Abstract
A nano enhanced phase change material, NEPCM, was prepared by the addition of carbon nanotubes, CNTs, to butyl stearate. This study utilised single walled nano tube, multi-walled nanotube and modified multi-walled nanotube. Several kinds of surfactants were used to stabilise nano-particles in butyl stearate. Tetramethyl ethylene diamine (TEMED) was the best surfactant for enhancing the stability time of nano-fluid. The effect of CNTs concentration on the thermal properties of base fluid was studied and it was found that for all kinds of CNTs, the highest thermal conductivity of NEPCM was observed in weight fraction of 0.03. The modified-MWCNTs have the most enhancements in the butyl stearate thermal conductivity. It was found from differential scanning calorimetry tests, that modified-MWCNTs with 2 wt.% contents have the most effect on the latent heat of fusion of the base fluid. It was also observed that the SWCNT has a considerable effect on the melting point of PCM.
1. Introduction
Heat storage systems are utilised in the recovery and preservation of energy source and solar thermal systems [Citation1]. As a result of its high heat capacity and small temperature variation during the process of energy absorption and release, latent heat storage has been an interesting and effective approach for energy storage and recovery. In a latent heat storage system, energy is stored by melting a phase change material (PCM) and released during its freezing [Citation2].
Various PCMs have been investigated for application in latent heat storage systems [Citation3–6]. PCMs have been used widely, for heat storage in some systems such as heat pumps and solar systems [Citation7,Citation8]. In the last decade, many studies have been performed about the application of these materials for the cooling and heating of buildings, maintaining a comfortable temperature inside an automobile, Smart temperature adaptable textiles, heat exchangers and so on [Citation9–12].
Consequent upon their chemical stability, high latent heat and proper melting temperature, organic PCMs (paraffin and fatty acids) have been given a greater attention. PCMs have some disadvantages, one of which is low thermal conductivity. This affects the energy storing and releasing rates and also limits the PCM performance rate. Nano enhanced phase change materials, NEPCMs, are prepared by adding nano-particles to the phase-change materials, which can create changes in PCM characteristics such as thermal conductivity, latent heat, viscosity and phase change temperatures.
Carbon nano tubes (CNTs) are allotropes of carbon with a cylindrical nanostructure. Good thermal conductivity as well as lesser weight and lower density of the CNTs have made them ideal substances to improve heat storage performance. Therefore, CNTs have been used by researchers as potential enhancers in heat storage systems [Citation13]. Various investigations have been conducted in synthesising, and improving the thermo-physical properties like thermal conductivity, viscosity, specific heat, and so forth, of nano-fluids for thermal characterisation [Citation14–18].
There are two main kinds of nanotubes: single-walled carbon nanotubes (SWCNTs), consisting of a single rolled graphene sheet in diameter of 1-2nm, and multi-walled carbon nanotubes (MWCNTs), constituting several concentric graphene-cylinders [Citation19].
SWCNTs are significantly smaller in diameter compared to MWCNTs and so may have different thermal properties. Some studies have been reported in the field of enhancing the thermal conductivity of the PCM using the CNTs.
Tang et al. reported that the thermal conductivity of some organic PCMs greatly increased by incorporating multi-walled carbon nanotubes into the organic matrix [Citation20]. Cui et al. conducted an experiment to improve the thermal performance of soy and paraffin wax PCMs, by employing a CNT additive. They found that with the enhancement ratios of CNT/PCM in the mixture, the thermal conductivity of soy wax was enhanced but the latent heat did not decrease as the CNT contents increased [Citation21].
Choi et al. investigated the effect of carbon additives (MWCNT, graphite and graphene) on the heat conductivity of stearic acid as PCM. They observed that the thermal conductivity of PCM is enhanced by the addition of carbon additives. They also reported that adding poly vinyl pyrrolidone as a dispersion stabiliser into the nano PCM, enhanced the thermal conductivity of PCM [Citation22].
Kumaresan et al. prepared a nanofluid phase change material (NFPCM) by dispersing a small amount of multi-walled carbon nanotubes (MWCNT) in liquid paraffin. They reported that there was negligible change in the freezing/melting temperature of the NFPCM, but a small observable change in the latent heat values was observed. They also found that thermal conductivity increased with the increasing volume of the MWCNT [Citation13].
Nanoparticle size and geometry is an effective parameter affecting the thermal conductivity of nanoparticle-PCM composites. Warzoha and Fleischer used MWCNT with different diameters to improve the bulk thermal conductivity of an organic paraffin PCM. They found that nanoparticles with smaller diameters produced PCMs with lower thermal conductivities. This is in contrast with the effective medium approximations, which assumed that smaller nanoparticles have higher thermal conductivities [Citation23]. In fact, the enhancement in the thermal conductivity of bulk material depend on the geometric relationship between the nanoparticle fillers, the nanoparticle network morphology and heat flow rate at contacting nanoparticles junction when they diffuse through the bulk material [Citation24]. The results obtained by Warzoha showed that the interfacial thermal resistance between contacting nanoparticles decreases with increasing contact area. Nanoparticles with smaller diameter have lower contact area which leads to increased thermal resistance and so decreased thermal conductivity.
Shaikh et al. investigated the effect of three types of nanoparticles on the latent heat of a PCM. They added SWCNT, MWCNT and CNF (carbon nanofiber) into the wax and found that SWCNT had the most effect on the latent heat of PCM. The relatively larger latent energy can be attributed to the higher molecular density of SWCNT relative to other additives, as well as its large surface area [Citation25].
However, the literature review clearly indicates that few studies have so far been done concerning the use of SWCNT as an additive to enhance the thermal conductivity of PCM. In the present study, three types of CNTs, SWCNT, MWCNT and modified-MWCNT were used to improve the thermal conductivity of butyl stearate as PCM. Different surfactants were used to disperse nanoparticles within the PCM and produce a stable nanofluid. Differential scanning calorimetry (DSC) analysis was used to investigate the effect of adding nano particles on the thermal properties of PCM.
2. Experimental
2.1. Materials
Butyl stearate as PCM with melting point of about 21 °C was purchased from Merck Co. (Darmstadt, Germany). Triton X-100, 1-decanol, sodium dodecyl sulphate and tetra methyl ethylene diamine as surfactants were purchased from Merck Co.
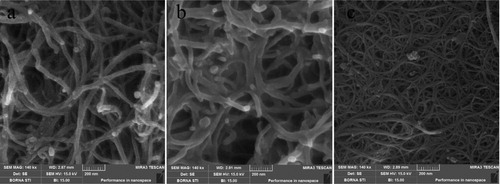
Single-walled carbon nanotube, multi-walled carbon nanotube and modified multi-walled carbon nano tube with specification listed in , were supplied from Cheap Tubes (Vermont, USA).
Table 1. Specification of the carbon nanotubes.
presents the SEM graphs of these CNTs.
2.2. Nanofluid preparation
For preparation of nano fluid samples, a certain amount of nanoparticles were added to 10 ml of butyl stearate in the liquid state and stirred vigorously by a mechanical stirrer for about 60 min. In order to properly disperse nanoparticles in fluid and obtain a stable suspension, 1 ml of a surfactant was added and sonicated continuously in an ultrasonic bath for a period of 2 h. During the preparation process, the temperature was maintained around 30 °C to ensure that the samples are kept above the melting point of butyl stearate.
Various samples were prepared with weight fractions of 1, 2, 3, 4, and 5% of SWCNT, MWCNT and modified-MWCNT nanoparticles.
2.3. Measurement of thermal properties
The thermal conductivity of the prepared samples was measured by using the KD2 Pro analyser (Decan Devices Inc.), based on the transient line heat source method. The accuracy of data was ±0.01%. The scanning rate of the sensor is 90 measurement cycles in 1 s. Since the variation in the thermal conductivity of the PCMs with respect to temperature is a vital factor for thermal storage applications, the thermal conductivity of nanofluids was measured at different temperatures.
Thermal properties including melting temperature (Tm) and latent heat capacity (Ls) of samples were measured using a DSC instrument (DSC1 Stare System, Mettler Toledo, Greifensee, Switzerland). The DSC measurements were performed at a heating rate of 10 °C/min and in a temperature range of −20 to 100 °C.
3. Results and discussion
3.1. Nanofluid stability
The dispersion of carbon nanotubes in butyl stearate without any dispersant agent is very difficult. The activity and high energy of the nanoparticle within the surface atoms and the strong Van der Waal's forces existing between the nanoparticles causes agglomeration in the base fluid.
In this present work, four types of surfactants were used to disperse nanoparticles in the butyl stearate and to prepare a stable suspension. showed the stability time of the prepared nanofluids with MWCNTs in the presence of different surfactants. It was observed that tetramethyl ethylene diamine (TEMED) has the most effect on the enhancement of stability time of nanofluid. shows that the strong bipolar interaction between nitrogen in the amine group of the TEMED and carbon atoms in the nanoparticles, creates electrostatic repulsive forces and reduces Van der Waal's forces between nanoparticles. It reduces accumulation and agglomeration of nanoparticles which leads to enhanced sustainability time of suspension. Furthermore, the tetraethyl methylenediamine surfactant has a better structural compatibility with butyl stearates than other surfactants [Citation26].
The stability of the nanofluid prepared with modified-MWCNTs was also investigated. It was observed that among the surfactants, TEMED had the greatest effect on the sustainability time of modified nanoparticles. However, the effect of TEMED on the dispersion of modified-MWCNTs is weaker compared to the non-modified ones. The carbonyl functional groups of the CNTs, cause a weaker hydrogen bond between modified-MWCNTs and the amine group of the dispersant. It resulted to accumulated nanoparticles and reduced effect of TEMED on nanofluid stability.
3.2. Thermal conductivity
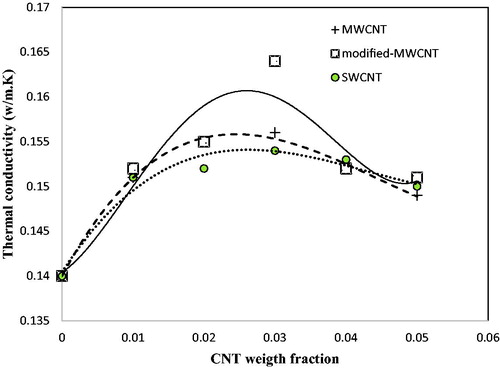
shows the thermal conductivity of the prepared nanofluids with different concentration of CNTs, at ambient temperature. It was observed that for all kinds of CNT nanofluids, the thermal conductivity increased with increasing nanoparticle weight fraction up to 0.03 and then decreased at higher contents. Enhancement of the nanoparticle concentrations in the nanofluid reduces the intermolecular free mobility of the particles. It caused instability of the suspension and agglomeration of the nanotubes. As a result, the thermal conductivity of the nanofluids was reduced.
It was also observed that the modified-MWCNTs have the most effect on the enhancement of the butyl stearate thermal conductivity. The results showed that the thermal conductivity of the nanofluid prepared with 3wt.% of the modified-MWCNTs is about 17 times higher than in the base fluid.
Carbon nanotubes have a regular hexagonal structure and have no functional group or another bond. The surface modification of CNTs is an approach that reduces the surface resistance and improves the interaction between nanoparticles and the base fluid. Therefore, the thermal conductivity of the nanofluid can be increased by surface modification of CNTs. Among the CNT types, single-walled carbon nanotubes have the least effect on the increasing thermal conductivity. As already mentioned, nanoparticles with smaller diameter have lower contact area that lead to increased thermal resistance, thereby decreasing thermal conductivity [Citation23].
Wang et al. were also found that adding MWCNT in a fatty acid PCM lead to increase thermal conductivity. They observed a 30% enhancement in thermal conductivity of MWCNT/palmitic acid with 1 wt.% of CNTs [Citation27].
Introducing the carbon nanotube in paraffin PCM s, also increases thermal conductivity. Kumaresan et al. prepared a composite of MWCNT and a liquid paraffin with different contents of CNT. They reported that increasing MWCNT volume fraction, increase the thermal conductivity of composite [Citation13]. Similar results were found in other works done by some researchers [Citation21,Citation22,Citation25].
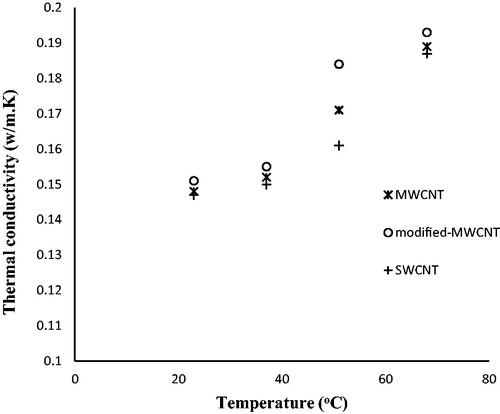
The effect of temperature on the thermal conductivity of the nanofluids prepared with 3wt.% of different kinds of CNTs is shown in . It was observed that for all CNT types, thermal conductivity increased with temperature. In fact, with increasing temperature, the number and intensity of contacts between fluid molecules and suspended particles increased. The thermal conductivity increased with increasing temperature.
3.3. Thermal analysis
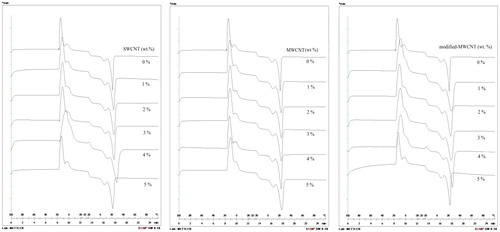
In order to investigate the effect of carbon nanotubes on the thermal properties of butyl stearate, DSC tests were performed. Some properties such as melting point, freezing point and latent heats of fusion and freezing, were obtained from DSC analysis. DSC curves of SWCNT, MWCNT and modified-MWCNT NEPCMs with content of nanoparticles are presented in .
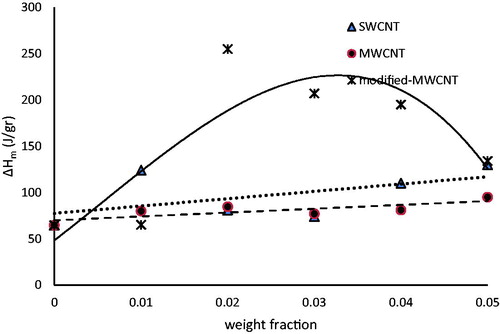
shows the thermal properties obtained from DSC graphs. It was seen that modified-MWCNTs have the most effect on the latent heat of fusion of the base fluid.
Table 2. Thermal specifications of NEPCMs obtained from DSC analysis.
As it can be seen in , heat of fusion increased with increasing modified-MWCNT concentration up to 2 wt.% and decreased at higher concentrations. It can be explained by the following thermodynamic equations: (ΔH = ΔU + VΔP&ΔU = TΔS-PΔV) [Citation28].
When modified-MWCNTs are added into the base fluid, the mixture tends to be in a chaotic state and entropy of the fluid is increased (ΔS > 0). For low contents of the nanoparticles in the fluid, the increase in volume is negligible and as a result TΔS > PΔV, and so ΔU > 0. Based on the relation ΔH = ΔU + VΔP, it can be concluded that ΔH > 0. Thus, increasing the nanoparticle up to 2%, enhances the latent heat of butyl stearate. For higher contents of nanoparticles, the volume change of the fluid is considerable and PΔV would be increased more than TΔS. Therefore, there will be a decrease in ΔU and ΔH. Also, the latent heat of melting decreases with increasing content of CNT, more than 2%.
It can be seen from that ΔHm is slightly increased with the content of MWCNTs and SWCNTs in nanofluid. The greater potential of interactions between nanoparticles and base fluid molecules in comparison with that of base material molecules is the cause of enthalpy enhancement [Citation29]. The ability of more dispersion and large surface area of SWCNT are effective parameters on latent heat increment [Citation25].
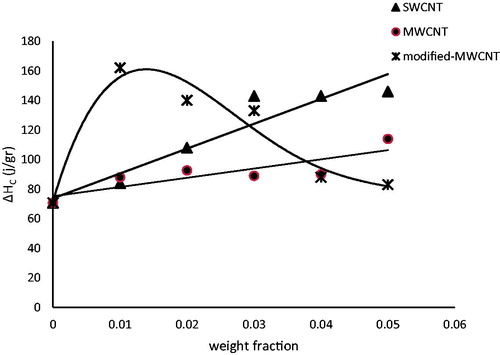
shows the latent heat of freezing for nanofluids for different types and concentrations of CNTs. A similar behaviour can be seen with the latent heat of fusion, for all types of nanofluids.
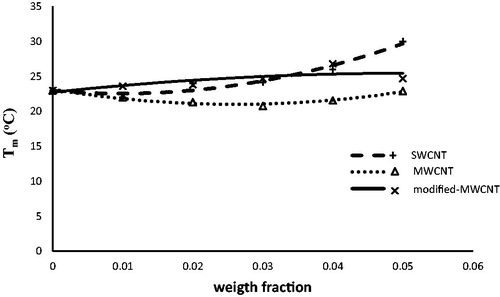
shows the variation of melting point temperature Tm of nanofluids. It can be seen that the SWCNT has a considerable effect on Tm. The addition of 5 wt.% SWCNT to the butyl stearate increases its melting point by about 30% whereas MWCNT and modified-MWCNT have no significant effect on the melting point of the nanofluid.
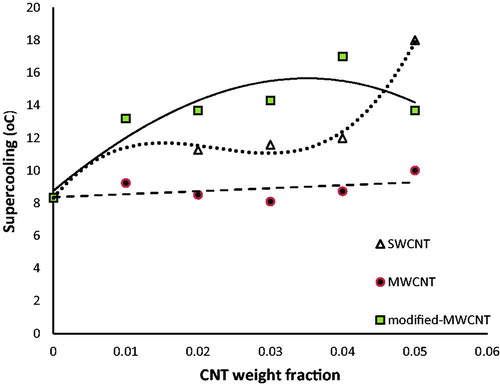
shows the supercooling of PCM with different contents of CNTs. It is observed a negligible change in supercooling when MWCNT was used, while in SWCNT and modified-MWCNT containing PCMs, it can be seen a considerable change in supercooling. Reducing the temperature of crystallization due to the presence of nanotubes has increased supercooling. Enhancement of nucleation is a factor that increases the crystallization temperature. Presence of very small nuclei cause to decrease crystallization temperature and thus increasing the supercooling [Citation30]. Carbon nanotube act as core of the nucleus and the nucleation is done around it. Due to the small size of the nanoparticles, the formed nuclei dissolve and so crystallization occurs in lower temperatures that lead to increase supercooling.
4. Conclusions
In this study, some carbon nanotubes were employed to improve the thermal properties of butyl stearate as a PCM. The thermal conductivity of NEPCM increased with increasing weight fraction of all CNTs (MWCNT, modified-MWCNT and SWCNT) up to 0.03. The highest thermal conductivity was related to NEPCM which contained modified-MWCNT. The latent heats of NEPCM (ΔHm&ΔHc) with 2 wt.% of modified-MWCNT had the highest values among all prepared samples. However, modified-MWCNT had no considerable effect on the melting point of PCM, but it increased with increasing mass fraction of SWCNT.
In general, it can be concluded that modified-MWCNTs in content of 2 wt.%, effectively improved the thermal conductivity and latent heat of butyl stearate without having any considerable effect on melting point. Therefore, butyl stearate containing 2 wt.% of the modified-MWCNT could be an appropriate NEPCM for thermal energy storage systems.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Dincer I, Rosen M. Thermal energy storage: systems and applications. 2002.
- Agyenim F, Hewitt N, Eames P, Smyth M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew Sustain Energy Rev. 2010;14(2):615–628.
- Nkwetta DN, Haghighat F. Thermal energy storage with phase change material—a state-of-the art review. Sustain Cities Soc. 2014;10:87–100.
- Kenisarin MM. Thermophysical properties of some organic phase change materials for latent heat storage. A review. Solar Energy. 2014;107:553–575.
- Liu M, Saman W, Bruno F. Review on storage materials and thermal performance enhancement techniques for high temperature phase change thermal storage systems. Renew Sustain Energy Rev. 2012;16(4):2118–2132.
- Rathod MK, Banerjee J. Thermal stability of phase change materials used in latent heat energy storage systems: a review. Renew Sustain Energy Rev. 2013;18:246–258.
- Kenisarin M, Mahkamov K. Solar energy storage using phase change materials. Renew Sustain Energy Rev. 2007;11(9):1913–1965.
- Shalaby SM, Bek MA, El-Sebaii AA. Solar dryers with PCM as energy storage medium: a review. Renew Sustain Energy Rev. 2014;33:110–116.
- Jamekhorshid A, Sadrameli SM. Application of phase change materials (PCMs) in maintaining comfort temperature inside an automobile. World Acad Sci Eng Technol Int J Chem Mol Nucl Mater Metall Eng. 2012;6(1):33–35.
- Mondal S. Phase change materials for smart textiles–an overview. Appl Therm Eng. 2008;28(11):1536–1550.
- Hosseini MJ, Rahimi M, Bahrampoury R. Experimental and computational evolution of a shell and tube heat exchanger as a PCM thermal storage system. Int Commun Heat Mass Transfer. 2014;50:128–136.
- Soares N, Costa JJ, Gaspar AR, Santos P. Review of passive PCM latent heat thermal energy storage systems towards buildings’ energy efficiency. Energy Buildings. 2013;59:82–103.
- Kumaresan V, Velraj R, Das SK. The effect of carbon nanotubes in enhancing the thermal transport properties of PCM during solidification. Heat Mass Transfer. 2012;48(8):1345–1355.
- Ding Y, Alias H, Wen D, Williams RA. Heat transfer of aqueous suspensions of carbon nanotubes (CNT nanofluids). Int J Heat Mass Transf. 2006;49:240–250
- Wen D, Ding Y. Effective thermal conductivity of aqueous suspensions of carbon nanotubes (carbon nanotube nanofluids). J Thermophys Heat Transf. 2004;18:481–485.
- Assael MJ, Metaxa I, Arvanitidis J, Christofilos D, Lioustas C. Thermal conductivity enhancement in aqueous suspensions of carbon multi-walled and double-walled nanotubes in the presence of two different dispersants. Int J Thermophys. 2005;26:647–664.
- He Y, Men Y, Liu X et al. Study on forced convective heat transfer of non-Newtonian nanofluids. J Therm Sci. 2009;1:20–26.
- Das SK, Putra N, Thiesen P, Roetzel W. Temperature dependence of thermal conductivity enhancement for nanofluids. J Heat Transf. 2003;125:549–552.
- Breuer O, Sundararaj U. Big returns from small fibers: a review of polymer/carbon nanotube composites. Polym Compos. 2004;25:630–641.
- Tang X, Hammel E, Reiter W. Carbon nanotube enhanced thermally conductive phase change material for heat dissipation. In Thermal Investigations of ICs and Systems, 2009. THERMINIC 2009. 15th International Workshop on (pp. 216–218). . IEEE; October 2009.
- Cui Y, Liu C, Hu S, Yu X. The experimental exploration of carbon nanofiber and carbon nanotube additives on thermal behavior of phase change materials. Solar Energy Mater Solar Cells. 2011;95(4):1208–1212.
- Choi DH, Lee J, Hong H, Kang YT. Thermal conductivity and heat transfer performance enhancement of phase change materials (PCM) containing carbon additives for heat storage application. Int J Refrig. 2014;42:112–120.
- Warzoha RJ, Fleischer AS. Effect of carbon nanotube interfacial geometry on thermal transport in solid–liquid phase change materials. Appl Energy. 2015;154:271–276.
- Foygel M, Morris R, Anez D, French S, Sobolev V. Theoretical and computational studies of carbon nanotube composites and suspensions: electrical and thermal conductivity. Phys Rev B 2005;71(10):104201.
- Shaikh S, Lafdi K, Hallinan K. Carbon nanoadditives to enhance latent energy storage of phase change materials. J Appl Phys. 2008;103(9):094302.
- Zhang S, Wu JY, Tse CT, Niu J. Effective dispersion of multi-wall carbon nano-tubes in hexadecane through physiochemical modification and decrease of supercooling. Sol Energy Mater Sol Cells. 2012;96:124–130.
- Wang J, Xie H, Xin Z, Li Y, Chen L. Enhancing thermal conductivity of palmitic acid based phase change materials with carbon nanotubes as fillers. Sol Energy. 2010;84(2):339–344.
- Yang Y, Luo J, Song G, Liu Y, Tang G. The experimental exploration of nano-Si3N4/paraffin on thermal behavior of phase change materials. Thermochim Acta. 2014;597:101–106.
- Choi SUS, Zhang ZG, Yu W, Lockwood FE, Grulke EA. Anomalous thermal conductivity enhancement in nanotube suspensions. Appl Phys Lett. 2001;79(14):2252–2254.
- Uzan AY, Kozak Y, Korin Y, Harary I, Mehling H, Ziskind G. A novel multi-dimensional model for solidification process with supercooling. Int J Heat Mass Transf. 2017;106:91–102.