?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The objective of this work is to develop cost-effective, reliable, and large-scale production of metallic nanoparticles (NPs) by adopting green chemistry principles for industrial applications. In that view, we have studied the phytochemical reducibility of silver nitrate by making use of Calotropis Procera (Ait.) R. Br root extract, and based on its medicinal properties, an attempt was made to evaluate the therapeutic potentials of silver (Ag) NPs containing this plant extract towards the clinical strains of bacteria. The optimization studies on reducing the potentials were done considering the concentration, pH, temperature, and reaction period of both the extract and the metal precursor. The nanoarchitecture elements were interpreted using visual, spectroscopic, and microscopic analyses cohorting the antibacterial potentials towards the clinically significant strains. The antimicrobial activity exercised by these Ag NPs towards 10 different strains of medically important bacteria at a given concentration was proved to be significant. The antimicrobial potential was further validated quantitatively, and the MIC/MBC concentration values were determined. Finally, the cytotoxicity of Ag NPs when tested against the HEPK cell line indicated that the metal-phytochemical moiety exhibited the maximum therapeutic efficacy and thereby paving the way for the development of disruptive technologies in the field of nanomedicine.
1. Introduction
In recent years, the increased incidence and emergence of antibiotic resistance has not only threatened the hospital environment but also remaining a global economic menace [Citation1]. The major contributor to this public health concern is attributed by bacteria (∼54%), envisioned to exceed the global mortality rate triggered by cancer. As a defense mechanism, various antimicrobials have been formulated against bacteria, fungi, viruses, protozoa, etc. to impede essential cellular processes either to inhibit or eliminate the microbial attacks. But their efficiency is compromised due to the microbial strategies striving for their survival and also inheriting the same for their progeny. A collection of pathogens termed ESKAPE (Enterococcus faecalis, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species) were found to be the most susceptible strains in the course of acquiring antibiotic resistance [Citation2,Citation3]. Alongside, the resistance pattern towards multiple classes of antibiotics are due to their indiscriminate use and was best studied in microbes such as Escherichia coli. It was reported that the spontaneous mutation and horizontal gene transfer remains to be the principal mechanism conferring antimicrobial resistance property [Citation4]. As a result, the quantity of antibiotics intake gets reduced via alteration in the target sequence and efflux pumps. In the process of striking the resistance mechanism, novel antibiotics have been developed and actively been pursued but those formulations were decrypted by the microcosmonauts.
On the other hand, the metallic nanoparticles (NPs) that exhibit improved properties based on their size, distribution, architecture, chemical assembly, colloidal stability and biocompatibility have been devised to counter balance this resistance pattern via nanocomposite assembly and surface modification [Citation5]. In addition, the NP’s high surface area to volume ratio allows easy interaction with other particles or living cells and thereby bringing about the desired effect. So, the optical properties of nanoforms of noble metals namely gold, silver, platinum, etc. were employed in biomedical and therapeutic applications [Citation6]. Meanwhile, the metallic NPs have low colloidal stability which tends them to aggregate in the biological environment and to solve this problem, the usage of chemicals in the synthesis protocol has been replaced with phytochemical moieties of indigenous plant such as Calotropis procera and the surface modification of which increases the biocompatibility of these materials [Citation7].
C. procera (Ait.) R. Br. belongs to the Asclepiadaceae family and is an extensively grown plant in India and other warm, dry places due to the very interesting medicinal properties that are used in many ayurvedic formulations and as a traditional system of medicines. The leaves are perceived as a valuable antidote for snakebite, sinus, rheumatism, mumps, etc. and the root barks are used to treat a variety of illnesses such as leprosy, menorrhagia and high fever [Citation8]. They are traditionally used to treat diarrhoea, jaundice and the best remedy for skin diseases and for that reason, various reports on the pharmacological activities of the plant namely antihelminthic, antidiabetic, antimalarial, antimicrobial, anticancer and hepato-protective are being developed [Citation9–11].
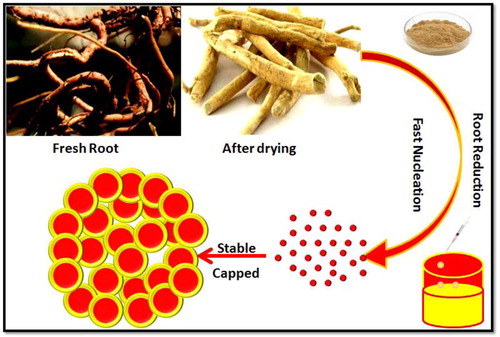
The surface-modified NPs with pharmacologically active phytochemicals of Calotropis sp. would challenge conventional drugs encountering clinically significant strains [Citation12]. Herein, we report a tailored phyto-based silver NPs (AgNPs) using Calotropis root extract, where the characterisation and bactericidal activity experiments were carried out using sophisticated analytical techniques and clinically significant bacterial isolates to highlight the biological activity of synthesised system. The schematic representation of AgNPs from Calotropis root extract is shown in .
2. Experimental detail
2.1. Materials
Fresh roots of C. procera plant were obtained from a local farm in Chennai (Lat. 10°7′12″ N; Long. 77°33′0″ E), Tamilnadu, India. Silver nitrate (AgNO3 ≥ 99.8%; AR grade) was procured from Sigma-Aldrich (Bengaluru, India) and used without further purification. All the glassware used was cleansed using chromic acid (HiMedia, Mumbai, India), sterilised using an autoclave and dried in a hot air oven. All the experiments were performed in triplicates and the results are expressed as mean ± SD (standard deviation) of all concentrations.
2.2. Preparation of Calotropis root extract
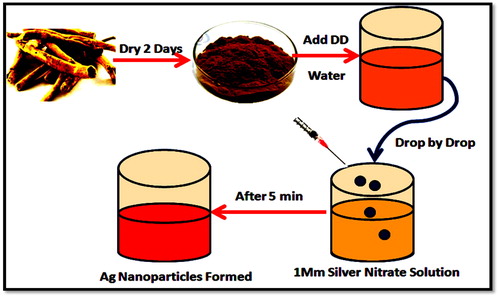
For the preparation of Calotropis root extract, the fresh and healthy Calotropis roots obtained were thoroughly washed and dried in shade for 2–3 days, finely chopped into 50 g size followed by converting into fine powder using a blender. A specified quantity of the powdered sample was boiled along with 150 mL of deionised water (Millipore, Model 8200, Germany) for 20 min, filtered through muslin cloth followed by Whatman No. 1 filter paper (Merck, Mumbai, India). The phytochemical analysis of filtered root extract was done using the standard procedures [Citation13]. The filtered Calotropis root extract was used within 4 h for synthesising the AgNPs until which it was stored in a refrigerator.
2.3. Green synthesis of AgNPs
In a typical synthesis protocol, an aqueous solution of 1 mM AgNO3 prepared was used for the synthesis of AgNPs and for that, the root extract was added dropwise to 1 mM AgNO3 at 1:10 ratio and left for about 5–10 min under the stirring condition at various temperatures (100, 150 and 200 °C) and observed for the changes in colour. This experiment was performed in dark to prevent the photolytic effect of the metal precursor. Further, the separation of AgNPs from the dispersion medium was carried out by centrifugation (10,000 rpm/15–20 min). The supernatant was discarded, and the pellet was washed several times with deionised water, dried, powdered and stored at 4 °C for further experimentation ().
2.4. Characterisation studies
UV–Vis spectrophotometer (Varian Cary 300, Agilent, CA, USA) was used to monitor the concentration of AgNPs, where the spectra measured in the wavelength range of 300–800 nm using silver nitrate as control and the absorbance maxima typical for AgNPs was recorded. Further detailing on the crystal structure, phase and texture of the synthesised AgNPs was monitored using a Rigaku MultiFlex X-ray diffractometer (Rigaku, Tokyo, Japan) through Cu Kα rays at a diffraction angle (2θ) from 20° to 100°. The mean size of AgNPs was calculated taking the full width at half maximum (FWHM) of (111) plane using the Debye–Scherrer equation,
where K is the Scherrer constant (0.9); λ is the wavelength of the X-rays; β is the FWHM and θ is the Bragg’s angle in radians.
The functional groups responsible for the reduction of silver to stable nanosilver was detected using Fourier-transform infrared (FTIR) spectrophotometer (FT/IR 4600, JASCO, Japan). The analysis was performed using KBr pellets (1:100) and the spectrum recorded in the range from 400 to 4000 cm−1 at 4 cm−1 resolution. To determine the morphology and arrangement of AgNPs, a drop of AgNPs was dispersed onto a carbon-coated copper grid, dried and examined using Zeiss Supra 55 (Carl Zeiss, Germany) at an accelerating voltage of 25 kV. The size of the particles and its distribution were determined using ImageJ software (National Institutes of Health) measuring atleast 200 particles. The elemental composition pertaining to the purity of nanosilver was evidenced using the Energy-dispersive X-ray spectroscopy (EDX) (Oxford instruments X-MAX). The hydrodynamic diameter of the synthesised nanostructures was validated using Malvern zeta sizer Nano ZS (Malvern Instruments, Worcestershire, UK) 90 using deionised water as a dispersing agent at neutral pH.
2.5. Antibacterial study—In vitro approach
2.5.1. Agar diffusion method
The antibacterial efficacy of AgNPs was determined using the agar diffusion method [Citation14] against clincally significant bacteria namely Salmonella typhi, Shigella flexneri, Bacillus subtilis, E. coli, S. aureus, Enterococcus sp., K. pneumoniae, P. aeruginosa, Staphylococcus epidermidis, and E. faecalis. The strains used are typical representatives of two large bacterial taxonomical lineages and were obtained from a private clinical laboratory in India. Prior to the assay, each bacterium was refreshed on nutrient agar stocks and fresh overnight grown suspensions in nutrient broth were preferred. The inoculum size of each strain from overnight suspensions was prepared using 0.8% NaCl and the turbidity adjusted to OD600 = 0.1. Sterile disks were loaded with 5, 102, 550, 100 µL of AgNPs (1 mg/mL) and control disk with the 1 mM solution of AgNO3. The inoculated plates were incubated at 37 °C for 24 h for any inhibitory effect and at the same visualised and measured the extent of inhibition using the calipers.
2.5.2. Determination of minimum inhibitory concentration and minimum bactericidal concentration
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of phyto-fabricated AgNPs were analysed following CLSI 2015 guidelines [Citation15]. The MIC test was performed in a 96-well microtiter plate using a standard broth microdilution method and MBC test on the Mueller–Hinton Agar (MHA) plates. The bacterial inoculums were adjusted to the concentration of 106 CFU/mL. For the MIC test, 100 µL of the synthesised AgNPs stock solution (1000 µg/mL) was added and diluted twofold in 100 µL of MHA started from column 1 to column 10. Column 1 of the microtiter plate contained the highest concentration of AgNPs, while column 10 contained the lowest concentration. Columns 11 and 12 served as negative and positive controls respectively containing only medium and medium with bacterial inoculums. Each well of the microtiter plate was added with 30 µL of the resazurin solution (0.02% w/v) and incubated at 37 °C for 24 h [Citation16]. Any change in colour was observed namely blue or purple colour indicated no growth, while pink or colourless indicated bacterial growth. The lowest concentration of antibacterial agent inhibiting the bacterial growth was considered as its MIC value. MBC test was performed by plating the suspension from each dilution of microtiter plates into the MHA plate. The plates were incubated at 37 °C for 24 h, where the lowest concentration with no visible growth on the MHA plate was considered as its MBC value.
2.6. Cytotoxicity studies—MTT assay
The cytotoxic effect of phyto-fabricated AgNPs was tested against HEPK (Keratinocytes) cells procured from NCCS, Pune, India. The HEPK cells were seeded in a 96-well plate at a density of 1 × 104 cells per well in 200 μL of Dulbecco’s modified eagle’s medium containing 10% foetal bovine serum and 1% penicillin–streptomycin antibiotics and were cultured at 5% CO2 and 37 °C for 24 h. The growth medium in the wells was replaced after 24 h with medium containing AgNPs (0.78–100 μg/mL) and incubated for another 24 h. The medium was removed thereafter and replaced with 100 μL of medium containing MTT reagent or 3(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide and incubated for 4 h. The unreduced MTT was aspirated and 200 μL of dimethyl sulfoxide was added to each well to dissolve the MTT formazan crystals. The content was mixed properly, and absorbance measured at 595 nm in an ELISA microplate reader (Invitrogen Bioservices, Bengaluru, India) [Citation17]. The test was for the comparison, TGF-β was used as positive control and the cells without any treatment served as the negative control.
3. Results and discussion
The green chemistry principles remain to be one of the promising strategies to override the hurdles associated with conventional methods in the event of NPs synthesis. The biological extract particularly plants origin has been the most sought after reducing agent for its non-toxic and rich metabolic content. Accordingly, the root extract prepared from the C. procera plant was phytochemically probed prior to the NPs synthesis.
The phytochemical screening of C. procera root for the presence of secondary metabolites was investigated using ethanolic and aqueous extract, respectively, and the constituents identified based on the polarity, colour intensity and precipitates formed (). The presence of phytochemical constituents was indicated in terms of scores of +, ++ and +++ using standardised values based on the prepared root extract. A score of ‘+’ corresponds to 0.01 to 0.1%, ‘++’ corresponds to 0.1 to 0.3% and ‘+++’ corresponds to more than 0.3% based on the dry weight of plant material. It is quite obvious from , the root extract of C. procera showed the presence of alkaloids scoring ‘+’, saponins, flavonoids, sterols and triterpenoids scoring ‘++’ and cardiac glycosides with ‘+++’ score which is in agreement with the previous reports [Citation18]. Subsequently, the extraction efficiency was higher when ethanol was used compared to the aqueous extract that showed slight to the mere presence of above-mentioned secondary metabolites. Thus, the ethanolic extract was retained for fabricating the AgNPs.
Table 1. Phytochemical screening of C. procera root extract.
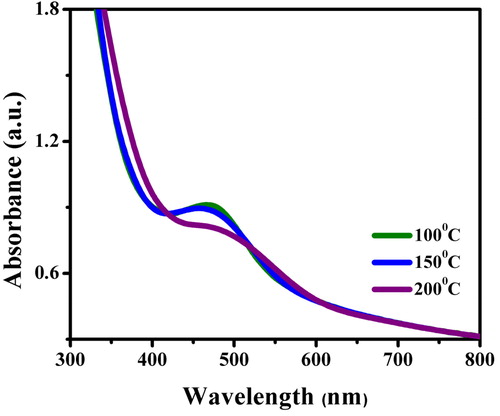
The synthesis and stability of the reaction mixture were monitored from the colour change and UV–Vis spectra. There was a gradual change in colour from yellowish-green to golden yellow and finally yellowish-brown colloid within 10 min of the addition of extract. This clearly indicated the initiation of the reduction of silver salt by the phytochemical moieties to the nano form. Secondly, the spectral analysis focussed on the wavelength range of 400–460 nm () with its absorbance maxima (λmax) at 455 nm attributed to the typical SPR property of silver (Ag0) at 100 °C [Citation19]. A few hours later, no further colour change was observed indicating a complete reduction of silver salts. Moreover, the AgNPs thus formed remained stable showing a very little aggregation. Meanwhile, the NPs synthesised at increased temperatures namely 150 and 200 °C showed little broadening inferring agglomeration of particles. Besides the broadening of plasmon bands, the absorption tail was found prominent at longer wavelengths and at higher temperatures indicative of size distributions of NPs [Citation20]. The time taken for AgNPs synthesis was much less and this fact can be explained taking into account the role of bio-molecules such as flavonoids and phenolic compounds present in the extract that might have provided the high reductive ability [Citation21].
Figure 3. UV–Visible spectrum of AgNPs synthesised using the root extract of C. procera at different temperatures of 100, 150 and 200 °C.
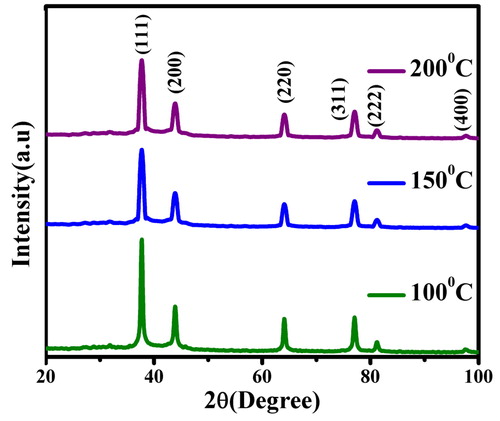
The powdered XRD remains one of the best analytical techniques to examine the crystal nature and purity of a compound after 12 h for different temperatures. The prepared AgNPs showed diffraction patterns () with prominent peaks at 2θ = 38.21°, 44.43°, 64.32°, 77.65° and 81.72°, 98.2° that matched well with (111), (200), (220), (311) and (222), (400) planes of Ag typical for FCC symmetry (JCPDS 04-0783) at all temperatures. The highest peak intensity of (111) plane with narrow FWHM at all temperatures illustrated the crystalline nature of synthesised AgNPs [Citation22]. The average crystallite size of AgNPs was calculated using the Debye–Scherrer equation and found to be ∼22 nm at 100 °C. It is at this tiniest scale, the particles tend to nucleate, grow and multiply with their lowest energy (111) which corroborates well with the previous reports.
Figure 4. Comparison of the powdered XRD patterns of AgNPs synthesised using the root extract of C. procera at different temperatures.
3.1. FTIR spectral analysis
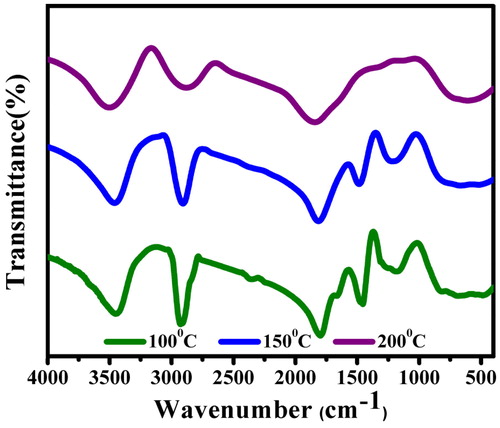
The bio-molecular interaction of the root extract in the event of nanosilver reduction was identified using FTIR analysis (), where the spectrum demonstrated prominent peaks around 3482, 2911, 2841, 2352, 1794, 1459, 1151 and 830 cm−1. There are stretching vibrations at 3482 cm−1 (strong, broad O‒H), 2911 cm−1 (strong N‒H stretch, amine), 2841 cm−1 (medium C‒H stretch, alkane), 1794 cm−1 (around 1780 cm−1 C = O stretch, carboxylic acid), 1459 cm−1 (around 1450 cm−1 C‒H bend), 1151 cm−1 (weak O‒H stretch, alcohol) and 830 cm−1 (medium C = C bend, alkene). This indicates the presence of amine, phenolic, alcoholic aromatics from the root extract of C. procera involved as reducing and/or capping agent in the AgNPs synthesis [Citation23–24]. However, the FTIR spectrum of AgNPs prepared by boiling the extract to 200 °C highlighted peaks only at 3482, 2911 and 1794 cm−1 attributing to stretching vibrations of O‒H, N‒H and C‒H, respectively. As the metal NPs in the colloidal form are easily influenced by van der Waals forces of attraction, the possibility of coagulation is much faster at higher temperatures (150 and 200 °C). It was presumed that these moieties behave as steric or electrostatic barriers around the particle surface conferring a highly dispersive nature of particles [Citation25].
3.2. Scanning electron microscopy and Energy-dispersive X-ray spectroscopy (EDX) analysis
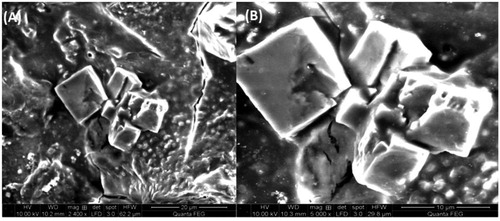
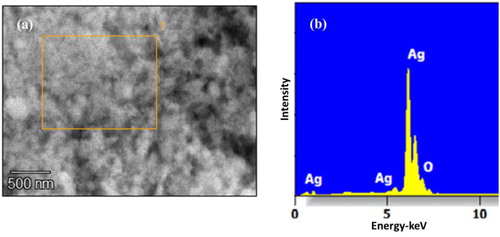
Electron microscopy is a useful tool that bestows the topographical insights namely size, shape and distribution of particles. In this accord, the fabricated NPs revealed elliptical to nearly spherical and square shaped particles () in the size range of 38–44 nm. The variable shapes of particles may be correlated with different sizes. When resolved, the NPs were not found in direct contact even within the aggregates, indicating the stabilisation of particles by the capping agent. The EDAX spectra recorded in showed that the chemical composition of colloid constituted pure silver with 75.2% as its weight percentage.
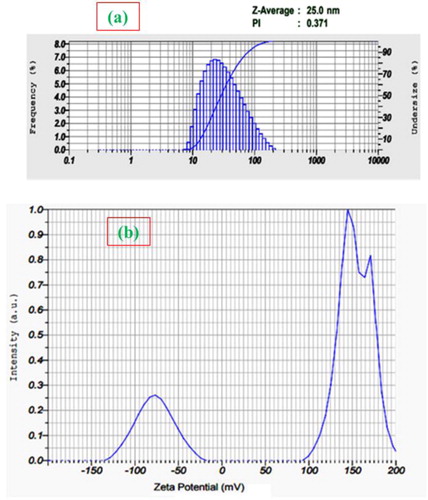
3.3. Dynamic light scattering (DLS) and zeta potential analysis
The DLS technique was used to measure the size distribution of NPs (aggregation) in deionised water and based on this, the DLS measurements shown in indicates the dispersive nature of NPs at physiological temperatures. The AgNPs apparent size distribution towards smaller value (20–30 nm) was found consistent with the XRD studies. This may be explained in terms of the enhancement of strong interparticle electrostatic interactions and chaperone-like activity of phytochemicals. Alongside, the measurements of zeta potential () gave information about the stability of dispersed NPs and the observation of AgNPs higher zeta potential value in between −50 and −100 mV correlating to higher stability of their dispersions [Citation26] and the possibility of strong electrostatic interaction between the NPs and phytochemical moieties.
3.4. Antibacterial activity
The obtained results of antibacterial activity testing for AgNPs are shown in . From the analysis of results, the AgNPs exhibited significant antibacterial action in a dose-dependent fashion for all bacteria under optimised laboratory conditions (pH 7; 37 °C; 1 mM). For the same bacterial concentrations, the increase of NPs concentration resulted in an increased bactericidal effect. This trend could be observed as there were fewer NPs that encountered each bacterial cell at their lowest concentration. Among the tested strains, E. coli and P. aeruginosa exhibited maximum zone of inhibition (ZoI) of 17 mm as evident from the zone size followed by S. flexneri (16 mm), S. typhi (15 mm) and K. pneumoniae (15 mm). The comparison between the ZoI of bacteria exposed to different concentrations of AgNPs showed significant differences at 10, 25, 50 and 100 μg concentrations in an increasing trend when statistically analysed using one-way analysis of variance (ANOVA; p < 0.05). This was substantiated by running a control test using only AgNO3 which exhibited lower growth inhibition than the AgNPs.
Table 2. Concentration-dependent antimicrobial susceptibility of AgNPs towards clinically significant pathogens.
Secondly, upon analysing the antibacterial effect observed between groups using independent t-test (t = 0.0381; df = 8; p > 0.05), not much significant difference in the inhibitory effect could be appreciated when the same AgNPs concentration was treated against Gram-positive and Gram-negative bacterial strains (N = 5). This clearly indicates that the highest toxicity is observed for the highest AgNPs concentration (100 μg) irrespective of bacterial strains. This bactericidal effect may be attributed to the binding affinity of silver ions with various bacterial cell components namely DNA and protein causing injury to the cell resulting in the cytoplasmic outpour [Citation27]. It is also found that the damage incurred by AgNPs in the DNA might influence bacterial metabolic processes such as respiration by combining with oxygen and sulfhydryl (S‒H) groups on the cell wall, which consumes the energy currency ATP, ultimately leading to cell death [Citation28].
Prior to the preliminary antibacterial screening of AgNPs using agar diffusion test, MIC and MBC were performed to validate the antibacterial potential. The recorded MIC values at which no visible growth of test bacterial strains is found are presented in . This range of concentration was selected prior to an initial antimicrobial screening where the inhibitory phenomenon was most prominent. The antimicrobial effect for AgNPs towards a panel of clinical isolates was appreciated by the amount of fluorescent Resorufin produced in proportion to the viable bacterial cell concentrations. Resazurin (7-hydroxy-3H-phenoxazin-3-one 10-oxide) is a blue dye that gets irreversibly reduced to Resorufin producing pink fluorescence by the action of oxidoreductases indicative of viable cells. However, in the non-viable cells, this Resorufin is further reduced to a colourless and a non-fluorescent molecule, hydroresorufin [Citation29]. Among the tested pathogens, S. flexneri, E. coli, P. aeruginosa and E. faecalis were found more sensitive to AgNPs with a MIC value of 3.12 μg/mL followed by S. typhi and B. subtilis with an MIC value of 6.25 μg/mL. Whilst S. aureus, Enterococcus sp., K. pneumoniae and S. epidermidis were found resistant to AgNPs among the tested strains showing a MIC value of 12.5 μg/mL. This difference in MIC values could have resulted due to differences in their metabolic activity. In addition, the tendency of bacteria to form films might limit the accessibility of fluorophores to bacteria within the biofilm preventing the emission of fluorescence signal [Citation30].
Table 3. MIC and MBC measurements of AgNPs treated clinical pathogens.
Subsequently, the MBC values for the respective strains exposed to different concentrations of AgNPs are shown in . This parameter determines the lowest concentration of antimicrobial, in this case, AgNPs that brings about bacterial cell death. The growth pattern of bacterial colonies upon sub-culturing AgNPs treated strains was observed. Among the tested strains S. aureus, Enterococcus sp., K. pneumoniae and S. epidermidis showed an MBC value of 25 μg whereas S. typhi and B. subtilis showed MBC value at 12.5 μg followed by S. flexneri, E. coli, P. aeruginosa and E. faecalis with an MBC value 6.25 μg. This ensured the complete protection of bacteria at higher concentrations namely 50 and 100 μg. The higher potency of AgNPs as mentioned above (MBC @ 6.25 μg) might attribute to a local dosage effect, where these NPs could simultaneously encounter a bacterium in a tiny area [Citation31]. This superior performance of AgNPs was well reflected in MIC and MBC showing a fourfold strengthened bactericidal effect.
3.5. Cytotoxicity assay
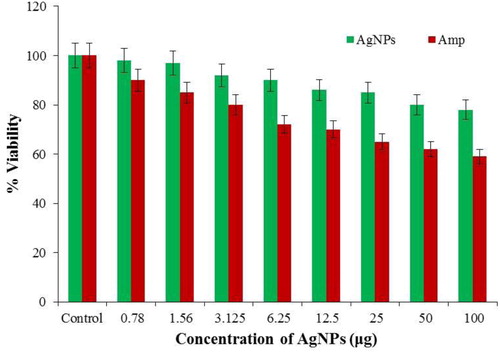
The success of any antibacterial agents for clinical applications depends on the level of toxicity induced to the cells. In this study, cytotoxicity of phyto-fabricated AgNPs was explored against HEPK keratinocytes using MTT assay and from the results, the phyto-fabricated AgNPs as compared against the positive control of TNF-β are found to be non-toxic () to mammalian cells with significant antibacterial activity against clinically important bacteria. A possible explanation could be due to the difference in the composition of the cell membrane of bacteria and human cells. The bacterial cell membrane predominantly consists of peptidoglycan layer comprising as many saccharide units and whose hydrolysis of bonds brings about cell death. As for the mechanism of action is concerned, the antibiotic permeates the bacterial cell wall and inhibits enzymes (transpeptidases and carboxypeptidases) responsible for the synthesis of peptidoglycan layer snuffing out bacterial cell [Citation32].
Figure 9. Comparison of the cytotoxic effects of phyto-fabricated AgNPs and Amp (positive control) against HEPK cell line (Values were expressed as mean ± SD of the experiment performed in triplicate. Statistical significance was considered when p < 0.05).
It was inferred from earlier reports that β-lactam antibiotics mimics d-alanylalanine peptide fragment, an enzyme-substrate that facilitates the binding of penicillin-binding proteins (PBPs). These PBPs are found anchored in the cell membrane and are involved in the cross-linking of the bacterial cell wall. The antibiotic irreversibly binds to the active site of PBP disrupting cell wall synthesis. As there is a complete absence of such membranous architecture and enzyme machinery in human cells, antibiotics could not harm them [Citation33].
4. Conclusion
In conclusion, we indicate a simple, cost-effective and eco-friendly approach towards the synthesis of AgNPs from Calotropis root extract and explored its therapeutic potential. Typical nano characteristics of silver were achieved at optimised laboratory conditions. The therapeutic efficiency of AgNPs against clinical pathogens was found significant as evident from the ZoI (agar diffusion method), MIC and MBC. The mechanism of action of AgNPs towards Gram-positive and Gram-negative strains remained unbiased at a given concentration which varied significantly with that of the antibiotics. Further, the phyto-fabricated AgNPs were found to be not cytotoxic to the HEPK cells even at higher concentrations (100 μg) rather toxic to bacterial strains treated at the same concentration. This could be one such strategy to combat antimicrobial resistance wherein AgNPs could diffuse easily within biofilms and encounter them. Hence, the AgNPs could be a promising candidate to design non-toxic functionalised NPs which can be used in antimicrobial, anticancer, and drug delivery applications.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Suresh Sagadevan
Dr. Suresh Sagadevan is currently working as a Senior Research Fellow in Nanotechnology & Catalysis Research Centre (NANOCAT), University of Malaya, Malaysia. He has authored 12 international book series and 15 book chapters. He has published more than 250 research papers in the National and International journals. He is a member of many professional bodies at the national/international level. He is the editor/ editorial board member/ reviewer for various high impact factor journals. He also has 2 filed patents to his credit.
Selvaraj Vennila
Selvaraj Vennila is working as a Project Fellow in the Department of Nanoscience and Technology, Alagappa University, Karaikudi, Tamil Nadu, India. She has published Ten papers in International Journals. She has also presented papers in various International/National conferences. Her field of research includes the green synthesis of metal and metal oxide nanoparticles for biomedical applications.
Lakshmipathy Muthukrishnan
Dr. Lakshmipathy Muthukrishnan is currently working as a Research Associate at CSIR-Central Leather Research Institute (CSIR-CLRI), Chennai, Tamil Nadu, India. His research interests include viral diagnostics using ELISA, RT-PCR; metal nanoparticle synthesis using microbes and plant products and their application against MDR strains and cancer (nanomedicine); animal cell culture, toxicity studies and microscopy (AFM); leather process technology. He has won several other awards as well for the best student and best oral presentation.
K. Gurunathan
Prof. K. Gurunathan, BOYSCAST(DST, India) Fellow, Brain Pool Scientist (KOFTS, S. Korea), Selected for LEAP program ( NIEPA, New Delhi) and Rashtriya Nirman Rathna Awardee, EGSI, Delhi heading the Dept. for Nanoscience & Technology, Alagappa University, Karaikudi-630 003, INDIA since 2008. He published 120 papers in peer-reviewed journals and presented 250 papers in National/International conferences. He has guided 4 Ph.D. scholars and guiding more than 7 Ph.D. scholars.
Won Chun Oh
Prof. Won-Chun Oh is a Full Professor in the Department of Advanced materials and engineering at Hanseo University in Korea and School of Materials Science and Engineering at Anhui University of Science and Technology in China. And, he is a guest professor in some Universities in China, Thailand, and Indonesia. His current research fields are nanostructured materials such as metal/nanocomposite, graphene materials and metal nanoparticles, and their catalytic applications for future energy sources and green chemical technologies. He is the author or a co-author of 693 papers published in domestic and international journals and speeches on the conference as Special lecture, Plenary lecture, and Keynote lecture speaker.
Suriati Paiman
Dr. Suriati Paiman is currently working as an associate professor at the Physics Department, Faculty of Science, Universiti Putra Malaysia. Her researches are in the area of compound semiconductors nanostructures, nanoelectronics, and condensed matter physics. She is a member of many professional bodies at the national/international level. She has published numerous papers on semiconductors nanowires and has been the reviewer of the Nanotechnology, Journal of Crystal Growth and other peered review journals.
Faruq Mohammad
Dr. Faruq Mohammad, at present working as an Assistant Professor at Surfactant Research Chair, Department of Chemistry, King Saud University, Saudi Arabia. He obtained his Ph.D. from the Department of Environmental Toxicology, Southern University and A&M College, USA during May 2011. So far, he has edited 2 books and published about 70 journal articles, 5 review articles, and 15 book chapters. He is the reviewer for various high impact factor journals.
Hamad A. Al-Lohedan
Prof. Hamad A. Al-Lohedan has more than 37 years of an extremely active and productive career in the field of surfactant, colloids, and interface chemistry. His keen interest in research has rewarded him in the publication of a good and large number of research articles in Journals (about 270) with high impact factors. Currently, he is also the Supervisor of the Surfactant Research Chair and the guide of the colloid and interfaces research group at King Saud University.
Ainil Hawa Jasni
Dr. Is Fatimah currently working as Professor and Head, Department of Chemistry, Universitas Islam Indonesia, Sleman, Yogyakarta, Indonesia. She has published numerous papers in internationally reputed journals. She is a member of many professional bodies at the national/international level. She is the editorial board member/ reviewer for various high impact factor journals. Her field of research includes synthesis and characterization of composite and Nanocomposite for catalysis and photocatalysis.
Is Fatimah
Ainil Hawa Jasni is a Ph.D. student in Biology at National Defence University of Malaysia (NDUM). She received her BSc. (Biotechnology) from Monash University and pursued her MS.C degree in Biology at NDUM. Her research interest includes electrospinning of eco-biomaterials, microbiology, molecular biology, recombinant DNA technology, and enzyme-based Biosensor.
Kuppan Sivaranjan
Dr. Kuppan Sivaranjan is currently working as a Senior Lecturer in the Department of Chemistry, Faculty of Science and Mathematics, Universiti Pendidikan Sultan Idris, Tanjong Malim, Perak, Malaysia. He has published more than Six papers in the internationally reputed journals and one book chapter. His field of research includes catalysis, bimetallic nanoparticles, graphene-based bimetallic nanocomposite for catalytic and bio applications.
Prasanna Kumar Obulapuram
Mr. Prasanna Kumar Obulapuram, at present pursuing his Ph.D. (Pharmaceutics) at the Advanced Drug Delivery Platform Research Unit, Department of Pharmacy and Pharmacology, School of Therapeutic Sciences, University of the Witwatersrand, Johannesburg, South Africa. His research interests are in the development of polymers for biomedicine, tissue engineering, stem cell technologies, Nano-biotechnology, etc.
References
- Morens DM, Fauci AS. Emerging infectious diseases: threats to human health and global stability. PLoS Pathog. 2013;9(7):e1003467.
- Mulani MS, Kamble EE, Kumkar SN, et al. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol. 2019;10:539.
- Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197(8):1079–1081.
- Chen J, Novick RP. Phage-mediated intergeneric transfer of toxin genes. Science. 2009;323(5910):139–141.
- Beyth N, Houri-Haddad Y, Domb A, et al. Alternative antimicrobial approach: nano-antimicrobial materials. Evid-Based Complementary Altern Med. 2015;2015:1–16.
- Akram FE, El-Tayeb T, Abou-Aisha K, et al. A combination of silver nanoparticles and visible blue light enhances the antibacterial efficacy of ineffective antibiotics against methicillin-resistant Staphylococcus aureus (MRSA). Ann Clin Microbiol Antimicrob. 2016;15:48.
- Bairagi SM, Prashant G, Gilhotra R. Pharmacology of natural products: an recent approach on Calotropis gigantea and Calotropis procera. Ars Pharm. 2018;59(1):37–44.
- Rahimi M. Pharmacognostical aspects and pharmacological activities of Calotropis procera. Bull Environ Pharmacol Life Sci. 2015;4(2):156–162.
- Oloumi H. Phytochemistry and ethno-pharmaceutics of Calotropis procera. Ethno-Pharm Prod. 2014;1(2):1–8.
- Rasik AM, Raghubir R, Gupta A, et al. Kulshrestha DK healing potential of Calotropis procera on dermal wounds in Guinea pigs. J Ethnopharmacol. 1999; 68(1–3):261–266.
- Parrotta J. Healing plants of Peninsular India. Wallingford: CAB International, 2001; p. 944.
- Mohamed NH, Ismail MA, Abdel-Mageed WM, et al. Antimicrobial activity of latex silver nanoparticles using Calotropis procera. Asian Pac J Trop Biomed. 2014; 4(11):876–883.
- Trease GE, Evans WC. Pharmacognosy. 11th ed. Bailliere Tindall: Cassell and Collier Macmillan Publishers; 1989.
- Bauer AW, Kirby WM, Sherris JC, Turck, et al. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493–496.
- CLSI. M100-S25 performance standards for antimicrobial susceptibility testing; Twenty-fifth informational supplement; 2015.
- Khalifa RA, Nasser MS, Gomaa AA, et al. Resazurin microtiter assay plate method for detection of susceptibility of multidrug resistant Mycobacterium tuberculosis to second-line anti-tuberculous drugs. Egypt J Chest Dis Tuberc. 2013;62(2):241–247. 05.008.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Met. 1983;65(1–2):55–63.
- Shaker KH, Morsy N, Zinecker H, et al. Secondary metabolites from Calotropis procera (Aiton). Phytochem Lett. 2010;3(4):212–216.
- Shankar SS, Rai A, Ahmad A, et al. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275(2):496–502.
- Ahmad A, Mukherjee P, Senapati S, et al. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B Biointerfaces. 2003;28(4):313–318.
- Terenteva EA, Apyari VV, Dmitrienko SG, et al. Formation of plasmonic silver nanoparticles by flavonoid reduction: A comparative study and application for determination of these Substances. Spectrochim Acta A Mol Biomol Spectrosc. 2015;151:89–95.
- Raveendran P, Fu J, Wallen SL. Completely green synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125(46):13940–13941.
- Sivakumar J, Premkumar C, Santhanam P, et al. Biosynthesis of silver nanoparticles using Calotropis gigantea leaf. African J Basic Appl Sci. 2011;3(6):265–270.
- Raman C. Green synthesis of silver nanoparticles using Calotropis gigantea and their potential mosquito larvicidal property. Int J Pure Appl Zool. 2014;2:128–137.
- Mulvaney P. Surface plasmon spectroscopy of nanosized metal particles. Langmiur. 1996;12(3):788–800.
- McGown DN, Parfitt G, Willis E. Stability of non-aqueous dispersions. I. The relationship between surface potential and stability in hydrocarbon media. J Colloid Sci. 1965;20(7):650–664.
- Yan J, Abdelgawad AM, El-Naggar ME, et al. Antibacterial activity of silver nanoparticles synthesized In-situ by solution spraying onto cellulose. Carbohydr Polym. 2016;147:500–508.
- El-Naggar NE-A, Hussein MH, El-Sawah AA. Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Sci Rep. 2017;7(1):10844.
- Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42(4):321–324.
- Grant SS, Hung DT. Persistent bacterial infections, antibiotic tolerance, and the oxidative stress response. Virulence. 2013;4(4):273–283.
- Zarei M, Jamnejad A, Khajehali E. Antibacterial effect of silver nanoparticles against four foodborne pathogens. Jundishapur J. Microbiol. 2014;7:8720–8723.
- Scheffers DJ, Pinho MG. Bacterial cell wall synthesis: new insights from localization studies. Microbiol Mol Biol Rev. 2005;69(4):585–607.
- Mobley H. How do antibiotics kill bacterial cells but not human cells? Sci Am. 2006;294:98.