ABSTRACT
Introduction
Oleanane-type pentacyclic triterpenes named glycyrrhetinic acids (GAs) featuring a C-30 carboxylic acid group, are extracted from the licorice (Glycyrrhiza uralensis). Numerous biological properties of GA have been reported and have attracted researchers from all over the world in recent years due to the peculiar GA scaffold-based semisynthetic cytotoxic effects.
Areas Covered
This review represents the applications of semisynthetic derivatives of GA for the development of future cancer treatments. Included in the review are important structural features of the semisynthetic GAs crucial for cytotoxic effects.
Expert opinion
Numerous semisynthetic GA derivatives illustrated excellent cytotoxic effects toward various cancer cells. Notably the C-3(OH) at ring A along with C30-CO2H at ring E as vital structural features, make GA very appealing as a lead scaffold for medicinal chemistry, since these two groups permit the creation of further chemical diversity geared toward improved cytotoxic effects. Furthermore, numerous GA derivatives have been synthesized and indicate that compounds featuring cyanoenone moieties in ring A, or compounds having the amino group or nitrogen comprising heterocycles and hybrids thereof, illustrate more potent cytotoxicity. Furthermore, GA has a great capability to be conjugated with other anticancer molecules to synergistically enhance their combined cytotoxicity.
1. Introduction
The challenges of discovering new drug candidates which can treat various diseases having minor or negligible effects is a huge task. Despite the establishment of drug candidates to treat challenging diseases viz., cancer, HIV/AIDS, hypertension, malaria, and diabetes, these diseases continually effect world populations with notable associated-mortalities [Citation1]. Plant Natural Products (NPs) have been an inspiring source for the establishment of drug candidates to treat cancer and microbial diseases [Citation1–4]. Subsequently, numerous extracts of medicinal plant are employed today as prescription drugs in various developed countries viz., Germany, UK, France, and China [Citation1]. Notably, plants produce approximately a quarter of all FDA and/or the EMA drugs, with a few examples viz., Morphine and Paclitaxel. In addition, around a third of FDA-approved drugs in the last two decades are NPs or NP scaffold-based derivatives [Citation1].
Organic compounds synthesized by plants play a crucial role in the drug development [Citation4–9] and among the biologically useful natural molecules, pentacyclic triterpeniods have been intensively investigated for their intriguing medicinal and pharmacological effects [Citation10,Citation11]. However, these pentacyclic triterpeniods illustrated only weak activities in terms of the biological effects of their molecular targets. For this reason, these natural products have been employed as important templates for the preparation of more potent derivatives [Citation11].
Glycyrrhetinic acid (GA), a glycyrrhizin (also called glycyrrhizic acid) aglycon, is widely present in licorice roots (Glycyrrhiza glabra and G. uralensis). Furthermore, licorice was also employed in Persian traditional medicine to treat respiratory infections, peptic ulcers, tremor, and gastritis [Citation12,Citation13]. In ancient times, licorice was employed to treat cough and asthma [Citation14,Citation15], lung diseases [Citation15,Citation16], chest diseases [Citation15,Citation17], liver diseases [Citation15,Citation18], stomach diseases [Citation15,Citation16], intestine diseases [Citation15,Citation16], indigestion [Citation15,Citation16], arterial diseases [Citation15,Citation19], urinary tract infections [Citation15,Citation20], kidney pain [Citation15,Citation18], expulsion of kidney stones [Citation15,Citation21], ulcers [Citation15,Citation22,Citation23], bladder diseases [Citation15,Citation22,Citation23], fever [Citation15,Citation16,Citation23], and eye diseases [Citation15,Citation18,Citation23].
Licorice root extract had important applications in traditional medicine and illustrated recently significant effects toward the coronaviruses along with other viruses [Citation24,Citation25]. Quite recently, Sand et al. [Citation26] showed that licorice possesses significant antiviral effects toward SARS-CoV-2. Moreover, GA demonstrated in vitro antiviral effects and was reported to decrease replication of VV, VSV, NDV, and HSV-1 [Citation27]. In addition, GA was reported to illustrate antiviral effects toward the SARS coronavirus, herpesviruses, flaviviruses, hepatitis C virus, and influenza virus [Citation28]. The ACE2 enzyme plays a significant role in the COVID-19 infection because this enzyme is the COVID-19 entry point. Subsequently, ACE2 is part of the RAAS and molecules that upregulated this, exibit the ACE via the aldosterone receptor (MR) and plasma aldosterone. Notably, activation of MR could shield organs from binding with the COVID-19. GA is reported to inhibit the 11βHSD2 enzyme along with MR activation in organs where the expression of this enzyme occurs especially in the lungs [Citation29]. In another report, GA is reported to have a direct mineralocorticoid potential which is achieved via binding to mineralocorticoid receptors [Citation30] and blocking of the 11βHSD2 enzyme [Citation31]. Very recently, Ding et al. [Citation32] reported that the GA derivative, diammonium glycyrrhizinate along with vitamin C is used to treat COVID-19.
Excessive consumption of licorice on the other hand can give rise to hypertension and hypokalemia and these outcomes are linked with enhanced activation of hypoaldosteronism and renal mineralocorticoid receptors. GA is one of the active ingredients of the licorice, and acts on the 11β‐HSD2 enzyme to cause it to induce pseudohyperaldosteronism, that transforms active glucocorticoid cortisol to cortisone (inactive form) [Citation33]. Because of any excessive use of licorice and the above-mentioned significant adverse clinical effects, physicians suggested the essentiality to inform in a comprehensive and detailed dietary manner coupled with drug reports to the patients experiencing hypertension and hypokalemia problems the importance of this [Citation33].
GA is reported to demonstrated a wide range of significant biological effects viz., anti-inflammatory [Citation34,Citation35], hepatoprotective [Citation36,Citation37], antiviral [Citation38,Citation39], cytotoxic effects [Citation40,Citation41]. In addition, GA is reported to treat liver diseases [Citation42] and diabetes [Citation43]. Moreover, GA synthetic derivatives exhibited anti-inflammatory effects [Citation44], anti-HCV effects [Citation45], and antimicrobial effects [Citation46]. GA shows remarkable cytotoxic effects toward lung cancer [Citation41], hepatocellular cancer [Citation47], pituitary adenoma cells [Citation48], glioblastoma cells [Citation49], and prostate cancer [Citation50]. In addition GA is also reported to possess in vivo anticancer effects [Citation51,Citation52] along with apoptotic properties in various tumor cell lines [Citation50,Citation53,Citation54]. Based on both the GA cytotoxic effects and GA as an important scaffold, numerous studies have been published to prepare synthetic GA derivatives in order to get more potent molecules. A literature survey revealed that very few reviews have been published on the cytotoxic effects of GA derivatives [Citation55,Citation56]. The current review is focused on the cytotoxic effects of the most active GA synthetic derivatives (IC50: ≤10 µM or percentage inhibition >90%).
2. Cytotoxic effects of glycyrrhetinic acid (GA) synthetic derivatives
2.1. Ring A modified derivatives
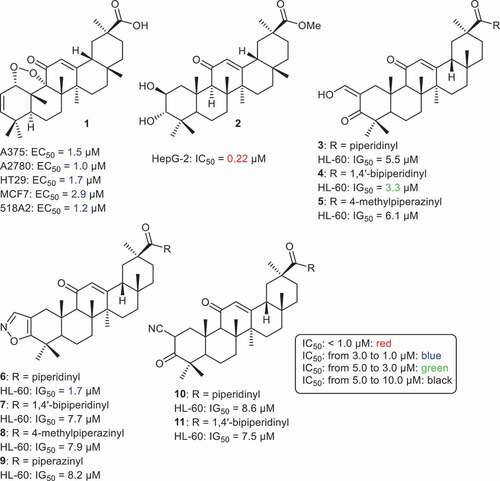
Serbian et al. synthesized the GA endo-peroxide derivative 1 () which was found to possess significant cytotoxic effects toward A375, A2780, HT29, MCF7, 518A2, and A375 with IC50 values ranging from 1.0 (A2780) to 2.9 (MCF7) [Citation57]. In another report, Jun et al. [Citation58] prepared GA analogue 2 which has an additional hydroxyl group at C-3, and which displayed significant cytotoxic effects toward the HepG-2 cancer cell line with IC50: 0.22 µM. Gao et al. [Citation59] prepared GA analogs 3–11, all of which possessed cytotoxic effects toward leukemia cells (HL-60) in which the IC50 ranged from 1.7 to 8.6 µM. Moreover, compounds 4 and 6 showed significant effects toward HL-60 with IC50’s of 3.4 and 1.7 µM, respectively.
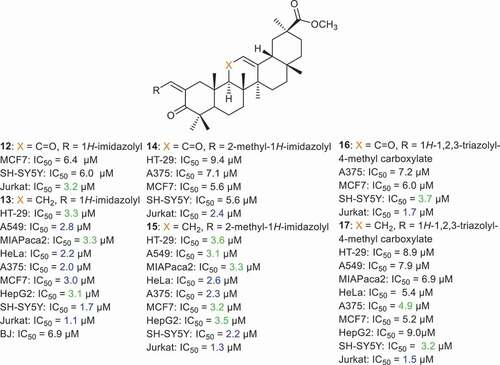
Alho et al. [Citation60] prepared a large library of GA derivatives 12–17 () through insertion of heterocycles viz., 1H-imidazolyl, 2-methyl-1H-imidazolyl, and 1H-1,2,3-triazolyl-4-methyl carboxylate conjugated with the α,β-unsaturated ketone of ring A. These compounds possess cytotoxic effects toward pancreas cancer (MIAPaCa 2), cervix cancer (HeLa), melanoma (A375), breast cancer (MCF7), hepatocellular cancer (HepG2), neuroblastoma (SH-SY5Y), and acute T cell leukemia (Jurkat) cells with IC50 values ranging from 1.1 to 9.4 µM. Among the tested compounds, compound 13 bearing the 1H-imidazolyl group in ring A was the most effective GA analog toward Jurkat cells with an IC50: 1.1 µM, which is 96-times more effective than the parent GA. In addition, this compound was also effective toward HT-29, A549, MIAPaca2, HeLa, A375, MCF7, and HepG2 with IC50’s ranging from 1.7 to 3.3 µM. Moreover, compounds 15–17 had potent effects toward Jurkat cells with IC50 of 1.3, 1.7, and 1.5 µM, respectively [Citation60].
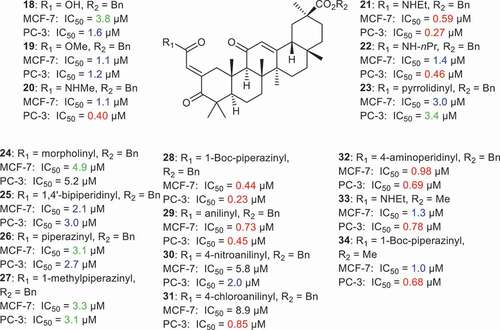
Various ring A-modified GA derivatives 18–34 () bearing an exocyclic α,β-unsaturated carbonyl group were prepared and found to possess cytotoxic effects toward prostate (PC-3) and breast cancer (MCF-7) with IC50 values ranging from 0.23 to 8.9 µM [Citation61]. Furthermore, compound 21 featuring an ethylamide group illustrated better cytotoxic effects (MCF-7: IC50: 0.59 µM; PC-3: IC50: 0.27 µM) than compound 20 with the corresponding methylamide group (MCF-7: IC50: 1.1 µM; PC-3: IC50: 0.40 µM) and n-propylamide bearing compound 22 (MCF-7: IC50: 1.4 µM; PC-3: IC50: 0.46 µM). This may indicate, that the number of carbons in the alkyl chain of the amide play a crucial role in the cytotoxic effects. Moreover, various nitrogen heterocycles and aromatic amines were inserted at ring A and as an illustration, compound 28 featuring a 1-Boc-piperidin-4-yl group turned out to be the most potent compound toward the PC-3 and MCF-7 cancers, with IC50: 0.23 and 0.44 and μM, respectively. In addition, GA derivatives 29 and 32 featuring and anilinyl and 4-aminoperidinyl group respectively, showed potent effects toward PC-3 and MCF-7 with IC50 < 1.0 µM. On the other hand, compounds 31, 33, and 34 were significantly effective toward PC-3 with IC50: 0.85, 0.78, 0.68 µM, respectively [Citation61].
2.2. Glycyrrhetinic acid (GA) derivatives with ring A enones
2.2.1. GAs with non-enolizable cyanoenones in ring A
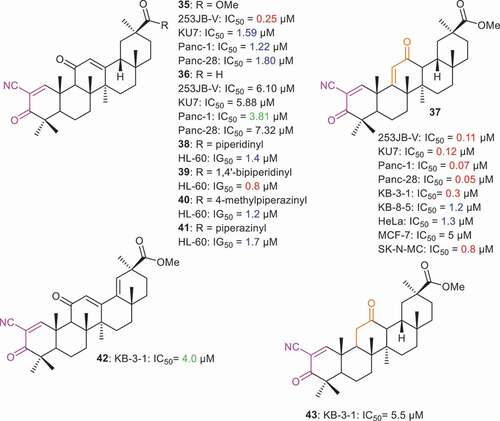
Chadalapaka et al. [Citation62] prepared cyanoenone derivatives 35 and 36 () which turned out to be significantly active toward 253JB-V, KU7, Panc-1, Panc-28 with IC50 values ranging from 0.25 µM to 1.80 µM. Notably, compound 35 possesses very potent effects toward bladder cancer (253JB-V: IC50: 0.25 µM). On the other hand, compound 37 demonstrated very potent effects toward four cancer cell lines with IC50 values ranging from 0.05 µM to 0.12 µM. Notably, this latter compound was most effective toward the pancreatic cancer cells (Panc-1: IC50 = 0.07 µM and Panc-28: IC50 = 0.05 µM). The effects of the ring C functional group also play a crucial role in the reactivity of these compounds. For instance, compound 37 has an [9(11)-en-12-one while compounds 35 and 36 have the 12-en-11-one core system. Gao et al. [Citation59] prepared compounds 38–41 and all these compounds illustrated significant effects toward leukemia cells (HL-60) in which IG50 ranged from 0.8 to 1.7 µM. Moreover, it was found that compounds having the 1,4ʹ-bipiperidinyl group possess better effects with IG50: 0.8 µM. Literature revealed that diterpenes with cyanoenones in ring A also illustrated similar cytotoxic effects compounds 38–41 [Citation63]. Salomatina et al. [Citation64] reported that GA derivatives 37, 42 and 43 possess cytotoxic effects toward epidermoid cancer cells (KB-3-1) with IC50 0.3, 4.0, and 5.5. µM, respectively.
Logashenko et al. [Citation65] reported that compound 37 demonstrated potent cytotoxic effects toward epidermoid cancer (KB-3-1: IC50 = 0.3 µM; KB-8-5: IC50 = 1.2 µM), HeLa cervical cancer (HeLa: IC50 = 1.3 µM) and neuroblastoma cells (SK-N-MC: IC50 = 0.8 µM). The same authors further reported that compound 37 was able to sensitize epidermoid cancer cells and other multi drug resistant cancer cells at micromolar concentrations. Compound 37 triggers an intrinsic apoptotic pathway to induce cell death. Moreover, the authors compared the antiproliferative activity of compound 37 with CDDO-Me, a known drug used for the treatment of kidney disease and different types of solid tumors. Compound 37 was reported as an effective anticancer agent compared to CDDO-Me by disrupting the mitochondrial membrane potential and activating caspase signaling. It is noteworthy that the starting material to synthesize compound 37 is readily available in natural plant root extracts of Glycyrrhiza glabra [Citation65].
2.2.2. Glycyrrhetinic acid (GA) derivatives with enones in ring A
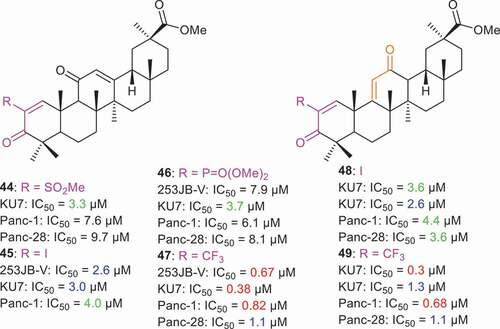
Chadalapaka et al. [Citation62] prepared a small library of ring A enone glycyrrhetinic acid analogs 44–49 () having a C-2 substitution. Additionally, these compounds have either a ring C 9(11)-en-12-one or 12-en-11-one core system. Notably, 2-trifluoromethyl derivatives 47 and 49 illustrated potent effects toward 253JB-V, KU7, Panc-1, Panc-28 with IC50 values ranging from 0.3 µM to 1.3 µM. Moreover, compound 47 with ring C 12-en-11-one core, possesses better effects toward KU7 compound 49 with the corresponding ring C 9(11)-en-12-one system (IC50: 0.38 vs 1.3 µM). In contrast, the 2-iodo substituted analogs 45 and 49 were less active than the 2-trifluoromethyl derivatives and the IC50 values for these two compounds ranged from 2.6 µM to 4.4 µM. In addition, the 2-methanesulfonyl derivative (compound 44) and 2-dimethylphosphonyl derivative (compound 46) also possessed higher IC50 values than the corresponding 2-trifluoromethyl and 2-iodo substituted analogs [Citation62].
2.3. Glycyrrhetinic acid (GA) ring A pseudo seco derivatives
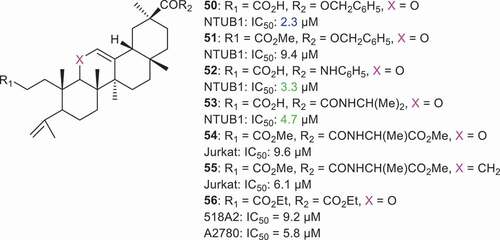
Lin et al. [Citation66] synthesized pseudo seco GA derivatives 50–53 () and screened them for cytotoxic effects toward bladder cancer cell (NTUB1). Interestingly, compounds 50, 52, and 53 illustrated significant cytotoxic effects with IC50: 2.34, 3.3, and 4.7 µM, respectively. Lin et al. determined the effect of compound 53 on the induction of apoptosis in bladder carcinoma cells (NTBU1). Thus, the authors treated the NTBU1 cells with different concentrations (25, 50, and 75 µM) of compound 53 along with cisplatin (positive control) and N-acetylcysteine (NAC). Data revealed that compound 53 treatment increased the population of NTBU1 cells in the sub-G1 phase and also reduced the population of cells in the S phase of the cell cycle. This indicates that compound 53 facilitates the exit of NTBU1 cells from the cell cycle and is a sign of apoptotic induction. Similarly, it was found that NAC and hydrogen peroxide (H2O2) led to free radical generation to induce the p53 activation which in turn triggers apoptosis. Inactivation of p53 led to tumorigenesis and chemoresistance in cells. The level of the phosphorylated form of p53 increased in NTUB1 cells upon treatment by compound 53 [Citation66].
In another report, Alho et al. [Citation67] prepared GA pseudo analogs 54 and 55 and tested them for activity toward various cancer cells. Compound 55 illustrated significant effects toward Jurkat cells with an IC50: 6.1 µM and its activity was 55-fold higher than the parent GA. On the other hand, compound 54 also showed good cytotoxic effects toward Jurkat cells (IC50: 9.6 µM). The authors further studied the efficacy of compound 55 on cell cycle arrest and apoptosis in the Jurkat cell line. Thus, the Jurkat cells were treated at IC50 concentrations and the results obtained after 24 hr for compound 55 treatment increased the Jurkat cell population at the S phase, however after 48 hr and 72 hr, this effect was completely abolished. DNA fragmentation was noticed by the authors after 72 hr of compound 55 treatment. Cell cycle arrest at the S phase induced by compound 55 indicates that cells underwent apoptosis. Annexin V/propidium iodide staining of cells clearly illustrates the respective stages of apoptotic events. The authors used these staining method to evaluate the apoptotic induction effect of compound 55 in Jurkat cells. Alho et al. reported that compound 55 (6.1 µM and 12.2 µM) treatment increased the early apoptotic population dose dependently. However, compound 55 did not increase the Jurkat cells population at the later apoptotic state and this result coincides with the cell cycle arrest effect of compound 55 [Citation67]. Compound 56 illustrated cytotoxic effects toward 518A2 (IC50 = 9.2 µM) and A2780 (IC50 = 5.8 µM) [Citation68].
2.4. Glycyrrhetinic acid (GA) C-30 amides
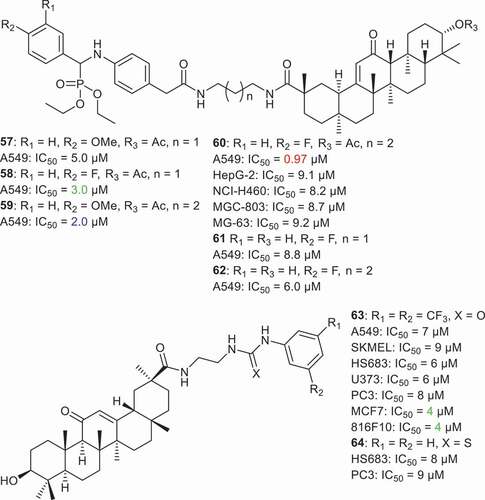
Jin et al. [Citation69] synthesized a small library of GA C-30 amide derivatives 57–62 () comprising the aminophosphonate moiety and screened them for their cytotoxic effects toward various cancer cells. Compound 60 was found to possess better effects toward NCI-H460 (lung), HepG-2 (hepatoma), MGC-803 (gastric), MG-63 (osteosarcoma), and A549 (lung) cell lines in which the IC50 ranged from 6.25 to 9.11 μM. On the other hand, the remaining compounds were not effective and their IC50 values were above 10 μM. In addition, compounds 57–62 illustrated significant effects to the NF-κB in A549 cells in which the IC50 ranged from 0.97 to 8.8 μM. Notably, compound 60 had potent effects with IC50 0.97 μM. An SAR study showed that the number of methylene groups as well the acetyl group at C-3 play a crucial role in the activity because the differences between compounds 58, 60 and 60, 62 is due to methylene and acetyl groups.
Activation of nuclear factor kappa B (NF-kB) is well established in tumor progression and spread. Jin et al. studied the inhibitory effect of molecule 60 on NF-kB expression in A549 cells. The outcome of their study found that compound 60 suppressed TNF-α induced NF-kB activation in a dose dependent manner. The authors showed furthermore that the molecular mechanism of molecule 60 inhibition of NF-kB activation occurred by down regulation of the phosphorylated forms of IκBα and IKKα/β [Citation69]. Acquiring of cisplatin resistance by solid tumors is a major limitation from a clinical point of view. Thus, compounds which can overcome cisplatin resistance are considered to be ideal drug candidates for solid tumor treatment. Jin et al. tested the cytotoxic effects of molecule 60 on cisplatin-resistant (SK-OV-3/CDDP) and cisplatin sensitive (SK-OV-3) cancer cells. The results indicate that compound 60 is able to sensitize the SK-OV-3/CDDP cells significantly and has a resistance factor value of 0.92. In addition, Jin et al analyzed the mode of cell death induced by compound 60 in A549 cells by flow cytometry. After a 24 hr treatment of A549 cells with compound 60 it was shown to exert a remarkable dose dependent apoptosis. Compound 60 (20 μM) treatment induced early (19.5%) and late (39.7%) apoptosis in A549 cells. To ensure further apoptosis in A549 cells upon treatment of molecule 60, the authors stained the nucleus of A549 cells with Hoechst 33258 after a 24 hr treatment period. Compound 60 was found to damage the nucleus considerably by hyper condensation which is a clear indication of apoptosis [Citation69]. Lallemand et al. [Citation70] screened two C-30 amides viz., 63 and 64 toward various cancer cells. Notably, compound 63 displayed cytotoxic effects toward A549, SKMEL, HS683, U373, PC3, MCF7 and 816F10 cancer cells with IC50 ranging from 4.0 to 9 µM. On the other hand, the thioketo derivative 64 was only active toward HS683 and PC3 cancer cells with IC50 of 8 and 9 µM, respectively.
2.5. Glycyrrhetinic acid (GA) C-30 aromatic esters
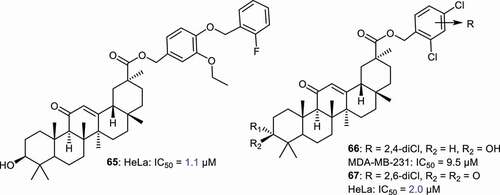
Zheng et al. [Citation71] prepared GA derivatives 65–67 () bearing C-30 ester function with halogenated aromatic rings and screened these toward breast cancer (MDA-MB-23) and cervical cancer cells (HeLa). Compounds 65 and 67 illustrated potent effects toward HeLa cells, with IC50: 1.1 and 2.0 μM, respectively. Notably, both compounds possess better effects than the standard doxorubicin (IC50: 2.4 μM) and gefitinib (IC50: 6.7 μM). On the other hand, compounds 65 and 67 were not active toward MDA-MB-23 while compound 66 achieved good cytotoxic effects toward MDA-MB-23 with IC50: 9.5 μM.
2.6. Conjugates of GA with bioactive molecules
2.6.1. Conjugates of GA with amino acids
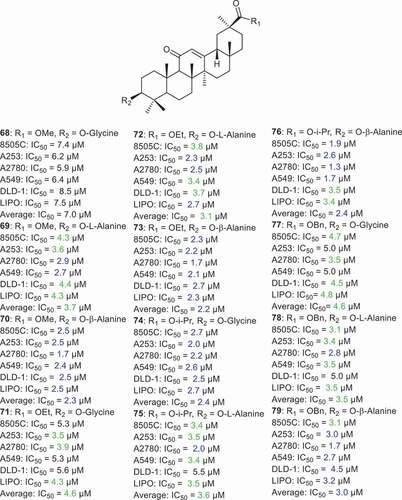
Schwarz et al. [Citation72] prepared a series of GA derivatives 68–79 (), which were with amino acids viz., glycine and alanine, subsequently. It was found that these compounds illustrated cytotoxic effects toward 8505 C, A253, A2780, A549, DLD-1, and LIPO with IC50 ranging from 1.7 to 8.5 µM. Notably, compound 76, which has a β-alanine at C-3 and an isopropyl group at C-30, possessing potent cytotoxic effects toward ovarian cancer (A2780: IC50 = 1.3 µM) along with compounds 70, 73, and 79, which showed effects toward A2780 with IC50 = 1.7 µM. In addition, compound 76 illustrated potent effects toward lung (A549) and thyroid cancer (8505 C) with IC50 = 1.7 and 1.9 µM, respectively. Moreover, compounds 70 (methyl moiety at C-30 and β-alanine at C-3), 73 (ethyl moietyat C-30 and β-alanine at C-3) and 74 (isopropyl moiety at C-30 and β-alanine at C-3) displayed potent effects toward 8505 C, A253, A2780, A549, DLD-1, and LIPO with IC50 _<3.0 µM [Citation72].
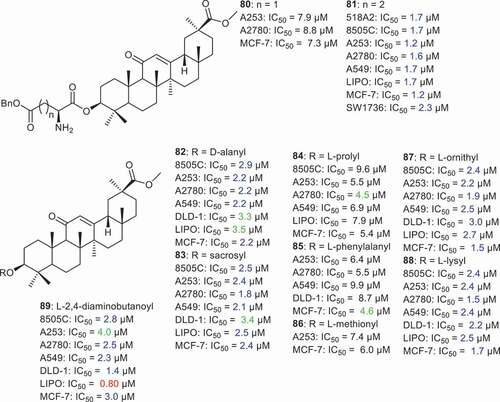
Csuk et al. [Citation73] prepared GA derivatives 80 and 81 () being coupled with benzylated aspartic and glutamic acids afterward. Compound 80 having the benzylated aspartic acid moiety, illustrated good cytotoxic effects toward A253, A2780, and MCF-7 with IC50: 7.9, 8.8, and 7.3 µM, respectively. On the other hand compound 81 bearing the benzylated glutamic acid, displayed potent effects toward 518A2, 8505 C, A253, A2780, A549, LIPO, MCF-7 and SW1736 in which the IC50 ranged from 1.2 to 2.3 µM. In another report [Citation74], the same group prepared GA-amino acid coupled derivatives (compounds 82–89) including D-alanine, sarcosine, L-proline, L-phenylalanine, L-methionine, L-ornithine, L-lysine, and 2,4-diaminobutyric acid. Notably, compounds having sarcosine (83), L-ornithine (87) and L-lysine (88) illustrated potent effects toward 8505 C, A253, A2780, A549, LIPO and MCF-7 with IC50 ≤ 3 µM. On the other hand, GA derivatives bearing L-proline (84), L-phenylalanine (85) and L-methionine (86) were less active on cancer cells mentioned above with IC50 values between 4.5 to 9.9 µM. More interestingly, compound 89, having the 2,4-diaminobutyric acid as the amino acid, displayed very potent effects toward liposarcoma (LIPO: IC50: 0.80 µM) and colon cancer (DLD-1: IC50: 1.4 µM).
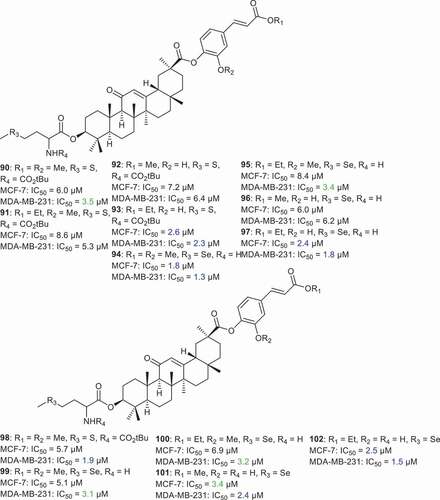
Li et al. [Citation75] prepared GA derivatives 90–102 () which were coupled with amino acids (l-selenomethionine and l-methionine) at C-3. In addition, some isoferulic acid and trans-4-hydroxycinnamic acid derivatives were inserted at C-30. It was found that compounds 90–102 possess cytotoxic effects toward breast cancer cell lines (MCF-7, MDA-MB-231) with IC50 between 1.3 and 8.6 µM. Notably, compound 94 bearing l-selenomethionine (as the amino acid at C-3) and isoferulic acid methyl ester at C-30, illustrated potent effects toward MCF-7 and MDA-MB-231 with IC50: 1.8 and 1.3 µM, respectively. In addition, compounds 97, 98, and 102 were significantly effective toward MDA-MB-231 cancer cells with IC50: 1.8, 1.9, and 1.5 µM, respectively. On the other hand, compounds 93, 97, and 102 displayed significant effects toward MCF-7 cancer cells with IC50: 2.6, 2.4, and 2.5 µM, respectively.
2.6.2. Conjugates of GA with cinnamic acid derivatives
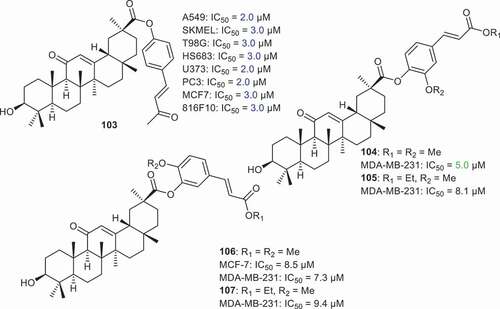
Lallemand et al. [Citation70] prepared compound 103 () which was coupled with vanillylidenacetone at C-30 and illustrated significant effects toward A549, SKMEL, T98G, HS683, U373, PC3, MCF7 and 816F10 cancer cells with IC50 ≤ 3. Notably, this compound demonstrated some significant effects toward A549, U373 and PC3 with IC50: 2.0 μM. Li et al. [Citation75] prepared GA derivatives 104–107 which were coupled with isoferulic acid and trans-4-hydroxycinnamic acid derivatives at C-30. It was found that these compounds viz., 104–107 possess cytotoxic effects toward the breast cancer cell lines (MDA-MB-231) with IC50 between 5.0 and 9.5 µM while only compound 106 was active toward MCF-7 with IC50: 8.5 µM.
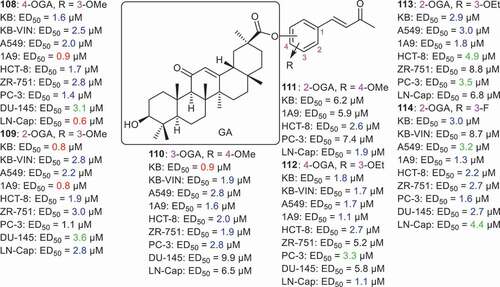
Tatsuzaki et al. [Citation76] coupled GA with dehydrozingerone (DZ) via esterification of C-30 to form the conjugate GA–DZ series (108–114) () all of which illustrated cytotoxic effects toward KB, KB-VIN, A549, 1A9, HCT-8, ZR-751, PC-3, DU-145, and LN-Cap with the ED50 ranging from 0.6 to 8.8 µM. Moreover compound 111 having the 2-OGA ester along with 4-OMe group, was less potent than compounds 108–110, which demonstrated potent cytotoxic effects toward most of the cancer cells with ED50: <3.0 µM. Based on cancer cell selectivity, conjugate 108 bearing both a 4-OGA ester and 3-OMe group, illustrated the highest effects toward ovarian (1A9: ED50: 0.9 µM) and prostate (LN-Cap: ED50: 0.6 µM) cells. On the other hand, compound 109 was very effective toward KB and 1A9 with ED50: 0.8 µM while compound 110 was very potent against KB cells with ED50: 0.9 µM.
2.7. Glycyrrhetinic acid (GA) glycosides
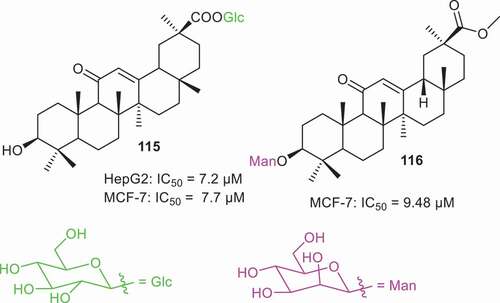
Natural triterpene glycosides have been reported from numerous natural sources and these compounds possess a diverse range of sugar units in their structures. In addition, numerous triterpene glycosides have been prepared via different synthetic methodologies. Of interest to the current authors is the fact that triterpene glycosides are reported to possess a wide range of biological effects [Citation77]. Dai et al. [Citation78] prepared GA glycoside 115 () which has a glucopyranoside at the C-30 position. Biological studies revealed that this compound possesses good cytotoxic effects toward the liver cancer (HepG2: IC50: 7.2 µM) and breast cancer (MCF-7: IC50: 7.7 µM). In another report, Schwarz et al. [Citation79] prepared GA glycoside 116 having a mannoside unit at the C-3 position. This glycoside viz., 116 possesses cytotoxic effects against MCF-7 cells with IC50: 9.4 µM.
2.8. Glycyrrhetinic acid (GA) C-3 amino derivatives and related compounds
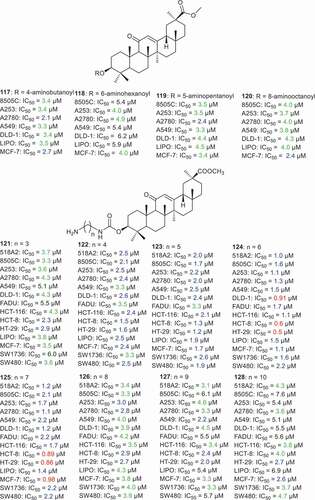
Csuk et al. [Citation74] esterified the C-3 hydroxyl group of GA with various acyl groups with terminal amino groups including 4-aminobutanoyl, 6-aminohexanoyl, 5-aminopentanoyl, and 8-aminooctanoylmethyl to afford compounds 117–120 (). It was found that compounds 117–120 illustrated almost similar effects toward 8505 C, A253, A2780, A549, DLD-1, LIPO and MCF-7 cancer cells in which the IC50 ranged from 2.1 to 6.2 µM. In another report, the same authors [Citation80] synthesized aminoalkyl substituted GA analogs 121–128 and they all possessed cytotoxic effects toward 14 human cancer cells with IC50 ranging from 0.5 to 7.6 µM. Moreover, compounds 124 and 125 were the most active among the tested compounds toward 518A2, 8505 C, A253, A2780, A549, DLD-1, FADU, HCT-116, HCT-8, HT-29, LIPO, MCF-7, SW1736, and SW480 with IC50 ranging from 0.5 to 2.2 µM. Moreover, compound 124 was significantly active toward the three colon cancer cell lines (DLD-1: IC50 = 0.91 µM; HCT-8: IC50 = 0.6 µM; HT-29: IC50 = 0.5 µM). In addition, compound 125 was more active toward colon (HCT-8: IC50 = 0.89 µM; HT-29: IC50 = 0.86 µM), liposarcoma (LIPO: IC50 = 1.4 µM) and breast (MCF-7: IC50 = 0.98 µM) cancer cells.
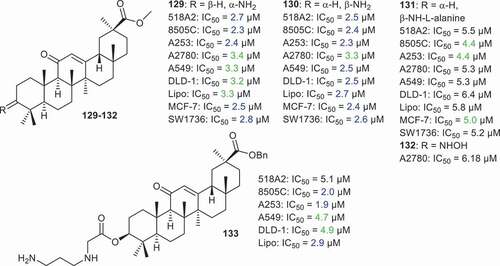
Csuk et al. [Citation68] prepared GA derivatives 129–133 () and tested them against various cancer cells. Among them, compound 133, having a benzyl at C-30 and the 3-N-(3-aminopropyl)glycyl group at C-3, demonstrated significantly potent effects against neck cancer (A253: IC50 = 1.9 µM) along with good cytotoxic effects toward 518A2, 8505 C, A549, DLD-1 and LIPO and showed IC50 values from 2.0 to 5.1 µM. In addition, the C-3 amino derivatives 129 and 130 also illustrated significant effects toward 518A2, 8505 C, A253, A2780, A549, DLD-1, LIPO, MCF-7, and SW1736 with IC50 ranging from 2.3 to 3.4 µM.
2.9. Miscellaneous
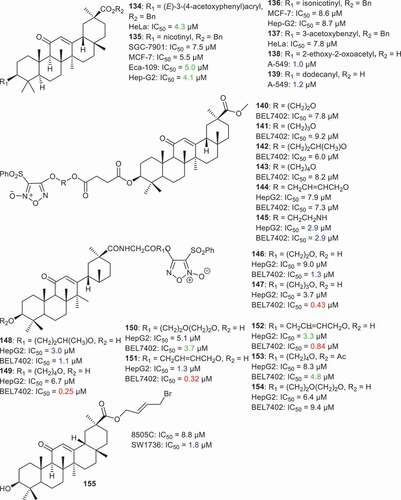
Wang et al. [Citation81] prepared GA derivatives 134–137 () having aromatic acyl groups at C-3. The in vitro anticancer activities of the new GA derivatives were tested toward breast cancer (MCF-7), gastric cancer (SGC-7901), cervical cancer (HeLa), esophageal cancer (Eca-109), and hepatoma cancer (Hep-G2). It was found that compound 135 having the nicotinyl group at C-3, presented the better inhibitory effects toward gastric cancer (SGC-7901: IC50 = 7.5 µM), breast cancer MCF-7 (MCF-7: IC50 = 5.5 µM), esophageal cancer (Eca-109: IC50 = 5.0 µM), and hepatoma cancer (Hep-G2: IC50 = 4.1 µM). Yadav et al. [Citation82] prepared compounds 138 and 139 via insertion of aliphatic acyl groups at C-3 and these illustrated significant cytotoxic effects toward lung cancer (A549) with IC50: 1.0 and 1.2 µM, respectively.
Lai et al. [Citation83] prepared furan-based nitric oxide (NO)-releasing analogs of GA 140–154 and all were found to possess cytotoxic effect toward hepatocellular cancer cells (HepG2, BEL-7402) with IC50 ranging from 0.25 to 9.2 µM. Notably, compound 149 having a furan nitric oxide unit at C-30, illustrated almost four times better effects toward BEL-7402 cells than the usual standard Adriamycin (149: 0.25 µM; Adriamycin: 0.90 µM). In addition, compounds 147, 151, and 152 also demonstrated better effects toward BEL-7402 cells in which the IC50: values were 0.43, 0.32 and 0.84 µM, respectively. Furthermore, compound 145 illustrated significant effects toward broth hepatocellular cells (HepG2, BEL-7402) with IC50 < 3.0 µM. In the report, Csuk et al. [Citation84] prepared the C-30 alkyl derivative 155 which illustrated potent cytotoxic effects toward SW1736 cells with IC50 = 1.88 μM.
2.10. Glycyrrhetinic acid (GA) derivatives as Pin1 inhibitors
2.10.1. Conjugates of GA with benzo[d]imidazole
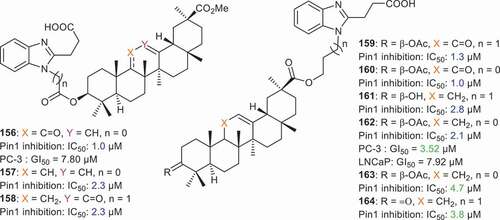
Cancer manifestation depends on numerous signaling pathways in order to sustain proliferation and peptidyl-prolyl isomerase NIMA-interacting-1 (Pin1) constitutes a unified signaling hub which is exploited by cancer cells in order to inactivate tumor suppressors and to activate oncogenes. Li et al. [Citation85] prepared conjugates of GA with 3-(1H-benzo[d]imidazol-2-yl)propanoic acid via the C-3 hydroxyl group of GA. Compounds 156–158 () all possessed potent Pin1 inhibition with IC50 < 2.4 µM. In addition, compound 156 bearing a C-11 carbonyl group, was also active toward prostate cancer cells (PC-3) with GI50 values of 7.8 µM. Compounds 159–164 were prepared by introducing the benzimidazole group to C-30 of GA. Furthermore, these compounds also illustrated significant Pin1 inhibition with IC50 ranging from 1.0 to 4.7 µM. Among these conjugates, compound 162 was quite effective toward prostate cancer cells (PC-3: GI50 = 3.52 μM and LNCaP: GI50 = 7.92 μM) [Citation85].
3. Expert opinion
The structural diversity and complexity of natural molecules are derived from numerous fascinating multistep enzymatic conversions. Natural products present themselves as attractive scaffolds for an intriguing drug discovery process because of the complex nature and structural chemical diversity of these molecules. Inspired by the interesting biosynthetic routes which create complex natural products, numerous authors prepared GA semisynthetic derivatives via chemical diversification. This review on semi-synthetic derivatives of GA demonstrates that numerous chemical modifications have been accomplished on the GA scaffold, which showed significant cytotoxic effects toward various cancer cells. In addition, GA is a very interesting model of skeletal chemical diversity as this compound has a C-3 hydroxyl group, C-11 keto moiety and a C-30 carboxylic acid.
Based on the cytotoxic effects of GA derivatives, the C-3 hydroxyl group turned out to be critical in retaining the cytotoxicity. In addition, insertion of an additional amino group, amino acid or a nitrogen-comprising group was crucial for enhancing the cytotoxic effects. Introduction of exocyclic α,β-unsaturated carbonyl group to ring A bearing extra nitrogen comprising groups including ethyl amine, propylamine, 1-boc-piperazine, piperazine, aniline, and 4-aminoperidine surprisingly increased the cytotoxic effects even further. On the other hand, the activities were decreased when the C-3 hydroxyl group was oxidized to the keto group. Notably some substituents in ring A play a crucial role in enhancing cytotoxic effects. Interestingly, cytotoxic effects were dramatically increased after insertion of a cyano (CN) or trifluoromethyl (CF3) group at C-2 of ring A. Furthermore, expansion or cleavage (pseudo seco compounds) of ring A did not influence cytotoxicity. In addition, glycosylation at C-3 and C-30 did not effect the cytotoxicity so much either. The authors strongly suggest that future research should focus on preparation of GA derivatives featuring enones in ring A (especially cyanoenones) along with the introduction of heterocyclic ring systems and amino-comprising alkyl groups at C-3 and C-30.
Esterification/amidation of the C-30 carboxylic acid group should form serious synthetic plans to increase the cytotoxicity at another level. Notably, the conjugate compounds of GA with other bioactive molecules dramatically enhances cytotoxic effects. Clinical investigations have demonstrated that the combination strategy (combination of drugs) has a better cytotoxic effect than single molecule [Citation86,Citation87]. This may be due to a synergistic effect via divergent modes of actions of multiple drugs. However, the administration of drugs could be difficult because of differences in drug biodistribution and solubility [Citation87]. Biological active carrier-materials may solve this issue. Numerous research investigations have shown that GA derivatives could efficiently inhibit the invasion and migration of various cancer cells as angiogenesis inhibitor, cellular signal transduction inhibitor and cytokines inhibitors [Citation87–89]. Based on these results, future research should focus on to combine GA with other anticancer drugs in order to develop more potent anticancer drugs, which act in a multi-target way. Another important aspect for future research that should be investigated includes the biological role of GAs as monotherapy versus combination therapy as anticancer agents.
The total syntheses of GA is relatively insignificant because the cost-to-benefits ratio constitute a deterrent factor to furnish sufficient quantities of GA for the chemical and pharmaceutical industries. It is common knowledge that any chemical conversion and pharmaceutical application of GA-derivatives is dependent on the supply of raw material. Consequently, the modification of extraction protocols is a critical step to increase the yields of these types of pentacyclic triterpenes. Numerous protocols have been developed for GA extraction and purification from licorice [Citation90–97]. Nevertheless, these orthodox solvent based purification strategies need large amounts of solvent, are time consuming and have relatively low target product yields. Moreover, conventional extraction and purification procedures pose health risks. Therefore, it is the strong suggestion of the current authors that it is necessary to establish productive, economically viable, efficient, and ecofriendly strategies for the extraction and purification of GA.
Quite recently is has been reported that a man died after eating black licorice in excessive quantities over a period of 2 weeks and thus any side effects should be carefully monitored and addressed in detail [Citation98]. Excessive consumption of licorice can give rise to hypertension and hypokalemia and these outcomes are linked with enhanced activation of both block of 11HSD2 and mineralocorticoid receptors. One possibility of mitigating these side effects is addition of spironolactone, which antagonizes the mineralocorticoid effect of licorice [Citation99]. Moreover, natural GA is reported to illustrate anticancer effects. However, the general use of this molecule in cancer treatment has a serious limitation due to its poor bioavailability and hydrophobicity [Citation100]. Various GA synthetic compounds have been prepared bearing exocyclic α,β-unsaturated carbonyl groups in ring A and having additional nitrogen comprising polar groups viz., ethyl amine, propylamine, 1-boc-piperazine, piperazine, aniline, and 4-aminoperidine. In addition, some other polar groups such as cyano (CN) or trifluoromethyl (CF3) have also been introduced at C-2 of ring A along with amino-comprising alkyl groups at C-3 and C-30. Notably, the conjugate compounds of GA with other bioactive polar molecules have also been prepared. The current strongly opinions are that the polar groups (mentioned above) which when inserted into the GA skeleton or via conjugation with GA, will effectively improve the solubility and bioavailability of GA synthetic deriavtives.
Moreover, pharmacodynamic and pharmacokinetic data were unfortunately not available for the most potent GA derivatives and consequently these most active derivatives should be carefully checked for further detailed investigations. While it is often difficult to propose effective regulatory decisions due to the lack of pharmacodynamic and pharmacokinetic data being available, this deficiency in the knowledge domain has to be addressed in the future. We believe that GA can be logically chemically modified to be geared toward becoming more potent as cytotoxic GA compounds. The chemical procedures for the chemical modifications of GA are valuable, but these strategies have at times been complicated and sometimes followed very harsh reaction conditions. We strongly believe that the ‘next generation’ of GA derivatives should involve new short and efficient synthetic protocols supported by more comprehensive anticancer evaluation assays and new standards to prove their true efficiency.
Article highlights
Glycyrrhetinic acid (GA) has great potential as a scaffold to generate an intriguing chemical diversity of new structures.
This review outlines the GA semisynthetic derivatives in the establishment of GAs for cytotoxic potential
Modifying GA at the C3-OH, and C30-CO2H functional groups greatly enhances their cytotoxic effects
GA derivatives featuring cyanoenone, amino group, or nitrogen comprising heterocycles illustrated better cytotoxic effects
Conjugates of GAs with various bioactive molecules is discussed
GA derivatives illustrate great potential to treat various cancer
This box summarizes key points contained in the article.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Thomford NE, Senthebane DA, Rowe A, et al. Natural products for drug discovery in the 21st century: innovations for novel drug discovery. Int J Mol Sci. 2018;19:1578.
- Ashrafizadeh M, Zarrabi A, Orouei S, et al. Recent advances and future directions in anti-tumor activity of cryptotanshinone: a mechanistic review. Phytother Res. 2021;35:155–179.
- Ashrafizadeh M, Najafi M, Makvandi P, et al. Versatile role of curcumin and its derivatives in lung cancer therapy. J Cell Physiol. 2020;235:9241–9268.
- Sultani HN, Morgan I, Hussain H, et al. Access to new cytotoxic triterpene and steroidal acid-TEMPO conjugates by Ugi multicomponent-reactions. Int J Mol Sci. 2021;22:7125.
- Salminen A, Lehtonen M, Suuronen T, et al. Terpenoids: natural inhibitors of NF-kappaB signaling with anti-inflammatory and anticancer potential. Cell Mol Life Sci. 2008;65(19):2979–2999.
- Lee KH. Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach. J Nat Prod. 2010;73:500–516.
- Nobili S, Lippi D, Witort E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365–378.
- Rao GV, Kumar S, Islam M, et al. Folk medicines for anticancer therapy-a current status. Cancer Ther. 2008;6:913–922.
- Hussain H, Green IR, Ali I, et al. Ursolic acid derivatives for pharmaceutical use: a patent review (2012-2016). Expert Opin Ther Pat. 2017;27(9):1061–1072.
- Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68.
- Kuo RY, Qian K, Morris-Natschke SL, et al. Plant-derived triterpenoids and analogues as antitumor and anti-HIV agents. Nat Prod Rep. 2009;26:1321–1344.
- Bahmani M, Rafieian-Kopaei M, Jeloudari M, et al. A review of the health effects and uses of drugs of plant licorice (Glycyrrhiza glabra L.) in Iran. Asian Pac J Trop Dis. 2014;4:S847–S849.
- Zargaran A, Zarshenas MM, Mehdizadeh A, et al. Management of tremor in medieval Persia. J Hist Neurosci. 2013;22:53–61.
- Hort A. Theophrastus: enquiry into plants and minor works on odours and weather signs. London: Harvard University Press; 1961. p. 13 (Chapter IX).
- Fiore C, Eisenhut M, Ragazzi E, et al. A history of the therapeutic use of liquorice in Europe. J Ethnopharmacol. 2005;99:317–324.
- Marcellus. Marcelli De medicamentis. Lipsiae, liber: Teubneri; 1889. p. XIV, 7; XVI, 103; XX, 108; XXVI, 63; XXIX, 11; XXX, 24.
- Toletanum G. Abub. Rhazae lib. De pestilentia ad Mansorem finis. Cremona: Librum Divisionium; 1544. p. 371–374.
- Von Sontheimer J. Grosse Zusammenstellung ueber die Kraefte der bekannten einfachen Heil-und Nahrungsmittel von Abu Mohammed Abdallah Ben Ahmed aus Malaga bekannt unter dem Namen Ebn Baithar. Stuttgart: Aus dem Arabischen uebersetzt. Hallberger’sche Verlagshandlung; 1842.
- Scribonius. Scribonii largi compositiones. Leipzig: Teubner; 1983. p. LXXV, LXXXVI.
- Plinius, 1875 and 1897. C. Plini Secundi Naturalis historiae. Lipsiae, libri: Teubneri; XXXVII, XI, 119; XXII, 11; XXV, 43.II, IV.
- Celsus. A. Cornelii Celsi De medicina libri octo. Lipsiae: Teubneri; 1859. p. 20, 6.
- Avicenna. Liber canonis De Medicinis cordialibus Cantica De Removendis nocumentis in regimine sanitatis De syrupo acetoso. Iuntas, Venetiis. 1562;II(2):437.
- Hussain H, Green IR, Shamra U, et al. Therapeutic potential of glycyrrhetinic acids: a patent review (2010-2017). Expert Opin Ther Pat. 2018;28(5):383–398.
- Huang W, Chen X, Li Q, et al. Inhibition of intercellular adhesion in herpex simplex virus infection by glycyrrhizin. Cell Biochem Biophys. 2012;62:137–140.
- Cinatl J, Morgenstern B, Bauer G, et al. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046.
- Van De Sand L, Bormann M, Alt M, et al. Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses. 2021;13:609.
- Pompei R, Flore O, Marccialis MA, et al. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature. 1979;281:689–690.
- Fiore C, Eisenhut M, Krausse R, et al. Antiviral effects of Glycyrrhiza species. Phytother Res. 2008;22:141–148.
- Murck H. Symptomatic protective action of glycyrrhizin (Licorice) in COVID-19 Infection? Front Immunol. 2020;11:1239.
- Armanini D, Lewicka S, Pratesi C, et al. Further studies on the mechanism of the mineralocorticoid action of licorice in humans. J Endocrinol Invest. 1996;19:624–629.
- Armanini D, Karbowiak I, Funder JW. Affinity of liquorice derivatives for mineralocorticoid and glucocorticoid receptors. Clin Endocrinol. 1983;19:609–612.
- Ding H, Deng W, Ding L, et al. Glycyrrhetinic acid and its derivatives as potential alternative medicine to relieve symptoms in nonhospitalized COVID-19 patients. J Med Virol. 2020;2200–2204. DOI:https://doi.org/10.1002/jmv.26064
- Sontia B, Mooney J, Gaudet L, et al. Pseudohyperaldosteronism, liquorice, and hypertension. J Clin Hypertens. 2008;10:153–157.
- Wang CY, Kao TC, Lo WH, et al. Glycyrrhizic acid and 18-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-B through PI3K p110 and p110 inhibitions. J Agric Food Chem. 2011;59:7726–7733.
- Kao TC, Shyu MH, Yen GC. Glycyrrhizic acid and 18-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3 signaling and glucocorticoid receptor activation. J Agric Food Chem. 2010;58:8623–8629.
- Chen S, Zou L, Li L, et al. The protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. PLoS One. 2013;8:e53662.
- Jeong HG, You HJ, Park SJ, et al. Hepatoprotective effects of 18-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: inhibition of cytochrome P450 2E1 expression. Pharmacol Res. 2002;46:221–227.
- Hardy ME, Hendricks JM, Paulson JM, et al. 18β-glycyrrhetinic acid inhibits rotavirus replication in culture. Virol J. 2012;9:96.
- Wang LJ, Geng CA, Ma YB, et al. Synthesis, biological evaluation and structure-activity relationships of glycyrrhetinic acid derivatives as novel anti-hepatitis B virus agents. Bioorg Med Chem Lett. 2012;22:3473–3479.
- Huang YC, Kuo CL, Lu KW, et al. 18-glycyrrhetinic acid induces apoptosis of HL-60 human leukemia cells through caspases-and mitochondria-dependent signaling pathways. Molecules. 2016;21:872.
- Huang RY, Chu YL, Huang QC, et al. 18β-Glycyrrhetinic acid suppresses cell proliferation through inhibiting thromboxane synthase in non-small cell lung cancer. PLoS One. 2014;9:e93690.
- Wu SY, Wang WJ, Dou JH, et al. Research progress on the protective effects of licorice-derived 18β-glycyrrhetinic acid against liver injury. Acta Pharmacol Sin. 2021;42:18–26.
- Li ZY, Tung YT, Chen SY, et al. Novel findings of 18β‐glycyrrhetinic acid on sRAGE secretion through inhibition of transient receptor potential canonical channels in high‐glucose environment. Biofactors. 2019;45:607–615.
- Wang K, Zhang Y, Cao Y, et al. Glycyrrhetinic acid alleviates acute lung injury by PI3K/AKT suppressing macrophagic Nlrp3 inflammasome activation. Biochem Biophys Res Commun. 2020;532:555–562.
- Zhang KX, Wang PR, Chen F, et al. Synthesis and Anti-HCV Activities of 18β-Glycyrrhetinic Acid Derivatives and Their In-silico ADMET analysis. Curr Comput Aided Drug Des. 2021;(in print): DOI:https://doi.org/10.2174/1573409916666200827104008
- Shen P, Zhang J, Zhu Y, et al. Microbial transformation of glycyrrhetinic acid derivatives by Bacillus subtilis ATCC 6633 and Bacillus megaterium CGMCC 1.1741 P. Bioorg Med Chem. 2020;28:115465.
- Satomi Y, Nishino H, Shibata S. Glycyrrhetinic acid and related compounds induce G1 arrest and apoptosis in human hepatocellular carcinoma HepG2. Anticancer Res. 2005;25:4043–4047.
- Wang D, Wong HK, Feng YB, et al. 18beta-Glycyrrhetinic acid induces apoptosis in pituitary adenoma cells via ROS/MAPKs-mediated pathway. J Neurooncol. 2014;116:221–230.
- Li S, Zhu JH, Cao LP, et al. Growth inhibitory in vitro effects of glycyrrhizic acid in U251 glioblastoma cell line. Neurol Sci. 2014;35:1115–1120.
- Shetty AV, Thirugnanam S, Dakshinamoorthy G, et al. 18-glycyrrhetinic acid targets prostate cancer cells by down-regulating inflammation-related genes. Int J Oncol. 2011;39:635–640.
- Lee CS, Kim YJ, Lee MS, et al. 18-Glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity. Life Sci. 2008;83:481–489.
- Hibasami H, Iwase H, Yoshioka K, et al. Glycyrrhetic acid (a metabolic substance and aglycon of glycyrrhizin) induces apoptosis in human hepatoma, promyelotic leukemia and stomach cancer cells. Int J Mol Med. 2006;17:215–219.
- Yang JC, Myung SC, Kim W, et al. 18-Glycyrrhetinic acid potentiates Hsp90 inhibition-induced apoptosis in human epithelial ovarian carcinoma cells via activation of death receptor and mitochondrial pathway. Mol Cell Biochem. 2012;370:209–219.
- Gao Z, Kang X, Ju Y, et al. Induction of apoptosis with mitochondrial membrane depolarization by a glycyrrhetinic acid derivative in human leukemia K562 cells. Cytotechnology. 2012;64:421–428.
- Bing X, Gao-Rong W, Xin-Yu Z, et al. An overview of structurally modified glycyrrhetinic acid derivatives as antitumor agents. Molecules. 2017;22:924.
- Csuk R. Recent developments in the synthesis of antitumor-active glycyrrhetinic acid derivatives. Mini-Rev Org Chem. 2014;11:253–261.
- Serbian I, Wolfram RK, Fischer L, et al. Hydroxylated boswellic and glycyrrhetinic acid derivatives: synthesis and cytotoxicity. Mediterr J Chem. 2018;7(4):286–293.
- Jun H, Yang W, Chang-Qi Z. Synthesis and anti-tumor activity of opened A-ring modified 18 beta-glycyrrhetinic acid derivatives. Chem J Chin Univ. 2010;31:1762–1768.
- Gao Y, Guo X, Li X, et al. The synthesis of glycyrrhetinic acid derivatives containing a nitrogen heterocycle and their antiproliferative effects in human leukemia cells. Molecules. 2010;15:4439–4449.
- Alho DPS, Salvador JAR, Cascante M, et al. Synthesis and antiproliferative activity of novel heterocyclic glycyrrhetinic acid derivatives. Molecules. 2019;24(766):1–21.
- Huang M, Gong P, Wang Y, et al. Synthesis and antitumor effects of novel 18β-glycyrrhetinic acid derivatives featuring an exocyclic α,β-unsaturated carbonyl moiety in ring A. Bioorg Chem. 2020;103:104187.
- Chadalapaka G, Jutooru I, McAlees A, et al. Structure-dependent inhibition of bladder and pancreatic cancer cell growth by 2-substituted glycyrrhetinic and ursolic acid derivatives. Bioorg Med Chem Lett. 2008;18:2633–2639.
- Yang LF, Xing Y, Xiao JX, et al. Synthesis of cyanoenone-modified diterpenoid analogs as novel bmi-1-mediated antitumor agents. ACS Med Chem Lett. 2018;9(11):1105–1110.
- Salomatina OV, Markov AV, Logashenko EB, et al. Synthesis of novel 2-cyano substituted glycyrrhetinic acid derivatives as inhibitors of cancer cells growth and NO production in LPS-activated J-774 cells. Bioorg Med Chem. 2014;22:585–593.
- Logashenko EB, Salomatina OV, Markov AV, et al. Synthesis and pro-apoptotic activity of novel glycyrrhetinic acid derivatives. ChemBioChem. 2011;12:784–794.
- Lin K-W, Huang A-M, Hour T-C, et al. 18β-Glycyrrhetinic acid derivatives induced mitochondrial-mediated apoptosis through reactive oxygen species-mediated p53 activation in NTUB1 cells. Bioorg Med Chem. 2011;19:4274–4285.
- Alho DPS, Salvador JAR, Cascante M, et al. Synthesis and antiproliferative activity of novel A-ring cleaved glycyrrhetinic acid. Molecules. 2019;24:2938.
- Csuk R, Schwarz S, Siewert B, et al. Synthesis and antitumor activity of ring A modified glycyrrhetinic acid derivatives. Eur J Med Chem. 2011;46:5356–5369.
- Jin L, Zhang B, Hua S, et al. Glycyrrhetinic acid derivatives containing aminophosphonate ester species as multidrug resistance reversers that block the NF-κB pathway and cell proliferation. Bioorg Med Chem Lett. 2018;28:3700–3707.
- Lallemand B, Chaix F, Bury M. N-(2-{3-[3,5-bis(trifluoromethyl)phenyl]ureido}ethyl)-glycyrrhetinamide (6b): a novel anticancer glycyrrhetinic acid derivative that targets the proteasome and displays anti-kinase activity. J Med Chem. 2011;54:6501–6513.
- Zheng Q-X, Wang R, Xu Y, et al. Design, preparation and studies regarding cytotoxic properties of glycyrrhetinic acid derivatives. Biol Pharm Bull. 2020;43:102–109.
- Schwarz S, Csuk R. Synthesis and antitumor activity of glycyrrhetinic acid derivatives. Bioorg Med Chem. 2010;18:7458–7474.
- Csuk R, Schwarz S, Kluge R. Improvement of the cytotoxicity and tumor selectivity of glycyrrhetinic acid by derivatization with bifunctional amino acids. Arch Pharm. 2011;344:505–513.
- Csuk R, Schwarz S, Siewert B, et al. Synthesis and cytotoxic activity of methyl glycyrrhetinate esterified with amino acids. Z Naturforsch B. 2012;67:731–746.
- Li Y, Feng L, Song ZF. Synthesis and anticancer activities of glycyrrhetinic acid derivatives. Molecules. 2016;21(199):1–20.
- Tatsuzaki J, Taniguchi M, Bastow KF. Anti-tumor agents 255: novel glycyrrhetinic acid-dehydrozingerone conjugates as cytotoxic agents. Bioorg Med Chem. 2007;15:6193–6199.
- Aminin DL, Menchinskaya ES, Pisliagin EA, et al. Anticancer activity of sea cucumber triterpene gycosides. Mar Drugs. 2015;13:1202–1223.
- Dai L, Li J, Yang J, et al. Enzymatic synthesis of novel glycyrrhizic acid glucosides using a Promiscuous bacillus glycosyltransferase. Catalysts. 2018;8(615):1–11.
- Schwarz S, Siewert B, Xavier NM, et al. A “natural” approach: synthesis and cytoxicity of monodesmosidic glycyrrhetinic acid glycosides. Eur J Med Chem. 2014;72:78–83.
- Csuk R, Schwarz S, Kluge R, et al. Synthesis and biological activity of some antitumor active derivatives from glycyrrhetinic acid. Eur J Med Chem. 2010;45:5718–5723.
- Wang R, Li Y, Huai X-D, et al. Design and preparation of derivatives of oleanolic and glycyrrhetinic acids with cytotoxic properties. Drug Des Dev Ther. 2018;12:1321–1336.
- Yadav DK, Kalani K, Khan F, et al. QSAR and docking based semi-synthesis and in vitro evaluation of 18-glycyrrhetinic acid derivatives against human lung cancer cell line A-549. Med Chem. 2013;9:1073–1084.
- Lai Y, Shen L, Zhang Z, et al. Synthesis and biological evaluation of furoxan-based nitric oxide-releasing derivatives of glycyrrhetinic acid as anti-hepatocellular carcinoma agents. Bioorg Med Chem Lett. 2010;20:6416–6620.
- Csuk R, Schwarz S, Siewert B. Conversions at C-30 of glycyrrhetinic acid and their impact on antitumor activity. Arch Pharm. 2012;345:223–230.
- Li K, Ma T, Cai J, et al. Conjugates of 18β-glycyrrhetinic acid derivatives with 3-(1H-benzo[d]imidazol-2-yl)propanoic acid as Pin1 inhibitors displaying anti-prostate cancer ability. Bioorg Med Chem. 2017;25:5441–5451.
- Hostetler BJ, Uchakina ON, Ban H, et al. Treatment of hematological malignancies with glycyrrhizic acid. Anticancer Res. 2017;37(3):997–1004.
- Su X, Wu L, Hu M, et al. Glycyrrhizic acid: a promising carrier material for anticancer therapy. Biomed Pharmacother. 2017;95:670–678.
- Smolarczyk R, Cichon T, Matuszczak S, et al. The role of glycyrrhizin, an inhibitor of HMGB1 protein, in anticancer therapy. Arch Immunol Ther Exp. 2012;60(5):391–399.
- Kohlschütter J, Michelfelder S, Trepel M. Drug delivery in acute myeloid leukemia. Expert Opin Drug Deliv. 2008;5(6):653–663.
- Pan X, Liu H, Jia G, et al. Microwave-assisted extraction of glycyrrhizic acid from licorice root. Biochem Eng J. 2000;5(3):173–177.
- Tian M, Yan H, Row KH, et al. Extraction of glycyrrhizic acid and glabridin from Licorice. Int J Mol Sci. 2008;9:571–577.
- Charpe TW, Rathod VK. Extraction of glycyrrhizic acid from licorice root using ultrasound: process intensification studies. Chem Eng Process. 2012;54:37–41.
- Cirillo G, Curcio M, Parisi OI, et al. Molecularly imprinted polymers for the selective extraction of glycyrrhizic acid from liquorice roots. Food Chem. 2011;125(3):1058–1063.
- Jang S, Lee AY, Lee AR, et al. Optimization of ultrasound-assisted extraction of glycyrrhizic acid from licorice using response surface methodology. Integr Med Res. 2017;6(4):388–394.
- Shabkhiz MA, Eikani MH, Sadr ZB, et al. Superheated water extraction of glycyrrhizic acid from licorice root. Food Chem. 2016;210:396–401.
- Hedayati A, Ghoreishi SM. Supercritical carbon dioxide extraction of glycyrrhizic acid from licorice plant root using binary entrainer: experimental optimization via response surface methodology. J Supercrit Fluid. 2015;100:209–217.
- Wang Q, Fu SMB. Development of multi-stage countercurrent extraction technology for the extraction of glycyrrhizic acid (GA) from licorice (Glycyrrhiza uralensis Fisch). Biochem Eng J. 2004;21(3):285–292.
- Edelman ER, Butala NM, Avery LL, et al. Case 30-2020: a 54-year-old man with sudden cardiac arrest. N Engl J Med. 2020;83:1263–1275.
- Sabbadin C, Bordin L, Donà G, et al. Licorice: from Pseudohyperaldosteronism to Therapeutic Uses. Front Endocrinol. 2019;10:484.
- Wang X, Tan Y, Zhang Y, et al. The novel glycyrrhetinic acid–tetramethylpyrazine conjugate TOGA induces anti-hepatocarcinogenesis by inhibiting the effects of tumor-associated macrophages on tumor cells. Pharmacol Res. 2020;161:105233.