-PEG5k-OMe data sets using Prism 8.
Results
AuNP characterization
Synthesis and characterization of physicochemical properties of PEI-stabilized AuNPs (Au-PEI25kBs) were fully investigated and reported in recent publications (Cho et al. Citation2019, Citation2020). The PEI-stabilized AuNP were characterized in terms of their size (via DLS, TEM), size distribution (using TEM, single particle inductively coupled plasma mass spectrometry, electrospray differential mobility analysis), optical properties (surface plasmon resonance (SPR) by UV-Vis), and surface functionality (by X-ray photoelectron spectroscopy, attenuated total reflectance-FTIR). Briefly, Au-PEI25kB were freshly prepared as described earlier (Cho et al. Citation2019). As shown in , Au-PEI25kB had a diameter of (22.8 ± 0.3) nm (z-average hydrodynamic size (dz)) as obtained from DLS measurements (), (+18.6 ± 0.7) mV zeta potential (z-p), and exhibited a surface plasmon resonance (SPR) band at 522 nm (by UV-Vis spectroscopy (). On the other hand, Au-PEG5k-OMe exhibited dz = (38.4 ± 0.6) nm (), a z-p of −19.4 ± 2.8) mV and a maximum SPR band at 521 nm (). In a representative colloidal stability test of the AuNPs, we evaluated the shelf-life of Au-PEI25kB and Au-PEG5k-OMe. Both freshly prepared AuNPs were stored for a year under ambient conditions (at (20–25)°C, away from direct light). The size was monitored by DLS and the absorption by UV-Vis (, dotted lines). The tested Au-PEI samples showed minimal changes in their hydrodynamic size (Δdz = 2 nm) and almost identical maximum SPR bands (Δλmax = 1 nm) after 1 year, demonstrating their stable behavior under ambient conditions without the aid of excipients. Au-PEG5k-OMe, the comparison material used in this study, also showed stable shelf-life without any significant changes in either size or SPR as shown in .
Figure 1. z-average sizes (a,c) and SPR bands (b,d) of initially prepared Au-PEI25kB and Au-PEG5k-OMe (black circles), and 1-year-aged (shelf-life) Au-PEI25kB and Au-PEG5k-OMe (red dots) obtained from DLS and UV-Vis measurements, respectively. Colloidal stability of Au-PEI25kB (e) and Au-PEG5k-OMe (f) in FETAX media by measuring SPR bands (UV-Vis) over 96 h. Insets in panel (e,f) are representing no morphological changes over time (left; 0h, middle; after 48 h, right: after 96 h) for both AuNPs.
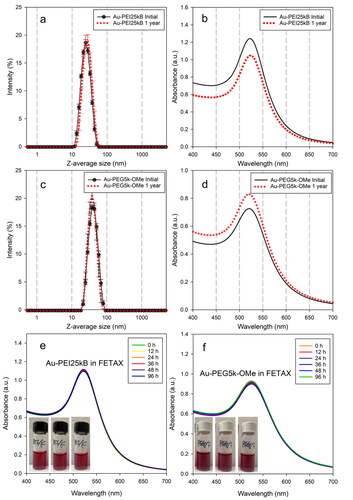
As a representative stability test of AuNPs for this study, the colloidal behavior of Au-PEI25kB and Au-PEG5k-OMe in FETAX media over time was assessed. Both AuNPs exhibited almost identical SPR bands in UV-Vis absorbance spectra for up to 96 h (), and without any visible morphological changes such as agglomeration (from particle-particle interactions) or aggregation (from core-to-core fusion) in this media (, insets in panels e and f), which suggest sufficient colloidal stability of test materials for the FETAX study. Moreover, in our previous study (Cho et al. Citation2020), a wider range of physiologically relevant environmental conditions was explored for Au-PEI25kB, such as the biological medium (PBS, M9, DMEM, BSA/DMEM), pH (1 ∼ 12), temperature (20 °C ∼ 70 °C), and lyophilization-reconstitution cycling (Cho and Hackley Citation2018). These results confirm that Au-PEI25kB is very stable and is appropriate for biological applications; therefore, no further stability studies for of Au-PEI25kB were performed in the present investigation.
The surface coverage of Au-PEI25kB (mass ratio of PEI to Au) was determined by TGA (described in SM). The mass ratio of PEI to Au in the Au-PEI conjugate was 4.89 ± 0.13 (one standard deviation), which was converted to a PEI/Au molar ratio of 21.6 (calculated using 42 as the unit molar mass of PEI) in Au-PEI25kB. This value indicates that ≈ 24.5% of the initially applied PEI mass was conjugated after the reduction of AuIII. No further characterization of Au-PEG5k-OMe other than size, SPR, shelf-life, and FETAX media stability, was necessary given that (1) numerous biological applications of Au-PEGs were been reported previously as described above and (2) Au-PEG5k-OMe is used mostly for comparison purposes to Au-PEI25kB as a potential nanoscale negative control.
Cytotoxic effect of Au-PEI25kB on various cell types
Chinese hamster ovary (CHO)-K1 cells were previously reported to be a sensitive cell type to 20 nm AuNPs inducing high levels of toxicity (Vetten et al. Citation2013). Therefore, the cytotoxic effect of Au-PEI25kB was evaluated using CHO-K1 () as a basic normal cell line via the MTS assay (see SM). HeLa, () and human alveolar adenocarcinoma (A549 (), cancer cell lines were tested as well. The data presented in and show that Au-PEI25kB reduced cell viability in a dose-dependent manner. The estimated doses (µmol/L of Au and Cd) for causing 50% lethality (LD50) in CHO-K1, HeLa, and A549 cells after 24 h were obtained by approximating the viability data as a continuous concentration-response curve by performing a non-linear fit (). The LD50 values indicate that the particle toxicity of Au-PEI25kB toward the tested mammalian cell lines was significantly greater than that of CdSO4 on a molar basis (positive control; pink bars in ). In addition, Au-PEG5k-OMe, which is a neutral functionalized AuNP, showed negligible toxicity in all three tested cell lines than Au-PEI25kB. Notably, due to the stability of Au-PEI25kB in the test media, no significant changes were observed in the color or morphology before or after the MTS assay (data not shown). The data showed significant toxic effects in the tested cell lines, demonstrating a cytotoxicity trend consistent with that reported previously (Cho et al. Citation2021) using similar material (prepared in different batches).
Figure 2. Representative cell toxicity data. The MTS assay results showing viability of (a) CHO-K1, (b) HeLa, and (c) A549 cell line after 24 h exposure to Au-PEI25kB (black), Au-PEG5k-OMe (green), and CdSO4 (pink, used as a positive control). The insets indicate the expanded range of low concentrations used to treat cells from (0 to 50) µmol/L.
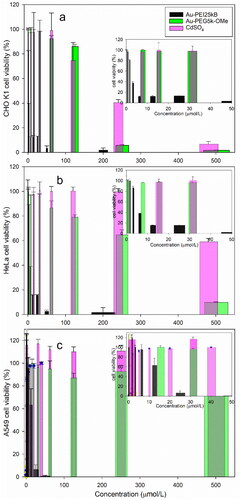
Table 1. 50% lethality (LD50, µmol/L) of tested materials in CHO-K1, HeLa, and A549 cells after 24 h.
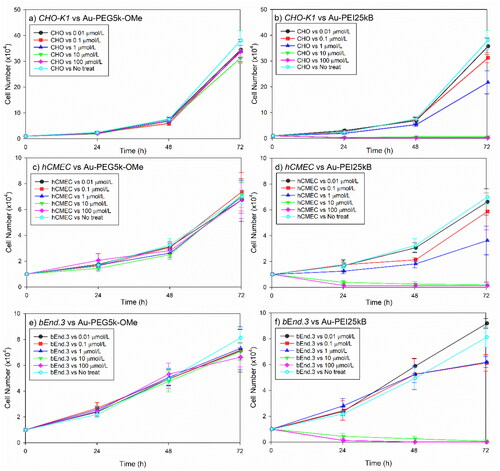
Based on the data from the MTS assay, we performed systematic AuNP-induced cellular toxicity studies using a cell count assay with CHO-K1 and two additional types of endothelial cell lines. Au-PEG5k-OMe did not affect growth in CHO-K1 cells, human cerebral microvascular endothelial cells (hCMEC), or mouse brain endothelial cells (bEnd.3) (, three left panels). In contrast, Au-PEI25kB induced toxicity in all cell lines at doses above 1 µmol/L after 1 d of treatment (, three right panels). Overall, in vitro studies with test materials showed consistent presence or absence of efficacy depending on the surface of AuNPs to all five cells in common, regardless of the cell types or assay methods. Because of these notable and distinct cell responses to type of AuNPs, we conducted an advanced toxicity analysis using the FETAX system.
Figure 3. Comparative mammalian cell survival chart corresponding to various concentrations (0.–100 µmol/L) of Au-PEG5k-OMe and Au-PEI25kB. Chinese hamster ovary cells ((a,b); CHO-K1), human cerebral microvascular endothelial cells ((c,d); hCMEC), and mouse brain endothelial cells ((e,f); bEnd3) were tested. Notably the cell growth did not decrease with Au-PEG5k-OMe treatment compared to the no-treatment control (three left panels), whereas the Au-PEI25kB-treated cells exhibited significantly compromised growth (three right panels). Error bars indicate one standard deviation.
Treatment-mediated FETAX: embryonic toxicity and teratogenicity of Au-PEI25kB and Au-PEG5k- OMe
We used the FETAX method to determine the potential toxicity and teratogenicity of Au-PEI25kB and Au-PEG5k-OMe. Owing to the lack of any previous information regarding the embryo toxicity of our newly synthesized gold NPs, range determination experiments were performed before the definitive FETAX method was used. The temporal morphology of Xenopus laevis embryos (often referred to as larvae in toxicology) after treatment with serially diluted Au-PEI25kB, PEI25kB, Au-PEG5k-OMe or PEG5k-OMe was evaluated (). Au-PEI25kB at 2500 µmol/L (stock; prepared by addition of 20× FETAX medium) exhibited a red wine color, and the embryos in this medium were lysed and turned white with black spots within 12 h. Similar results were observed with 67 µmol/L Au-PEI25kB in the FETAX medium. At 9.5 µmol/L of Au-PEI25kB, embryos showed no lethality but exhibited 94% malformation (mean; from surviving embryos) at stage 45 (96 hpf). PEI25kB alone (without gold) induced 100% lethality before entering the tailbud larva at the concentration of 192 µmol/L. In contrast, Au-PEG5k-OMe and PEG5k-OMe did not cause embryonic toxicity compared to the untreated control, even at the highest tested concentration. Note that at this concentration the FETAX media appears red upon a bright, yellowish background of embryos. We did not test higher concentrations of Au-PEG5k-OMe because 2500 µmol/L was the highest concentration produced by our synthesis procedure. The malformations induced by Au-PEI treatment included eye defects, bent axis, edema (head, heart, and abdomen), aberrant coiling of the intestines (with spectrum), monsters (no clear head and axis structure). The gut coiling defects was the most common malformation among the phenotypes noted above (, ).
Figure 4. Range determination experiment. Top left corner: Temporal morphology Xenopus laevis embryo after treatment with serially diluted Au-PEI25kB, PEI alone, Au-PEG5k-OMe, PEG alone, and no treatment. (a) The Au-PEG5k-OMe 96 h post-fertilization (hpf) panel shows the embryos at stage 45, prior to anesthesia/euthanasia/fixation, which were washed once with 1x FETAX medium, and subsequently anesthetized to evaluate hemorrhage. Note the lighter wine color of the medium after transferring the embryos to the null-FETAX medium (+MS222). All scale bars: 1 mm. (b) Examples of malformations, including eye defects, bent axes, edema (head, heart, and abdomen), miscoiled intestines (with a spectrum of mild to severe cases), and monsters (with no clear head or axis structure). The malformations shown are the counted features in c (also in ). All scale bars: 1 mm. (c) Concentration-dependent survival and malformation rates. The values indicate the mean and standard deviation. (I) Range-determining test (96 hpf) results obtained using 20 embryos per group, and the tests were repeated three times. Notably, no survival was observed at 13 and 384 µmol/L of Au-PEI and PEI, respectively. (II) Definitive FETAX results. One-way ANOVA (nonparametric Kruskal–Wallis test) test. (i) p = 0.0049, (ii) p = 0.0032, (iii) p < 0.0001, and (iv) p < 0.0001. Kolmogorov–Smirnov test (t-test; nonparametric) for (v) p > 0.99, (vi) p = 0.10, (vii) p > 0.99, (viii) p = 0.10 significant (alpha = 0.05)? Kolmogorov–Smirnov results: (v) D = 0.33, (vi) D = 1.00, (vii) D = 0.33, and (viii) D = 1.00. None of the results are significant at the p < 0.05 level.
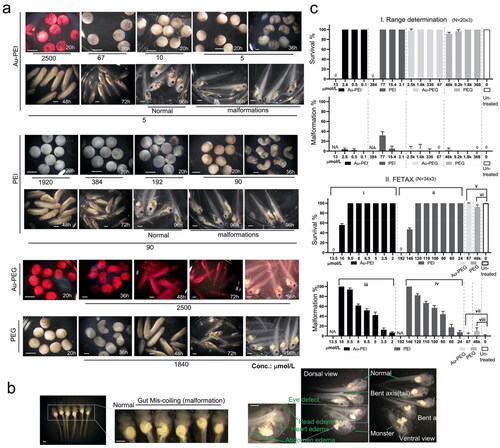
Table 2. Survival and malformation of stage 45 embryos 96 h post-treatment with the compounds.
The range-determination test (96 hpf) using 20 embryos per group showed no survival at 13 or 384 µmol/L of Au-PEI25kB or PEI25kB, respectively (). Furthermore, 3.3 ± 2.9% and 31.7 ± 7.7% malformations were observed when 2.6 µmol/L of Au-PEI25kB and 77 µmol/L of PEI25kB were used respectively. The results indicated that 2.6–13 µmol/L and 77–384 µmol/L are the ranges of interest for Au-PEI25kB and PEI25kB, respectively, for future LC50 and EC50 analysis.
For the FETAX results (, ), embryos treated with 13.5 µmol/L Au-PEI25kB showed 100% mortality. At 10 µmol/L and 9.5 µmol/L Au-PEI25kB, (56 ± 2.4)% and 100% treated embryos survived, respectively. The surviving embryos showed malformations at a rate of (94 ± 2.4)% and 100% with 9.5 µmol/L and 10 µmol/L Au-PEI25kB, respectively. Furthermore, (6.9 ± 1.4)% of embryos treated with 2 µmol/L Au-PEI25kB exhibited malformations. On the other hand, exposure to Au-PEG5k-OMe appeared to have little or no effect on the embryos. Only (3 ± 2.5)% of the embryos showed malformations even at 67 µmol/L(twice the complete lethality concentration of Au-PEI25kB). These malformations include 1% of cardiac edema and 2% of the mis-coiled gut, which was also found in untreated control embryo with a 1% rate (). The PEG5k-OMe-treated embryos also exhibited a malformation rate of only (9.8 ± 5.6)%. However, the results from both treatments were not statistically significant (Wilcoxon signed-rank test) when compared to the untreated control group that showed a (1 ± 1.4)% malformation rate. The 96 hpf median lethal and teratogenic concentrations were deduced using the 4PL equation (). The median lethal concentration (LC50) for Au-PEI25kB was 10.01 µmol/L, which is markedly lower (higher toxicity) than for CdCl2, (for comparison only; the referred values are cited from literature) LC50 = 32 µmol/L (Sunderman, Plowman, and Hopfer Citation1991). The FETAX test shows a positive conclusion when the teratogenic index (TI) value is ≥1.2 and a negative conclusion when the TI value is ≤1.0 (Mouche, Malesic, and Gillardeaux Citation2017). The TI of Au-PEI25kB was 1.45, which is regarded as positive teratogenicity, although it is not comparable to the value of 8.6 for CdCl2 (Sunderman, Plowman, and Hopfer Citation1991). The TI of PEI25kB was 1.45, which is the same as that for Au-PEI25kB. To determine the minimum concentration inhibiting growth (MCIG), we measured the head-to-tail tip lengths of the chemically treated embryos (Figure S2(a)). The minimum tested concentration of 2 µmol/L still had a mild body length shortening effect, indicating that the MCIG is lower than this concentration. However, the MCIG for PEI25kB was approximately >10 and <20 because the mean body length for 10 µmol/L was not statistically different from that of the control, but treatment with 20 µmol/L did show statistically significant body length affects.
Table 3. Median lethal concentration (96 hpf LC50) and median effective (teratogenic (TC50)) concentration (96 hpf EC50) calculated using 4PL.
Avidity/permeability of Au-PEI25kB to jelly coat, chorionic sheath, and plasma membrane
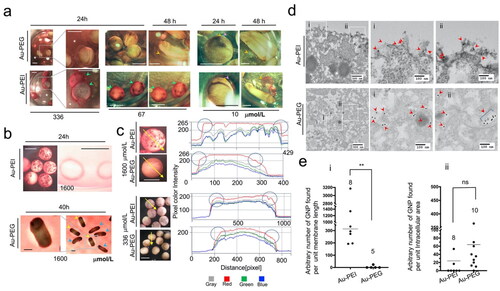
In our initial experiments, the jelly coat turned red; therefore, we hypothesized that Au-PEI25kB could be taken up into the jelly coat and very importantly, remain ‘stable’ so that the characteristic red color is retained (The red color is from the SPR band). In addition, the chorionic sheath (vitelline membrane (VE), also known as the fertilization envelope) is known to be a protective barrier against external substances. Given the steep slope of the survival curve corresponding to the embryos exposed to concentrations of Au-PEI25kB or PEI25kB, we postulated a threshold effect for the membranous structures and examined the membranes to confirm our speculation. Au-PEI25kB showed a strong affinity for the embryo jelly coat (), as evident from the red color-coated embryo surface. The red color of the media disappeared as if it was filtered by the embryo jelly coat at high concentrations such as 336 µmol/L and 67 µmol/L (). At lower concentrations (10 µmol/L) of Au-PEI25kB, where the media did not display a red color, the jelly coat appears to concentrate the AuNPs producing a red color, with the color becoming more prominent in the hatching stage when the jelly plus VE peeled off from the tailbud larva. On the other hand, Au-PEG5k-OMe did not exhibit these features, suggesting the amine-containing PEI component is necessary for Au particles to accumulate in the jelly coat. Next, we examined the VE using L-cysteine treated/de-jellied embryos (). We observed the attachment of Au-PEI25kB on a polystyrene plastic dish (). The use of Au-PEG5k-OMe, which was not toxic in our test, displayed embryo survival through the tailbud stage when an empty space in chorion forms. This result confirmed that the VE indeed functions as a blockade (), and is supported by the reduced red color observed within the VE boundary. Because the VE acts as a shield against Au-PEG5k-OMe, we next wanted to know whether there was any affinity of AuNPs for VE. During several experiments with de-jellied embryos, a dense red lining was observed at the periphery of the Au-PEI25kB-treated embryos. We analyzed these digital images using pixel color assessment in Zeiss Zen Blue 3.1 software (). The red pixels on the embryo-crossing lines were quantified based on their intensities. The boundaries of the Au-PEI25kB-treated embryos showed red pixels with greater intensity, whereas embryos treated with Au-PEG5k-OMe did not, indicating that Au-PEI25kB is attracted to VE whereas Au-PEG5k-OMe is not. This analysis is supported by the study on changes of the vitelline surface upon fertilization, in which the overall charge of the vitelline surface turns more negative (Cooper and Bedford Citation1971). We speculate the positively charged Au-PEI obviously binds much more efficiently to the negatively charged vitelline membrane than the neutral Au-PEG. Next, we wanted to know whether AuNPs reach the intracellular space. Thus, we performed TEM analysis to locate the AuNPs directly at the subcellular level (). Unfortunately, we could not find AuNPs in the embryos at the various stages analyzed (20, 48, and 96 hpf) using TEM. Therefore, we attempted to utilize a more direct and simpler setup involving a 4 h treatment with the highest AuNP concentration on VE-intact or VE-peeled eight-cell-stage embryos (5 hpf). We located AuNPs in sections of the VE-peeled embryos, but not from the VE-intact ones (data not shown). The TEM image analysis showed that a significant amount of Au-PEI25kB was present on the extracellular side of the plasma membrane compared to Au-PEG5k-OMe, the majority of which was found in the intra-cellular space. However, the number of AuNPs in intracellular spaces was statistically indistinguishable between the two groups, indicating that both types of AuNPs undergo similar cellular uptake, but Au-PEI25kB shows avidity to the cytoplasmic membrane alone.
Figure 5. (a) Au-PEI with avidity for the jelly coat. The panels show the embryos treated with Au-PEG5k-OMe or Au-PEI25kB. One-day-used FETAX medium with high-concentration Au-PEI25kB (336 µmol/L) became cleared (asterisk; compare with Au-PEG5k-OMe particles) because the colloidal Au-PEI25kB, unlike Au-PEG5k-OMe, had been bound/trapped/concentrated in the jelly coats (green arrowheads) of the dead embryos. The squared regions in the first columns of the panels are magnified in the second columns. Also, at a lower concentration (67 µmol/L) of Au-PEI, although the medium has much lighter wine color (more perceivable in the same concentration Au-PEG group), the embryos suffered complete lethality and had dark red surface within 24 h (green arrowheads) indicating the Au-PEI attraction to jelly coat occurred within 24 h post-treatment. Larvae in this dish did not become darker red when seen at the 48 h indicating further attraction of the AuNPs to the jelly coat, and that the jelly layer was saturated with NPs or no more AuNPs were left in the medium. When treated with 10 µmol/L Au-PEI25kB, the embryo jelly coat (pink arrowheads) absorbed AuNPs in the layer, and turned red, whereas the jelly coat of the Au-PEG5k-OMe group remained transparent no color in obvious contrast. During the hatching period (48 h), the VE (yellow arrowheads)/jelly (pink arrowheads), double-layer coat peels off the larvae tethering on the cement gland of the tail bud embryo, which shows an obvious red color with Au-PEI25kB, but not with Au-PEG5k-OMe, indicating that the colored layer is not the larval surface, but the envelope and coat. Scale bars: 1 mm. (b) De-jellied embryos. A large filtration effect of the jelly layer on Au-PEI was observed. The FETAX method included L-cysteine treatment that removed the jelly coat but left the VE intact. Au-PEI25kB (1600 µmol/L) attached to the bottom of the plastic dish from which dead embryos were removed at 24 h. Note the ring-shaped dense red stains surrounding the embryos. These polystyrene-attached AuNPs were not observed with Au-PEG5k-OMe (data not shown). Instead, at 40 h, as the tail bud larvae became longer and curled inside the VE, a space developed between the VE and embryonic body, and a red color-free area was observable in this space, indicating that Au-PEG5k-OMe cannot undergo simple diffusion through the VE. The hatched embryos do not have this space. The absence of red stain condensation along the edge of this space indicated little or no attachment of Au-PEG5k-OMe onto the VE. Hatched embryos (blue arrowheads, with burst VE); pre-hatching embryos (yellow arrowheads, intact VE). Scale bars: 1 mm. (c) Line pixel assessment of 24 h old embryos treated with Au-PE25kB or Au-PEG5k-OMe following de-jellying. Left four panels: Photographs showing embryos treated with 1600 µmol/L and 336 µmol/L of Au-PEI25kB and Au-PEG5k-OMe, respectively, with a crossing line drawn on top. Using Zen blue 3.1 software (Zeiss), color intensities of pixels on the embryo-crossing line were measured. Right panels: intensity charts for the four separate colors. Note that the edges of the Au-PEI25kB-treated embryo boundaries exhibited more intense red pixels, whereas the centers of the Au-PEG5k-OMe-treated embryo boundaries included stronger red pixels than the edges (circles). This indicated that Au-PEI25kB accumulated on the VE, whereas Au-Au-PEG does not. (d) TEM results of mid-blastula embryos treated with a high concentration (1600 µmol/L) of Au-PEI25kB or Au-PEG5k-OMe. Note that these embryos lacked the VE. Four-hour treatment with AuNPs. First column: 5000× (low magnification), and the second and third columns: 20000× (high magnification). Zoomed-in images of the two square regions (i,ii) in the first column panels. Arrowheads: AuNPs observed in the images. Note: Au-PEI25kBs were observed along the plasma membrane (first row panels). Au-PEG5k-OMes were observed in the cytoplasm. (e) Scatter plot of the AuNP location frequency. Each dot represents a scanned TEM image wherein the intracellular area and membrane length were measured, and the number of AuNPs was counted. The counted number of AuNPs were divided by the area or length (arbitrary value by Image J), and then multiplied with 1000× (chart ii) or 100 (chart i) to decrease the decimal number, which is represented here as a single dot. A nonparametric t-test was performed for a small number of samples. Kolmogorov–Smirnov D test results: **: p = 0.0016, Kolmogorov–Smirnov D = 1.000, (the results are significantly different at the p < 0.05 level); ns: not significant, p = 0.1203, Kolmogorov–Smirnov D = 0.5250 (the results are not significantly different at the p < 0.05 level). Notably, the sum of the arbitrary lengths of the plasma-membrane in all scanned images was 64.351 for Au-PEI25kB and 31.764 for Au-PEG5k-OMe. However, the numbers of peri-extra-membranous AuNPs were 466 and 2, respectively, indicating higher probability of the extracellular lining of Au-PEI25kB on the plasma membrane, whereas a very low chance was observed for Au-PEG5k-OMe.
Injection-mediated FETAX
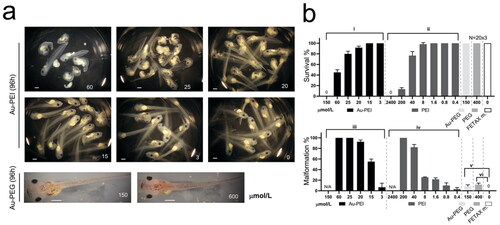
As Au-PEI25kBs exhibited significant avidity to the VE and the Au-PEG5k-OMes failed to simply diffuse into VE-protected larvae, we attempted to circumvent these mechanical factors and analyzed the direct cytotoxic effect of the compounds via microinjection (). The commonly used maximum volume for microinjection into a one-cell stage embryo is 10 nL for gene function studies using Xenopus laevis embryos as specimens. This minimal volume of liquid introduction does not disrupt the ratio of the embryonic cell body volume to the number of total mRNAs or proteins, which is crucial for proper development (Leibovich et al. Citation2020). Because of this limitation, we prepared a more concentrated stock of Au-PEI25kB and Au-PEG5k-OMe. A minimal amount of distilled water was used to re-suspend the AuNPs, and the highest product concentration was 15 000 µmol/L (6× concentrated from the batch, 2500 µmol/L used for Tr-FETAX). The highest dose utilized was 10 nL of 15 000 µmol/L ().
Figure 6. (a) Inj-FETAX. Morphology of the AuNP-injected embryos. Upper 6 panels: Au-PEI-injected embryos, showing lethality/malformation at high to low doses when examined at 96 post-fertilization (hpf). Lower 2 panels: Au-PEG5k-OMe-injected embryos exhibited no lethality even at the highest dose (150 µmol/L). In contrast, less than half of the maximum dose (60 µmol/L) of Au-PEI25kB caused more than 50% lethality. The injection volume limit permitted the injection of a maximum dose of 150 µmol/L (shown in (a) Au-PEG5k-OMe; left bottom panel). Notably, this concentration was rather high, as the wine-colored Au-PEG dispersed in the head, thorax, and tail was visible through the semi-transparent body. Even surprisingly, eight out of ten embryos with extreme dose (600 µmol/L; 40nL injection) in over-testable volume showed no obvious defect. (b) Survival and malformation rate chart. FETAX was performed three times independently, with 20 embryos comprising each group. FETAX m.: injection with FETAX medium alone. Embryos injected with decreasing dose of the Au-PEI25kB but not of the Au-PEG5k-OMe exhibited gradual increasing survival rate and decreasing malformation rate. One-way ANOVA (nonparametric Kruskal–Wallis test) test. (i) p = 0.0049, (ii) p = 0.0032, (iii) p < 0.0001, (iv) p < 0.0001, and (v) p < 0.0001. Kolmogorov–Smirnov test (t-test; nonparametric) for (v) p = 0.10 and (vi) p = 0.10, significant (alpha = 0.05)? Kolmogorov–Smirnov results: (v) D = 1.00 and (vi) D = 1.00. The results are not significant at the p < 0.05.
The standardized unit for the dose employed in the injection drug test is grams per body weight. However, Xenopus embryos are too small compared to mice or humans for body weight measurements, although the body volume information is available. For direct comparison with Tr-FETAX, we used the molar concentration per body volume (mol/µL). This value represents the approximate total number of AuNPs remaining in the embryonic body, which is equivalent to the molar concentration of the treated AuNPs (in Tr-FETAX) in the medium if AuNPs can undergo simple diffusion through the embryonic body. This diffusion of injected AuNPs (in Inj-FETAX) occurs instantly before the first cleavage that drives the embryo to the two-cell stage. This is evidenced by the observation of not only head-to-tail but also the left-right spread of wine color in the embryo with the injection of a high concentration AuNPs as shown in (600 μmol/L Au-PEG injected 96h embryo). If the diffusion is slow and confined to only the injected area, then the 96 h embryo will show only partially localized wine color at this later stage, according to the fate map of the Xenopus laevis embryo. Traditionally, the volume of a fertilized embryo is 1 µL (approximate volume at 24 hpf until neurula, stage 20), which increases to 2 µL during stage 30 at 35 hpf and, becomes 4 µL during stage 45 at 96 hpf (Riebsell and Hausen Citation1991; Leibovich et al. Citation2020). We chose a one-cell-stage embryo (1 µL, stage 1) as the standard because microinjection was performed at this stage with 10 nL volume of 15 000 µmol/L AuNPs as 150 µmol/L treatment in Tr-FETAX (because 10 nL is 1/100 of 1 µL; the injected AuNPs will be diluted 100-fold in the embryo).
The injection volume limit permitted the injection of a maximum dose of 150 µmol/L (left lower panel in ; Au-PEG5k-OMe). Notably, Au-PEI25kB administration induced significant toxicity and teratogenicity, depending on the concentration introduced ().
Although the injection of more than 10 nL is not recommended to guarantee normal development, a 40 nL injection of 15 000 µmol/L stock (regarded as 600 µmol/L in the embryo) was tested on 10 embryos to evaluate the effects. Surprisingly, eight of these embryos showed no distinctive malformation or decreased viability at 96 hpf, whereas the remaining two embryos showed malformations including bent axis, gut coiling defects, and craniofacial hypotrophy (data not shown). However, further investigation into the toxicity arising due to Au-PEG administration was not possible because it is difficult to interpret whether the malformations result from the injection of an excessive amount of solution or the injected substance itself.
In embryos injected with Au-PEI25kB, the 96 hpf survival was below 50% (mean) at a dose of 60 µmol/L but was over 70% (mean) at 25 µmol/L. At a dose of 15 µmol/L, the survival rate was 100% and the malformation rate among the surviving embryos decreased to 50%. PEI25kB alone exhibited a similar pattern of toxicity and malformation rate based on the dose change. As per our calculations, a PEI to the gold molar ratio of 21.6 (obtained from TGA) consists of one molar particle of Au-PEI25kb. Although water-soluble free PEI25kB showed slightly lower embryo toxicity (based on LC50 values) compared to Au-PEI25kB, we considered the stoichiometric unit PEI number of free PEI25kB versus Au-PEI25kB, and the actual toxicity of free PEI25kB can be regarded higher than that bound in Au-PEI25kB (LC50, 62.14 µmol/L × 21.6 = 216.2 µmol/L (concentration of PEI moieties on the surface of Au-PEI25kB; )).
Table 4. Median lethal concentration (96 hpf LC50) and median effective (teratogenic (TC50)) concentration (96 hpf EC50) calculated using 4PL.
The survival and malformation data are presented in . The median lethal and median teratogenic concentrations for Au-PEI25kB and PEI25kB alone are listed in . The Au-PEG5k-OMe and PEG-injected embryos showed malformation rates ≤10% and 15%, respectively, and these differences from the control were not statistically significant (Wilcoxon test, p > 0.05).
Table 5. Survival and malformation of stage 45 embryos 96 h post injection of the compounds.
The body length of chemically injected embryos at 96 hpf was measured to determine the MCIG (Figure S2(a)). The minimum doses of 3 µmol/L and 0.4 µmol/L Au-PEI25kB and PEI25kB, respectively, induced a mild body length shortening effect, indicating that the MCIG is at a lower concentration window.
Discussion
The results of our study demonstrate that Au-PEI25kB has high toxicity and teratogenicity, whereas Au-PEG5k-OMe has almost no toxicity ( and and ). The embryo toxicity of Au-PEI25kB was very high and comparable to that of cadmium ions, as shown by LC50 values (). A comparison of the LC50 values of Au-PEI25kB and PEI indicated that Au-PEI25kB showed 14-fold higher toxicity than that of PEI alone. However, based on the molar ratio of the Au core and surface PEI moiety (Note: the stoichiometry of 10.01 µmol/L of Au-PEI25kB (Au core) is equivalent to 216 µmol/L of unit PEI (surface)), the actual toxicity of Au-PEI25kB was1.5-fold lower than that of PEI25kB alone ().
In general, AuNP itself is known to be nontoxic, therefore we hypothesized that the noticeable toxicity of Au-PEI25kB is due to the coating material, PEI25kB. This result suggests that the active positive charge of PEI in Au-PEI25kB is effective in disrupting the viability of embryos as well as in the free PEI ().
In addition to embryo toxicity, both Au-PEI25kB and PEI25kB had teratogenic potential (). The TI of CdCl2 is 8.6 and the standard criterion to indicate positive teratogenicity is TI ≥1.2 (Mouche, Malesic, and Gillardeaux Citation2017). The TI of 1.45 for both Au-PEI25kB and free PEI25kB indicates that these compounds are teratogenical though their TIs are much lower than that of CdCl2. Notably, the teratogenic effects of Au-PEI25kBand free PEI25kB are comparable, indicating that the LC50:EC50 ratios remain the same even when the respective LC50 and EC50 values are in different ranges. This indicates that both materials have the same capability to cause malformations even at a low number of particles/molecules, without causing death within their unique concentration windows. The mechanisms underlying death or malformation caused byAu-PEI25kB and free PEI25kB with two different material structures may be the same when treated in media.
Fortunately, the material we used is characterized by a strong SPR absorption band when colloidally stable, resulting in a visibly wine-red color, which enabled us to observe that Au-PEI25kB has significant avidity to three different membranous bio-structures, including the glycoprotein meshwork present in jelly coat and VE, and the lipid bilayer of the cytoplasmic membrane (). We also demonstrated that Au-PEG5k-OMe cannot penetrate efficiently through the VE. This blockade effect was represented by the standard FETAX test ( lower two panels). The survival curves of embryos treated with Au-PEI and PEI had steep slopes; therefore, the 95% credential range for LC50 was wide (). Indeed, there was a threshold effect between the 100% lethal concentration and 100% survival concentration (Figure S2(b)). Therefore, we analyzed the extent of the embryotoxicity and teratogenic potentials of Au-PEI25kB or Au-PEG5k-OMe when present in the cytoplasmic space. We performed injection-mediated FETAX, and unlike the zebrafish toxicity test in which the injection is administered into the yolk sac (Schubert et al. Citation2014), we injected directly into the cytoplasm of one-cell-stage embryos, as performed in gene function studies using Xenopus laevis embryos. We could have used the pico or nanogram unit level for injection. However, this would have prevented the intuitive comparison between the two different FETAX methods. Moreover, in medicine, units of grams/body weight are widely used. Therefore, we chose to use moles/body volume as units. For the Inj-FETAX results, we obtained a gradual slope for the lethal concentration (Figure S2(b)), suggesting that the threshold-like nature of the lethality curve may arise due to the membrane effect.
The LC50 and EC50 from Inj-FETAX were comparable but different to those from Tr-FETAX, indicating that the embryotoxic or teratogenic effect of a substance might differ when introduced internally or treated externally, and also differ depending on the material structure. The median lethal concentration (toxicity readout; LC50) and median effective concentration (teratogenicity readout; EC50) of Au-PEI25kB were lower in Tr-FETAX ( and ), whereas those for free PEI25kB were lower in Inj-FETAX. Thus, we inferred that the greater toxicity (lethal) effect of Au-PEI25kB originates from the dysfunction of the cell surface moiety rather than cytosolic effects. On the other hand, for the free PEI25kB, the toxicity was higher when functioning internally than externally. Based on our study observations, the following questions arise:(1) Does Au-PEI, a bulky positively charged surface attributed to a shell of PEI chains surrounding a gold core (i.e. a nest of PEI chains), attach to the plasma membrane without interference when treated externally?; (2) when it is already in the cytosol following injection, does the colloid show reduced diffusion due to the interaction with other anionic subcellular compartments? Because of the similar charge states of Au-PEI25kB and PEI25kB, a few other critical characteristics of the Au-PEI25kB such as particle size, particle shape, physical status as a suspension, and morphology of PEI chains surrounding the gold core (unlike free PEI chains) can be responsible for the lower toxicity of Au-PEI25kB than free branched PEI. The difference in aqueous properties between a soluble polymeric material (PEI) and a colloidal material (Au-PEI)in a specific physiological environment may result in differential toxic effects. Tracing and comparing the intracellular behaviors of the two materials could be an interesting approach for future studies. Analysis of pathways activated in hypoxia might also be informative because the accumulation of Au-PEI25kB along the vitelline membrane (or plasma membrane) observed in this study may function as an oxygen blocker. The PEI polymer is known to induce cytotoxicity, although the underlying mechanism has not been fully characterized (Fischer et al. Citation1999, Citation2002; Florea Citation2002; Neu, Fischer, and Kissel Citation2005). The disruption of the mitochondrial membrane potential results in several types of apoptosis involving caspase-3, -9, and cytochrome C (Cyt c) (Martin et al. Citation1996; Mandal et al. Citation2002), although this feature could be the consequence of the trigger, rather than the trigger itself.
These differences in toxicity and teratogenicity are intriguing, although they cannot be currently interpreted, as there is little available information on the mechanism underlying the toxicity of AuNPs. Even though these two FETAX methods cannot be directly compared in that the threshold-like effect is not seen in Inj-FETAX, they can still be referenced to each other.
Overall, embryo toxicity and teratogenic studies have done little to distinguish structure-dependent mechanistic details of toxicity between PEI and Au-PEI. However, the present study revealed that Au-PEI exhibited clear toxicity in several types of cells and embryos, along with persistent colloidal stability in the media. Au-PEI exhibited significantly higher toxicity compared to Au-PEG. This has confirmed the possible critical role of AuNPs surface charge on toxicity and suggests a pathway to develop reference materials that can be utilized as controls in nanotoxicology assays.
Conclusion
To the best of our knowledge, this is the first study to report an analysis of in-medium, pan-annually stable colloidal AuNPs, which enable in vitro and in vivo toxicity to extend from the more restricted 24–72 h observation window to 96 h and longer. The embryotoxicity and teratogenicity of these highly stable materials, Au-PEI25kB and Au-PEG5k-OMe, were tested using the material controls PEI and PEG through the well-established FETAX method. The results revealed the high toxicity and teratogenic potential of Au-PEI25kB and a negligible toxicity/teratogenicity of Au-PEG5k-OMe. The positively charged Au-PEI25kB had severely affected embryo development, whereas the charge-neutral Au-PEG5k-OMe had no significant effect. To circumvent any shielding effect of the vitelline envelope that prevents the biochemical interaction of Au-PEI25kB, which causes an abrupt change in the survival rate with a slight change in concentration, we employed Inj-FETAX for the first time to examine toxicity and teratogenicity in an amphibian model, which has recently been adapted for the toxicity/teratogenicity assays of soluble pollutants or drugs in zebrafish. In addition to the toxicity/teratogenicity of Au-PEI25kB and absence of these characteristics in Au-PEG5k-OMe, the survival curves of Au-PEI25kB per unit concentration became gradual, supporting the use of the new method as a reference tool for standard FETAX. Due to the clearly expressed difference in toxicity and teratogenicity between Au-PEI25kB and Au-PEG5k-OMe along with their excellent colloidal stability in physiological conditions, these materials may be considered as metrological reference materials in nanotoxicology assays for both in vitro and in vivo studies. This work is the first demonstration that would enable comparisons between test materials and controls to be ‘apples-to-apples’ comparisons from a size perspective and could later improve the toxicity assay protocols for nanotoxicology. To achieve this, a comprehensive validation by inter laboratory study including a wide range of cells will have to be performed prior to their standardization as nanoscale viability assay controls. In addition, the possible role of material properties underlying their cytotoxic activity other than surface charge (particle size, shape, density, etc.) will have to be explored in further mechanistic biochemical studies.
Supplemental Material
Download MS Word (474.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Additional information
Funding
Notes
1 The identification of any commercial product or trade name does not imply endorsement or recommendation by the National Institute of Standards and Technology.
References
- Ahamed, M., M. J. Akhtar, M. A. M. Khan, Z. M. Alaizeri, and H. Alhadlaq. 2021. “Facile Synthesis of Zn-Doped Bi2O3 Nanoparticles and Their Selective Cytotoxicity towards Cancer Cells.” ACS Omega 6 (27): 17353–17361. doi:10.1021/acsomega.1c01467.
- Arvizo, R. R., O. R. Miranda, M. A. Thompson, C. M. Pabelick, R. Bhattacharya, J. D. Robertson, V. M. Rotello, et al. 2010. “Effect of Nanoparticle Surface Charge at the Plasma Membrane and beyond.” Nano Letters 10 (7): 2543–2548. doi:10.1021/nl101140t.
- Asharani, P. V., Y. Lianwu, Z. Gong, and S. Valiyaveettil. 2011. “Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos.” Nanotoxicology 5 (1): 43–54. doi:10.3109/17435390.2010.489207.
- Bacchetta, R., N. Santo, U. Fascio, E. Moschini, S. Freddi, G. Chirico, M. Camatini, et al. 2012. “Nano-Sized CuO, TiO(2) and ZnO Affect xenopus laevis Development.” Nanotoxicology 6 (4): 381–398. doi:10.3109/17435390.2011.579634.
- Bonfanti, P., A. Colombo, F. Orsi, I. Nizzetto, M. Andrioletti, R. Bacchetta, P. Mantecca, et al. 2004. “Comparative Teratogenicity of Chlorpyrifos and Malathion on Xenopus laevis Development.” Aquatic Toxicology 70 (3): 189–200.
- Bose, T., D. Latawiec, P. P. Mondal, and S. Mandal. 2014. “Overview of Nano-Drugs Characteristics for Clinical Application: The Journey from the Entry to the Exit Point.” Journal of Nanoparticle Research 16 (8): 2527. doi:10.1007/s11051-014-2527-7.
- Boussif, O., F. Lezoualc’h, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix, J. P. Behr, et al. 1995. “A Versatile Vector for Gene and Oligonucleotide Transfer into Cells in Culture and in-Vivo-Polyethylenimine.” Proceedings of the National Academy of Sciences 92 (16): 7297–7301. doi:10.1073/pnas.92.16.7297.
- Browning, L. M., K. J. Lee, T. Huang, P. D. Nallathamby, J. E. Lowman, and X.-H. Nancy Xu. 2009. “Random Walk of Single Gold Nanoparticles in Zebrafish Embryos Leading to Stochastic Toxic Effects on Embryonic Developments.” Nanoscale 1 (1): 138–152. doi:10.1039/b9nr00053d.
- Cebrián, V., F. Martín-Saavedra, C. Yagüe, M. Arruebo, J. Santamaría, and N. Vilaboa. 2011. “Size-Dependent Transfection Efficiency of PEI-Coated Gold Nanoparticles.” Acta Biomaterialia 7 (10): 3645–3655. doi:10.1016/j.actbio.2011.06.018.
- Chae, J.-P., M. S. Park, Y.-S. Hwang, B.-H. Min, S.-H. Kim, H.-S. Lee, M.-J. Park, et al. 2015. “Evaluation of Developmental Toxicity and Teratogenicity of Diclofenac Using Xenopus Embryos.” Chemosphere 120: 52–58. doi:10.1016/j.chemosphere.2014.05.063.
- Chen, Z., L. Zhang, Y. He, and Y. Li. 2014. “Sandwich-Type Au-PEI/DNA/PEI-Dexa Nanocomplex for Nucleus-Targeted Gene Delivery in Vitro and in Vivo.” ACS Applied Materials & Interfaces 6 (16): 14196–14206. doi:10.1021/am503483w.
- Cho, K., X. Wang, S. Nie, Z. G. Chen, and D. M. Shin. 2008. “Therapeutic Nanoparticles for Drug Delivery in Cancer.” Clinical Cancer Research 14 (5): 1310–1316. doi:10.1158/1078-0432.CCR-07-1441.
- Cho, E. C., J. Xie, P. A. Wurm, and Y. Xia. 2009. “Understanding the Role of Surface Charges in Cellular Adsorption versus Internalization by Selectively Removing Gold Nanoparticles on the Cell Surface with a I-2/KI Etchant.” Nano Letters 9 (3): 1080–1084. doi:10.1021/nl803487r.
- Cho, T. J., R. I. MacCuspie, J. Gigault, J. M. Gorham, J. T. Elliott, and V. A. Hackley. 2014. “Highly Stable Positively Charged Dendron-Encapsulated Gold Nanoparticles.” Langmuir 30 (13): 3883–3893. doi:10.1021/la5002013.
- Cho, T. J., J. M. Pettibone, J. M. Gorham, T. M. Nguyen, R. I. MacCuspie, J. Gigault, V. A. Hackley, et al. 2015. “Unexpected Changes in Functionality and Surface Coverage for Au Nanoparticle PEI Conjugates: implications for Stability and Efficacy in Biological Systems.” Langmuir 31 (27): 7673–7683. doi:10.1021/acs.langmuir.5b01634.
- Cho, T. J., and V. A. Hackley. 2018. Assessing the Chemical and Colloidal Stability of Functionalized Gold Nanoparticles. Gaithersburg, MD: National Institute of Standards and Technology. doi:10.6028/NIST.SP.1200-26.
- Cho, T. J., J. M. Gorham, J. M. Pettibone, J. Liu, J. Tan, and V. A. Hackley. 2019. “Parallel Multi-Parameter Study of PEI-Functionalized Gold Nanoparticle Synthesis for Bio-Medical Applications: part 1—a Critical Assessment of Methodology, Properties, and Stability.” Journal of Nanoparticle Research 21 (8): 188. doi:10.1007/s11051-019-4621-3.
- Cho, T. J., J. M. Gorham, J. M. Pettibone, J. Liu, J. Tan, and V. A. Hackley. 2020. “Parallel Multiparameter Study of Pei-Functionalized Gold Nanoparticle Synthesis for Biomedical Applications: Part 2. Elucidating the Role of Surface Chemistry and Polymer Structure in Performance.” Langmuir 36 (46): 14058–14069. doi:10.1021/acs.langmuir.0c02630.
- Cho, T. J., V. Hackley, F. Yi, D. LaVan, V. Reipa, A. Tona, B. Nelson, C. Sims, and N. Farkas. 2021. Preparation, Characterization, and Biological Activity of Stability-Enhanced Polyethyleneimine-Conjugated Gold Nanoparticles (Au-PEI@NIST) for Biological Application. Gaithersburg, MD: National Institute of Standards and Technology. doi:10.6028/NIST.SP.1200-29.
- Cooper, G. W., and J. M. Bedford. 1971. “Charge Density Change in the Vitelline Surface following Fertilization of the Rabbit Egg.” Journal of Reproduction and Fertility 25 (3): 431–436. doi:10.1530/jrf.0.0250431.
- Daniel, M. C., and D. Astruc. 2004. “Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology.” Chemical Reviews 104 (1): 293–346. doi:10.1021/cr030698+.
- Dayal, N., M. Thakur, P. Patil, D. Singh, G. Vanage, and D. S. Joshi. 2016. “Histological and Genotoxic Evaluation of Gold Nanoparticles in Ovarian Cells of Zebrafish (Danio rerio).” Journal of Nanoparticle Research 18 (10): 291. doi:10.1007/s11051-016-3549-0.
- De, M., P. S. Ghosh, and V. M. Rotello. 2008. “Applications of Nanoparticles in Biology.” Advanced Materials 20 (22): 4225–4241. doi:10.1002/adma.200703183.
- Dedeh, A., A. Ciutat, M. Treguer-Delapierre, and J.-P. Bourdineaud. 2015. “Impact of Gold Nanoparticles on Zebrafish Exposed to a Spiked Sediment.” Nanotoxicology 9 (1): 71–80. doi:10.3109/17435390.2014.889238.
- Ding, Y., Z. Jiang, K. Saha, C. S. Kim, S. T. Kim, R. F. Landis, V. M. Rotello, et al. 2014. “Gold Nanoparticles for Nucleic Acid Delivery.” Molecular Therapy 22 (6): 1075–1083. doi:10.1038/mt.2014.30.
- Elbakry, A., E.-C. Wurster, A. Zaky, R. Liebl, E. Schindler, P. Bauer-Kreisel, T. Blunk, et al. 2012. “Layer-by-Layer Coated Gold Nanoparticles: size-Dependent Delivery of DNA into Cells.” Small 8 (24): 3847–3856. doi:10.1002/smll.201201112.
- Fischer, D., T. Bieber, Y. Li, H. P. Elsässer, T. Kissel. 1999. “A Novel Non-Viral Vector for DNA Delivery Based on Low Molecular Weight, Branched Polyethylenimine: effect of Molecular Weight on Transfection Efficiency and Cytotoxicity.” Pharmacologyresearch 16 (8): 1273–1279.
- Fischer, D., A. von Harpe, K. Kunath, H. Petersen, Y. Li, T. Kissel. 2002. “Copolymers of Ethylene Imine and N-(2-Hydroxyethyl)-Ethylene Imine as Tools to Study Effects of Polymer Structure on Physicochemical and Biological Properties of DNA Complexes.” Bioconjugate Chemistry 13 (5): 1124–1133.
- Florea, B. I., C. Meaney, H. E. Junginger, and G. Borchard. 2002. “Transfection Efficiency and Toxicity of Polyethylenimine in Differentiated Calu-3 and Nondifferentiated COS-1 Cell Cultures.” AAPS PharmSci 4 (3): 1–11. doi:10.1208/ps040312.
- Fort, D. J., and R. R. Paul. 2002. “Enhancing the Predictive Validity of Frog Embryo Teratogenesis Assay–Xenopus (FETAX).” Journal of Applied Toxicology 22 (3): 185–191.
- Geffroy, B., C. Ladhar, S. Cambier, M. Treguer-Delapierre, D. Brèthes, and J.-P. Bourdineaud. 2012. “Impact of Dietary Gold Nanoparticles in Zebrafish at Very Low Contamination Pressure: The Role of Size, Concentration and Exposure Time.” Nanotoxicology 6 (2): 144–160. doi:10.3109/17435390.2011.562328.
- Hamley, I. W. 2003. “Nanotechnology with Soft Materials.” Angewandte Chemie 42 (15): 1692–1712. doi:10.1002/anie.200200546.
- Haque, E., and A. C. Ward. 2018. “Zebrafish as a Model to Evaluate Nanoparticle Toxicity.” Nanomaterials 8 (7): 561. doi:10.3390/nano8070561.
- Hill, A. J., H. Teraoka, W. Heideman, and R. E. Peterson. 2005. “Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity.” Toxicological Sciences 86 (1): 6–19. doi:10.1093/toxsci/kfi110.
- Hu, C. M. J., and L. F. Zhang. 2009. “Therapeutic Nanoparticles to Combat Cancer Drug Resistance.” Current Drug Metabolism 10 (8): 836–841. doi:10.2174/138920009790274540.
- Hu, C., Q. Peng, F. Chen, Z. Zhong, and R. Zhuo. 2010. “Low Molecular Weight Polyethylenimine Conjugated Gold Nanoparticles as Efficient Gene Vectors.” Bioconjugate Chemistry 21 (5): 836–843. doi:10.1021/bc900374d.
- Hu, Q., F. Guo, F. Zhao, G. Tang, and Z. Fu. 2017. “Cardiovascular Toxicity Assessment of Poly (Ethylene Imine)- Based Cationic Polymers on Zebrafish Model.” Journal of Biomaterials Science 28 (8): 768–780.
- Hunter, A. C. 2006. “Molecular Hurdles in Polyfectin Design and Mechanistic Background to Polycation Induced Cytotoxicity.” Advanced Drug Delivery Reviews 58 (14): 1523–1531. doi:10.1016/j.addr.2006.09.008.
- Katz, E., and I. Willner. 2004. “Integrated Nanoparticle-Biomolecule Hybrid Systems: Synthesis, Properties, and Applications.” Angewandte Chemie International Edition 43 (45): 6042–6108. doi:10.1002/anie.200400651.
- Kim, K., H. B. Lee, J. W. Lee, H. K. Park, and K. S. Shin. 2008. “Self-Assembly of Poly(Ethylenimine)-Capped Au Nanoparticles at a Toluene-Water Interface for Efficient Surface-Enhanced Raman Scattering.” Langmuir 24 (14): 7178–7183. doi:10.1021/la800733x.
- Kim, E. J., J. H. Yeum, H. D. Ghim, S. G. Lee, G. H. Lee, H. J. Lee, S. I. Han, et al. 2011. “Ultrasmall Polyethyleneimine-Gold Nanoparticles with High Stability.” Polymer Korea 35 (2): 161–165. doi:10.7317/pk.2011.35.2.161.
- Kim, J. A., C. Åberg, G. de Cárcer, M. Malumbres, A. Salvati, and K. A. Dawson. 2013a. “Low Dose of Amino-Modified Nanoparticles Induces Cell Cycle Arrest.” ACS Nano. 7 (9): 7483–7494. doi:10.1021/nn403126e.
- Kim, K. T., T. Zaikova, J. E. Hutchison, and R. L. Tanguay. 2013b. “Gold Nanoparticles Disrupt Zebrafish Eye Development and Pigmentation.” Toxicology Science 133 (2): 275–288.
- Kramer, G., H. M. Buchhammer, and K. Lunkwitz. 1998. “Investigation of the Stability of Surface Modification by Polyelectrolyte Complexes – Influence of Polyelectrolyte Complex Components and of Substrates and Media.” Colloids and Surfaces a-Physicochemical and Engineering Aspects 137 (1-3): 45–56.
- Kratz, F., I. A. Müller, C. Ryppa, and A. Warnecke. 2008. “Prodrug Strategies in Anticancer Chemotherapy.” ChemMedChem. 3 (1): 20–53. doi:10.1002/cmdc.200700159.
- Lee, M. Y., S. J. Park, K. Park, K. S. Kim, H. Lee, and S. K. Hahn. 2011a. “Target-Specific Gene Silencing of Layer-by-Layer Assembled Gold-Cysteamine/siRNA/PEI/HA Nanocomplex.” ACSnano 5 (8): 6138–6147.
- Lee, Y., S. H. Lee, J. S. Kim, A. Maruyama, X. Chen, and T. G. Park. 2011b. “Controlled Synthesis of PEI-Coated Gold Nanoparticles Using Reductive Catechol Chemistry for siRNA Delivery.” Journal of Controlled Release 155 (1): 3–10.
- Leibovich, A., T. Edri, S. L. Klein, S. A. Moody, and A. Fainsod. 2020. “Natural Size Variation among Embryos Leads to the Corresponding Scaling in Gene Expression.” Developmental Biology 462 (2): 165–179.
- Lynch, I., H. Bouwmeester, H. Marvin, A. Casey, G. Chambers, M. Berges, and M. Clift. 2009. “First Approaches to Standard Protocolsand Reference Materials for the Assessment of Potential Hazardsassociated with Nanomaterials.” NanoImpactNet; Deliverable 1.4 under the European Commission’s Seventh Framework Programme, NMP4-CA-2008-218539, Grant Agreement 218539 for Project NanoImpactNet.
- Maeda, H. 2001. “The Enhanced Permeability and Retention (EPR) Effect in Tumor Vasculature: The Key Role of Tumor-Selective Macromolecular Drug Targeting.” In Advances in Enzyme Regulation, edited by G. Weber, Vol. 41, 189–207. Oxford: Pergamon-Elsevier Science Ltd.
- Mandal, D., P. K. Moitra, S. Saha, and J. Basu. 2002. “Caspase 3 Regulates Phosphatidylserine Externalization and Phagocytosis of Oxidatively Stressed Erythrocytes.” FEBS Letters 513 (2–3): 184–188.
- Martin, S. J., D. M. Finucane, G. P. Amarante-Mendes, G. A. O'Brien, and D. R. Green. 1996. “Phosphatidylserine Externalization during CD95-Induced Apoptosis of Cells and Cytoplasts Requires ICE/CED-3 Protease Activity.” The Journal of Biological Chemistry 271 (46): 28753–28756.
- Moghimi, S. M., P. Symonds, J. C. Murray, A. C. Hunter, G. Debska, and A. Szewczyk. 2005. “A Two-Stage Poly(Ethylenimine)-Mediated Cytotoxicity: Implications for Gene Transfer/Therapy.” Molecular Therapy 11 (6): 990–995. doi:10.1016/j.ymthe.2005.02.010.
- Mouche, I., L. Malesic, and O. Gillardeaux. 2017. “FETAX Assay for Evaluation of Developmental Toxicity.” Methods in Molecularbiology 1641: 311–324.
- Nations, S., M. Wages, J. E. Cañas, J. Maul, C. Theodorakis, and G. P. Cobb. 2011. “Acute Effects of Fe2O3, TiO2, ZnO and CuO Nanomaterials on Xenopus laevis.” Chemosphere 83 (8): 1053–1061. doi:10.1016/j.chemosphere.2011.01.061.
- Neu, M., D. Fischer, and T. Kissel. 2005. “Recent Advances in Rational Gene Transfer Vector Design Based on Poly(Ethylene Imine) and Its Derivatives.” Journal of Gene Medicine 7 (8): 992–1009.
- Niemeyer, C. M. 2001. “Nanoparticles, Proteins, and Nucleic Acids: Biotechnology Meets Materials Science.” Angewandte Chemie International Edition 40 (22): 4128–4158. doi:10.1002/1521-3773(20011119)40:22<4128::AID-ANIE4128>3.0.CO;2-S.
- Nelson, B. C., E. J. Petersen, B. J. Marquis, D. H. Atha, J. T. Elliott, D. Cleveland, S. S. Watson, et al. 2013. “NIST Gold Nanoparticle Reference Materials Do Not Induce Oxidative DNA Damage.” Nanotoxicology 7 (1): 21–29. doi:10.3109/17435390.2011.626537.
- Note, C., S. Kosmella, and J. Koetz. 2006. “Poly(Ethyleneimine) as Reducing and Stabilizing Agent for the Formation of Gold Nanoparticles in w/o Microemulsions.” Colloids and Surfaces a-Physicochemical and Engineering Aspects 290 (1-3): 150–156.
- Pan, Y., A. Leifert, D. Ruau, S. Neuss, J. Bornemann, G. Schmid, W. Brandau, et al. 2009. “Gold Nanoparticles of Diameter 1.4 nm Trigger Necrosis by Oxidative Stress and Mitochondrial Damage.” Small 5 (18): 2067–2076. doi:10.1002/smll.200900466.
- Pyshnaya, I. A., K. V. Razum, J. E. Poletaeva, D. V. Pyshnyi, M. A. Zenkova, and E. I. Ryabchikova. 2014. “Comparison of Behaviour in Different Liquids and in Cells of Gold Nanorods and Spherical Nanoparticles Modified by Linear Polyethyleneimine and Bovine Serum Albumin.” BioMed Research International 2014: 908175.
- Reznickova, A., N. Slavikova, Z. Kolska, K. Kolarova, T. Belinova, M. Hubalek Kalbacova, M. Cieslar, et al. 2019. “PEGylated Gold Nanoparticles: Stability, Cytotoxicity and Antibacterial Activity.” Colloids and Surfaces A 560: 26–34. doi:10.1016/j.colsurfa.2018.09.083.
- Riebsell, M., and P. Hausen. 1991. The Early Development of Xenopus laevis: An Atlas of the Histology. Berlin: Verlag Der Zeitschrift Für Naturforschung.
- Roebben, G., S. Ramirez-Garcia, V. A. Hackley, M. Roesslein, F. Klaessig, V. Kestens, I. Lynch, et al. 2011. “Interlaboratory Comparison of Size and Surface Charge Measurements on Nanoparticles Prior to Biological Impact Assessment.” Journal of Nanoparticle Research 13 (7): 2675–2687. doi:10.1007/s11051-011-0423-y.
- Rosi, N. L., and C. A. Mirkin. 2005. “Nanostructures in Biodiagnostics.” Chemical Reviews 105 (4): 1547–1562. doi:10.1021/cr030067f.
- Schubert, S., N. Keddig, R. Hanel, U. Kammann. 2014. “Microinjection into Zebrafish Embryos (Danio rerio) – a Useful Tool in Aquatic Toxicity Testing?” Environmental Sciences Europe 26: 32.
- Singh, P., S. Pandit, V. R. S. S. Mokkapati, A. Garg, V. Ravikumar, and I. Mijakovic. 2018. “Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer.” International Journal of Molecular Sciences 19 (7): 1979. doi:10.3390/ijms19071979.
- Song, W.-J., J.-Z. Du, T.-M. Sun, P.-Z. Zhang, and J. Wang. 2010. “Gold Nanoparticles Capped with Polyethyleneimine for Enhanced siRNA Delivery 5.” Small 6 (2): 239–246. doi:10.1002/smll.200901513.
- Sullivan, M. M. O., J. J. Green, and T. M. Przybycien. 2003. “Development of a Novel Gene Delivery Scaffold Utilizing Colloidal Gold-Polyethylenimine Conjugates for DNA Condensation.” Gene Therapy 10 (22): 1882–1890. doi:10.1038/sj.gt.3302083.
- Sun, X. P., S. J. Dong, and E. K. Wang. 2005. “One-Step Preparation of Highly Concentrated Well-Stable Gold Colloids by Direct Mix of Polyelectrolyte and HAuCl4 Aqueous Solutions at Room Temperature.” Journal of Colloid and Interface Science 288 (1): 301–303.
- Sunderman, F. W., Jr., M. C. Plowman, and S. M. Hopfer. 1991. “Embryotoxicity and Teratogenicity of Cadmium Chloride in Xenopus laevis, Assayed by the FETAX Procedure.” Annals Inclincallaboratoryscience 21 (6): 381–391.
- Sztandera, K., M. Gorzkiewicz, and B. Klajnert-Maculewicz. 2019. “Gold Nanoparticles in Cancer Treatment.” Molecular Pharmaceutics 16 (1): 1–23. doi:10.1021/acs.molpharmaceut.8b00810.
- Tao, Y., Z. Li, E. Ju, J. Ren, and X. Qu. 2013. “Polycations-Functionalized Water-Soluble Gold Nanoclusters: A Potential Platform for Simultaneous Enhanced Gene Delivery and Cell Imaging.” Nanoscale 5 (13): 6154–6160. doi:10.1039/c3nr01326j.
- Taylor, U., S. Klein, S. Petersen, W. Kues, S. Barcikowski, and D. Rath. 2010. “Nonendosomal Cellular Uptake of Ligand-Free, Positively Charged Gold Nanoparticles.” Cytometry Part A 77A (5): 439–446.
- Thomas, M., and A. M. Klibanov. 2003. “Conjugation to Gold Nanoparticles Enhances Polyethylenimine’s Transfer of Plasmid DNA into Mammalian Cells.” Proceedings of the National Academy of Sciences 100 (16): 9138–9143. doi:10.1073/pnas.1233634100.
- Truong, L., K. S. Saili, J. M. Miller, J. E. Hutchison, and R. L. Tanguay. 2012. “Persistent Adult Zebrafish Behavioral Deficits Results from Acute Embryonic Exposure to Gold Nanoparticles.” Comparative Biochemistry and Physiology Part C 155 (2): 269–274. doi:10.1016/j.cbpc.2011.09.006.
- Truong, L., S. C. Tilton, T. Zaikova, E. Richman, K. M. Waters, J. E. Hutchison, R. L. Tanguay, et al. 2013. “Surface Functionalities of Gold Nanoparticles Impact Embryonic Gene Expression Responses.” Nanotoxicology 7 (2): 192–201. doi:10.3109/17435390.2011.648225.
- Truong, L., T. Zaikova, N. M. Schaeublin, K.-T. Kim, S. M. Hussain, J. E. Hutchison, R. L. Tanguay, et al. 2017. “Residual Weakly Bound Ligands Influence Biological Compatibility of Mixed Ligand Shell, Thiol-Stabilized Gold Nanoparticles.” Environmental Science 4 (8): 1634–1646. doi:10.1039/C7EN00363C.
- Vetten, M. A., N. Tlotleng, D. Tanner Rascher, A. Skepu, F. K. Keter, K. Boodhia, L. A. Koekemoer, C. Andraos, R. Tshikhudo, and M. Gulumian. 2013. “Label-Free in Vitro Toxicity and Uptake Assessment of Citrate Stabilised Gold Nanoparticles in Three Cell Lines.” Particlefibre Toxicology 10: 50.
- Wang, Y., J. E. Q. Quinsaat, T. Ono, M. Maeki, M. Tokeshi, T. Isono, K. Tajima, et al. 2020. “Enhanced Dispersion Stability of Gold Nanoparticles by the Physisorption of Cyclic Poly(Ethylene Glycol).” Nature Communications 11 (1): 6089. doi:10.1038/s41467-020-19947-8.
- Whitesides, G. M. 2005. “Nanoscience, Nanotechnology, and Chemistry.” Small 1 (2): 172–179. [Database] doi:10.1002/smll.200400130.
- Williams, J. R., J. R. Rayburn, G. R. Cline, R. Sauterer, and M. Friedman. 2015. “Effect of Allyl Isothiocyanate on Developmental Toxicity in Exposed xenopus laevis Embryos.” Toxicology Reports 2: 222–227.
- Zhang, Z., K. Wen, C. Zhang, F. Laroche, Z. Wang, Q. Zhou, Z. Liu, J. P. Abrahams, and X. Zhou. 2020. “Extracellular Nanovesicle Enhanced Gene Transfection Using Polyethyleneimine in HEK293T Cells and Zebrafish Embryos.” Frontier Bioengineering and Biotechnology 8: 448.
