Abstract
Ischemic stroke results in severe disabilities due to extensive cellular loss and the resulting impairment of brain functions. Current methods for regenerating brain tissue are ineffective. Stroke treatment requires innovative therapeutic techniques that are both safe and effective. For neuronal repair, a promising alternative is using a hydrogel-based tissue engineering technique that delivers neurotrophic cytokines and cells to injured sites. However, the limited encapsulation effectiveness, less in vivo cell survival ratio and cytokine loss make this strategy difficult to implement. We aim to design a biomaterial that can efficiently construct a matrix enriching the survival of cells and minimizing loss in vivo cytokines to overcome these constraints. We report the development of genipin conjugated sericin hydrogels (Gen-SH) with a high porous morphology and a moderate swelling rate utilizing sericin, a natural silk protein. In vitro, Gen-SH aids in the attachment and development of neurons. Our results indicate that sericin is inherently neuroprotective and neurotrophic, branching and publicizing axon extension and avoiding hypoxia-induced cell death in primary neurons. Notably, the breakdown products of Gen-SH inherit these capabilities, saving the expense of cytokines. Furthermore, we show that the Lkb1–Nuak1 pathway is required for this neurotrophic impact, whereas the Bcl-2/Bax protein ratio is necessary for the neuroprotective effect. Transplanted in vivo, Gen-SH has a high percentage of cell survival and promotes cell proliferation. Taking all this information into account, it's clear that Gen-SH can serve as a viable carrier for treatment and care for ischemic stroke healing, both in terms of delivering neuronal cells and protecting them from oxidative damage.
1. Introduction
Stroke is the world's fifth-most prominent cause of mortality, behind heart disease, cancer and respiratory diseases [Citation1–3]. Nearly 75 to 80% of all strokes are ischemic, which occurs when blood flow to the brain is disrupted, depriving neurons of oxygen and glucose, resulting in cell death and behavioural impairment [Citation4–7]. Ischemic stroke treatments are few despite their relevance. Intravenous tissue-type plasminogen activator (t-PA) revolutionized the management of acute ischemic stroke more than two decades ago, providing a new therapeutic intervention for a devastating neurological disorder [Citation8–10]. However, the short treatment window can only assist a few patients because of the quick treatment window. Other therapies are essentially supportive, such as breathing, blood pressure management, reduction of cerebral oedema and infection prevention. Stroke-induced damage to brain tissue still cannot be reversed by any currently available therapy [Citation11–14]. Approaches such as tissue engineering have been suggested as a viable option. It is usual to use hydrogels with various neuroprotective agents and stem cells to restore damaged neural tissue [Citation15–17]. However, the low in vivo cell survival, limited encapsulation effectiveness and a considerable decrease in the activity of neurotrophic factors make this technique difficult to implement [Citation18]. Identifying a biomaterial with inherent neurotrophic activity while also being appropriate for the fabrication of a hydrogel that improves in vivo cell survival may be a method to address these disadvantages [Citation19–21].
Hyaluronan/methylcellulose hydrogel, alginate, PLA/PLGA and matrigel are synthetic natural polymers materials studied for their potential use in repairing neural tissue [Citation15]. Though they may appear to be neuroprotective, these substances are neurotoxic and have their own set of drawbacks [Citation22]. While the HAMC and alginate hydrogels are ineffective in adhering to cells, the degraded PLA/PLGA products modify host tissue pH [Citation23–25]. The origin of matrigel, derived from the tumour cell basement membrane [Citation26–31], is relevant to its potential use in medicine [Citation32]. As a result, a biopolymer with intrinsic neurotrophic activity must also be natural degradation, and cell-adhesive properties must be neural biocompatible without causing adverse side effects on neural redevelopment [Citation33].
Fibrin and sericin are the two main protein components of silkworm silk. Compared to fibroin, sericin has recently been researched in regenerative medicine and tissue engineering [Citation34–36]. Sericin behaves like an amorphous material with a 63% random coil structure [Citation26]. As a natural protein, sericin exhibits attractive properties, including good biocompatibility, mild immunogenicity [Citation37], biodegradability [Citation38], promoting cell adhesion [Citation39] and proliferation activity [Citation28]. Sericin itself can form hydrogels, films and sponges, representing an exciting biomaterial for tissue engineering and regenerative medicine [Citation31]. Nevertheless, the major challenge in the biomedical applications of sericin is its amorphous structure and the resulting poor mechanical performance [Citation1, Citation40–42]. The mechanical properties of sericin materials could not meet the application requirement. Sericin membrane is brittle after air drying and easily solubilized in normal saline [Citation32]. Thus, it is necessary to improve sericin's mechanical performance and stability to expand its application in biomedical materials [Citation43]. In addition to its significant number of hydroxyl, carboxyl and amino groups, sericin contains 17–18 amino acids [Citation44]. Serine and Glycine, which are pioneers of chlorophyll II and tryptophan ethanolamine, are prevalent among the breakdown metabolites of sericin [Citation45–47]. It is hypothesized that sericin and its degradation products may have neuron-favourable activity because of their diverse biological activities, such as antioxidation and antibacterial properties, and their ability to promote cell migration and differentiation [Citation48]. Previously, we discovered that sericin is inherently cell adhesive and can be conjugated by glutaraldehyde to construct a facile injectable hydrogel, which provides a foundation for advanced assessment of whether a sericin injectable hydrogel might provide a carrier for neural tissues development [Citation49].
Cross-linked sericin hydrogels have been synthesized using genipin, a biocompatible cross-linker (Gen-SH). Scientists have looked at the Gen-SH mechanical and physical characteristics. In vitro studies have examined the Gen-SH compatibility with primary cortical neurons. We have uncovered sericin’s intrinsic neurotrophic function and the underlying molecular mechanisms [Citation50]. This work encountered the sericin protein neuroprotective impacts and Gen-SH breakdown results in an in vitro model mimicking treatment and care of stroke injury. This compound's ability to transfer in vivo B35 neuronal cells and enhance cell survival ratio has been studied. Gen-SH with inherent neurotrophic and neuroprotective properties is an efficient cell delivery system for in vivo brain tissue repair.
2. Materials and methods
2.1. Fabrication of Gen-SH and degraded products of the Gen-SH
The cocoon silks spun by the domestic silkworm Bombyx mori have been used in textiles for thousands of years (at least 5000 years). Silk has long been a vital textile stock due to its unique feel, luster, dyeability, tensile strength and elasticity, good thermal stability, hygroscopic nature and microbial resistance. For almost two decades, the regenerated liquid silk fibroin (SF) solution has been extensively applied to the research and development of various forms of biomaterials, such as drug/enzyme delivery systems, regenerated fibres, artificial skin, porous matrix or 3D scaffolds, biomimetic nanofibrous scaffolds, and a platform for transistors, and various classes of photonic devices, due to its biocompatibility. Extraction of the sericin protein from the 140-cocoon silk (Department of Neurology, Wenling First People's Hospital, Wenling-317500, China) using the LiBr (6 M LiBr aqueous solution (55 mL) extraction procedure described earlier [Citation51–53]. Afterwards, the mixture was centrifuged to remove any remaining sericin solution and for another 30 min at room temperature to create the Gen-SH. After a range of sericin-to-genipin ratios and reaction periods were tested, the 6:1 sericin:genipin ratio and the 30 min cross-linking reaction time were identified [Citation54–56].
2.2. Characterizations and mechanical behaviour
SEM-(JSM-7800F) was employed to detect the microscopic morphology of the hydrogels after lyophilization. FTIR spectra were recorded on PerkinElmer Spectrum Two Pharmaceutical System. Hydrogels' mechanical properties were measured using a Discovery Hybrid Rheometer 3 (TA, Instruments, New Castle, DE). Before the measurement, the hydrogel precursor solutions were mixed with a volume of 280 μL and applied to a temperature control stage at 37 °C.
2.3. Swelling behavior of the Gen-SH
The freshly fabricated injectable hydrogels were measured (Wf), consuming an electronic weighing balance to determine the generation swelling ratio (TA, DHR-2). In the oven, these hydrogels were then dried. Their dry weight (Wf′) was measured. The newly generated hydrogels were balanced for 24 hrs at 37 °C in 50 mL of PBS to calculate the equilibrium swelling ratio. We weighed (We) and dried (We′) these swollen hydrogels to get their dry weights. The equations can determine the swelling ratio of hydrogel during formation (f-swelling) and equilibrium (e-swelling).
2.4. In vitro degradation of the Gen-SH
A PBS solution heated to 37 °C was used to soak the hydrogels, formed in moulds 5 mm in width and 10.2 mm in size. Every day, a new show on PBS aired. Throughout the experiment, the hydrogels were removed and reweighed several times. We divided the original dry mass by the difference in subsequent dry groups determined at the specified dates to determine deterioration.
Sericin and genipin solutions were combined in a 50 mL tube to produce the Gen-SH deteriorated products. This was done to acquire the Gen-SH degraded products. PBS (45 mL) was mixed into the glass tube after gelling for 30 min. Shaking the tube at 200 rpm, the temperature was maintained at 37 °C. Later, after four weeks of fermentation, the samples were filtered and collected by filter (0.22-µm). The BCA assay was used as earlier reported to determine the new Gen-SH degradation product concentrations. A culture medium was used to alter the concentration of the new Gen-SH degradation products.
2.5. CCK-8 assay and live and dead staining
Ninety-six-well plates with Gen-SH prepared at the bottom of each well were used, and the cells growing in them were washed three times. CCK-8 solution was combined with DMEM, then incubated for three hrs at 37 °C in the incubator. The plates were then washed and used to determine the absorbance at 450 nm (Spark multimode microplate reader, Switzerland). The live and dead staining was carried out (Biovision, CA, USA). A 96-well plate was used to seed primary neurons [Citation57–61]. Fluorescence microscopy was used immediately after 24 or 48 hrs of treatment, and the cells were stained for 15 min at 37 °C (Olympus IX71, Japan). Dojindo Japan's CCK-8 test was conducted [Citation62–64].
2.6. Long-term B35 cell growth on the Gen-SH
Gen-SH was placed in the bottom of the wells, and B35 cells were plated at a density (1 × 105 cells/well) on 24-well plates and 96-well plates for the 20 days RPMI-1640 medium with 2% FBS. A compound microscope was used to view the cells in the 24-well plates at various periods (Keyence, Itasca, IL). The B35 cells in the 96-well plate were treated to the CCK-8 experiment designated above to get the growth curvature.
2.7. Cytoskeleton F-actin staining
Sterilized Gen-SH seed primary neurons on a 35 mm glass culture plate. Cells were grown for 48 hrs before being fixed for 15 min in 4% paraformaldehyde. 4′,6-diamidino-2-phenylindole (DAPI), and Phalloidin-Rhodamine stained F-actin and cell nuclei, respectively. A confocal laser microscope was used to see the labelled cells (Nikon A1Si, Japan).
2.8. Oxygen glucose deprivation
The OGD method was utilized in the manner described earlier. A glucose-free bicarbonate buffer was used to cultivate isolated primary mouse cortical neurons for 1 h at 37 °C/4% CO2 and 96% N2. The OGD- neurons treated were incubated for 24 hrs in the specified solution. To perform the CCK-8 test and the live and dead assay, the cells were collected and put into a 96-well plate. As previously mentioned, a commercially available kit (Jiancheng, Nanjing, China) was used to detect the release of lactate dehydrogenase (LDH).
2.9. Analysis of branching of primary neurons and axon extension
The fetal mouse brain was used to separate and cultivate primary neurons. After isolated primary neurons were plated at a density of 1 × 102 cells/mm2 onto a 24-well plate for 4–6 h, the sericin solution (0.05 mg/mL) was added to the wells along with the neurobasal medium described above. The neurons were cultured in the neurobasal medium containing sericin (0.05 mg/mL) for 4 days.
The neurons in the 24-well plate were imaged at different time points. Images were taken of the neurons in the 48-well plates at various time intervals. Individual neuron axon length and axon branch counts were measured with ImageJ and performed by hand. Neurons were placed in 24-well plates at a density (1 × 104 cells/well), and WZ4003 was introduced to block Nuak1 activity 4–6 hrs after being seeded. The neurobasal media was replenished after 24 hrs of WZ4003 incubation. On day 4, the length and branching of axons were measured.
2.10. Western blot and RT-PCR
Trizol, ThermoScript Real Time-PCR System was used for RT-PCR (Invitrogen, CA, USA). Primer Premier 5.0 was used to create the primers that were ultimately used. As previously mentioned, Western blot analyses were carried out. This test reagent measures the amount of protein in cell lysates (Pierce, USA). After SDS-PAGE was done, it transferred the samples to nitrocellulose membranes (Amersham, Piscataway, NJ, USA). Anti-actin, anti-Bcl-2 and anti-Bax antibodies were used to block and incubate the membranes (Santa Cruz Technology, CA, USA). A Luminescent Image Analyzer was used to examine the blots.
2.11. B35 cells generation
An enzyme called SalI/XhoI was used to insert the GFP gene into the pFB-Neovector. PFB-NeoGFP was co-transfected into the 293 T cell lines to see how it affects GFP expression. The retrovirus-infected supernatant was collected. The retrovirus was inoculated into B35 cells for 48 hrs. It took four weeks to grow the monoclonal cells in the presence of G418 added in the medium.
2.12. Assessment of cell survival in the Gen-SH
The hydrogels were seeded with GFP-expressing B35 cells at a density (1 × 104 cells/each well). They were placed in the lower abdomen of nude mice after 24 hrs of growth in hydrogels containing B35 cells. The hydrogels were extracted from the dead mice on days 1, 2, 3, 5, 7 or 10 and analyzed. For cell collection, one-half of each separated hydrogel sample was digested in 0.25% trypsin solution and used for imaging (Keyence, Itasca, IL). The GFP-positive cells in the cell suspension were resuspended in PBS and counted. PI staining was used to test the vitality of GFP-positive cells. The cells were immediately examined using a Calibur FACS flow cytometer (SYSMEX, Japan).
2.13. Statistical analysis
All experiments were performed at least three times independently, and the results were presented as mean ± standard deviation (SD). Comparisons between any two groups were performed using a two-tailed unpaired Student’s t-test.
3. Results and discussion
3.1. Fabrication method and characterizations of a genipin cross-linked hydrogel (Gen-SH)
The sericin protein from the 140-cocoon silk using the chemically mild LiBr approaches previously reported to maintain sericin's integrity and biological activity [Citation65–67]. The natural cross-linker genipin was used to construct the cross-linked sericin hydrogel (). The SEM image reveals the clear morphology of the hydrogel (). Fourier transform infrared spectroscopy was used to study the Gen-secondary SH's structure (FTIR). The amide absorption bands in the infrared spectrum were typical of proteins and polypeptides. It was common to employ the amide I and II bands for protein secondary structure analysis. As for amide, the β-sheet absorption is around 1630 cm−1, the α-helix is roughly 1655 cm−1 and the random coil is approximately 1645 cm−1. Cross-linked sericin hydrogel showed an amide peak of 1648 cm−1 compared to 1645 cm−1 for pure sericin powder (). The conjugated sericin hydrogel showed a peak of 1530 cm−1 for the amide group, derived from the -NH bending the and -CN stretching vibration, comparable to the pure sericin sample (), and the findings indicate that conjugation does not have a substantial mechanical impact [Citation68].
Figure 1. The cross-linking method for constructing a genipin conjugated by sericin hydrogels (Gen-SH).
Figure 2. Physicochemical characterizations of a genipin conjugated by sericin hydrogels (Gen-SH). (A) FT-IR spectra of Gen-SH and sericin. (B) The Gen-SH image presents the porous structure of a Gen-SH examined by SEM. Scale bar 50 µm.
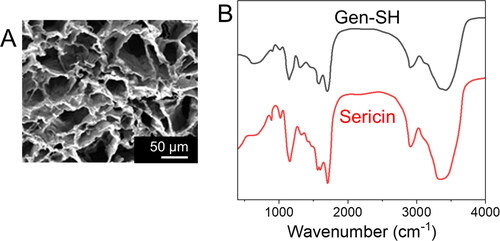
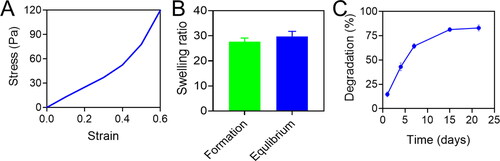
Compared to brain tissue (11–23 kPa), the modulus compressive of the Gen-SH was 1.034 ± 0.032 kPa, which may facilitate the hydrogel's integration into the surrounding brain tissue. Next, we tested the Gen-ability SH's to swell. To demonstrate the hydrogel's modest capacity to expand, the ratio of swelling during hydrogel formation (25.9 ± 1.7; was like the rate of swelling during equilibrium (29.1 ± 2.0). This minimal swelling is ideal for in-vivo transplantation since it reduces extrusion damage [Citation69]. A closer look at the Gen-microstructure SHs revealed that it had a very porous structure (), which would make it easier for cells growing on the Gen-SH to exchange oxygen, nutrients and growth factors with the surroundings. Gen-SH was destroyed after four weeks in PBS at 37 °C, according to in vitro degradation study ().
3.2. Effectively attach neuronal cells and growth on the Gen-SH
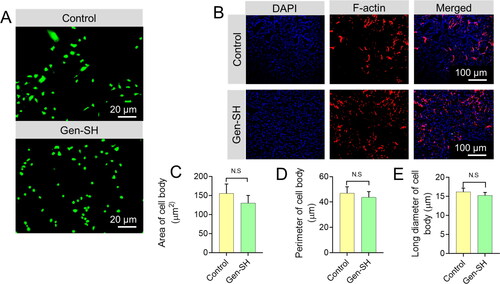
Next, we examined how primary cortical neurons adhered to the Gen-SH and how their morphology changed over time. Neuron protein, a particular marker for neurons, was used to determine the purity of the separated primary neurons, which reached 90% after six days of culture (). The primary neurons attached to the Gen-SH surface are much like the growth dish's control cells (). Primary neurons planted onto the Gen-SH exhibited the same cell spreading area, length and breadth as neurons developing on cell culture plates, according to confocal microscopy examination of F-actin in the cytoskeleton ().
Figure 4. (A) The representative images demonstrate the morphology of primary cortical neurons planted on a Gen-SH after 2 days. (B) The representative confocal images showing the phalloidin-rhodamine-staining for DAPI staining and F-actin Scale bar 20 μm. (C–E) Quantify the cell body area, perimeter and long diameter. N.S. Non-significant.
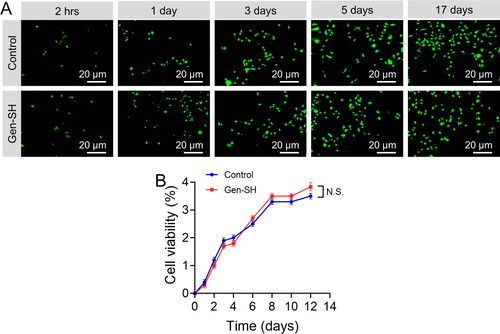
A biomaterial's ability to support long-term cell development is critical and essential. A neuron B35 cells were utilized to examine in vitro proliferation and long-term cell survival ratio in the Gen-SH. B35 cells adhered effectively to this Gen-SH, as was shown with primary neurons. For almost 17 days, Gen-SH aided inefficient cell development (). This experiment, which compares cell viability to controls on a culture plate, confirmed that cells grown on the Gen-SH had vitality equivalent to controls (). As a result, our findings show that Gen-SH aids in cell proliferation and long-term viability in culture.
3.3. Biocompatibility
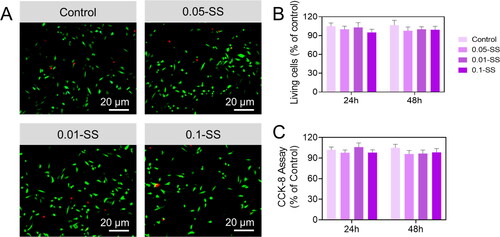
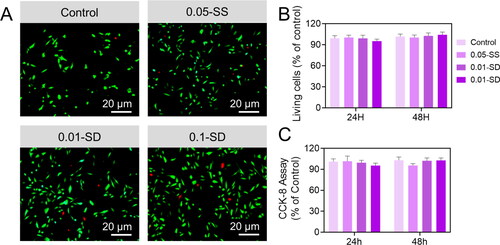
In vivo transplantation, cells interact with molecules derived from the Gen-breakdown SH products. As part of our investigation into Gen-SH biocompatibility, we investigated the characteristics of sericin protein and degraded Gen-SH derivatives on primary neurons (). At various doses (0.01 mg/mL, 0.05 mg/mL and 0.10 mg/mL), the sericin protein or Gen-SH degradation products were applied to the fetal primary cortical neurons. C shows that the cell viability of cells treatment with either the sericin or the Gen-SH degradation derivatives was unaffected by either treatment. Based on these findings, the sericin hydrogel, which includes the sericin and the Gen-SH degradation derivatives, may be ideal for in vivo neural tissue repair.
3.4. Oxygen glucose deprivation (OGD)
Using an OGD setting typically utilized in vitro to mimic significant pathological abnormalities occurring during an ischemic stroke, we then tested the results of sericin protein. We degraded Gen-SH derivatives on primary mouse neurons. Compared to OGD PBS controls, the cell viability in the primary neurons treated with high concentrations of sericin solution (0.05 mg/mL or 0.10 mg/mL) was enhanced by 34.12% and 25.1%, respectively. However, treated cells with a minimum dosage of sericin protein (0.01 mg/mL) exhibited OGD-induced cell death at a ratio comparable to OGD control. To confirm the sericin protein's protective benefits, the study examined the discharge of lactate dehydrogenase (LDH). Compared to the OGD controls, sericin dosages of 0.05 mg/mL and 0.10 mg/mL significantly decreased neurons to LDH release by 45.2% and 47.2%, respectively, according to cell viability data (). We conducted the same tests with the Gen-SH deteriorated products to see if they had the same protective effects on neurons as the Gen-SH degraded products. OGD damage resulted in less cell death and LDH release when these products were employed at 0.01–0.10 mg/mL ().
Figure 8. Neuroprotective properties of the sericin oxygen-glucose deprivation (OGD) injury. (A) The neurons were treated with PBS, BSA and the sericin solution (SS) Live and Dead staining. (B) Quantifications of the ratio of live cells. (C) CCK-8 analysis. (D) LDH release.
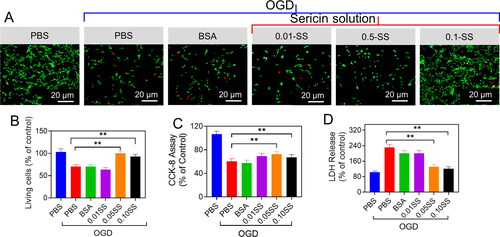
Figure 9. Neuroprotective properties of the sericin oxygen-glucose deprivation (OGD) injury. (A) The neurons treated with PBS, BSA and the Gen-SH degraded derivatives (SD) Live and Dead staining. (B) Quantifications of the ratio of live cells. (C) CCK-8 analysis. (D) LDH release. (E and F) Respective Bax/Bcl-2 mRNA level.
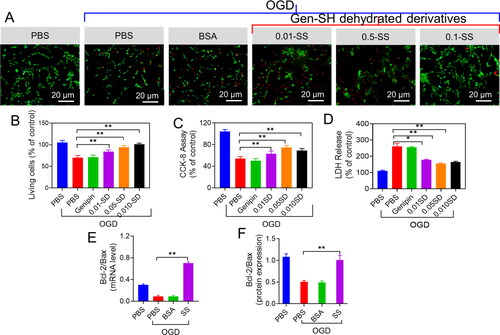
Because apoptosis is a significant factor in OGD-induced cell death, we investigated if sericin protein may increase cell viability by antagonizing apoptosis. After 24 hrs of OGD exposure, we measured Bcl-2 and Bax. For example, the Bcl-2 to Bax ratio in the treated cells with sericin protein was roughly 17-folds more significant than in the treated cells with BSA and PBS, two commonly used indicators of apoptosis (). Sericin administration resulted in a 74% increase in the Bcl-2 to Bax protein ratio 24 hrs after treatment compared to PBS or BSA treatment (). Sericin and Gen-SH degradation products have been shown to help primary neurons survive and maintain their cellular integrity in the absence of oxygen or glucose, suggesting that Gen-SH may also provide neuronal protection during brain tissue healing by suppressing cell death.
3.5. Primary neurons branching and axon extension of primary neurons
Axons, a specialized membrane prolongation from neuron bodies, grow and branch to provide a crucial anatomical substrate for successful neural regeneration. Sericin protein was used to treat primary cortical neurons obtained from mice brains to examine if it impacted neuron morphology. This therapy (0.05 mg/mL) extended the length of neurons by 38% and increased the number of branches per cell by roughly 70% (). This was achieved by increasing the concentration of sericin in the axons from 103.8 to 144.0 µm ().
Figure 10. (A) Percentage of axon branches and length of the neurons. (B and C) The mRNA genes levels of Lkb1 and Nuak1 in the primary neurons 48 hrs after the revealed treatments. (D) The axon length of the primary neurons’ treatments with WZ4003 or DMSO.
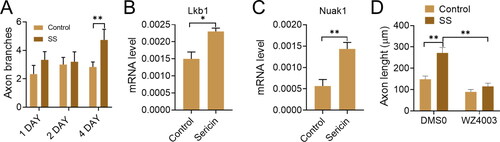
We next determined the molecular mechanism of how sericin promotes axon extension and branching. This pathway regulates the development and branching of the terminal axon. Hence it was decided to explore whether sericin affected the amounts of primary neurons Nuak1 and Lkb1 mRNA. According to the mRNA levels of Nuak1 and Lkb1 after sericin therapy, the sericin therapy may enhance Nuak1 and Lkb1 activity by improving their transcription, as shown in C. A Nuak1 specific inhibitor (WZ4003) was used to verify the sericin promotes axon effect via the Nuak1 and Lkb1 kinase pathways. The use of WZ4003 virtually eliminated axon extension augmentation by sericin (). These findings show that sericin activates the Nuak1 and Lkb1 kinase pathways, promoting axons outgrowth.
3.6. Assessment of neuronal cell survival
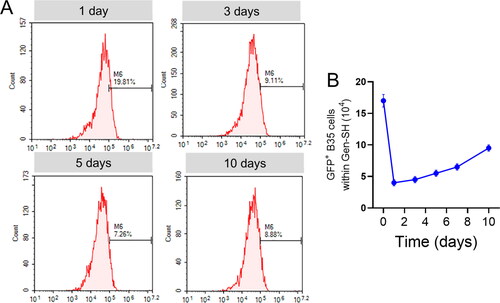
Hydrogels' capacity to transport foreign cells is critical since mature neurons are nonrenewable, and endogenous neuronal stem cells are limited in their ability to heal damaged tissue. To evaluate the Gen-ability SH's to transfer cells in vivo, we created GFP-expressing neuronal B35 cells (). Because primary neurons are resistant to plasmid transfection, we could not produce GFP-expressing neurons. It was then subcutaneously injected into the lesser abdomen hollow of the mice with the help of a GFP-expressing B35 cell line (). Even after 10 days, the positive GFP- in the Gen-SH in vivo transplanted cells were alive by flow cytometry analysis in the vast majority (88–94%) (). According to the results of this study, around 25% of the GFP cells supplied through directly injected after 24 hrs transplantation, and just 6% of cells left viable in reported trials were still alive. In addition, the number of viable cell lines in the Gen-SH grew during the incubation period, more than doubling transplantation after 10 days, implying that the transplant cells may multiply in vivo inside the Gen-SH environment ().
4. Conclusion
We effectively reported the development of genipin conjugated sericin hydrogels (Gen-SH) with a high porous morphology and a moderate swelling rate utilizing sericin, a natural silk protein. In vitro, Gen-SH aids in the attachment and development of neurons. Our results suggest that sericin is inherently neuroprotective and neurotrophic, branching and publicizing axon extension and avoiding hypoxia-induced cell death in primary neurons. Remarkably, the breakdown products of Gen-SH inherit these capabilities, saving the expense of cytokines. Furthermore, we exhibit that the Lkb1-Nuak1 pathway is required for this neurotrophic impact, whereas the Bcl-2/Bax protein ratio is necessary for the neuroprotective effect. Transplanted in vivo, Gen-SH has a high percentage of cell survival and promotes cell proliferation. The ability to endorse in vivo proliferation and long-term cell survival and the Gen-SH designed physicochemical characteristics and bioactivities emanating from sericin protein, and hydrogel derivates contribute to the Gen-SH potency and suitability for the treatment and care for ischemic stroke rehabilitation.
Disclosure statement
The authors declare no competing financial interest.
References
- Sharma R, Kwon S. New applications of nanoparticles in cardiovascular imaging. J Exp Nanosci. 2007;2(1-2):115–126..
- Huang Y, Zhu C, Xie R, et al. Green synthesis of nickel nanoparticles using fumaria officinalis as a novel chemotherapeutic drug for the treatment of ovarian cancer. J Exp Nanosci. 2021;16(1):368–381..
- Traversa E, Mecheri B, Mandoli C, et al. Tuning hierarchical architecture of 3D polymeric scaffolds for cardiac tissue engineering. J Exp Nanosci. 2008;3(2):97–110..
- Liu Z, Ran Y, Xi J, et al. Polymeric hybrid aerogels and their biomedical applications. Soft Matter. 2020;16(40):9160–9175.
- Hu D, Yang Y. Tennis rehabilitation training-assisted paclitaxel nanoparticles in treatment of lung tumor. J Chem. 2020;2020:4916726.
- González-Nieto D, Fernández-Serra R, Pérez-Rigueiro J, et al. Biomaterials to neuroprotect the stroke brain: a large opportunity for narrow time windows. Cells. 2020;9(5):1074.
- Ghasemi S, Alavian K, Alavian F. Nanoparticle-based gene therapy intervention for stroke treatment: a systematic review. Curr Gene Ther. 2020;20(5):373–382.
- Obermeyer JM, Ho E, Gracias A, et al. Influencing neuroplasticity in stroke treatment with advanced biomaterials-based approaches. Adv Drug Deliv Rev. 2019;148:204–218.
- Olaru D-G, Olaru A, Kassem GH, et al. Toxicity and health impact of nanoparticles. Basic biology and clinical perspective. Rom J Morphol Embryol. 2019;60:787–792.
- Zhang L, Xu M, Zhu M, et al. Study on the therapeutic and nursing effect of thrombus targeted thrombolysis mediated by recombinant plasminogen activator modified nanoparticles on stroke patients. Mat Express. 2021;11(7):1024–1030.
- Bernardo-Castro S, Albino I, Barrera-Sandoval ÁM, et al. Therapeutic nanoparticles for the different phases of ischemic stroke. Life. 2021;11(6):482.
- Kakar S, Smorenburg J. Intranasal administration of chitosan-nanoparticles conjugated with imipramine and its effect on stroke-induced secondary neurodegeneration: a research protocol, undergrad. URNCST J. 2021;5(10):1–7.
- Chen L, Gao X. The application of nanoparticles for neuroprotection in acute ischemic stroke. Ther Deliv. 2017;8(10):915–928.
- Li H, Yang Z, Tang Q, et al. Embolic stroke model with magnetic nanoparticles. ACS Appl Mater Interfaces. 2021;13(37):43993–44001.
- Dong X, Gao J, Su Y, et al. Nanomedicine for ischemic stroke. Int J Mol Sci. 2020;21(20):7600.
- Poellmann MJ, Bu J, Hong S. Would antioxidant-loaded nanoparticles present an effective treatment for ischemic stroke? Nanomedicine (Lond). 2018;13(18):2327–2340.
- Zhao Y, Li D, Zhu Z, et al. Improved neuroprotective effects of gallic acid-loaded chitosan nanoparticles against ischemic stroke. Rejuvenation Res. 2020;23(4):284–292.
- Bao Q, Hu P, Xu Y, et al. Simultaneous blood-brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano. 2018;12(7):6794–6805.
- Jeong JH, Kang SH, Kim DK, et al. Protective effect of cholic acid-coated poly lactic-co-glycolic acid (PLGA) nanoparticles loaded with erythropoietin on experimental stroke. J Nanosci Nanotechnol. 2019;19(10):6524–6533.
- Lv W, Xu J, Wang X, et al. Bioengineered boronic ester modified dextran polymer nanoparticles as reactive oxygen species responsive nanocarrier for ischemic stroke treatment. ACS Nano. 2018;12(6):5417–5426.
- Deng G, Ma C, Zhao H, et al. Anti-edema and antioxidant combination therapy for ischemic stroke via glyburide-loaded betulinic acid nanoparticles. Theranostics. 2019;9(23):6991–7002.
- Nucci MP, Filgueiras IS, Ferreira JM, et al. Stem cell homing, tracking and therapeutic efficiency evaluation for stroke treatment using nanoparticles: a systematic review. World J Stem Cells. 2020;12(5):381–405.
- Amani H, Habibey R, Shokri F, et al. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci Rep. 2019;9:1–15.
- Huang L, Wang J, Huang S, et al. Polyacrylic acid-coated nanoparticles loaded with recombinant tissue plasminogen activator for the treatment of mice with ischemic stroke. Biochem Biophys Res Commun. 2019;516(2):565–570.
- Nayak S, Shet VB, Rao CV, et al. Performance evaluation and emission characteristics of a 4 stroke diesel engine using green synthesized silver nanoparticles blended biodiesel. Mater Today Proc. 2018;5(2):7889–7897.
- Qiang G, Lee I. Kinetics of swelling/shrinking rearrangement of a self-aggregated nanohydrogel at solid/liquid interfaces. J Exp Nanosci. 2011;6(5):509–520..
- Arizaga A, Ibarz G, Piñol R, et al. Encapsulation of magnetic nanoparticles in a pH-sensitive poly(4-vinyl pyridine) polymer: a step forward to a multi-responsive system. J Exp Nanosci. 2014;9(6):561–569..
- Wang S, Zhang C, Chang Q. Synthesis of magnetic crosslinked starch-graft-poly(acrylamide)-co-sodium xanthate and its application in removing heavy metal ions. J Exp Nanosci. 2017;12(1):270–284..
- Goswami S, Bajpai J, Bajpai AK. Calcium alginate nanocarriers as possible vehicles for oral delivery of insulin. J Exp Nanosci. 2014;9(4):337–356..
- Jamshidi P, Ma P, Khosrowyar K, et al. Tailoring gel modulus using dispersed nanocrystalline hydroxyapatite. J Exp Nanosci. 2012;7(6):652–661..
- Park KM, Choi JH, Bae JW, et al. Nano-aggregates using thermosensitive chitosan copolymers as a nanocarrier for protein delivery. J Exp Nanosci. 2009;4(3):269–275..
- Blanco S, Peralta S, Morales ME, et al. Hyaluronate nanoparticles as a delivery system to carry neuroglobin to the brain after stroke. Pharmaceutics. 2020;12(1):40.
- Zamanlu M, Eskandani M, Barar J, et al. Enhanced thrombolysis using tissue plasminogen activator (tPA)-loaded PEGylated PLGA nanoparticles for ischemic stroke. J Drug Deliv Sci Technol. 2019;53:101165.
- Hu X, Zhou X, Li Y, et al. Application of stem cells and chitosan in the repair of spinal cord injury. Int J Dev Neurosci. 2019;76:80–85.
- Mohebbi S, Nezhad MN, Zarrintaj P, et al. Chitosan in biomedical engineering: a critical review. Curr Stem Cell Res Ther. 2019;14(2):93–116.
- Lin PH, Dong Q, Chew SY. Injectable hydrogels in stroke and spinal cord injury treatment: a review on hydrogel materials, cell–matrix interactions and glial involvement. Mater Adv. 2021;2(8):2561–2583.
- Zhang Y, Shi N, He L, et al. Silk sericin activates mild immune response and increases antibody production. J Biomed Nanotechnol. 2021;17(12):2433–2443.
- Nishida A, Yamada M, Kanazawa T, et al. Sustained-release of protein from biodegradable sericin film, gel and sponge. Int J Pharm. 2011;407(1-2):44–52.
- Arango MC, Montoya Y, Peresin MS, et al. Silk sericin as a biomaterial for tissue engineering: a review. Int J Polym Mater Polym Biomater. 2021;70(16):1115–1129.
- Li T, Yan G, Bai Y, et al. Papain bioinspired gold nanoparticles augmented the anticancer potency of 5-FU against lung cancer. J Exp Nanosci. 2020;15(1):109–128..
- Pourabadeh A, Mirjalili M, Shahvazian M. Modification of silk fibroin nanofibers scaffold by PAMAM dendrimer for cell culture. J Exp Nanosci. 2020;15(1):297–306..
- Noorisafa F, Razmjou A, Emami N, et al. Surface modification of polyurethane via creating a biocompatible superhydrophilic nanostructured layer: role of surface chemistry and structure. J Exp Nanosci. 2016;11(14):1087–1109..
- Sonamuthu J, Cai Y, Liu H, et al. MMP-9 responsive dipeptide-tempted natural protein hydrogel-based wound dressings for accelerated healing action of infected diabetic wound. Int J Biol Macromol. 2020;153:1058–1069..
- Dai W, Yang Y, Yang Y, et al. Material advancement in tissue-engineered nerve conduit. Nanotechnol Rev. 2021;10(1):488–503.
- Hasanpour A, Esmaeili F, Hosseini H, et al. Use of mPEG-PLGA nanoparticles to improve bioactivity and hemocompatibility of streptokinase: in-vitro and in-vivo studies. Mater Sci Eng C Mater Biol Appl. 2021;118:111427.
- Suryawanshi R, Kanoujia J, Parashar P, et al. Sericin: a versatile protein biopolymer with therapeutic significance. Curr Pharm Des. 2020;26(42):5414–5429.
- Zhang Q, Fan A, Fu J, et al. Precise engineering of iron oxide nanoparticle-encapsulated protein hydrogel: implications for cardiac toxicity and ultrasound contrast agents. Process Biochem. 2021;102:296–303.
- Luo L, Zang G, Liu B, et al. Bioengineering CXCR4-overexpressing cell membrane functionalized ROS-responsive nanotherapeutics for targeting cerebral ischemia-reperfusion injury. Theranostics. 2021;11(16):8043–8056.
- Wang Z, Zhang Y, Zhang J, et al. Exploring natural silk protein sericin for regenerative medicine: an injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci Rep. 2014;4:1–11.
- Sung H, Huang R, Huang LLH, et al. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J Biomed Mater Res. 1998;42(4):560–567.
- Li X, Wang L, Xiao G, et al. Adhesive tape-assisted etching of silk fibroin film with LiBr aqueous solution for microfluidic devices. Mater Sci Eng C Mater Biol Appl. 2021;118:111543..
- Sashina ES, Bochek AM, Novoselov NP, et al. Structure and solubility of natural silk fibroin. Russ J Appl Chem. 2006;79(6):869–876..
- Sohn S, Strey HH, Gido SP. Phase behavior and hydration of silk fibroin. Biomacromolecules. 2004;5(3):751–757..
- Dong P, Wang H, Xing S, et al. Fluorescent magnetic iron oxide nanoparticle encapsulated protein hydrogel against doxorubicin-associated cardiotoxicity and for enhanced cardiomyocyte survival. J Biomed Nanotechnol. 2020;16(6):922–930.
- Bari E, Perteghella S, Faragò S, et al. Association of silk sericin and platelet lysate: premises for the formulation of wound healing active medications. Int J Biol Macromol. 2018;119:37–47.
- Kalantari K, Mostafavi E, Afifi AM, et al. Wound dressings functionalized with silver nanoparticles: promises and pitfalls. Nanoscale. 2020;12(4):2268–2291..
- Kalaiarasi G, Subarkhan MM, Fathima Safwana CK, et al. New organoruthenium(II) complexes containing N, X-donor (X = O, S) heterocyclic chelators: synthesis, spectral characterization, in vitro cytotoxicity and apoptosis investigation. Inorg Chim Acta. 2022;535:120863..
- Mohamed Kasim MS, Sundar S, Rengan R. Synthesis and structure of new binuclear ruthenium(II) arene benzil bis(benzoylhydrazone) complexes: investigation on antiproliferative activity and apoptosis induction. Inorg Chem Front. 2018;5(3):585–596..
- Mohamed Subarkhan MK, Ramesh R, Liu Y. Synthesis and molecular structure of arene ruthenium(II) benzhydrazone complexes: impact of substitution at the chelating ligand and arene moiety on antiproliferative activity. New J Chem. 2016;40(11):9813–9823..
- Sathiya Kamatchi T, Mohamed Subarkhan MK, Ramesh R, et al. Investigation into antiproliferative activity and apoptosis mechanism of new arene Ru(ii) carbazole-based hydrazone complexes. Dalton Trans. 2020;49(32):11385–11395..
- Subarkhan MKM, Ramesh R. Ruthenium(II) arene complexes containing benzhydrazone ligands: synthesis, structure and antiproliferative activity. Inorg Chem Front. 2016;3(10):1245–1255..
- Jiao G, He X, Li X, et al. Limitations of MTT and CCK-8 assay for evaluation of graphene cytotoxicity. RSC Adv. 2015;5(66):53240–53244..
- Ye G, Tao L, Ma C, et al. Influences of CCK-8 on expressions of apoptosis-related genes in prefrontal cortex neurons of morphine-relapse rats. Neurosci Lett. 2016;631:115–121..
- Yu S, Zhang X, Tan G, et al. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr Polym. 2017;155:208–217..
- Kwak HW, Lee KH. Polyethylenimine-functionalized silk sericin beads for high-performance remediation of hexavalent chromium from aqueous solution. Chemosphere. 2018;207:507–516..
- Kalita H, Hazarika A, Kandimalla R, et al. Development of banana (Musa balbisiana) pseudo stem fiber as a surgical bio-tool to avert post-operative wound infections. RSC Adv. 2018;8(64):36791–36801..
- Wu P, Liu Q, Li R, et al. Facile preparation of paclitaxel loaded silk fibroin nanoparticles for enhanced antitumor efficacy by locoregional drug delivery. ACS Appl Mater Interfaces. 2013;5(23):12638–12645..
- Huang W, Ling S, Li C, et al. Silkworm silk-based materials and devices generated using bio-nanotechnology. Chem Soc Rev. 2018;47(17):6486–6504..
- Dorishetty P, Dutta NK, Choudhury NR. Silk fibroins in multiscale dimensions for diverse applications. RSC Adv. 2020;10(55):33227–33247..