ABSTRACT
Introduction: Malnutrition refers to both over- and undernutrition and results from a disruption in energy balance. It affects one in three people worldwide and is associated with increased morbidity and mortality. The intestinal microbiota represents a newly identified factor that might contribute to the development of malnutrition, as it harbors traits that complement the human metabolic and endocrine capabilities, thereby influencing energy balance.
Areas covered: In the current review, we aim to give a comprehensive overview on the microbiota, its development and its possible influence on energy balance, with emphasis the role of short-chain fatty acids. We also consider microbial characteristics associated with obesity and undernutrition and evaluate microbial manipulating strategies. The PubMed database was searched using the terms: ‘gastrointestinal microbiota’, ‘volatile fatty acids’, ‘malnutrition’, ‘undernutrition’, ‘obesity’, ‘insulin resistance’, ‘prebiotics’, ‘probiotics’, ‘antibiotics’ and ‘fecal microbiota transplantation’.
Expert commentary: Microbiota make important contributions to the regulation of energy balance, whereas microbial disturbances might predispose to malnutrition. If we manage to manipulate the microbiota to our benefit, it could lead to preventive or therapeutic strategies targeting malnutrition.
1. Introduction
By definition, malnutrition results in adverse effects on tissue or body form, function, and clinical outcome. Malnutrition refers to a state in which there is an excess, deficiency, or imbalance of energy, proteins, or other nutrients [Citation1]. It therefore covers two broad groups: undernutrition (which includes stunting, wasting, underweight, and specific micronutrient deficiencies) and caloric overnutrition (presenting as obesity or overweight) [Citation2]. Currently, one in three people worldwide is malnourished: 1.9 billion adults are overweight and 462 million adults are underweight [Citation2]. It must be noted that over- and undernutrition are not mutually exclusive, and can coincide in the same community, household, and even individual. For example, specific micronutrient deficiencies and muscle wasting can occur in obese individuals. In the current review, we specifically address malnutrition in the context of disturbances in energy balance. Obesity typically results from an excess of energy intake in comparison to energy requirement. The well-known obesity epidemic is thought to be explained by the increased availability and affordability of high-calorie foods, combined with the adoption of a sedentary lifestyle [Citation3]. In contrast, (caloric) undernutrition results from energy intake that is too low to meet energy requirements. This is due to either an absolute food shortage or a considerable decrease in appetite. Undernutrition can also result from an increase in energy expenditure due to disease. Both obesity and undernutrition are associated with increased morbidity and mortality. In order to properly prevent or treat malnutrition, a thorough understanding of all contributing factors is needed.
In the last years, increasing attention is being directed toward the role of the intestinal microbiota in the development of malnutrition. The intestinal microbiota consist of trillions of microorganisms that reside within the human gut. These microorganisms have coevolved with the human race and have acquired traits that complement human metabolic capabilities, thereby actively influencing host energy balance [Citation4]. Accordingly, multiple epidemiological studies have demonstrated microbial compositional patterns that are specific to both obesity and undernutrition [Citation5,Citation6]. In addition, studies using murine models have demonstrated a causal relation between the intestinal microbiota and weight regulation [Citation7,Citation8]. The precise pathways through which microbiota influence host energy balance are complex, diverse, and remain poorly understood. However, key players that mediate these pathways are thought to be microbial metabolites, such as short-chain fatty acids (SCFAs) [Citation9]. These SCFAs are produced through bacterial fermentation of otherwise indigestible polysaccharides, oligosaccharides, and proteins. SCFAs can be incorporated in glucose- and lipid synthesis and can function as signaling molecules in several metabolic and endocrine processes [Citation9]. If a disrupted microbial composition is indeed instrumental in the development of malnutrition, microbial manipulation would offer an interesting field of research, contributing to a new range of therapeutic and preventive strategies. The microbial-manipulating strategies that are currently being investigated include pre-, pro-, and antibiotics and fecal microbiota transplantation (FMT).
In this review, we aim to provide a comprehensive overview of the gut microbiota, its development, and its potential role in energy balance with specific emphasis on SCFAs. We also evaluate the microbial characteristics associated with both obesity and undernutrition and review the microbial-manipulating strategies currently at hand. Finally, we provide expert commentary and a 5-year view on the subject.
2. Microbial development
Recently, it was reported that a low-abundance placental microbiome may already be present in utero [Citation10]. It must be noted, however, that detection of bacterial species in placental samples might have resulted from contamination, rather than in utero colonization [Citation11]. Actual colonization of the human gut is initiated during birth. This vertical transmission from mother to child could be considered the first, natural occurring microbiota transplant and is evidenced by the considerable differences in neonatal microbial composition depending on delivery mode [Citation12]. Whereas the gut microbiota of newborns delivered by cesarean section is enriched with oral and skin commensals, the microbiota from vaginally delivered newborns correlate more to the microbiota in the mother’s vaginal canal and gastrointestinal tract [Citation12]. During the first 3 years of life, this compositional difference gradually decreases and the microbiota develop into an adultlike state. This is characterized by a substantial increase in diversity and decrease in interindividual variability [Citation12,Citation13]. During maturation, microbial composition is thought to be particularly prone to external influences, which may affect the child’s energy balance later in life. For instance, delivery mode by cesarean section might predispose children to obesity [Citation14]. Moreover, children who were treated with antibiotics early in life (which possibly affected their microbial composition) had increased risk of becoming overweight and suffering autoimmune diseases later on [Citation15,Citation16].
Around the age of 4 years, the adultlike gut microbiota will contain up to 1013 microorganisms, consisting of approximately 160 species per individual [Citation17]. The number of gut microbes roughly equates to the number of somatic cells in the human body [Citation18] and harbors a collective genetic material that vastly outnumbers the human genome [Citation17]. Of the microbial genes, approximately 99% is bacterial, whereas the rest is archaeal, eukaryotic, and viral [Citation17]. The bacterial phyla typically dominating the adult human gut are Firmicutes and Bacteroidetes, followed by Actinobacteria and Proteobacteria [Citation12]. Precise microbial composition is highly individual specific [Citation19,Citation20]. It is influenced by host genetics [Citation21] and age [Citation13], in addition to a number of environmental factors such as gastrointestinal physiology, geographical location [Citation13,Citation22], housemates [Citation23], comorbidity, medication use, and diet [Citation19,Citation24].
Despite the large interindividual differences, vast collections of bacterial species and genes are shared among unrelated individuals, suggesting the existence of a core microbiome encoding indispensable metabolic traits [Citation17]. Turnbaugh et al. proposed that deviation from the core microbiome may predispose to different physiological states like obesity [Citation25].
3. Diet and the microbiota
When regarding the intestinal microbiota and energy balance, it should be noted that diet plays a vital role. Diet influences both energy balance and microbiota directly. It influences microbial composition by determining which nutrients become available to the microbes residing in the gut and thus which microbes thrive or regress. Additionally, dietary components may alter the intestinal microenvironment favoring some bacteria over others. Furthermore, the diet might be capable of introducing new foodborne microbes to the gut [Citation26]. Finally, the microbiota can modulate energy extraction from the diet by fermentation of otherwise indigestible nutrients.
The influence of diet on the gut microbiota was first illustrated by the microbial differences found between individuals with distinct dietary patterns. A Western diet is typically low in fiber and rich in animal protein, sugars, and saturated fat. This diet correlated with lower microbial richness and diversity and higher proportions of Firmicutes and Proteobacteria [Citation13,Citation22]. In contrast, a non-Western diet, typically dominated by fiber and plant polysaccharides, correlated with higher microbial richness and diversity and higher proportions of Bacteroidetes and Actinobacteria [Citation13,Citation22]. The presence of Prevotella specifically seems to be highly associated with fiber intake [Citation13,Citation22,Citation26]. These microbial differences were far less prominent in breastfed children, underlining the influence of diet rather than geographical location or hygiene [Citation22]. Furthermore, functional assessment of the metagenomes showed a distinction in metabolic capacity between Western versus non-Western microbiota. This parallels the distinction found between carnivorous and herbivorous mammalian microbiota and is thought to represent the functional adaptation of microbiota to available nutrients [Citation13].
Although microbial composition remains largely stable over time, major dietary shifts induce microbial adaptations [Citation19,Citation26,Citation27]. In a cohort study of 800 insulin-resistant subjects, dietary changes affected both postprandial glucose responses and microbiota composition underscoring the potential causality of gut microbiota composition [Citation27]. With regard to short-term effects, subjecting healthy volunteers to a purely animal-based diet led to a shift in their microbial composition within 24 h after the diet reached the gut. One possible explanation for this finding could be that high-fat diet drives the growth of bile acid–resistant bacteria and reduces polysaccharide-fermenting bacteria. The microbial composition changed back to normal 2 days after the diet ended [Citation26]. The functional adaptation of microbiota to a specific diet was further exemplified by an in vitro study with a model of the proximal colon inoculated with microbiota from either obese or healthy humans. It showed that substrate type determined which of the two microbial compositions had the highest fermenting capacity. Whereas the obesity-associated microbiota produced more SCFAs in response to galacto-oligosaccharides and lactulose, the opposite was true for pectin and fibers [Citation28].
In conclusion, long-term dietary patterns modulate bacterial composition and gene expression, likely in an attempt to optimize nutrient utilization. Furthermore, bacterial compositional changes can be rapidly established by major dietary shifts, favoring bacterial species that may be best equipped to degrade the newly introduced dietary components. Therefore, if we manage to manipulate the microbial composition in a beneficial way to target either obesity or undernutrition, a corresponding diet would likely help to maintain the favorable composition over a longer period of time.
4. Microbiota and energy balance
Although diet plays an important role in microbial composition and its capacity to affect energy balance, several rodent studies affirmed that microbiota can influence body composition irrespective of the diet. Bäckhed et al. [Citation29] first showed that germ-free mice (lacking a microbiota of their own) had 40% less total body fat than conventional mice. This was despite the fact that both groups were fed the same polysaccharide-rich mouse food and that the germ-free mice even seemed to consume more food. When cecal microbiota from the conventionally raised mice was introduced to the germ-free mice, their body fat increased to levels similar to those of the conventional mice [Citation29]. This microbiota-dependent increase in body fat could in part be attributed to the production of SCFAs [Citation29]. By producing readily absorbable SCFAs from nonabsorbable macronutrients, the microbiota promote energy harvest. Moreover, microbiota and SCFAs influence glucose- and lipid metabolism and thereby energy storage. Furthermore, it has been demonstrated that the microbiota influence bile acid metabolism either directly or via SCFAs. Bile acids, in turn, can influence glucose homeostasis and facilitate lipid uptake. SCFAs have also been implicated in the regulation of fatty acid oxidation, which contributes to energy expenditure. Additionally, SCFAs likely affect energy intake by interacting with the central nervous system either directly or via the regulation of several hormones. Finally, the microbiota and SCFAs mediate host immunity and inflammation, which is often associated with obesity and insulin resistance. The ways in which the microbiota might influence energy balance are described in more detail in the following subsections with emphasis on the role of SCFAs.
4.1. Production of SCFAs
Acetate, propionate, and butyrate make up >90% of all SCFAs and are present within the colonic lumen in an average molar ratio of 3:1:1 [Citation30]. The production of SCFAs depends on a combination of substrate availability, intracolonic microenvironment, and microbial composition [Citation31]. Furthermore, there is a high degree of microbe–microbe collaboration involved in the fermentation of indigestible nutrients. Whereas some bacteria are potent fermenters themselves, others facilitate fermentation by metabolizing the inhibiting by-product H2. For instance, it was shown that co-colonization of germ-free mice with the methanogenic archaeon Methanobrevibacter smithii as well as the saccharolytic bacterium Bacteroides thetaiotaomicron resulted in a significant increase in host adiposity, compared to mono-colonization with either one [Citation32]. Moreover, after SCFAs have been formed, they are subjected to a high intracolonic interconversion due to microbial cross-feeding [Citation33]. This shows that interventional studies with only one type of SCFA should be interpreted with caution as the observed effects might be due to other SCFAs after conversion in the gut.
Once SCFAs are produced, they are absorbed rather efficiently by the colonocytes, leaving only 5–10% of SCFAs in fecal matter [Citation9]. SCFAs have been said to provide approximately 10% of the human daily energy requirements. However, as stated earlier, SCFA production is highly variable [Citation34]. Colonocytes especially largely depend on SCFA oxidation as its primary form of energy supply. Consequently, colonocytes of germ-free mice have been demonstrated to be extremely energy deprived [Citation35] and SCFA production is almost fully reduced in these animals [Citation36]. Of the three SCFAs, butyrate is the primary SCFA influencing the bowel epithelium. Butyrate promotes cell proliferation and differentiation [Citation37], stimulates apoptosis [Citation38], and relieves oxidative stress in the intestinal wall [Citation39], altogether stimulating mucosal health.
4.2. Glucose metabolism
By producing SCFAs, the microbiota affect glucose- and lipid metabolism. Propionate, specifically, is a substrate for intestinal [Citation40] and hepatic [Citation33] gluconeogenesis and accounts for 69% of total body glucose production in mice [Citation33]. Furthermore, SCFAs can regulate glucose metabolism by acting as signaling molecules. Butyrate and propionate were shown to induce intestinal gluconeogenesis in rats [Citation40]. It was believed that intestinal gluconeogenesis and the subsequent increased portal glucose sensing ultimately led to the improved glucose tolerance, improved insulin sensitivity, and decreased fasting glucose levels exhibited by the butyrate- and propionate-fed rats. These beneficial effects were absent in genetic intestinal neoglucogenesis-deficient mice or in rats that underwent portal denervation [Citation40].
Furthermore, SCFAs are thought to activate AMP-activated protein kinase (AMPK) in liver and muscle tissue. AMPK detects cellular energy depletion and promotes catabolic, rather than anabolic metabolism [Citation41]. AMPK induces peroxisome proliferator-activated receptor gamma coactivator (PGC)-1α. PGC-1α in turn regulates several transcriptional factors such as peroxisome proliferator-activated receptor (PPAR)-α, PPAR-γ, PPAR-δ, and farnesoid X receptor (FXR), all of which regulate glucose-, lipid-, and cholesterol metabolism [Citation42–Citation46]. Furthermore, AMPK activation reduces hepatic expression of genes involved in gluconeogenesis, like glucose-6-phosphatase [Citation46]. Interestingly, both PPARα and PPARγ have been used as pharmaceutical substrates for lipid-lowering (fibrates) and insulin-sensitizing (thiazolidinediones) drugs.
Finally, SCFAs stimulate the secretion of gut hormones glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) from the enteroendocrine L-cells in rodents. This is likely mediated through the binding of SCFAs to G-protein-coupled receptor (GPR)-41 and GPR-43 [Citation47–Citation49]. GLP-1 and PYY are incretin hormones that positively influence glucose homeostasis through the stimulation of a glucose-dependent insulin release and inhibition of glucagon release from the pancreas [Citation44]. Increase in fasting PYY and postprandial insulin and glucose concentrations were also found in humans who were rectally infused with acetate. Proximal colonic infusion in the same study showed no effect, however [Citation50]. Perry et al. [Citation51] demonstrated that acetate induced glucose-stimulated insulin secretion in rats independent of GLP-1, through parasympathetic activation. The chronic postprandial hyperinsulinemia resulted in hyperphagia, obesity, and insulin resistance [Citation51]. These findings are in contrast with studies that observed vinegar (rich in acetate) to have a beneficial effect on satiety, body composition, and glucose homeostasis in humans [Citation52,Citation53]. In summary, SCFAs regulate intestinal and hepatic gluconeogenesis. They also mediate glucose homeostasis, possibly by stimulating the release of gut-derived incretin hormones and insulin or parasympathetic activation.
4.3. Lipid metabolism
Once SCFAs are transported to the liver, acetate and to a lesser extend butyrate are used as substrates in cholesterol synthesis and lipogenesis [Citation33]. As mentioned earlier, through the activation of AMPK, SCFAs also regulate lipid- and cholesterol synthesis [Citation42–Citation46]. In addition, dietary SCFAs reduced white adipose tissue (WAT) mass and adipocyte size and induced peripheral insulin sensitivity in mice, while food intake and physical activity remained unaffected. There was generally a shift from lipogenesis to fatty acid oxidation. These effects were shown to be PPARγ dependent and PGC-1α independent [Citation42]. The reduction in WAT mass and adipocyte size is remarkable, as PPARγ expression has shown to induce adipocyte differentiation and adipogenesis in other studies [Citation54]. Moreover, through the activation of PGC-1α, SCFAs induce fatty acid oxidation and thermogenesis in brown adipose tissue (BAT), thereby increasing energy expenditure in mice [Citation45]. van der Beek et al. [Citation50] also demonstrated increased fatty acid oxidation after rectal acetate administration in men. Furthermore, SCFAs stimulate leptin secretion in adipocytes [Citation55]. Leptin, a prominent satietogenic hormone, also increases fat oxidation in an AMPK-dependent manner in both liver and muscle tissue [Citation56].
Paradoxically, whereas the microbiota’s main metabolites, SCFAs, were shown to increase AMPK and PGC-1α activation, this was also increased in germ-free mice that lack microbiota and are typically SCFA-depleted [Citation41]. The first study evaluating the role of the gut microbiota in lipid metabolism was performed by Bäckhed et al. [Citation29], comparing germ-free and conventional mice. They found that the presence of microbiota in conventional mice suppressed intestinal fasting-induced adipocyte factor (Fiaf, also called angiopoietin-like 4, ANGPTL4). The suppression of Fiaf by the microbiota led to increased lipoprotein lipase (LPL) expression and increased triglyceride uptake and deposition in adipocytes [Citation29]. SCFAs, particularly propionate, may also directly induce LPL activity [Citation57]. Conversely, germ-free mice demonstrated an increased Fiaf expression and were protected from diet-induced obesity [Citation29]. They had higher levels of AMPK and PGC-1α, leading to an increase in free fatty acid oxidation [Citation41].
In summary, the microbiota and SCFAs have been demonstrated to influence lipid metabolism in multiple ways, some of which might be contradictory. Although the precise pathways and their relative contribution to net energy balance are not entirely understood, there seems to be a trend toward reducing lipid and carbohydrate production and enhancing fatty acid oxidation [Citation44]. These changes could increase energy expenditure, decrease liver fat accumulation and circulating free fatty acids, and improve glucose tolerance.
4.4. Bile acid metabolism
Primary bile acids are synthesized in the liver from cholesterol and excreted in the duodenum to assist the absorption of fat-soluble vitamins and dietary lipids. Those primary bile acids, that are not reabsorbed in the ileum, enter the colon and are microbially converted to secondary bile acids [Citation58]. There is a bidirectional relationship between bile acids and the intestinal microbiota. Colonic bile acids can regulate microbial composition as some microbes, like Clostridium difficile, are susceptible to bile acid toxicity, while others require bile acids for growth [Citation58]. In reverse, bile acid metabolism can be influenced by the microbiota. As stated earlier, SCFAs influence AMPK and PGC-1α, which regulate the expression of FXR [Citation44]. FXR in turn regulates bile acid synthesis through negative feedback [Citation58]. Furthermore, the microbiota metabolize FXR ligands, thus promoting FXR signaling. Accordingly, Sayin et al. [Citation59] showed that germ-free mice had reduced FXR signaling and increased bile acid pools. FXR was also implicated in lipid and glucose regulation. However, whether this influence is beneficial remains unclear and might be dependent on host diet or site of FXR signaling [Citation58]. Another bile acid receptor is GPR TGR5, which increases thermogenesis and energy expenditure in BAT [Citation60]. Both FXR and TGR5 are expressed on enteroendocrine L-cells and regulate GLP-1 synthesis: TGR5 induces GLP-1 synthesis [Citation60], whereas FXR activation reduced it [Citation61]. Thus, the intestinal microbiota may ultimately influence lipid uptake and host glucose metabolism through its effects on bile acids.
4.5. Appetite regulation
When regarding energy balance, appetite (an important determinant of energy intake) cannot be overlooked. Appetite is regulated by the central nervous system which responds to a variety of peripheral neuronal and chemical signals [Citation62]. These signals are integrated in the hypothalamic arcuate nucleus (ARC) which can then exert both orexogenic and anorexogenic effects. Orexogenic neurons in the ARC express neuropeptide Y (NPY) and agouti-related protein (AgRP). Anorexogenic effects of the ARC are mediated by neurons containing proopiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) [Citation9,Citation62,Citation63].
As described earlier, SCFAs stimulate the secretion of satietogenic hormones such as leptin, GLP-1, and PYY, which seem to increase satiety by acting upon NPY/AgRP and POMC/CART within the ARC [Citation9,Citation62,Citation63]. Furthermore, GLP-1 and PYY inhibit gastric emptying and increase afferent vagal nerve firing to the hypothalamus, further promoting satiety [Citation63].
The effects of microbiota and SCFAs on appetite regulation were investigated in a study comparing mice fed a high-fat diet supplemented with highly fermentable oligofructose-enriched inulin to supplementation with corn starch [Citation64]. Inulin supplementation led to increased colonic SCFAs, decreased energy intake and adiposity, and increased neuronal activation within the ARC, suggesting a decrease in appetite. However, no significant differences in GLP-1 were observed [Citation64]. Therefore another study was conducted to see whether the apparent appetite reducing effects of fermentable carbohydrates could indeed be caused by SCFAs directly, independent of GLP-1 [Citation65]. They found that 3% of colonic- and intravenously infused 11C acetate was taken up by the brain, thus crossing the blood–brain barrier. Also, intraperitoneal injection of acetate resulted in reduced food intake without significant differences in GLP-1 and PYY. Finally, they observed increased neuronal activity within the ARC similar to their previous study and found a fourfold increase in POMC and suppression of AgRP, which would ultimately have a satietogenic effect on the host [Citation65]. These results are in accordance with studies that found a satietogenic effect of acetate (administered as vinegar) on healthy humans [Citation52], but inconsistent with the parasympathetically-induced hyperphagia found in acetate-infused rats [Citation51]. Thus, SCFAs might reduce appetite and food intake either indirectly through the effects of several hormones, or by directly acting on the hypothalamus.
4.6. Immunity and inflammation
The intestinal microbiota are essential in the development and maintenance of the immune system. This is evidenced by studies using germ-free mice, which showed impaired development of gut-associated lymphoid tissue and antibody production as well as hypoplastic mesenteric lymph nodes. Consequently, these animals are more susceptible to infection [Citation66]. Furthermore, the microbiota may protect the host from invading pathogenic microbes by competition for mucosal attachment sites and nutrient availability. However, microbiota may also promote inflammation by secreting pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS) [Citation4]. PAMPs bind toll-like receptors that induce pro-inflammatory responses which might result in a condition termed ‘metabolic endotoxemia’ [Citation67]. Metabolic endotoxemia is featured by a chronic low-grade inflammatory state and is often associated with obesity and insulin resistance [Citation67,Citation68]. Butyrate is thought to enhance the intestinal barrier function by facilitating tight junctions, which is theorized to reduce the uptake of PAMPs [Citation4,Citation69]. However, this theory is contested as LPS, for instance, is typically taken up through chylomicrons [Citation4]. In addition, SCFAs seem to promote anti-inflammatory colonic regulatory T cells in mice [Citation70]. Finally, in vitro studies incubating human omental adipose tissue with propionate demonstrated a downregulation of several pro-inflammatory cytokines and chemokines [Citation57]. This further demonstrates the anti-inflammatory effects of SCFAs.
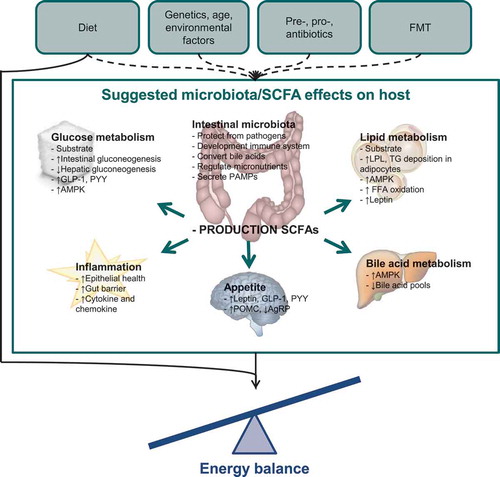
In conclusion, the ways through which the microbiota influence energy balance are numerous and include both direct and indirect pathways, which sometimes seem to contradict each other. The relative contribution of each microbiota-mediated pathway to net metabolism remains unclear and could vary depending on circumstance. A summary of the ways in which microbiota and SCFAs might influence host metabolism and energy balance is depicted by . It should be noted that most evidence is generated by in vitro studies and by animal experiments under standardized conditions. Whether these results hold any clinical relevance and to what extent they may be extrapolated to a human population needs to be further elucidated. Therefore, human studies are needed to affirm these biochemical reactions between the human host and its microbiota. Knowledge of these mechanistic pathways is a requirement for the development of more targeted microbial-manipulating strategies.
Figure 1. Suggested effects of the microbiota and short-chain fatty acids on energy balance.
In this figure, the various ways through which the microbiota and SCFAs might influence the host are depicted.Dashed lines: factors that might influence microbiota composition and metabolic capacity.Solid lines: factors that might influence energy balance.FMT: fecal microbiota transplantation; SCFA: short-chain fatty acids; GLP-1: glucagon-like petide-1; PYY: peptide YY; AMPK: AMP-activated protein kinase; PAMP: pathogen-associated molecular pattern; LPL: lipoprotein lipase; TG: triglyceride; FFA: free fatty acid; POMC: proopiomelanocortin; AgRP: agouti-related protein.
5. Microbial composition in both obesity and undernutrition
Previously, we have described the major mechanistic pathways through which the intestinal microbiota might influence host metabolism and energy balance. Here we summarize the general microbial compositional patterns that have been associated with obesity and undernutrition.
5.1. Microbial characteristics in obesity
Obesity, resulting from a continuously positive energy balance, forms a major health threat due to its high association with noncommunicable diseases such as type 2 diabetes mellitus, cardiovascular disease, and osteoarthritis [Citation2]. During the past years, many researchers have attempted to characterize the obesity-associated microbiota; however, inconsistent results have been published.
The obesity-associated microbiota is thought to have an increased capacity for energy harvest from the diet. This is attributed to the relative enrichment in specific genes encoding the breakdown of indigestible proteins and carbohydrates into SCFAs [Citation32]. Furthermore, fecal energy content in obese mice was lower, which could point to a more efficient energy uptake in obese subjects [Citation32]. The increased capacity for energy harvest is a trait that was found to be transmissible from one individual to another by FMT. Germ-free mice receiving fecal microbiota from obese donors suffered a significantly greater increase in body fat than mice receiving from lean donors. Meanwhile, chow consumption did not differ significantly between groups [Citation8,Citation32].
By comparing the microbiota of lean and obese individuals, a decrease in both microbial diversity and richness has generally been demonstrated in obese subjects [Citation5,Citation25,Citation71]. Furthermore, an increase in the Firmicutes:Bacteroidetes (F:B) ratio has been associated with obesity in mice [Citation32,Citation72] and humans [Citation25,Citation71]. Although some studies did not find a difference in F:B ratio [Citation73], or even found an inverse F:B ratio [Citation74], they did find significantly more SCFAs in obese subjects compared with lean subjects [Citation73,Citation74]. Fernandes et al. [Citation73] also found that the amount of fecal SCFAs did positively correlate to the F:B ratio and found an inverse association between Bacteroidetes and body mass index (BMI). Moreover, a progressive increase in Bacteroidetes was associated with voluntary weight loss in a dietary intervention study following 12 obese subjects over the course of a year [Citation5]. This further suggests a relationship between the major bacterial phyla and bodyweight. In another interesting study, 30 obese individuals undergoing Roux-en-Y gastric bypass (RYGB) surgery were followed [Citation75]. At baseline, they were found to have less Bacteroides and Prevotella species compared to lean controls. Abundance of Bacteroides, Prevotella, and Escherichia coli increased after surgery and were found to be inversely correlated to body weight, BMI, and body fat mass during follow-up. Furthermore, abundance of the Firmicute Faecalibacterium prausnitzii was significantly lower in obese diabetic subjects than in obese nondiabetic and lean subjects. F. prausnitzii has often been described to possess anti-inflammatory properties and indeed, levels of F. prausnitzii increased after RYGB along with an improvement of inflammatory markers. It is important to realize that abundancy shifts could in part be due to dietary and anatomy changes after surgery [Citation75]. Mucin-degrading Akkermansia muciniphila represents another species that was found to be negatively correlated to weight gain and inflammatory markers in high-fat fed mice [Citation76].
The diverse results found when characterizing the obesity-associated microbiota might be caused by the wide variety in the selection of study subjects, study design, and outcome measures. Moreover, the high interindividual variability in microbial composition and the various interactions with environmental factors such as diet could also diversify the results of these studies. Nonetheless, a decrease in diversity and increase in F:B ratio has often been reported. The suggested relationship between higher SCFA concentrations and obesity that was suggested [Citation32,Citation73,Citation74] is interesting, because interventional studies with SCFAs demonstrated that SCFAs generally have a beneficial effect on energy balance and may even cause subjects to lose weight [Citation40,Citation42,Citation45]. Perhaps the relative abundances of SCFAs are key in the net metabolic effect or there are other microbial-mediated pathways that also influence energy balance. This needs to be further investigated.
5.2. Microbial characteristics in undernutrition
Whereas microbial composition is widely being studied in relation to obesity and insulin resistance, studies concerning microbiota and undernutrition are relatively scarce. An undernutrition-associated microbiome may yield less capacity to harvest and utilize nutrients. It may additionally have a stronger satietogenic effect.
In mouse studies, it was shown that the microbiota of undernourished neonatal mice had lower overall bacterial richness and diversity compared to well-nourished control mice [Citation77]. The undernutrition-associated microbiota was distinct across the ileum, cecum, and colon. It contained significantly lower proportions of Bacteroidetes and higher proportions of Verrucomicrobia, particularly mucin degrading A. muciniphila [Citation77]. In a mouse model for leukemia-induced cachexia, a decrease in Lactobacillus reuteri and L. gasseri was shown compared to healthy controls. Interestingly, supplementation of these Lactobacillus species resulted in a reduction in atrophy markers and inflammatory cytokines [Citation78].
Human studies investigating the relation between the microbiota and undernutrition mainly focused on severely undernourished children from developing countries. Comparing the microbiota of undernourished and well-nourished Bangladeshi children, undernourished microbiota was significantly less diverse. It contained less Bacteroidetes and significantly more potentially pathogenic Proteobacteria, including Klebsiella, Escherichia, and Neisseria [Citation6]. Malnourished Bangladeshi children were also shown to have relatively immature microbial compositions [Citation79]. The relation between childhood undernutrition and immature gut microbial patterns was also found in Malawian children [Citation7,Citation80]. Transplantation of feces from undernourished children into germ-free mice resulted in significantly greater weight loss and impaired growth than transplantation from healthy children. These results depended on a typical Malawian diet [Citation7,Citation80].
In the developed world, undernutrition is observed in a variety of demographic groups, among which are the (institutionalized) older adults. Older adults suffer from physiological changes in appetite (anorexia of aging), gastrointestinal physiology, and immune function all contributing to undernutrition [Citation81]. In addition, undernutrition in older adults often coincides with (chronic) diseases like neoplasms, leading to cachexia. Furthermore, the aging microbiota diminishes in diversity and there is an increase in interindividual variability [Citation82]. Altogether, similar to the infant microbiota, the aged microbiota might be more prone to external disrupting factors. Claesson et al. [Citation24] investigated the microbiota of community-dwelling and institutionalized older adults. They established that microbial composition was correlated to Minimal Nutritional Assessment (MNA) score in both groups. They did not report any specific bacterial species that might be related to the MNA score and could not find a relation between BMI and F:B-ratio. Furthermore, they found that fecal SCFAs were lower in subjects with higher frailty scores [Citation24].
Another population that is particularly characterized by undernutrition, is patients suffering from anorexia nervosa (AN). Mack et al. [Citation83] compared the microbial composition of AN patients before and after weight gain to normal weight controls. They found that AN patients at baseline had significantly lower levels of Bacteroidetes compared to controls. During weight gain, abundance of Bacteroidetes decreased even further, whereas Firmicutes abundances increased. Actinobacteria and Verrucomicrobia abundances were also higher in AN patients, and Verrucomicrobia levels did decrease after weight gain [Citation83]. Moreover, the AN microbiota gained significantly in diversity and richness after weight gain, although this effect may in part be diet dependent [Citation83].
In conclusion, studies exploring the role of intestinal microbiota in undernutrition have been conducted in diverse patient groups. Also, undernutrition may be differently defined among studies. Whereas some define undernutrition by weight loss, others do so by low BMI or a combination of the two. Other quantifiers of undernutrition may include low body fat mass, low caloric intake or low body circumferences or questionnaires such as the MNA. Therefore, it should be noted that results from one study should not be extrapolated to undernutrition in general and microbial influences may differ among groups or types of undernutrition. However, undernutrition does seem to be associated with a reduction in bacterial diversity and richness as might be the case with the obesity-associated microbiota. Furthermore, some researchers have reported less Bacteroidetes in relation to undernutrition, but a consistently higher F:B ratio was not described.
6. Microbiota-manipulating interventions
Since the intestinal microbiota have been identified as a new contributing factor in the development of obesity and undernutrition, interventional studies have emerged to target the microbiota. Microbiota-manipulating interventions may aim to promote specific beneficial bacterial species or repress others. The former can be done by introducing live bacteria into the gut (probiotics) or by introducing nondigestible carbohydrates that are selectively fermented and promote beneficial bacteria (prebiotics). Synbiotics refer to a combination treatment with both pre- and probiotics. Repressing harmful bacteria can be established by treating subjects with antibiotics, like vancomycin. There are also studies attempting to radically alter the entire microbial composition by transferring the total range of microbiota from a healthy individual to a malnourished individual. This is done by FMT.
6.1. Pre-, pro-, and antibiotics
The research into pre- and probiotics has generated diverse results, which may be explained by the various types and doses of pre- and probiotics used, as well as baseline microbial composition of the subjects and differences in subject phenotype. The clinical use of pre- and probiotics extends to the treatment of several disorders like diarrhea and constipation, inflammatory bowel disease, cardiovascular disease, and the prevention of malignancies [Citation84]. In the context of malnutrition, pre- and probiotics may promote micronutrient synthesis and uptake, mediate inflammation, stimulate either weight gain or loss, promote insulin sensitivity, and affect appetite [Citation84].
Lactobacillus and Bifidobacterium strains are most researched for their probiotic properties. However, new probiotic strains are rapidly emerging. It should be noted that metabolic capabilities are highly strain specific and may even differ between two strains from the same species [Citation85]. Common prebiotics include resistant starch, inulin, galacto-oligosaccharides, and fructo-oligosaccharides. Ideally, prebiotics should be unaffected by the upper gastrointestinal tract, should be nonabsorbable, and confer their health benefits by stimulating the growth and activity of beneficial microbial species.
Metagenomic studies have shown that several bacterial species, like L. reuteri, harbor genes encoding micronutrient synthesis. This is especially true for the group of B vitamins [Citation85,Citation86]. Furthermore, pro- and prebiotics can promote absorption of minerals like magnesium, iron, and calcium [Citation87–Citation89]. This may be due to increased SCFA production, which decreases intestinal pH and promotes epithelial health leading to increased mineral solubility and uptake [Citation90]. Studies examining the use of pre- and probiotics in (micronutrient) undernutrition are currently lacking. Demonstrated beneficial effects are limited to the treatment of undernourished children from developing countries [Citation91].
In contrast, the role of pre- and probiotics in obesity and insulin resistance is more extensively researched. Parnell and Reimer [Citation92] demonstrated that oligosaccharide supplementation caused weight loss, improved glucose regulation and decreased self-reported food intake in overweight adults. The observed effects were associated with reduced ghrelin and enhanced PYY production. In line with these findings, Cani et al. [Citation93] found reduced hunger rates, increased postprandial GLP-1 and PYY, and decreased postprandial glucose responses in healthy volunteers treated with prebiotics. Probiotics, especially Lactobacillus strains, were also found to beneficially affect body weight, glucose- and lipid metabolism, and inflammatory status [Citation94–Citation97].
Beneficial effects of antibiotics on metabolic and inflammatory state were demonstrated in animal trials [Citation98,Citation99], but were far less evident in human studies. Although 1-week treatment with vancomycin of 57 obese, prediabetic men did lead to significant microbial differences (reduction in diversity and Firmicutes and increase in potentially pathogenic Proteobacteria) [Citation100,Citation101], no beneficial effects on insulin sensitivity, inflammation or gut permeability were demonstrated (101). Vrieze et al. [Citation100] even found a negative effect on insulin sensitivity.
6.2. Fecal microbiota transplantation
Although metabolic disorders encompass a relatively new indication for FMT, the practice itself is over a thousand years old. It was described to cure food poisoning and severe diarrhea during the Dong-Jin dynasty in the fourth century in China [Citation102]. Later, in the sixteenth century during the Ming dynasty, it was also used for fever, pain, vomiting, and constipation. By that time FMT was euphemized and referred to with statements such as ‘yellow soup’ [Citation102]. Ralph Lewin reported in 1999 that Bedouins recommended fresh camel feces as a remedy for bacterial dysentery, a method validated by German soldiers in Africa during World War II [Citation103]. Finally, in 1958, FMT was first documented in modern medicine by Eiseman et al. [Citation104]. It was found to be an effective treatment for pseudomembranous enterocolitis, which was likely caused by C. difficile infection. In 2013 the first randomized controlled trial was published comparing FMT to vancomycin in the treatment of C. difficile infection [Citation105]. Whereas vancomycin only cured 31% of patients, FMT cured 81% of patients after a single infusion and 94% of patients after repeated infusion [Citation105]. Other possible indications for FMT include irritable bowel syndrome, inflammatory bowel disease, constipation, and metabolic syndrome [Citation106].
To our knowledge, Vrieze et al. [Citation107] conducted the first randomized controlled FMT trial in patients with metabolic syndrome. They found that fecal transplantation from lean to obese individuals resulted in increased insulin sensitivity and improved gut-microbial diversity. The amount of fecal SCFAs decreased after transplantation. Note that this does not necessarily reflect decreased SCFA production, as it could also point to more efficient SCFA uptake and utilization. Especially since the abundance of several known butyrate-producing bacteria (including Roseburia intestinalis and Eubacterium hallii) increased compared to autologous transplantation. No differences in diet, resting energy expenditure, or counter-regulatory hormones were found after autologous or allogeneic FMT [Citation107]. Currently, there are seven trials registered at clinicaltrials.gov investigating the effect of FMT on either obesity or insulin resistance.
No studies investigating the effects of FMT on undernutrition have been published to our knowledge and none are registered at ClinicalTrials.gov. However, a case study reported significant weight gain after a successful treatment for recurrent C. difficile infection. The subject developed new-onset obesity (BMI increase from 26 to 34.5 kg/m2) after FMT from a healthy, obese donor [Citation108]. Additionally, our research group is currently conducting studies on the effects of FMT on energy homeostasis and satiety in anorexia nervosa patients and in patients suffering from cancer-related sarcopenia.
There are some practical and safety issues concerning FMT that need to be clarified before FMT can be regularly applied in a clinical setting. These include optimal method for donor selection and screening, optimal feces handling (aerobic or anaerobic), optimal administration form (fresh, frozen, or encapsulated), optimal administration mode (oral, duodenal, or rectal), optimal fecal volume, and effect durability. Effect durability would determine the need to repeat FMT in order to reach the desired effect. Using fecal samples from the FMT study by Vrieze et al. [Citation107], coexistence of donor and recipient strains was assessed [Citation109]. The durability of donor-specific strains varied across recipients and lasted for at least 3 months, although donor-recipient similarity did gradually decrease. Furthermore, different colonization and durability rates were observed between recipients of a single donor, suggesting that specific donor-recipient compatibility might be relevant. It was also found that new strains more easily colonize the recipient gut if the species is already present before FMT [Citation109]. FMT-related adverse events are rare, generally mild and self-limiting, and mostly constitute gastrointestinal discomfort. Serious adverse events that were reported included bacteremia, perforations, and death and occurred in patients who were severely ill prior to FMT [Citation106].
Altogether, there is currently little evidence on the optimal method for FMT and the beneficial effects of FMT on nutritional state. Additional studies are needed to determine the possible therapeutic role of FMT. However, preliminary results are promising.
7. Expert commentary
Over the last decades, the intestinal microbiota have intensively been investigated regarding its relation to energy balance and malnutrition. The increase in microbiota studies was catalyzed by the emergence of culture-independent sequencing techniques, which made it possible to identify microbial composition more easily and relate this to health and disease. Although the capacity of the microbiota to influence energy balance is indisputable, the precise mechanisms remain unclear. Attempting to elucidate these mechanisms, we anticipate a shift from studies examining microbial composition to studies examining the entire metagenome. Mapping the collective metabolic capacity of the microbiota rather than its taxonomic composition is likely far more informative and will give greater insights into the microbiota’s effect on its host, especially, since two strains of the same taxonomic species can hold different traits.
Furthermore, when characterizing the microbiota specific to either over- or undernutrition, it is important to realize that most studies map the fecal microbiota rather that the colonic mucosal microbiota, which may differ substantially [Citation110]. However, conducting colonoscopies to obtain mucosal biopsies is not feasible while retaining sample sizes. Furthermore, it should be realized that the microbiota is not only determined by nutritional status but also by numerous other factors. Factors like genetics, comorbidity, medication, age, and diet should be taken into account when conducting epidemiological or interventional studies. In the same way, malnutrition is not solely caused by microbial perturbations but also by other biological and psychosocial factors. It stands to reason that therapies manipulating microbial composition will be most successful in individuals in whom the microbiota exert a relatively strong influence on energy balance. The challenge lies in identifying those individuals who are most susceptible to microbial manipulation.
In addition to a more optimal patient selection, optimal administration mode and dose for both FMT and pre- and probiotics need to be determined. Furthermore, in order to maintain any beneficial effect of microbiota-manipulating interventions, it should be prevented that the microbial composition shifts back to normal after the intervention has ended. This could possibly be done by repeating the intervention or by identifying dietary patterns that will maintain beneficial microbiota. Also, it needs to be determined whether the host becomes tolerant to the beneficial effects of the altered microbiota. This could be done by conducting longer follow-up trials. As long as the beneficial tendencies toward weight gain or loss are sustained over a longer period of time, microbiota manipulation might be sufficient to cause a clinically relevant outcome.
8. Five-year view
The field of microbiota research is rapidly evolving. During the next 5 years, we expect that more in vitro studies and animal experiments will be conducted, attempting to further clarify the precise effects of the microbiota on host energy balance. In human studies, there may be a shift from studies focusing on intestinal microbial composition to studies focusing on metagenomics and next-generation probiotics. It will be important to move away from small, cross-sectional studies toward larger prospective studies. Falony et al. [Citation111] estimated that a sample size of approximately 535 subjects per group would be needed to adequately asses the relationship between obesity and microbiota composition when one corrects for age, gender, and other confounding variables [Citation111]. Furthermore, whereas most observational studies are currently cross-sectional, more prospective studies will be able to illustrate a temporal relationship between the microbiota and disease states. More randomized controlled trials will be conducted. These will include trials investigating the effect of a specific diet on the microbiota (for instance, diets rich in specific micro- or macronutrients, food groups, food products, or restrictive diets). FMT trials will illustrate more clearly how to optimally conduct fecal transplantation as well as long-term effects. Currently, FMT studies have shown limited effectiveness. Furthermore, more probiotic strains will be identified and mapped and the delivery system of pre- and probiotics to the distal gut by pH-driven capsules will be improved. We anticipate that microbial interventions might become more personalized, targeting microbially susceptible individuals and taking into account environmental factors as well as baseline microbial composition. Finally, trials with longer follow-up will determine durability of any clinical effect.
Key issues
The intestinal microbiome contains an amount of bacteria that is approximately equal to the somatic cells in the human body and the bacterial genes vastly outnumber the human genome, encoding metabolic and endocrine traits that complement those of humans.
Diet is an important determinant of both nutritional state and the microbiome. An acute, dietary switch can affect microbial composition within 24 h. The microbiota likely adapt to a specific diet, optimizing fermentation and thus SCFA production.
The microbiota ferment otherwise indigestible nutrients, producing SCFAs, which can be used as lipid- or glucose substrate or can be oxidized by colonocytes, thus contributing to energy harvest.
The microbiota can influence energy storage and expenditure either directly or through several signaling molecules, among which are bile acids and SCFAs. The latter activate AMPK, which regulates several transcriptional factors such as PGC-1α, PPAR-α, PPAR-γ, PPAR- δ and FXR thus influencing glucose- and lipid metabolism.
SCFAs stimulate several hormones involved in lipid- and glucose metabolism (such as GLP-1, PYY and leptin) as well as appetite, thus affecting energy intake.
Specific microbial characteristics have been described for individuals with either obesity or undernutrition and generally pertain to a decrease in bacterial diversity and a shift in Firmicutes:Bacteroidetes ratio. Also, increased SCFA concentrations have been reported in relation to an obesity-associated microbial composition.
Several strategies have emerged to manipulate microbial composition, aiming to promote healthy energy balance. Although some promising results have been reported, evidence is scarce and optimal treatment strategies have yet to be established.
Future observational studies should include larger sample sizes, target well-defined patient groups and focus on metagenomics, rather than microbial composition.
Future interventional studies should target microbially susceptible individuals, correcting for environmental factors such as diet, co-morbidity and medication and should include longer follow-up time.
Declaration of interest
M Nieuwdorp is on the scientific advisory board of Caelus Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Lochs H, Allison SP, Meier R, et al. Introductory to the ESPEN Guidelines on Enteral Nutrition: terminology, definitions and general topics. Clin Nutr. 2006;25(2):180–186.
- What is malnutrition? Online Q&A. World Health Organisation, [cited 2017 Apr 18]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.
- Obesity and overweight: fact sheet. World Health Organisation, [cited 2017 Apr 18]. Available from: http://www.who.int/features/qa/malnutrition/en/.
- Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249.
- Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023.
- Monira S, Nakamura S, Gotoh K, et al. Gut microbiota of healthy and malnourished children in Bangladesh. Front Microbiol. 2011;2:228.
- Smith MI, Yatsunenko T, Manary MJ, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339(6119):548–554.
- Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214.
- Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–591.
- Aagaard K, Ma J, Antony KM, et al. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra265.
- Lauder AP, Roche AM, Sherrill-Mix S, et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 2016;4(1):29.
- Bäckhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(5):690–703.
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227.
- Darmasseelane K, Hyde MJ, Santhakumaran S, et al. Mode of delivery and offspring body mass index, overweight and obesity in adult life: a systematic review and meta-analysis. PLoS One. 2014;9(2):e87896.
- Ajslev TA, Andersen CS, Gamborg M, et al. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond). 2011;35(4):522–529.
- Clausen TD, Bergholt T, Bouaziz O, et al. Broad-spectrum antibiotic treatment and subsequent childhood type 1 diabetes: a nationwide Danish cohort study. PLoS One. 2016;11(8):e0161654.
- Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65.
- Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337–340.
- David LA, Materna AC, Friedman J, et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15(7):R89.
- Consortium HMP. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214.
- Goodrich JK, Waters JL, Poole AC, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799.
- de Filippo C, Cavalieri D, di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–14696.
- Song SJ, Lauber C, Costello EK, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013;2:e00458.
- Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184.
- Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484.
- David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563.
- Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–1094.
- Aguirre M, Jonkers DM, Troost FJ, et al. In vitro characterization of the impact of different substrates on metabolite production, energy extraction and composition of gut microbiota from lean and obese subjects. Plos One. 2014;9(11):e113864.
- Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723.
- Cummings JH, Pomare EW, Branch WJ, et al. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–1227.
- Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62(1):67–72.
- Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031.
- den Besten G, Lange K, Havinga R, et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol. 2013;305(12):G900–910.
- Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70(2):567–590.
- Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–526.
- Hoverstad T, Midtvedt T. Short-chain fatty acids in germfree mice and rats. J Nutr. 1772-1776;116(9):1986.
- Frankel WL, Zhang W, Singh A, et al. Mediation of the trophic effects of short-chain fatty acids on the rat jejunum and colon. Gastroenterology. 1994;106(2):375–380.
- Clarke JM, Young GP, Topping DL, et al. Butyrate delivered by butyrylated starch increases distal colonic epithelial apoptosis in carcinogen-treated rats. Carcinogenesis. 2012;33(1):197–202.
- Hamer HM, Jonkers DM, Bast A, et al. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr. 2009;28(1):88–93.
- De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96.
- Bäckhed F, Manchester JK, Semenkovich CF, et al. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104(3):979–984.
- den Besten G, Bleeker A, Gerding A, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64(7):2398–2408.
- Li X, Chen H, Guan Y, et al. Acetic acid activates the AMP-activated protein kinase signaling pathway to regulate lipid metabolism in bovine hepatocytes. Plos One. 2013;8(7):e67880.
- den Besten G, van Eunen K, Groen AK, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340.
- Gao Z, Yin J, Zhang J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517.
- Sakakibara S, Yamauchi T, Oshima Y, et al. Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A(y) mice. Biochem Biophys Res Commun. 2006;344(2):597–604.
- Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371.
- Psichas A, Sleeth ML, Murphy KG, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond). 2015;39(3):424–429.
- Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–11319.
- van der Beek CM, Canfora EE, Lenaerts K, et al. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci (Lond). 2016;130(22):2073–2082.
- Perry RJ, Peng L, Barry NA, et al. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213–217.
- Ostman E, Granfeldt Y, Persson L, et al. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr. 2005;59(9):983–988.
- Kondo T, Kishi M, Fushimi T, et al. Vinegar intake reduces body weight, body fat mass, and serum triglyceride levels in obese Japanese subjects. Biosci Biotechnol Biochem. 1837-1843;73(8):2009.
- Hong YH, Nishimura Y, Hishikawa D, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146(12):5092–5099.
- Xiong Y, Miyamoto N, Shibata K, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A. 2004;101(4):1045–1050.
- Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–343.
- Al-Lahham S, Roelofsen H, Rezaee F, et al. Propionic acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur J Clin Invest. 2012;42(4):357–364.
- Wahlstrom A, Sayin SI, Marschall HU, et al. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41–50.
- Sayin SI, Wahlstrom A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17(2):225–235.
- Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–177.
- Trabelsi MS, Daoudi M, Prawitt J, et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun. 2015;6:7629.
- Clercq N, Groen AK, Romijn JA, et al. Gut microbiota in obesity and undernutrition. Advances in Nutrition. 2016;7:1080–1089.
- de Silva A, Bloom SR. Gut hormones and appetite control: a focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver. 2012;6(1):10–20.
- Anastasovska J, Arora T, Sanchez Canon GJ. et al. Fermentable carbohydrate alters hypothalamic neuronal activity and protects against the obesogenic environment. Obesity (Silver Spring). 2012;20(5):1016–1023.
- Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611.
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323.
- Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 1761-1772;56(7):2007.
- Genton L, Cani PD, Schrenzel J. Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clin Nutr. 2015;34(3):341–349.
- Peng L, Li ZR, Green RS, et al. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9):1619–1625.
- Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573.
- Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546.
- Ley RE, Bäckhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075.
- Fernandes J, Su W, Rahat-Rozenbloom S, et al. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4:e121.
- Schwiertz A, Taras D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010;18(1):190–195.
- Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–3057.
- Schneeberger M, Everard A, Gomez-Valades AG, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643.
- Preidis GA, Ajami NJ, Wong MC, et al. Composition and function of the undernourished neonatal mouse intestinal microbiome. J Nutr Biochem. 2015;26(10):1050–1057.
- Bindels LB, Beck R, Schakman O, et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. Plos One. 2012;7(6):e37971.
- Subramanian S, Huq S, Yatsunenko T, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510(7505):417–421.
- Blanton LV, Charbonneau MR, Salih T, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351(6275):830.
- Wysokinski A, Sobow T, Kloszewska I, et al. Mechanisms of the anorexia of aging-a review. Age (Dordr). 2015;37(4):9821.
- Claesson MJ, Cusack S, O’Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–4591.
- Mack I, Cuntz U, Gramer C, et al. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci Rep. 2016;6:26752.
- Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics - a review. J Food Sci Technol. 2015;52(12):7577–7587.
- Saulnier DM, Santos F, Roos S, et al. Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. Plos One. 2011;6(4):e18783.
- Biesalski HK. Nutrition meets the microbiome: micronutrients and the microbiota. Ann N Y Acad Sci. 2016;1372(1):53–64.
- Whisner CM, Martin BR, Schoterman MH, et al. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double-blind cross-over trial. Br J Nutr. 2013;110(7):1292–1303.
- Sazawal S, Dhingra U, Hiremath G, et al. Effects of Bifidobacterium lactis HN019 and prebiotic oligosaccharide added to milk on iron status, anemia, and growth among children 1 to 4 years old. J Pediatr Gastroenterol Nutr. 2010;51(3):341–346.
- Scholz-Ahrens KE, Schaafsma G, van den Heuvel EG, et al. Effects of prebiotics on mineral metabolism. Am J Clin Nutr. 2001;73(2 Suppl):459S–464S.
- Sheridan PO, Bindels LB, Saulnier DM, et al. Can prebiotics and probiotics improve therapeutic outcomes for undernourished individuals? Gut Microbes. 2014;5(1):74–82.
- Onubi OJ, Poobalan AS, Dineen B, et al. Effects of probiotics on child growth: a systematic review. J Health Popul Nutr. 2015;34:8.
- Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 1751-1759;89(6):2009.
- Cani PD, Lecourt E, Dewulf EM, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90(5):1236–1243.
- Razmpoosh E, Javadi M, Ejtahed HS, et al. Probiotics as beneficial agents in the management of diabetes mellitus: a systematic review. Diabetes Metab Res Rev. 2016;32(2):143–168.
- Sun J, Buys N. Effects of probiotics consumption on lowering lipids and CVD risk factors: a systematic review and meta-analysis of randomized controlled trials. Ann Med. 2015;47(6):430–440.
- Mobini R, Tremaroli V, Stahlman M, et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in patients with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2017;19:579–589.
- Simon MC, Strassburger K, Nowotny B, et al. Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: a proof of concept. Diabetes Care. 1827-1834;38(10):2015.
- Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481.
- Membrez M, Blancher F, Jaquet M, et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. Faseb J. 2008;22(7):2416–2426.
- Vrieze A, Out C, Fuentes S, et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014;60(4):824–831.
- Reijnders D, Goossens GH, Hermes GD, et al. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo-controlled trial. Cell Metab. 2016;24(1):63–74.
- Zhang F, Luo W, Shi Y, et al. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107(11):1755; author reply p 1755-1756.
- Nieuwdorp M. Faecal microbiota transplantation. Br J Surg. 2014;101(8):887–888.
- Eiseman B, Silen W, Bascom GS, et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–859.
- van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415.
- Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect. 2016;92(2):117–127.
- Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916e917.
- Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis. 2015;2(1):ofv004.
- Li SS, Zhu A, Benes V, et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. 2016;352(6285):586–589.
- Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 1635-1638;308(5728):2005.
- Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–564.
