1. Introduction
The discovery and development of any given drug is invariably a lengthy and expensive process in the pharmaceutical industry. It starts with the identification and validation of the putative molecular/cellular drug target, followed by generally lengthy preclinical and clinical investigations, with the process ending in a series of regulatory approvals. It is without question that the use of experimental animal models serves to better understand the origins, pathology, and the overall nature of comparable diseases of humans. Likewise, animal models serve in the development of safe and effective treatments and cures of such diseases and/or associated symptoms. The success rates for drug approval by regulatory agencies remain dismally low; this is despite the successes of the Human Genome Project and other molecular biology approaches that have helped spawn the identification of a large number of promising drug targets [Citation1]. The process of novel drug discovery and development has been estimated to take 10 to 15 years and cost as much as >1 billion US dollars (depending on the area of therapeutic use) in order to get one drug approved by a regulatory agency and bring it to the market for human use [Citation2]. In the United States (US), new drugs are approved by the US Food and Drug Administration (US FDA) for human use and only then will such drugs get marketed. The US FDA is a regulatory agency organized through Title 21 of the US code of federal regulations created in 1906 to control the quality of food and drugs. Similar agencies in European countries and Japan are known as the European Medicines Agency (EMA) and the Pharmaceuticals and Medical Devices Agency (PMDA), respectively [Citation3].
Animal models for drug discovery and development have played an important role in the characterization of the pathophysiology of diseases and associated mechanisms of injury, drug target identification, and evaluation of novel therapeutic agents for toxicity/safety, pharmacokinetics, pharmacodynamics, and efficacy [Citation4]. The specific animal model selected for a given drug to be tested and developed depends on the goal of the specific study. The conventional use of animal models in drug discovery is to establish and provide evidence for non-clinical ‘proof-of-concept’ for the safety, efficacy, and target of interest for specific drug molecules [Citation5]. Determining safety and efficacy parameters are critical processes during the initial phases of drug discovery and development, and also for subsequent regulatory approval for marketing and human use. Furthermore, the use of animal models is vitally important in terms of developing clinical predictions of the expected results in humans.
Although the literature is full of excellent examples that support the above comments, current efforts to develop new and improved arrays of both small, free-radical quenching radioprotective molecules and large, complex bioengineered drug recombinant molecules that serve to mitigate acute myelosuppressive effects of various cancer drug therapies provide good, relevant examples [Citation6–8]. Nevertheless, animal models and the associated preclinical data generated are not inherently infallible in terms of ‘predictiveness,’ as evidenced by the high failure rate of promising new drugs that fail to successfully serve as the translational bridge between preclinical and clinical phase studies. For new anti-cancer agents under study, the estimated rate of successful translation ability from animal model-based studies to clinical evaluations is a low 8% or less [Citation9]. There are many reasons for these translational failures including, but certainly not limited to, ineffective molecular targets and targeting, undesirable PK/PD profiles, and less than adequate drug-dose response relationships [Citation10]. Keeping the above in mind, the limits of data coming from a given preclinical animal model need to be recognized and taken into account when moving the new drug into clinical trials.
Animal models of various human diseases are sometimes viewed by some individuals (experimentalists, clinicians, drug regulators, etc.) as being imprecise and too distant from the ‘human condition.’ Animal models have come under criticism for their ability to predict new drug toxicity, safety, and efficacy in humans [Citation11,Citation12]. This criticism is not entirely unwarranted, and is due to a number of clinical trial failures of new, promising chemical entities that were found largely on positive and encouraging preclinical data generated in different animal models (). Despite such concerns, preclinical animal models remain a core of preclinical drug research and development; as such, they remain essential for regulatory approval for human use. Nevertheless, the limitations of in vivo disease models often restrict the translation of information from preclinical to clinical settings in the development of drugs intended for human use.
Table 1. Important animal models used in biomedical research
Herein, we provide our opinion on the necessity of animal models for modern drug discovery, suggest possible solutions, and propose future directions for research requiring animal models for drug discovery.
2. Standard, commonly utilized animal models: inbred, outbred, and other specialized strains of laboratory animals
Despite the advent of ‘tailor-made,’ genetically modified research animals, initial drug testing generally employs standard, commonly available laboratory animals. The use of such laboratory animals is necessitated by current US FDA guidelines that instruct drug developers to evaluate new drugs for their safety and efficacy profiles prior to testing in humans. These test animals run the full gamut of both small and large mammalian species, including (but certainly not limited to) mice, rats, rabbits, guinea pigs, canines, pigs, sheep, and nonhuman primates. Both inbred and outbred lines of these laboratory animals are used commonly for testing purposes and represent polar opposites in terms of familial matings. By definition, inbred animals are very closely genetically related (essentially homozygous due to a high fraction of identical genetic loci) due to prolonged and selective inbreeding (e.g., >20 breeding generations of sibling to sibling matings or parent to sibling matings). With extremely prolonged inbreeding, isogenic strains have been produced and are comprised of individuals that are essentially clones of one another. Inbred and outbred strains of laboratory animals effectively serve different purposes: inbred animals provide uniformity of potential test targets (molecules/cells/organs/animals) for drug testing and statistical analyses; outbred animals provide for a spread analysis of drug action among the more genetically diverse test population at large. Using the two strains within a given experiment provides for optimal analytic and statistical opportunities for the initial study, drug safety, and efficacy.
It is important to not only use healthy animals, but also animals with induced diseases or genetic disorders for drug development. In brief, the significance that these basic laboratory animals have played and will continue to play in gaining a better understanding of a myriad of basic and fundamental biological processes cannot be overstated, e.g., screening of new antimicrobials, development of monoclonal antibodies, concepts of immune tolerance, etc. [Citation13].
3. Other animal models of utility for drug research and development: selectively bred mutant, chimeric, and parabiotic animals
Selectively bred, mutant animals have been developed and utilized by medical researchers in the pursuit of preventive and/or therapeutic options for patients beset with species-comparable physiological/medical conditions. These include conditions such as obesity (obese rodents), cutaneous syndromes (hairless rodents), immunological deficiencies (athymic nude mice), and premature aging, etc. [Citation14].
Chimeric animals are derived from the fusion of multiple zygotes in utero shortly after fertilization. These animals, or ‘chimeras,’ possess more than one genotype, hence have multiple, genomically distinct physiological systems. As such, these unique animals can be and have been utilized not only for basic biological research, but also for pharmaceutical research. Examples of the latter include the use of chimeric mice (mouse-human chimeras) to assess selected molecular aspects of human metabolism that have an identified glucuronide metabolite that selectively inhibits the sodium-glucose transporter system in mammalian cells and tissues [Citation15].
Parabiotic animals are surgically adjoined animals that share single, common physiological systems, most notably the circulatory system. The technique has been successfully employed in a variety of research areas, including studies of obesity, aging, stem cell research, tissue regeneration, diabetes, organ transplantation, tumor biology, endocrinology, etc., and all of these study areas have had an impact on drug discovery, research, and development. This model has been used to investigate several complicated physiological problems; however, important parameters of recovery and exchange kinetics are not well characterized, which limits its experimental use and interpretation of results [Citation16].
4. Genetically engineered animal models for drug discovery
Animal models of given diseases generally do not precisely mirror comparable disease states of humans. As such and recognizing this limitation, research pathologists have made a concerted effort over many decades to alter the biology of select animal models in order to produce more 'human-like' disease states in experimental animals. Certainly, rodent tumor models with patient-derived xenografts (PDX) fall into this category, as do animals transplanted with humanized immune systems. Animal models are also used as surrogates of human biology due to ethical and moral reasons for limiting the experimental use of humans in general, or under specific circumstances, limiting their donated organs, tissues, or cells (e.g., fetal tissues). Despite the latter, however, preclinical drug safety and efficiency testing of new drugs using rodents with transplanted human tissues, i.e., the humanized mouse model, is consideredby the majority of experimentalists to not only to be acceptable, but likely more appropriate than using standard, rodents for selected preclinical investigations. As such, immunodeficient animals engrafted with functional human cells or tissues are becoming more acceptable and conventional as preclinical animal models for studying a large number of human diseases [Citation17]. Such studies allow for functional drug analyses that are essential for effective clinical translation. Animal models have been developed to study the multi-step cancer metastasis to various target organs using xenograft models. A xenograft is a graft taken from one species and transplanted to another species. These technologies have been significantly advanced by the establishment of genetically modified immunodeficient mice during the last two decades. Investigation of several human diseases is progressing using humanized mouse models due to the enhanced capability of human tissue engraftment, as well as the capacity of transplanted tissues to assume near-normal organ/tissue functions (e.g., proliferative and differentiative capacities of transplanted hematopoietic tissues).
Genetic mutations trigger thousands of inherited diseases, and genetically humanized animal models can offer enormous tools to accelerate the development and validation of new medicinals, and perhaps personalized medicines as well. Such models often employ custom genetic alterations (genome editing or anti-sense-mediated exon skipping) that target specific mutations with the aim of restoring normal function of previously altered, dysfunctional proteins. In brief, this strategy provides the affected cells or tissues with the mutation, a functioning copy of the lost gene, or cDNA to reinstate the missing protein [Citation18]. Because such genomic interventions target human mutations, the availability of humanized animal models is of significant utility. Furthermore, the use of humanized animals will clearly be helpful in developing and exploiting (i.e., fulfilling the medical ‘dream’) personalized medicine.
The capability to introduce genes of interest into the germline genome has offered a powerful tool for both basic research and pharmaceutical drug development. Selected genes of interest can be introduced into the targeted genome by homologous recombination, allowing for: (a) creation of genomically unique experimental animals essential for studying the nature of human-specific genes or associated diseases, (b) deletion or introduction of select gene mutations in order to investigate their function, (c) insertion of genes of microbial origin to investigate their role in various pathogenic processes, and (d) insertion of reporter genes in order to study and monitor expression of genes of interest [Citation19].
Transgenic animals have a foreign gene introduced into their genome. Such animals are usually produced by DNA microinjection into the pronuclei of a fertilized egg that is subsequently implanted into the oviduct of the surrogate mother. Transgenic animals have become a key tool in functional genomics in order to generate models for human diseases and validate new drugs [Citation20]. Transgenesis includes the addition of foreign genetic information to animals and specific inhibition of endogenous gene expression. The knockout animals are transgenic that have a specific interest gene disabled are transgenic, and are widely used to investigate both normal gene function, as well as the analyses of patho-biological roles of select genes involved in various disease states [Citation21]. In addition, such transgene/knockout animal models are actively used in the development of new therapeutics and associated strategies.
Gene therapy tools for adding or inserting an exogenous DNA copy into the target cell nucleus or genome may lead to side effects, as insertional mutations or latent (post-translation) expression of proteins. The programmable nucleases use a ‘cut-and-paste’ approach to remove the defect and install the correct gene. An RNA-guided genome editing tool known as clustered regularly interspaced short palindromic repeats/CRISPR-associated nuclease 9 (CRISPR-Cas9) provides numerous advantages over the conventional gene therapy and demonstrates therapeutic promises [Citation22]. CRISPR-Cas9 can be used in several ways for therapeutic purposes. It can correct the mutations and rescue the disease phenotypes. It can also engineer pathogen genomes for therapeutic purposes or induce protective or therapeutic mutations in host tissues. It has demonstrated promise in cancer gene therapy by deactivating oncogenic virus and inducing oncosuppressor expressions. The fast-growing use of CRISPR-Cas9 technology is appearing as an effective tool for the characterization and treatment of various human diseases. With time, this technology may revolutionize gene therapy and become a versatile option for gene therapy.
5. Need of animal models for drug development for chemical, biological, radiological, and nuclear (CBRN) agents: US FDA Animal Rule
There are special situations where drugs or biologics need regulatory agency approval for human use without conducting efficacy studies in human volunteers. In 2002, the US FDA released a special provision to accelerate the development of medical countermeasures for which efficacy studies cannot be conducted in humans because it would be unethical to deliberately expose humans to lethal or permanently deleterious chemical, biological, radiological, or nuclear (CBRN) agents for testing the efficacy of drugs [Citation23]. The regulations that dictate the path for approval of medicinals under 21 CFR 314.600 to 314.650 (drugs) or 21 CFR 601.90 t0 601.95 (biologic products) are referred to as the US FDA Animal Rule [Citation23]. The latest version of this guidance document has been compiled by the Center for Drug Evaluation and Research (CDER) in collaboration with the Center for Biologics Evaluation and Research (CBER) of the US FDA. Approval of new drugs under the Animal Rule can be followed only if efficacy studies of such agents under development cannot be accomplished in human volunteers since the execution of such clinical studies in humans would be unethical ().
Figure 1. Identification and development of drugs for regulatory approval and human use. Such drug development involves several steps and takes a significant amount of time. BLA, biologics license applications; CBRN, chemical, biological radiological and nuclear; EUA, emergency use authorization; FDA, Food and Drug Administration; IND, investigational new drug; NDA, new drug application; PBDD, phenotype-based drug discovery; TBDD, target-based drug discovery
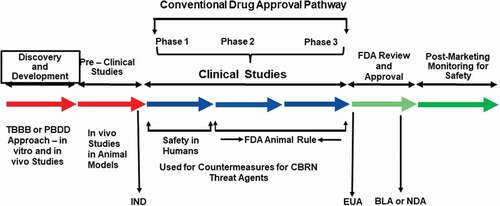
Under the Animal Rule, the US FDA depends on efficacy data from studies conducted in animal models for the effectiveness of the countermeasure only when: 1. there is a well-understood pathophysiological mechanism of the toxicity of the threat agent and its prevention or substantial mitigation by the countermeasure being developed, 2. the effect is demonstrated in more than one animal species and is expected to react with a response predictive for humans, unless the effect is demonstrated in a single animal species that is sufficiently well characterized as a model for predicting the response in humans, 3. the animal study endpoint is clearly related to the desired benefit in humans, generally the enhancement of survival or prevention of major morbidity, and 4. the data or information on the kinetics and pharmacodynamics of the product or other relevant data or information, in animals and humans, allows selection of an effective dose in humans [Citation23].
Substituting animals for human subjects for the assessment of drug efficacy was not intended to make it simpler to get regulatory approval for new agents, but rather as a way to circumvent the ethical and moral problem of exposing humans to potentially injurious levels of toxic substances simply for the purpose of testing a given drug’s efficacy. The reality is that the Animal Rule does not simplify the drug development process. It requires a significant amount of additional information regarding the animal model itself, the course and mechanism of the disease, and the mechanism of action of the agent being developed. Further, in the absence of conclusive human data, it becomes challenging to determine how a drug acts and why it works. Under such situations, it becomes difficult to generate conclusive data in animals to demonstrate the test drug’s effectiveness in humans. In addition, drugs assessed for efficacy under the Animal Rule still need to be investigated in human subjects, not only for safety/toxicity, but also to determine the appropriate drug dosing.
6. Approaches to advanced drug discovery and development
There are two different approaches to advance drug discovery and development: target-based drug discovery (TBDD) and phenotype-based drug discovery (PBDD). The former, TBDD, is referred to commonly as the molecular approach, while the latter, PBDD, is referred to as the physiology-based or empirical approach [Citation24,Citation25]. In a PBDD approach, the animal models play a more important role, as pharmacological actions are first identified in cells, tissues, or animal models without knowledge of specific molecular targets [Citation26]. The PBDD approach is reemerging as an alternative platform for drug discovery and is more dependent on studies conducted in animals. The majority of the drugs being developed for various indications employ the TBDD approach, and the PBDD approach is underutilized to some extent. A balanced drug discovery and development strategy might be a ‘hybrid’ type that is more dependent on TBDD for the primary drug identification and discovery via large-high throughput screening of candidate agents, and a secondary screening of those candidates utilizing the PBDD approach. This should be followed by a tertiary phase to pin down efficacious drugs that exactly target the specific indication [Citation27].
7. Expert opinion
Animal model-based scientific exploration remains a foundational pillar for the discovery and development of essential medicinals, designed and destined for the betterment of human health and condition. In practice, animal models have served, previously and currently, as a vital research tool in taking promising therapeutics from early phase preclinical studies to later clinical phase trials with humans. Highly successful translational studies are numerous and well documented; the advent of bioengineered recombinant growth factors and cytokines that serve to mitigate collateral normal tissue damage following radio and chemotherapeutic treatments have clearly and highly benefited from extensive preclinical safety and efficacy testing using both small and large animal models. Major medical advances in tissue transplantation would have been difficult, if not impossible, without the extensive use of appropriate animal models. Nevertheless, it is a basic improvement of a given preclinical animal model; its attributes and its experimental application are essential to improve the clinical ‘predictiveness’ of the model. Such ‘improvements’ could come in a variety of forms but would certainly include a better characterization of the animal model’s genome and selected drug-targets and improved algorithms for PK/PD data across species. Regardless, it is clear and unambiguous that preclinical animal-based studies often provide essential technical and scientific bases for subsequent and successful clinical translations in a number of key medical areas.
These animal models encompass not only an array of well-defined standard laboratory species and strains, but also a large number of experimental animals that have been selectively bred with tightly controlled husbandry that possess unique genetic, physiological, and anatomical features; features that, in aggregate, provide the experimentalist with additional testing opportunities.
These preclinical models can add great value to our knowledge of medicine and biology and the discovery and development of new drugs, provided such studies are conducted appropriately with precision. During the last few decades, investments in the development of new technologies using molecular biology tools and bioinformatics have been significantly higher compared with the investment in the development and improvement of predictive animal models for preclinical studies. There are three important components for improving both the quality and quantity of animal models: improving predictive features of existing models, refining the means in which animal models are used in decision-making, and investing in basic animal model research that has a clear focus on the development of clinically relevant, highly predictive models. The development of new models and the refinement of existing models are important. CRISPR-Cas9 provides numerous advantages over the conventional gene therapy and demonstrates therapeutic promises. This approach is definitely promising and demonstrates potential in cancer gene therapy by inducing oncosuppressor expressions and deactivating oncogenic virus, and may revolutionize the field of gene therapy. Further improvement can be made based on the experience gained.
In spite of significant progress in drug discovery and development strategies, the success rate of drugs during clinical investigations continues to be limited. One of the reasons for such drug failure is suboptimal preclinical data generated in various animal models for some indications to bridge the translational gap between the preclinical and clinical studies [Citation28]. It is not uncommon that animal-based data fail to accurately predict the full nature of a drug’s therapeutic efficacy, which is then often too low in clinical trials to obtain significant differentiation from a placebo. Although animal data help to prevent drugs with severe toxicity to be further developed, they do not predict subjective drug effects or idiosyncratic activities. This leads to high attrition rates during clinical development. Thus, the selection of a predictive and validated animal model is pivotal for overall success of drug discovery and development. Indications related to microbes or infectious diseases have the highest translational ability between animal and human studies. Adaptations in preclinical animal studies would be helpful to close the gap with the human situation, such as polypharmacy approach, larger number of animals in the different treatment arms with appropriate statistical power, differences in drug metabolism, the presence of specific unique human genotypes and the different comorbidities, especially for the aging clinical population. Though animal models are indispensable for drug development and approval, focusing on other approaches and the proper combination of animal models with these other approaches willdefinitely be helpful. In recent past, the process of drug discovery and development has been accelerated by computational biology, but it still remains a very challenging area.
Modern drugs are discovered and developed with the application of suitable and investigational animal models. In addition to vertebrate animals, some invertebrate animal models (e.g. Caenorhabditis elegans (nematode) and Drosophila melanogaster (fruit fly)) are drawing attention as drug discovery screening models [Citation29,Citation30]. There are some advantages with such models: lower cost, genetic amenability, high-throughput screening, and compatible culture conditions, as well as some disadvantages (misleading situation due to protein divergence between invertebrates and humans). Though invertebrate animal models are not perfect, they are a useful tool to bridge the gap between in vitro and preclinical vertebrate animal models [Citation31]. In recent past, the drug discovery process has shifted to in silico approaches, and such approaches are gaining ground with time. CRISPR is becoming a favorite tool for genome engineering for the drug discovery pipeline and helping in target identification to target validation, and supporting drug development. This technology will be refined with time and into a mature and future technology playing a vital role in genome engineering. In the future, there will be more novel and suitable animal models characterized to support cost-effective and efficient drug discovery process.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One referee is an employee of In Silico Biosciences. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgments
The opinions or assertions expressed herein are those of the authors and do not necessarily represent the opinions or policies of the Uniformed Services University of the Health Sciences, the Department of Defense, or the U.S. government. We are thankful to Ms. Alana Carpenter and Ms. Sara Nakamura-Peek for editing the manuscript.
Additional information
Funding
References
- Singh VK, Hanlon BK, Santiago PT, et al. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part III. Countermeasures under early stages of development along with ‘standard of care’ medicinal and procedures not requiring regulatory approval for use. Int J Radiat Biol. 2017;93:885–906.
- DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20–33.
- Tanimoto T, Tsubokura M, Mori J, et al. Differences in drug approval processes of 3 regulatory agencies: a case study of gemtuzumab ozogamicin. Invest New Drugs. 2013;31(2):473–478.
- Singh VK, Newman VL, Berg AN, et al. Animal models for acute radiation syndrome drug discovery. Expert Opin Drug Discov. 2015;10(5): 497–517.
- McGonigle P, Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol. 2014;87(1):162–171.
- Krumwieh D, Weinmann E, Siebold B, et al. Preclinical studies on synergistic effects of IL-1, IL-3, G-CSF and GM-CSF in cynomolgus monkeys. Int J Cell Cloning 1990;8 Suppl 1: 229–248; discussion 47-8.
- Singh VK, Seed TM. Entolimod as a radiation countermeasure for acute radiation syndrome. Drug Discov Today. 2021;26(1):17–30.
- Capizzi RL, Oster W. Chemoprotective and radioprotective effects of amifostine: an update of clinical trials. Int J Hematol. 2000;72:425–435.
- Seyhan AA. Lost in translation: the valley of death across preclinical and clinical divide – identification of problems and overcoming obstacles. Transl Med Commun. 2019;4(1):18.
- Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6:114–118.
- Arrowsmith J. Trial watch: phase III and submission failures: 2007-2010. Nat Rev Drug Discov. 2011;10(2):87.
- Arrowsmith J. Trial watch: phase II failures: 2008-2010. Nat Rev Drug Discov. 2011;10(5):328–329.
- Denayer A, Rtohe T, Van Roy M. Animal models in translational medicine: validation and prediction. New HorizonTransl Med. 2014;2:5–11.
- Lee H. Genetically engineered mouse models for drug development and preclinical trials. Biomol Ther (Seoul). 2014;22(4):267–274.
- Naritomi Y, Sanoh S, Ohta S. Chimeric mice with humanized liver: application in drug metabolism and pharmacokinetics studies for drug discovery. Drug Metab Pharmacokinet. 2018;33(1):31–39.
- Castellano JM, Palner M, Li SB, et al. In vivo assessment of behavioral recovery and circulatory exchange in the peritoneal parabiosis model. Sci Rep. 2016;6(1):29015.
- Ito R, Takahashi T, Ito M. Humanized mouse models: application to human diseases. J Cell Physiol. 2018;233(5):3723–3728.
- Aartsma-Rus A, van Putten M. The use of genetically humanized animal models for personalized medicine approaches. Dis Model Mech. 2019;13(2):dmm041673.
- Bouabe H, Okkenhaug K. Gene targeting in mice: a review. Methods Mol Biol. 2013;1064:315–336.
- Houdebine LM. Use of transgenic animals to improve human health and animal production. Reprod Domest Anim. 2005;40(4):269–281.
- Scherma M, Giunti E, Fratta W, et al. Gene knockout animal models of depression, anxiety and obsessive compulsive disorders. Psychiatr Genet. 2019;29(5):191–199.
- Leonova EI, Gainetdinov RR. CRISPR/Cas9 technology in translational biomedicine. Cell Physiol Biochem. 2020;54:354–370.
- Food US, Administration D. Guidance document: product development under the animal rule. 2015. cited 2018 Oct 20 Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf
- Swinney DC. Phenotypic vs. target-based drug discovery for first-in-class medicines. Clin Pharmacol Ther. 2013;93(4):299–301.
- Gintant GA, George CH. Introduction to biological complexity as a missing link in drug discovery. Expert Opin Drug Discov. 2018;13(8):753–763.
- Singh VK, Seed TM, Olabisi AO. Drug discovery strategies for acute radiation syndrome. Expert Opin Drug Discov. 2019;14(7):701–715.
- Vasaikar S, Bhatia P, Bhatia PG, et al. Complementary approaches to existing target based drug discovery for identifying novel drug targets. Biomedicines. 2016;4(4):27.
- van der Worp HB, Howells DW, Sena ES, et al. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7(3):e1000245.
- Tickoo S, Russell S. Drosophila melanogaster as a model system for drug discovery and pathway screening. Curr Opin Pharmacol. 2002;2(5):555–560.
- Artal-Sanz M, de Jong L, Tavernarakis N. Caenorhabditis elegans: a versatile platform for drug discovery. Biotechnol J. 2006;1(12):1405–1418.
- Segalat L. Invertebrate animal models of diseases as screening tools in drug discovery. ACS Chem Biol. 2007;2(4):231–236.
