?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nowadays, the expansion of nanoparticles as antimicrobial and anticancer agents is one of the most exciting methods for cancer therapy and microbial diseases that can vanquish the problems of drug resistance. In this research, the biosynthesis of silver nanoparticles (AgNPs) was stated using Summer Savory extract. Moreover, these AgNPs by UV-Vis spectroscopy, FTIR, XRD, FESEM, EDX, PSA, and zeta potential analysis were characterized, and by antimicrobial and anti-cancerous performance was evaluated. The maximum absorption peak at 472 nm by UV-Visible spectra confirmed the formation of Ag nanoparticles. The results obtained by FT-IR determined the presence of organic compounds such as amines, alkenes, alcohols, phenols, which performed significant roles in the fixating of these nanoparticles. The results obtained from FESEM, EDX, PSA, and Zeta Potential Analysis revealed that synthesized AgNPs had a hexagonal structure with a size ranging from 10 to 100 nm. The antimicrobial properties of the synthesized AgNPs were examined by Minimum Inhibitory Concentration, Minimum Bactericidal Concentration, and Minimum Fungal Concentration against five microbial strains included Klebsiella pneumonia, Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, and Saccharomyces cerevisiae. The results demonstrated that biosynthesized AgNPs had the most antimicrobial activity, especially against E. Coli. The anti-cancerous results of these nanoparticles through MTT assay demonstrated exceptional anticancer effects against K-562 and MCF-7 human cancer cell lines with IC50 values 50 and 200 µg/ml, respectively.
1. Introduction
Nanotechnology is the manipulation of material on an atomic, molecular, and supra-molecular measurement, and it also is a field of applied sciences and technology that covers a wide range of fields. The main issue is the inhibition of matter or devices less than one micrometer in diameter, usually about 1 to 1000 nanometers [Citation1–3]. There are different ways to produce nanoparticles, such as chemical, physical, and green synthesis methods. The green bioassays are more useful than other methods because of their non-toxicity, higher stability, and more valuable produced nanoparticles [Citation3–5]. Recent amazing progress in Nanotechnology and Nanoscience changed the methods of diagnosing, treating, and preventing various illnesses of humans [Citation6]. AgNPs is one of the most biotic and fascinating nanomaterials among metallic NPs. AgNPs are involved in many medical and therapeutic applications. AgNPs, due to their unique properties, have been remarkably effective in various fields of science, including biology, biomedicine, chemical engineering, environment, and many other fields of science. It also plays a significant role in nanotechnology and nanoscience, particularly in nanomedicine [Citation7–9]. Silver NPs have antimicrobial, antitumor, antioxidant, and anti-inflammatory properties, which have provided extensive open possibilities for the usage of these nanoparticles in biomedicine. [Citation7–10]. A schematic diagram representing various applications of AgNPs has been provided in .
The capability of microorganisms and organic compounds to reduce metal ions and stabilize them into nanoparticles forms the foundation of green synthesis of these nanoparticles. The green synthesis of different types of nanoparticles using a diverse array of plant extracts has been reported [Citation10–12]. One of the most essential herbs in Iran, which is native to different regions of the country, is Satureja sp [Citation13–16]. Satureja is a plant that is widely used in various food products. This herb is a one-year-old herbaceous plant that belongs to the family of mint ().
This plant is resistant to various weather conditions and has many medicinal properties, such as antioxidant, antifungal, antibacterial, and antiseptic properties. Moreover, it is an LDL cholesterol-lowering agent rich in oily and protein substances [Citation16–19]. Interestingly, from the 305 articles searched in the Scopus database between the years 1989 to 2018, 209 papers had focused on the summer savory effects [Citation19]. In this research, considering the mentioned therapeutic properties regarding the Summer Savory plant and the role and importance of AgNPs, the aqueous extract of Summer Savory was applied for the generation of AgNPs. The morphological, chemical and optical properties of synthesized AgNPs were investigated and characterized using a UV-Vis, FT-IR, EDX, XRD, FE-SEM, PSA, and Zeta Potential assays. Also, the Anti-Microbial performance of the green synthesized AgNPs was evaluated by MIC, MBC, and MFC methods against selected bacterial. The anticancerous performance of the synthesized AgNPs was analyzed through MTT colorimetric assay with exposing nanoparticles against K562 and MCF-7 human cancer cell lines.
2. Experimental section
2.1. Collection and investigation of summer savory plant
In this regard, Summer Savory plant was collected from Shiraz and Gachsaran (Southwestern, Iran). The genus of the collected plant was identified and confirmed by comparing it with herbarium specimen existing in the Department of Agriculture, Shiraz University (Iran). Plant samples were placed in sterile plastic bags, and they were brought to the laboratory for further processes.
2.2. Extraction of aqueous extract of SS and fabrication of Ag NP
Some chemical materials, such as the silver nitrate (AgNO3), were purchased from Merck Co (Germany). After washing the aerial parts of SS with deionized water, they were dried at room temperature for two weeks. Afterward, 20 grams of the dried plant of Summer Savory were powdered and poured into a glass beaker. Then, 100 mL of deionized water was added to a pure powdered plant and boiled for 20 min at 70 °C. In the following, the compound was cooled, and the aqueous extract using Whatman filter paper No.1 was filtered and stored at 4 °C in a dark bottle. Herein, for the biosynthesis of AgNPs, 5 mL of the obtained aqueous extract by sterile pipette was poured into a glass jar. Then, 95 ml of an aqueous solution of 1 mM AgNO3 was added. Finally, for future studies, this solution was placed in the room for 24 h at 37 °C.
2.3. Characterization
The formation, morphological and optical structure, particle size, constituent elements and functional group of biosynthesized silver nanoparticles, the nature of synthetic nanoparticles were investigated. The characterization of AgNPs was used using routine analysis such as Uv-Vis spectroscopy (ʎambda35, PERKIN Elmer, Germany), FTIR spectrometer (Bruker model Tensor II, Germany), X-ray diffractometer (XRD-7000, Shimadzu, Japan), FESEM (TESCAN-MIRA3, Czech Republic's), EDX (Tescan model S Max detector MIRA3), PSA (Malvern model MS1002, England) and zeta potential Analyzer (Horiba model SZ- 100, Japan).
2.4. Antibacterial activity
2.4.1. Reference strains and preparation of test samples
In this research, five microbial strains, including four bacterial strains (two g negative and two g positive) and one fungi spice, were used for antimicrobial performance. Microbial Strains were purchase from the Iranian Biological Resources Center (IBRC). five microbial strains included Klebsiella pneumonia ATCC 7881, Escherichia coli ATCC 33876, Enterococcus faecalis ATCC 6057, Staphylococcus aureus ATCC 6538, and Saccharomyces cerevisiae ATCC 9763. For the antimicrobial experiment of synthesized nanoparticles, the stock solution of silver nanoparticles had first to be prepared, and then the required concentrations were prepared. Silver nanoparticles were first dissolved in 5% Dimethyl Sulfoxide (DMSO) solution; thus the stock solution with a concentration of 20 mg L−1 was produced. Finally, different concentrations (7.8 ،15.62 ،31.25 ،62.5 ،125 ،250،500 ،1000 µg mL−1) of this stock solution were provided for antimicrobial testing.
2.4.2. Appraisement of antimicrobial of Ag NPs and summer savory extract
In this section, the antimicrobial activity of Ag NPs and Summer Savory extract was compared and determined against five selected microbial strains using Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC) and Minimum Fungal Concentration (MFC) methods. Herein, the related experiments were done corresponding to the dilution Instructions presented by the Clinical and Laboratory Standard Institute (CLSI) [Citation20]. The lowermost concentration of antimicrobial agents that prevented about 90 percent of the bacteria and fungi growth, respectively, after 24 h incubation was compared with the negative control of dimethyl sulfoxide (DMSO), and were defined as MIC, MBC, and MFC. To experiment antimicrobial of Summer Savory extract, sequential dilutions (7.8 ،15.62 ،31.25 ،62.5 ،125 ،250،500 ،1000 (µg/ml)) of Summer Savory extract were made ready and poured into a sterile 96-well microplate containing 90 microliters of Müller Hinton Broth (MHB) medium. In the following, the chosen microbial strains in the MHB attaining the turbidity of nearly 5 × 108 CFU mL−1 were cultured. In the next part, the microbial suspensions were accumulated for inoculation via diluting them to yield 5 × 106 CFU/mL. Then, 10 µL of each prepared microbial suspension was inseminated into microplates. Finally, after 24 h incubation, the optical density of microplates was measured using a microplate reader (Hyperion, model MPR4 Plus) at a wavelength of 600 nm. However, to investigate the effects of synthesized silver nanoparticles, first serial dilutions of the AgNPs (Similar to Summer Savory Extract) and control groups (Normal saline was used as negative control, and neomycin was considered positive control) were provided in 96-well microplates containing BHI medium. In the following, the bacteria and fungi suspension with the turbidity standard of 0.5 McFarland was added to microplates. Then, these microplates were incubated for 24 h at 37 °C. Finally, the optical density of plates, as stated above, was measured by a microplate reader (Hyperion, model MPR4 Plus) at 600 nm. For both synthesized compounds, this method was repeated three times. For the measurement of MBC and MFC (the lowermost concentration from the composition that leads off to no growth of bacteria and fungi, as MBC and MFC were considered, respectively.) experiment, each five selected microbial strains were cultured 24 h in BHI medium, afterward for each one of microorganisms a stock with a concentration of 105–106 CFU/mL was gathered. Then, 90 μL of diluted concentrations of compositions (7.8 to 1000 μg/mL) was added to a microplate containing 90 μg/ml BHI medium. Afterward, 10 μg/mL of any micro-suspension was added to each cell, and then Microplates were incubated within 24 h at a temperature of 37 °C. In the following, 10 μL of each prepared microbial suspension was added to a recently prepared BHI medium. To assess the microbial activity of each compound, microplates were incubated for 24 h at 37 °C.
2.5. Anticancer performance of silver nanoparticles and summer savory extract
The human cancer cell lines of K562 and MCF-7 were brought from the cancer center of Shiraz medical university, Iran. Various concentrations of green synthesized AgNPs, and Summer Savory extract were applied to study the anticancer performance on these human cell lines via exploring 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) method. 106 cells of K562 and MCF-7 cell lines were grown adequately in RPMI1640 on 96-well microplate and incubated with 2% carbon dioxide for 24 h at 37 °C. The microplates studied the cytotoxic performance of provided materials on the cell lines and were incubated with various concentrations (1, 10, 50, 100, 200, and 500 µg/mL) of AgNPs and Summer Savory extract. After completing the incubation time, micro-plates were entirely washed with phosphate buffer saline (PBS). In the following, micro-plate with MTT solution was treated and again, as in the previous step, it was incubated. Then, to dissolve the crystals formed via MTT solution, the micro-plates were treated with 100 ml of Dimethyl sulfoxide and were put on the rotary shaker so that the color of dissolved crystals could be expanded. The intensity of the color reaction was measured using a microplate reader at a wavelength of 570 nm to study the percentage of survivability of cell lines by the formula presented below.
The mentioned formula was used to determine the IC50 value of cells. In this test, Dimethyl Sulfoxide and was applied as control samples.
2.6. Statistical analysis
In this study, data analysis was performed using SPSS IBM computer software. To evaluate the results of biological experiments, the data were displayed in each group as mean ± standard deviation, and the significant difference between the mean groups was analyzed by One-Way Analysis of Variance (ANOVA), and then Tukey test was applied. In all tests, an average of 3 measurements was used for each group. The significant level in all statistical tests was considered less than 0.05 (p < 0.05). Excel software (2010) was also used to prepare the graphs.
3. Results and discussion
3.1. Characterization of synthesized nanoparticles
3.1.1. Visual observation and UV-Vis analysis
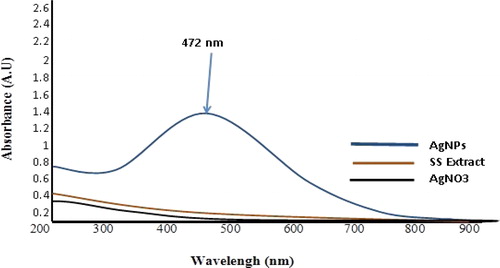
Metal ions such as Silver, Iron, Copper, and Gold due to the property of Surface Plasmon Resonance (SPR) of the optical properties, are unique [Citation21]. In this section, the formation of AgNPs was evaluated by a color change and UV-Vis assay. The change in reaction color from light yellow to dark brown after the addition of AgNO3 to the plant extract indicated the formation of AgNPs (). The generation of these nanoparticles happened due to the presence of active molecules in the Summer Savory plant extract, which reduced the Ag metal ions present in the reaction from Ag+ to Ag0. Several articles have reported this color change due to SPR stimulation [Citation15, Citation22,Citation23]. A characteristic peak for AgNPs using UV-Visible Spectrophotometric analysis was obtained at around λ 472 nm. The obtained spectrum, according to numerous papers and lectures, was related to the formation of AgNPs. According to these documents, the formation of the spectrum of AgNPs has been reported in the wavelength range between 400 to 500 nm [Citation15, Citation24].
3.1.2. Fourier transform infrared (FT-IR) spectroscopy
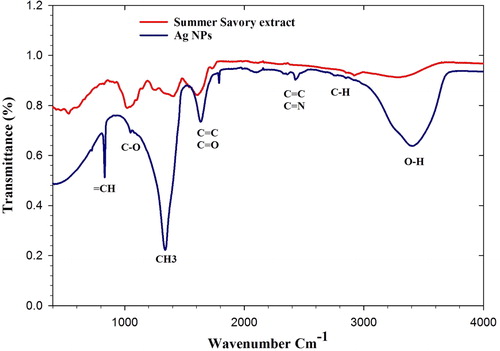
The purpose of the FT-IR experiment is the recognition of the possible functional groups of bio-molecules that are involved in capping, effective stabilization, and reduction of recently synthesized AgNPs [Citation15, Citation25]. FT-IR analysis of AgNPs was shown in and . In this figure, several distinct absorption peaks can be seen in the3405.88, 2427.61, 2359.46, 2100.99, 1788.81, 1635.08, 1338.55, 1048.96, and 832.54 cm−1areas. The peaks at 3405.88 cm−1 in the synthesized samples were related to the O-H stretching of phenols/alcohols [Citation15, Citation25] and N-H group of amide A [Citation15, Citation26]. The peak at 2427.61 cm−1 was related to the C-H group of Alkanes [Citation15, Citation25,Citation26]. The existing peaks at 2359.46 cm−1 to 2100.99 cm−1 were related to C≡N, and C≡C stretches in Aromatic/Aliphatic compounds [Citation25–28]. The peaks at 1788.81 cm−1 regions of 1635.08 cm−1 were related to the C = C stretch of Aromatic compositions [Citation29]. Another document has determined that these peaks are related to Amid I (C = O stretch) of proteins [Citation27]. The existing peak at 1338.55 cm−1 indicated the CH3 group of Sugars (carbohydrates) [Citation30]. The existing peak at 1048.96 cm−1 regions was related to the CO group of in-plane bending of carboxylic acids, alcohols, esters, ethers, and alkanes [Citation15]. Eventually, the existing peak at 832.54 cm−1 area was related to = CH stretch of Aromatic bi-cyclic monoterpenes, according to previous reports [Citation15, Citation31]. FT-IR Spectroscopy results have demonstrated the presence of various biomolecules that play a major role in the stabilizing of Ag NPs.
Table 1. FT-IR analysis of synthesized AgNPs.
3.1.3. X-Ray Diffraction (XRD) spectrometer
The crystalline structure of synthesized silver nanoparticles was confirmed using the XRD method. The results obtained from XRD assay for these nanoparticles were shown in and . Obtained results were demonstrated by the Hexagonal structure of AgNPs, showing that Ag was facing centered cubic (fcc). The X-ray patterns revealed that silver nanoparticles had different peaks at 2θ of about 29.448, 35.679, 38.395, 48.702, 55.649, 60.231, and 61.105 that could be attributed to the 221, 042, 111, 243, 003,030 and 442 orientations, respectively. These orientations were matched to the fcc phase of Ag0 ions. The formation of these peaks was due to the organic compounds present in the summer savory plant extract, which were responsible for reducing Ag ions and fixation of synthesized nanoparticles [Citation32]. These results were compared with synthesized AgNPs using various plant extracts, reported by other researchers reported a similar crystalline structure for AgNPs [Citation10, Citation33,Citation34].
Figure 5. XRD Analysis of synthesized Ag NPs by Summer Savory extract for determination of Ag crystals.
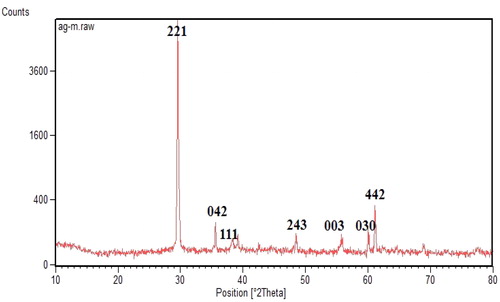
Table 2. Output data of XRD assay from synthesized AgNPs using summer savory.
The average crystalline size of the AgNPs was measured by EquationEquation (1)(1)
(1) (Debye-Scherrer equation):
(1)
(1)
In this Equation, D is the average crystalline size of synthesized nanoparticles, k is a geometric factor (0.9), λ is the wavelength of the X-Ray radiation source and β is the angular FWHM (full-width at half maximum) of the XRD peak at the diffraction angle θ [Citation35]. In this research, the average crystallite size of the biosynthesized silver nanoparticles was 69.2 nm using Summer Savory extract.
3.1.4. Field emission scanning electron microscope (FESEM)
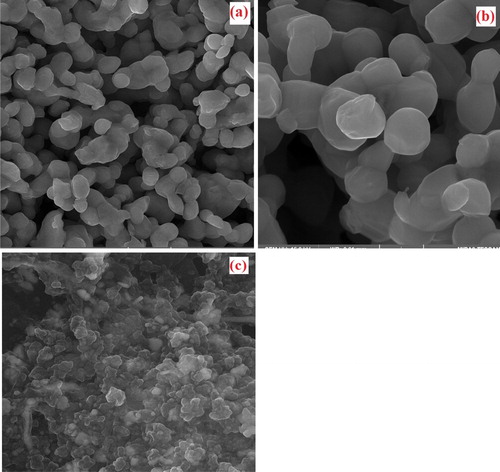
The microstructural and morphological analysis of synthesized silver nanoparticles was carried out by FE-SEM experiments. FE-SEM image from Ag nanoparticles is shown in . As observed from the figure, all particles were shown hexagonal and spherical crystal structure, and the diameter of AgNPs was in the range of 10 − 100 nm. The aggregation of Ag NPs was observed in FE-SEM Images, which could be due to the evaporation and removal of solvent used during sample preparation. Solvent removal causes electrostatic forces to bring Ag NPs closer together and aggregated [Citation36]. This factor has probably led to variations in the size of synthesized silver nanoparticles. According to the results obtained from the past researches, the silver nanoparticles were derived from different plant extracts, depending on the chemical composition of the plant extract and the concentration, and pH of the media can have various forms such as hexagonal, spherical and triangular [Citation15, Citation36–38].
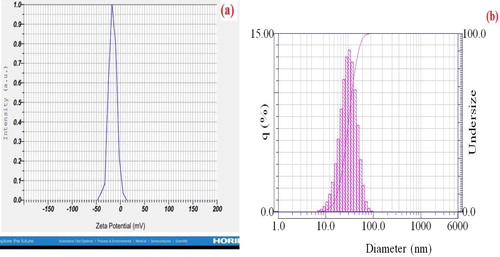
3.1.5. Particle size analyzer and zeta potential (PSA and Zeta)
The amount of size and the potential size of the synthesized silver nanoparticles by Summer savory extract were calculated using the PSA and Zeta method (). According to the obtained diagrams, the size of silver nanoparticles using PSA assay was in the range of 70 nm, and the zeta potential of these particles was about −15.8 mV. The negative potential value supports properties including good colloidal nature, long term stability, and high dispersity of green synthesized Ag nanoparticles [Citation39–41].
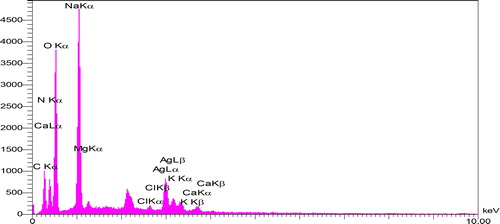
3.1.6. EDX spectroscopy
The purpose of the EDX experiment was to identify quantitive and qualitative elements in the reaction that may be involved in the synthesis of AgNPs. The EDX spectra from Ag NPs () also revealed and confirmed the establishment of Ag NPs. According to the results obtained from past researches, AgNPs due to their SRP has shown a typic optical absorption peak approximately at 3 keV [Citation21, Citation42]. EDX quantification of synthesized AgNPs () provided atomic percentages of 18.93% C, 42.42% O, 21.64% N, 7.70% Ag, 8.60% Na, 0.04% Cl, 0.11% Ca, 0.42% Mg and 0.14% K. According to the table, it can be stated that the presence of Silver and Oxygen elements in the composition indicated the formation of AgNPs, and also, the synthesized nanoparticles were in the form of silver oxide, Ag2O [Citation43,Citation44]. Also, the presence of carbon and oxygen elements in the composition can be related to the presence of polyphenolic compounds or any carbon or oxygenated composition found in the Summer Savory extract [Citation45]. The presence of nitrogen in the compound can be explained how that this ion most likely results from the decomposition of excess AgNO3 in a composition released during the reaction and synthesis of AgNPs. Other elements found in the composition, such as sodium, potassium, magnesium, and chlorine, are elements of the Summer Savory extract that play a critical role in enzymatic activities of reaction such as neutralizing the organic anions in the plant extract and stabilizing the pH between 7 and 8. In the meantime, the role of potassium and sodium is more important than other elements [Citation46]. Therefore, this is one of the major benefits of using plant extract to synthesized nanoparticles than using chemicals.
Table 3. Outcome of EDAX analysis for Ag NPs with Summer savory.
3.2. Antimicrobial performance of synthesized compounds
Silver nanoparticles, due to their high antimicrobial properties, are one of the most important metal nanoparticles in the treatment of microbial diseases. Today, the use of this nanoparticle has received much attention. It has a wide range of nanotechnology researches, especially in the field of medical sciences and against various microbial species. In this section, the antimicrobial performance of Summer Savory extract and bio-synthesized silver nanoparticles were evaluated against selected pathogenic microorganisms, including two G- (Escherichia coli and Klebsiella pneumonia) and two G+ (Staphylococcus aureus and Enterococcus faecalis) bacteria and one fungus spices (Saccharomyces cerevisiae). In and , the outcome of MIC, MBC and MFC evaluations for an aquatic extract of Summer Savory and green synthesized Ag NPs against diverse kinds of bacteria and fungi specie can be seen. In this regard, at high concentrations (500 to 1000 μgmL−1), all of the desired compounds had significant antimicrobial effects against selected microbial and fungi strains. Based on the obtained data, the aquatic extract of Summer Savory showed approximately the same MIC against all tested samples at a concentration of 250 μgmL−1. However, the MBC and MFC varied in the range of 250 to 500 μgmL−1. On the other, in the case of synthesized Ag NPs, different outcomes were obtained, where lower MIC, MBC, and MFC values were achieved compared with the aquatic extract of summer savory. Developed Ag NPs showed the highest MIC/MBC at a concentration of 62.5 µgmL−1 for gram-negative E.Coli bacterium. Concerning the mechanism of the antimicrobial effect of nanoparticles, although there is no comprehensive theory yet, different hypotheses have been proposed by researchers. One theory is that these nanoparticles attached to the outer surface of the microbial membrane, disrupt the permeability and cellular respiration of the microbial membrane and ultimately cause bacterial cell death [Citation3, Citation21, Citation40, Citation47]. Another theory mentioned that the size of NPs is effective in bacterial death. Proponents of this theory believed that the smaller size of the synthesized nanoparticles would cause more significant antimicrobial activity [Citation3, Citation21, Citation40, Citation48,Citation49]. Generally, it was observed that fabricated AgNPs through green protocol had a more potent inhibitory and lethal effect on the studied microorganisms than the aqueous extract of Summer Savory, which can be an effective antibiotic against pathogenic microorganisms.
Figure 9. Antibacterial and Antifungal performance of an aqueous extract of Summer Savory and green synthesized AgNPs derived from Summer Savory against (a) E.Coli, (b) Klebsiella pneumonia, (c) Enterococcus faecalis, (d) Staphylococcus aureus and (e) Saccharomyces cerevisiae, at diverse concentrations. Data are statistically meaningful at the p < 0.05 level.
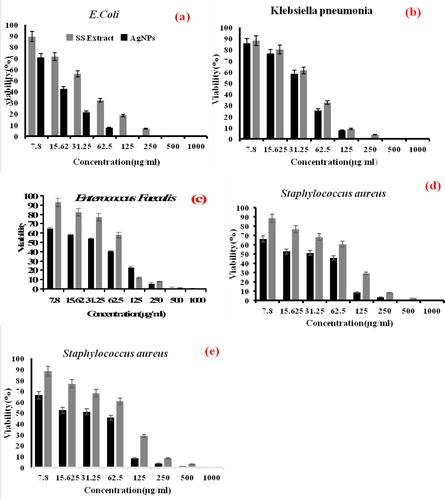
Table 4. Calculated MIC and MBC/MFC values of Summer Savory extract and green synthesized AgNPs.
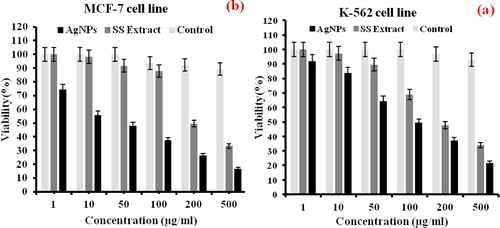
3.3. Cytotoxic effect of Ag NPs
Widespread cancer is one of the main reasons for mortality worldwide, which requires practical tumor-targeting strategies to minimize the death rate. Nowadays, the use of silver nanoparticles in the diagnosis and treatment of cancer-related diseases has attracted physicians, pharmacists, and researchers in these fields [Citation50,Citation51]. However, several articles have evaluated the anticancer effects of Satureja Species extract [Citation52–55]. Nevertheless, there was no article or report on the effect of synthesized silver nanoparticles by Summer Savory on selected cancer cell lines. In this research, the anticancer performance of Summer Savory extract and AgNPs was evaluated with the MCF-7 and K-562 human cancer cell lines by MTT experiment. As shown in , at high concentrations (200 and 500 μgmL−1), both compounds had a high cytotoxic effect against the target cell groups compared to the control sample. However, with decreasing concentration, the Anti-cancer effect of the substances has also decreased. Thus, at concentrations below 200 μgmL−1, the anticancer properties of Summer Savory extract on both cells have been significantly reduced. However, for synthesized silver nanoparticles, the antioxidant levels or cell survivability (IC50) were 50 and 100 μgmL−1, respectively, for the MCF-7 and K-562 human cancer cells. So, it can be deduced that synthesized silver nanoparticles had an anticancer effect on evaluated cell lines, and this cytotoxic effect on MCF-7 cancer cell was very significant, which highlighted the potential of developed nanoparticles as capable anticancer drugs. The results of this research were compared with previous researches on Satureja family plants. The results showed a potential anticancer effect of Summer Savory compared to other plants [Citation56,Citation57]. Many efforts have been made to identify the mechanism of the effect of AgNPs on different cancer cell lines. One of the most influential theories widely accepted has attributed the death of cancer cells, the potential production of free radicals by AgNPs, which prevents cell aggregation and, ultimately, cell death [Citation58–63]. The result of a study by Rahman et al. (2009) [Citation64] showed that the interaction between AgNPs and cancer cells could generate electrons that can lead to the production of ROS and, ultimately, cell death. Also, modification of AgNPs via green protocol could promote anticancer features of nanoparticles through a culmination of harmful radicals, which can attract protein and cause oxidative stress. These oxidative stress wills have partial or permanent damages on the functionality of proteins [Citation58, Citation65]. In another theory, the effects of nanoparticles on cellular protein function have been considered stating that AgNPs regulate the activity of DNA-dependent kinase enzymes involved in cellular damage [Citation65].
4. Conclusion
In this study, we tried to introduce a simple method, and in the shortest possible time to produce silver nanoparticles use the particular plant of summer Savory, which has received less attention. Concerns about the synthesis of AgNPs using chemical and physical methods such as the use of precursor chemicals and toxic solvents and generation of toxic using products have led to a new alternative approach, green synthesis. This eco-friendly technique incorporates the use of biological agents, plants or microbial agents as reducing and capping agents. Ag NPs synthesized using green chemistry suggest a novel and potential alternative to chemically and physically synthesized nanoparticles. Silver nanoparticles have antimicrobial, antitumor, antioxidant, and anti-inflammatory properties, which have provided extensive open possibilities for the usage of these nanoparticles in biomedicine. In previous studies, only one or two bacteria have been work, but in this presentation we have increased the range of antimicrobial functions and evaluated both bacterial and fungal. Eventually, its anticancer function has been reported to be complete. In this research, the green biosynthesis of AgNPs was investigated using Summer Savory extract. The synthesized AgNPs demonstrated a hexagonal structure with a size ranging from 10 to 100 nm, as observed by FESEM, PSA, Zeta Potential, and XRD assay. The possible functional groups from bio-molecules of Summer Savory extract that involved in capping, active stabilization, and bio-reduction of AgNPs, were detected using the FT-IR method. The EDX analysis determined the Ag, C, O, Na, and N elements as the main compositions of the present synthesized nanoparticles. The synthesized silver nanoparticles have shown antibacterial, antifungal, and anticancer activities against selected microorganisms, and two cell lines established their application in biomedicine. Therefore, it can be concluded that the green synthesis of AgNPs by Summer Savory extract was a relatively simple, completely practical, and also bio-environmental method. These nanoparticles not only could eliminate the disadvantages of chemical drugs, but also could be commercialized for mass production if their antimicrobial and anticancer effects on animal samples are examined.
Acknowledgments
This research was conducted in Fars Science and Technology Park, Shiraz. The author appreciates all the executives of this collection, especially the researchers of Borjobaro Fars Company. A sincere thanks to Dr. Alireza Keikhosravi and Dr. Ayoob Khosravi for their proofreading of this paper.
References
- Sriramulu M, Sumathi S. Fungal based synthesis of silver nanoparticles and their antimicrobial activity. Int J Chem Tech Res. 2017;10(1):367–377.
- Moore MN. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int. 2006;32(8):967–976.
- Majeed S, Danish M, Binti-Zahrudin AH, et al. Biosynthesis and characterization of silver nanoparticles from fungal species and its antibacterial and anticancer effect. Karbala Int J Mod Sci. 2018;4(1):86–92.
- Chahardoli A, Karimi N, Fattahi A. Biosynthesis, characterization, antimicrobial and cytotoxic effects of silver nanoparticles using Nigella arvensis seed extract. Iran J Pharm Res. 2017;16(3):1167–1175.
- Sudhasree S, Shakila Banu A, Brindha P, et al. Synthesis of nickel nanoparticles by chemical and green route and their comparison for biological effect and toxicity. Toxicol Environ Chem. 2014;96(5):743–754.
- Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications, and toxicities. REVIEW. Arabian J Chem. 2019;12(7):908–931.
- Vetchinkina E, Loshchinina E, Kupryashina M, et al. Green synthesis of nanoparticles with extracellular and intracellular extracts of basidiomycetes. PeerJ. 2018;6:e5237.
- Alarcon EI, Griffth M, Udekwu KI. Silver nanoparticle applications in the fabrication and design of medical and biosensing devices. New York: Springer; 2015.
- Wei L, Lu J, Xu H, et al. Silver nanoparticles: synthesis, properties, and therapeutic applications. Drug Discov Today. 2015;20(5):595–601.
- Zhang XF, Liu ZG, Shen W, et al. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. IJMS. 2016;17(9):1534.
- Marslin G, Siram K, Maqbool Q, et al. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials. 2018;11(6):940.
- Lee SH, Jun BH. Silver nanoparticles: Synthesis and application for nanomedicine. IJMS. 2019;20(4):865.
- Karimi N, Yari M, Ghasmpour HR. Identification, and comparison of essential oil composition and mineral changes in different phenological stages of Satureja hortensis L. Iranian J Plant Physiol. 2012;3(1):577–582.
- Valizadeh S, Fakheri T, Mahmoudi R, et al. Evaluation of antioxidant, antibacterial, and antifungal properties of Satureja hortensis essential oil. Biotech Health Sci. 2014;1(3):e24733.
- Rasaee I, Ghannadnia M, Baghshahi S. Biosynthesis of silver nanoparticles using leaf extract of Satureja hortensis treated with NaCl and its antibacterial properties. Microporous Mesoporous Mater. 2018;264:240–247.
- Mohammadhosseini M, Beiranvand M. Chemical composition of the essential oil from the aerial parts of Satureja hortensis as a potent medical plant using traditional hydrodistillation. J Chem Health Risks. 2012;3(4):43–54.
- Hazrati H, Saharkhiz MJ, Niakousari M, et al. Natural herbicide activity of Satureja hortensis L. essential oil nanoemulsion on the seed germination and morphophysiological features of two important weed species. Ecotoxicol Environ Saf. 2017;142:423–430.
- Nikaein F, Zibaeenezhad MJ, Babajafari SS, et al. The effects of Satureja hortensis L. dried leaves on serum sugar lipid profiles, hs-CRP, and blood pressure in metabolic syndrome patients: A double-blind randomized clinical trial. Iran Red Crescent Med J. 2017;19(1):e34931.
- Fierascu I, Dinu-Pirvu CE, Velescu BS, et al. Phytochemical profile and biological activities of Satureja hortensis L. A review of the last decade. Molecules. 2018;23(10):2458.
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard. 20th edition, M07-A10. Wayne, PA: CLSI; 2012. 68.
- Haytham Ibrahim MM. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J Radiat Res Appl Sci. 2015;8(3):265–275.
- Malinsky MD, Kelly KL, Schatz GC, et al. Chain length dependence and sensing capabilities of the localized surface plasmon resonance of silver nanoparticles chemically modified with alkanethiol self-assembled monolayers. J Am Chem Soc. 2001;123(7):1471–1482.
- Fayaz AM, Balaji KP, Kalaichelvan, Venkatesan R. Fungal based synthesis of silver nanoparticles—an effect of temperature on the size of particles. Colloids Surf B Biointerf. 2009;74:123–126.
- Pourmortazavi SM, Taghdiri M, Makari V, et al. Procedure optimization for green synthesis of silver nanoparticles by aqueous extract of Eucalyptus oleosa. Spectrochim Acta Mol Biomol Spectrosc. 2015;136:1249–1254.
- Jerković I, Giovanni Tuberoso CI, Kranjac M, et al. Characterization of Summer Savory (Satureja hortensis L.) honey by physico-chemical parameters and chromatographic/spectroscopic techniques (GC-FID/MS, HPLC-DAD, UV/VIS, and FTIR-ATR). Croat Chem Acta. 2015;88(1):15–22.
- Vanaja M, Gnanajobitha G, Paulkumar K, et al. Photosynthesis of silver nanoparticles by Cissus quadrangularis influence of physicochemical factors. J Nanostruct Chem. 2013;3:1–8.
- Suresh S, Karthikeyan S, Jayamoorthy K. FTIR, and multivariate analysis to study the effect of bulk and nano copper oxide on peanut plant leaves. J Sci Adv Metab Disord. 2016;1:343–350.
- Rajasekar P, Priyadharshini S, Rajarajeshwari T, et al. Bio-inspired synthesis of silver nanoparticles using Andrographis paniculata whole plant extract and their antimicrobial activity over pathogenic microbes. Int J Res Biomed Biotech. 2013;3:47–52.
- Raghunandan D, Bedre MD, Basavaraja S, et al. Rapid biosynthesis of irregularly shaped gold nanoparticles from macerated aqueous extracellular dried clove buds (Syzygium aromaticum) solution. Colloids Surf B Biointerf. 2010;79(1):235–240.
- Leopold LF, Leopold N, Diehl HA, et al. Quantification of carbohydrates in fruit juices using FTIR spectroscopy and multivariate analysis. J Spectrosc. 2011;26(2):93–104.
- Pavia DL, Lampman GM, Kriz GS, et al. Introduction to Spectroscopy, Cengage Learning. 4th Edition. 2008. ISBN-13: 978-0495114789.
- Roopan SM, Rohit Madhumitha G, Rahuman AA, et al. Low-cost and eco-friendly photosynthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crops Prod. 2013;43:631–635.
- Sedaghat S. Green biosynthesis of silver functionalized multi-walled carbon nanotubes, using Satureja hortensis L water extract and its bactericidal activity. J Nanoanalysis. 2017;4(1):59–64.
- Prakash P, Gnanaprakasam P, Emmanuel R, et al. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates . Colloids Surf B Biointerfaces. 2013;108:255–259.
- Dubey SP, Lahtinen M, Sillanpaa M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010;45(7):1065–1071.
- Prabu HJ, Johnson I. Plant-mediated biosynthesis and characterization of silver nanoparticles by leaf extracts of Tragia involucrata, Cymbopogocitronella, Solanum verbascifolium and Tylophora ovata. Karbala Int J Mod Sci. 2015;1(4):237–246.
- Chandran SP, Chaudhary M, Pasricha R, et al. Synthesis of gold nano triangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006;22(2):577–583.
- Aromal SA, Vidhu V, Philip D. Green synthesis of well-dispersed gold nanoparticles using Macrotyloma uniflorum. Spectrochim, Acta, Part A: Mol Biomol Spectrosc. 2012;85(1):99–104.
- Yardily A, Sunitha N. Green synthesis of iron nanoparticles using hibiscus leaf extract, characterization, antimicrobial activity. Int J Scientific Res Rev. 2019;8(7):32–46.
- Jyoti K, Baunthiyal M, Singh A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. Leaves and their synergistic effects with antibiotics. J Radiat Res Appl Sci. 2016;9(3):217–227.
- Mukherjee S, Chowdhury D, Kotcherlakota R, et al. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics. 2014;4(3):316–335.
- Magudapathy P, Gangopadhyay P, Panigrahi BK, et al. Electrical transport studies of Ag nanoparticles embedded in a glass matrix. Physica B. 2001;299(1–2):142–146.
- Da’na E, Taha A, Afkar E. Green synthesis of iron nanoparticles by Acacia nilotica pods extract and its catalytic adsorption, and antibacterial activities. Appl Sci. 2018;8(10):1922.
- Devatha CP, Kumar A, Katte SY. Green synthesis of iron nanoparticles using different leaf extracts for the treatment of domestic wastewater. J Clean Prod. 2016;139:1425–1435.
- Shahwan T, Abu Sirriah S, Nairat M, et al. Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem Eng J. 2011;172(1):258–266.
- Kanagasubbulakshmi S, Kadirvelu K. Green synthesis of iron oxide nanoparticles using Lagenaria siceraria and evaluation of its antimicrobial activity. Def Life Sci Jl. 2017;2(4):422–427.
- Mudasir AD, Ingle A, Rai M. Enhanced antimicrobial activity of silver nanoparticles synthesized by Cryphonectria sp. evaluated singly and in combination with antibiotics. J Nanomed. 2013;9(1):105–110.
- Sriram MI, Kanth SM, Kalishwaralal K, et al. Antitumor activity of silver nanoparticles in Dalton's lymphoma ascites tumor model. Int J Nanomed. 2010; 5:753–762.
- Mousavi SM, Hashemi SA, Ghasemi Y, et al. Green synthesis of silver nanoparticles toward bio and medical applications: a review study. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S855–S872.
- Thompson EA, Graham E, MacNeill CM, et al. Differential response of MCF7, MDA-MB-231, and MCF 10A cells to hyperthermia, silver nanoparticles, and silver nanoparticle-induced photothermal therapy. Int J Hyperthermia. 2014;30(5):312–323.
- Ranjitham AM, Suja R, Caroling G, et al. In vitro evaluation of antioxidant, antimicrobial, anticancer activities and characterization of Brassica oleracea. Var. Bortrytis. L synthesized silver nanoparticles. Int J Pharm Sci. 2013;5:239–251.
- Asadipour M, Amirghofran Z. Satureja hortensis induces cell death and inhibited cell cycle progression in K562 myelogenous and Jurkat T cell leukemia cell lines. J Immunoassay Immunochem. 2019;40(5):459–472.
- Cetojevic-Simin DD, Bogdanovic GM, Cvetkovic DD, et al. Antiproliferative and antimicrobial activity of traditional Kombucha and Satureja montana L. Kombucha. J Buon. 2008;13(3):395–401.
- Esmaeilbeig M, Kouhpayeh SA, Amirghofran Z. An investigation of the growth inhibitory capacity of several medicinal plants from Iran on tumor cell lines. Iran J Cancer Preven. 2015;8(5):e4032.
- Sharifi-Rad J, Sharifi-Rad M, Hoseini-Alfatemi SM, et al. Composition, cytotoxic and antimicrobial activities of Satureja intermedia C.A. Mey Essential Oil. IJMS. 2015;16(8):17812–17825.
- Narchin F, Larijani K, Rustaiyan A, et al. Phytochemical synthesis of silver nanoparticles by two techniques using Saturaja rechengri Jamzad Extract: Identifying and comparing in vitro anti-proliferative activities. Adv Pharm Bull. 2018;8(2):235–244.
- Erci F, Cakir-Koc R, Isildak I. Green synthesis of silver nanoparticles using Thymbra spicata L. var. spicata (zahter) aqueous leaf extract and evaluation of their morphology-dependent antibacterial and cytotoxic activity, Artificial Cells. Artif Cells Nanomed Biotechnol. 2018;46(sup1):150–158.
- Hamouda RA, Hussein MH, Abo-Elmagd RA, et al. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci Rep. 2019;9(1):13071.
- Xia T, Kovochich M, Brant J, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6(8):1794–1807.
- Ott M, Gogvadze V, Orrenius S, et al. Mitochondria, oxidative stress, and cell death. Apoptosis. 2007;12(5):913–922.
- Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009; 32(1):79–84.
- Martins D, Frungillo L, Anazzetti MC, et al. Antitumoral activity of L-ascorbic acid-poly-D, L-(lactide-coglycolide) nanoparticles containing violacein. Int J Nanomed. 2010;5:77–85.
- Venkatesan B, Subramanian V, Tumala A, et al. Rapid synthesis of biocompatible silver nanoparticles using aqueous extract of Rosa damascena petals and evaluation of their anticancer activity. Asian Pac J Trop Med. 2014;7:294–300.
- Rahman MF, Wang J, Patterson TA, et al. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol Lett. 2009;187(1):15–21.
- Dos Santos CA, Seckler MM, Ingle AP, et al. Silver nanoparticles: therapeutical uses, toxicity, and safety issues. J Pharm Sci. 2014;103(7):1931–1944.