?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Lack of efficacy of weight loss(WL) interventions is attributed in-part to low adherence to dietary/physical activity(PA) recommendations. However, some compensation may occur in PA as a response to energy restriction such as a decrease in non-exercise PA(NEPA) or non-exercise activity thermogenesis(NEAT). The current study aim was (1) to investigate whether adaptive thermogenesis(AT) in NEAT occurs after WL, and (2) to understand the associations of these compensations with WL. Ninety-four former athletes [mean±SD, age: 43.0±9.4y, BMI: 31.1±4.3 kg/m2, 34.0% female] were recruited and randomly assigned to intervention or control groups (IG, CG). The IG underwent a one-year lifestyle WL-intervention; no treatments were administered to the CG. PA was measured using accelerometery and NEAT was predicted with a model including sample baseline characteristics. AT was calculated as measuredNEAT4mo/12mo(kcal/d)–predictedNEAT4mo/12mo(kcal/d)-measuredNEATbaseline(kcal/d)–predictedNEATbaseline(kcal/d). Dual-energy x-ray absorptiometry was used to assess fat-free mass and fat mass. No differences were found in the IG for NEAT or NEPA after WL. Considering mean values, AT was not found for either group. The SD of individual response (SDIR) for AT was −2(4-months) and 24(12-months) (smallest worthwhile change = 87kcal/d), suggesting that the interindividual variability regarding AT in NEAT is not relevant and the variability in this outcome might reflect a large within-subject variability and/or a large degree of random measurement error. No associations were found between AT in NEAT and changes in body composition. Further studies are needed to clarify the mechanisms behind the large variability in AT observed in NEAT and related changes in NEPA to better implement lifestyle-induced WL interventions.
Highlights
No significant differences were found for non-exercise activity thermogenesis (NEAT) or non-exercise physical activity (NEPA) after the weight loss (WL) intervention;
Although a large variability was found for NEAT and NEPA, the interindividual variability regarding these outcomes is not relevant. The variability in these outcomes might reflect a large within-subject variability and/or a large degree of random measurement error;
Although no energy conservation was observed in NEAT after moderate WL (mean values), further studies are needed to clarify the mechanisms behind the large variability in adaptive thermogenesis observed in NEAT and related changes in NEPA to better implement lifestyle-induced WL interventions.
Trial registration: ClinicalTrials.gov identifier: NCT03031951.
Introduction
When retiring from their sports career, athletes struggle with maintaining their regular exercise habits, reducing drastically their energy expenditure (EE), while energy intake does not always show similar reductions (Stubbs et al., Citation2004). If this positive energy balance (EB) is maintained, weight gain is to be expected, leading to obesity and obese-related adverse health conditions (Griffin et al., Citation2016). Despite the increasing number of weight loss (WL) interventions globally, their lack of efficacy (i.e. lower-than-expected WL, weight regain) is still a matter of debate (Aronne et al., Citation2021). Although these discouraging results are mostly attributed to the lack of adherence to dietary and/or physical activity (PA) recommendations (Heymsfield et al., Citation2007), metabolic and behavioural adaptations my also occur as a response to a prolonged negative EB (Nunes et al., Citation2021).
As resting energy expenditure (REE) is the major contributor to total daily energy expenditure (TDEE), most investigations focused on exploring the lower-than-expected decreases in REE (i.e. adaptive thermogenesis – AT) following WL interventions (Nunes et al., Citation2021). Nevertheless, compensations in other EE components have been highlighted, particularly those related with regular PA habits (Levine et al., Citation1999).
PA (measured in minutes/day) is defined as “any bodily movement produced by skeletal muscles that results in energy expenditure”, being divided in (i) exercise, i.e. a planned and structured PA with a specific aim regarding physical fitness; and (ii) daily life activities, such as fidgeting, posture maintenance and non-specific ambulatory activities, which is considered non-exercise PA (NEPA) (Silva et al., Citation2018). Following REE, the energy expended in PA [PA-induced EE (PAEE)] is the most significant contributor to TDEE, representing the overall EE during movement activities (measured in kcal/day) (Silva et al., Citation2018). Similarly to PA, daily PAEE can also be further divided into: (i) the energy expended during exercise/sports practice [exercise-induced energy expenditure (EiEE)]; and (ii) the energy expended with activities that are not considered exercise (NEPA), defined as non-exercise activity thermogenesis (NEAT).
Therefore, PAEE can potentially play an important role toward the WL and long-term maintenance (Ostendorf et al., Citation2019). In fact, whereas REE and thermic effect of food provide similar levels of contribution to the TDEE variance, PAEE (specially NEAT) depicts a greater variation in TDEE within and between individuals (accounting for 5–50% of TDEE), due to the large variability in NEPA (von Loeffelholz & Birkenfeld, Citation2000). For this reason, assessing NEPA and/or NEAT in WL interventions may represent a unique opportunity to determine its potential impact on weight management.
Adopting a prolonged caloric restriction may also play an important role regarding the decrease in NEPA, and consequently NEAT (Martin et al., Citation2011; Redman et al., Citation2009). Considering these potential marked decreases in NEAT are associated with higher rates of WL, it is expected that such energy imbalances may lead to an increase in energy conservation processes (Silva et al., Citation2018). Therefore, along with the lack of adherence to the diet and/or exercise recommendations, these compensatory responses may play an important role in weight management, undermining the magnitude of WL and its maintenance.
Despite an increasing research interest in examining the mechanisms underlying the NEPA/NEAT responses to WL interventions, this relationship remains to be fully understood (Silva et al., Citation2018). Additionally, there is a research gap concerning the effects of a negative EB (through dietary-induced energy restriction) on changes in NEAT and related NEPA and how those changes may affect WL. Therefore, the aims of the present investigation were (1) to investigate whether adaptive thermogenesis in NEAT occurred after a moderate WL, and (2) to understand how these compensations were associated with the magnitude of WL.
Methodology
This investigation is part of the Champ4life project, a lifestyle WL intervention targeting inactive former elite athletes (Silva et al., Citation2020) and the main results of this programme are described in detail elsewhere (Silva et al., Citation2021). The programme comprised of a 1-year lifestyle intervention that consisted of a 4-month WL intervention and an 8-month WL maintenance period. The Champ4life project was approved by the Ethics Committee of the Faculty of Human Kinetics, University of Lisbon (Lisbon, Portugal) (CEFMH Approval Number: 16/2016) and was conducted in accordance with the Declaration of Helsinki for human studies from the World Medical Association (World Medical Association, Citation2008). The trial is registered at www.clinicaltrials.gov (clinicaltrials.gov ID: NCT03031951).
Lifestyle intervention
The IG underwent a self-determination-based intervention, consisting of educational weekly sessions targeting diet, eating behaviour, PA, and behaviour change domains. Participants were followed throughout the programme by a certified dietitian, to adjust their diet and perform a moderate caloric deficit (∼300 kcal.d−1). Regarding PA habits, the participants were only encouraged to increase their PA levels and to decrease the time spent in sedentary behaviour. For the 8-month weight maintenance period, nutritional appointments were held to create a neutral EB.
Body composition
Participants had their weight and height measured to the nearest 0.01 kg and 0.1 cm, using a weight scale and a stadiometer (SECA, Hamburg, Germany), respectively. Body Mass Index (BMI) was calculated as weight (kg) divided by the square of the height (m) and classified according to the World Health Organization (WHO) cutoffs (Weir & Jan, Citation2022). To assess total and regional fat mass (FM) and fat-free mass (FFM), dual-energy X-ray absorptiometry (DXA; Hologic Explorer-W, Waltham, MA, USA) was used, as described elsewhere (Park et al., Citation2002).
Physical activity (PA)
PA (min/day) was objectively measured using a tri-axial accelerometer (ActiGraph GT3X+, Pensacola, FL). Participants were asked to wear the accelerometer on the right side of the hip for 7 consecutive days and to only remove the sensor during sleep and water-based activities (e.g. bathing and swimming). The accelerometers were initialised on the morning of the assessment day and data were recorded in 15-s epochs and reintegrated into 60-s epochs and using a 100 Hz frequency. Periods of at least 60 consecutive minutes of zero counts were considered as non-wear time. A valid day was defined as having ≥600 min of monitor wear per day. Only participants with at least three valid days (with at least one weekend-day) were included in the analysis.
A logbook was given to the participants to record the exercise sessions (type and duration of the activities – start and end time). If participants were not able to use the accelerometer during the exercise session (e.g. water-based activities), this information should be stated in the logbook. The time spent in exercise was removed from the total PA to determine NEPA (PA in daily activities that are not considered exercise, min/day). By contrast, the time excluded for NEPA analysis plus registered information from structured PA in which the participants did not use the accelerometer was used to determine overall levels of exercise. Participants were also asked to record daily waking and sleeping hours, as well as the timings and reasons for not using the accelerometer.
Energy expenditure (EE) measures
Exercise-induced energy expenditure (EiEE) and non-exercise activity thermogenesis (NEAT)
The caloric expenditure (kcal/d) of both structured (exercise) and unstructured PA (NEPA) was calculated from Freedson Combination ’98 algorithm (Sasaki et al., Citation2011), which considers the Work-Energy Theorem and the Freedson ’98 equation to calculate EE under 1951 and above 1952 counts, respectively. The energy expended in NEPA, i.e. non-exercise activity thermogenesis (NEAT, kcal/d) was calculated by applying the algorithm over the time spent in non-exercise related activities. On the other hand, the EE of exercise (EiEE) was determined from the combination of the data excluded in the NEAT analysis and additional data of PA participants reported when the accelerometer was not used. The EiEE not recorded with the accelerometer was calculated using specific PA metabolic equivalents (METs) of the 2011 Compendium of Physical Activities (Ainsworth et al., Citation2011).
Resting energy expenditure (REE)
The MedGraphics CPX Ultima indirect calorimeter (MedGraphics Corporation, Breezeex Software, Italy) was used to measure breath-by-breath oxygen consumption (V̇O2) and carbon dioxide production (V̇CO2). The flow and volume were measured using a pneumotachograph calibrated with a 3L-syringe (Hans Rudolph, inc.TM). The assessment was performed in the morning, after an overnight fast. Before testing, participants were instructed about all the procedures and asked to relax, breathe normally, and not to sleep or talk during the evaluation. Participants underwent a resting period of ∼15 min, before the attachment of the calorimeter device to the mask. The exam duration was ∼30 min, where the lowest mean of 5 min of steady state (i.e. coefficient of variance ≤ 10% for V̇O2 and V̇CO2), between the 5 and the 25 min of REE assessment, with respiratory exchange ratio between 0.7 and 1.0, were considered for analysis.
Total daily energy expenditure (TEE)
TDEE was estimated as the sum of REE, NEAT and EiEE, divided by 0.9 (i.e. assuming the thermic effect of food accounts for 10% of TDEE) (Weststrate, Citation1993).
Statistics
Statistical analysis was performed using IBM SPSS statistics version 27.0 (IBM, Chicago, Illinois, USA). Linear mixed models included randomised group and time as fixed effects, with sex as a covariate, to assess primary and secondary outcomes for the impact of group, time (baseline– 0 months, post-intervention– 4 months, and follow-up– 12 months), and group-by-time interaction. The covariance matrix for repeated measures within subjects over time was modelled as Compound Symmetry. Model residual distributions were examined graphically, and by using the Kolmogorov–Smirnov test, and no data transformations were necessary. Differences-in-differences (DiD) were calculated between the IG and CG throughout time, calculated as the difference between changes for IG and changes for CG. To remove the effect of the hours spent with the accelerometer (usage time), NEPA was adjusted for daily wear time (percentage).
To evaluate the proportion of response in the IG, participants were classified as “responders” and “non responders” according to the typical error (TE) method proposed by Swinton et al. (Citation2018). TE was assessed by dividing the SD of the changes (difference between 4 or 12 months’ time point and the baseline) for the CG by . A “responder” is considered an individual who showed beneficial changes that were greater than TE (Swinton et al., Citation2018). Chi-square tests were performed to compare the response rates between IG and CG.
Multiple linear regression models were performed with the baseline characteristics of all participants to generate equations to predict NEAT, defined as:
Where wear time is considered the amount of time that a participant wore the accelerometer divided by 24 h. The generated equations were used to predict values for the aforementioned EE component after 4 months and at the end of the intervention (1-year). Adaptive thermogenesis (AT) was calculated by subtracting measured NEAT (through accelerometry) by predicted NEAT (equation model) and then subtracting the so called residuals (i.e. the difference between the measured and the predicted NEAT at baseline), such as:
Where negative values indicate a lower-than-expected decrease in NEAT considering the changes in body composition, i.e. the measured NEAT is lower than predicted NEAT, whereas positive values represent a change in NEAT equal to or greater than the predicted NEAT (measured NEAT higher than predicted NEAT). To understand if interindividual differences are present, the SD of individual response (SDIR) was calculated according to Atkinson and Batterham (Citation2015):
If the SDCG surpasses the SDIG, the SDIR formula was reversed and the SDIR was reported as a negative value (Bonafiglia et al., Citation2021). A positive SDIR suggests that there is evidence of interindividual differences in the outcome responses, while a negative SDIR indicates that these differences are inexistent, suggesting that the reported variability may be due to a large degree of random measurement error and/or within-subject variability (Bonafiglia et al., Citation2021). These values were compared to the smallest worthwhile change (SWC), calculated by multiplying 0.2 by the SD of the CG at baseline. A SDIR > SWC suggests meaningful interindividual differences, while a SDIR < SWC insinuates that interindividual differences are irrelevant (Atkinson & Batterham, Citation2015). Ninety-five percent confidence intervals (95%CI) were estimated by using the following equation (Hopkins, Citation2015):
All analyses were intention-to-treat including data from all participants who were randomly assigned. Sensitivity analyses were conducted out for analyses of NEAT and NEPA, by using imputation of missing data based on multivariate linear regression to simultaneously predict missing outcomes data from body composition measures and its changes over time. Statistical significance was set at p < 0.05 (2-tailed).
Results
The baseline characteristics of the Champ4Life participants are presented elsewhere (Silva et al., Citation2021). Briefly, 94 participants were included and randomly divided in two groups: intervention [N = 49; mean (SD): BMI = 31.7 (3.9) kg/m2, age = 42.4 (7.3) y, 35% females] and control group [N = 45; mean (SD): BMI = 30.5 (4.7) kg/m2, age = 43.6 (11.3) y, 33% females]. At the end, the dropout rate was ∼27.7%, being similar between groups (28.6% and 26.7% for the IG and CG, respectively).
After 4 months, participants from the IG achieved a greater WL [estimated difference (ED) from DiD = −4.7 kg (95% CI: −6.1 to −3.3; p < 0.001)] when compared to the CG. Considering body composition stores, the IG lost a greater amount of FM [FM (kg): ED = −3.8 kg (95%CI: −5.1 to −2.6) p < 0.001 and FM (%): ED = −2.6% (95%CI: −3.6 to −1.7) p < 0.001] and were able to maintain their FFM throughout time. During the follow-up period, weight and FM changes remained significant [weight: ED = −5.3 kg (95%CI: −6.9 to −3.8), p < 0.001; FM: ED = −4.1 kg (95%CI: −5.4 to −2.8) p < 0.001 and ED = −3.1% (95%CI: −4.1 to −2.1) p < 0.001]. No changes were observed in weight, FM or FFM for the CG (p > 0.05 for DiD).
The TE for WL was 1.72 kg, corresponding to a 1.88% change in weight. Thirty-one participants from the IG (75.6%) were classified as “responders” for WL, with differences in the proportion of responders in both groups (Chi-square test = 33.47, p < 0.001).
Values of the EE components and comparisons between groups and over time (baseline vs 4 months or baseline vs 12 months) are presented in . The values for REE were already presented and discussed in detail (Nunes et al., Citation2022).
Table 1. Estimated means of energy expenditure components.
A time*group interaction was found for EiEE and exercise between groups (p < 0.001). Participants from the IG showed an increase on exercise and EiEE at 4 months. However, after the follow-up period, the results were no longer significant. Although the IG achieved a ∼5% WL, no differences were found for NEPA nor NEAT over time.
The TE for NEPA was 57.7 min/day, corresponding to a change of 6.9%. Only 9 (18.4%) participants from the IG were classified as responders (i.e. with significant increases in NEPA), with no differences in the proportion of responders in both groups (Chi-square test = 0.548, p = 0.459).
Values for AT for NEAT are presented in .
Table 2. Adaptive thermogenesis for NEAT.
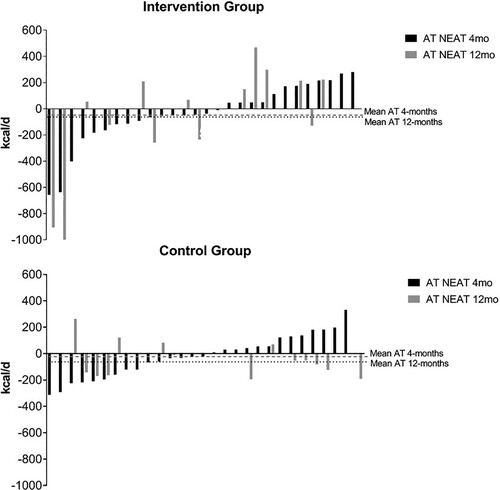
The IG did not show a lower-than-expected decrease for NEAT. No differences were found between groups. A negative SDIR (−2) was observed for AT in NEAT after 4 months, while a positive but smaller than the SWC value was found at 12 months (SDIR = 24; SWC = 87.0). The variability among participants is graphically presented in .
No associations were found (adjusted by group) between changes in NEPA and WL (kg: −0.166, p = 0.398; %: −0.245, p = 0.210), FM loss (kg and %) (kg: −0.135, p = 0.494; %: −0.072, p = 0.716) nor EB (kcal/d) (−0.137, p = 0.488) at 4-months. After 12 months the associations remained irrelevant (WL(kg): −0.092, p = 0.629; WL(%): −0.146, p = 0.443; FM loss (kg): −0.101, p = 0.596, FM loss (%): −0.136, p = 0.475; EB: −0.053, p = 0.780).
Similarly, AT in NEAT was not associated with the degree of WL (4 months: kg: −0.060, p = 0.762; %: −0.032, p = 0.873; 12 months: kg: −0.338, p = 0.079, %: −0.338, p = 0.079) nor EB (4 months: −0.126, p = 0.521, 12 months: −0.281, p = 0.148).
After performing single imputation for missing data, the results of sensitivity analyses for NEAT and NEPA were similar and are presented as supplementary file (table S1).
Discussion
Even though no energy conservation was found in NEAT after the Champ4Life intervention, large variability in this PAEE component was observed among individuals.
However, this heterogeneity in observed responses might reflect a large within-subject variability and/or a large degree of random measurement error (Atkinson & Batterham, Citation2015), as the SDIR was negative at 4 months. After 1 year, although the SDIR was positive, it did not exceed the SWC, suggesting that the interindividual variability regarding AT in NEAT is not relevant. In addition, no associations were found between the degree of energy conservation (AT) in NEAT and the magnitude of WL or changes in body composition stores.
Along with its well-known health-related benefits, PA seems to play an important role in weight management, potentiating WL and preventing weight regain (Swift et al., Citation2018). However, a suggestion is that during prolonged negative EB (through a caloric deficit and/or increasing exercise) some behavioural compensations may occur, such as decreases in NEPA (King et al., Citation2007). While this reduction in NEPA appears not to occur in most exercise-only interventions (Fedewa et al., Citation2017), some studies involving diet-only interventions reported a compensation in this component (de Groot et al., Citation1990; Racette et al., Citation1995). Nevertheless, the effects of a diet and/or an exercise intervention on NEPA/NEAT are still contradictory, as some authors found a decrease in NEPA/NEAT, while others reported no compensations in these components (Silva et al., Citation2018).
Despite the expected substantial decrease in PAEE due to a lowering in body mass with dieting (Levine et al., Citation2001; Ostendorf et al., Citation2019), decreases in NEPA will consequently lead to a decrease in NEAT. Thus, reducing NEPA might lead to a decrease in TDEE, affecting the initially created negative EB and consequently, the ability to lose weight. In our study, NEPA remained stable, even in participants that significantly increased their exercise (Riou et al., Citation2019) showing that this change did not lead to a decrease in NEPA. Contrary to other studies, the Champ4life programme was a Self-Determination Theory intervention, where participants were taught, through educational sessions, the benefits of increasing PA, not only by increasing exercise, but specifically by decreasing their time spent in sedentary behaviours and being more active (Silva et al., Citation2021). Therefore, in this intervention, we were not focused on delivering exercise sessions nor a personalised exercise plan, but rather giving simple strategies to increase PA and encourage participants to adapt those strategies according to their lives and routines. Thus, the authors believe that this strategy may have helped on maintaining a similar NEPA not only after WL but also during the follow-up period.
Nevertheless, higher-than-expected reductions in NEAT have been pointed out as a compensatory response to a caloric restriction and/or increasing exercise, undermining negative EB and, consequently, the ability to lose weight (Dhurandhar et al., Citation2015). The effect of caloric restriction is also known to play an important role in PAEE (i.e. NEAT and EiEE). According to Redman et al., which aimed to examine the metabolic and behavioural compensations in 4 intervention groups (control, 2 groups of diet-only, and a group with combined diet and exercise), NEAT was found to decrease only in the participants from the 2 diet-only groups (Redman et al., Citation2009). Similarly, the effect of caloric restriction was found to be linked to a substantial decrease in NEAT, independently of sex and age (Martin et al., Citation2011). These findings are in agreement with previous research demonstrating that caloric restriction may have a negative influence on PAEE, even during moderate caloric restriction interventions (Martin et al., Citation2011). In our investigation and comparably to NEPA, no differences were found throughout time in NEAT (mean values). The degree of compensation (i.e. AT) in this component was also studied through a predictive equation, similarly to what is usually used to assess AT in REE (Nunes et al., Citation2021).
Both groups did not show a significant AT for NEAT after 4 months. Also, at the end of the Champ4life programme, no differences were found between groups. Nevertheless, a huge variability among participants from both groups was found, emphasising the importance of analysing not only the mean responses but also the inter-individual variability in what concerns to outcomes from WL interventions. In fact, despite the Champ4life being well-succeeded on improving body composition (Silva et al., Citation2021), participants showed a large variability for the amount of WL and FM loss.
In our study, no associations were found between the degree of compensation on NEAT and the magnitude of WL and EB. In contrast, associations between changes in NEAT and body composition changes were reported in other studies (Herrmann et al., Citation2015; Martin et al., Citation2011). In line with this, it is suggested that individuals with larger decreases in NEAT or PAEE are generally those with high rates of weight regain (Herrmann et al., Citation2015). Even though our participants were well-succeeded in maintaining their weight reduced during the follow up period (Silva et al., Citation2021), a large variability was observed for changes in body composition. Due to the importance of examining the impact of inter-individual variability when considering the amount of WL after an intervention (Dent et al., Citation2020), further research on the inter-individual variability on NEAT is needed.
The magnitude of NEAT compensation might be affected by several factors, including either biological and non-biological factors (i.e. the type of study sample and methodology, the duration of the caloric restriction and the magnitude of WL). In terms of non-biological factors, a recent systematic review (Silva et al., Citation2018) has highlighted that studies that reporting decreases in NEAT were mostly those with higher magnitude of WL. Considering that our participants only lost a moderate amount of weight (∼5%), which is a smaller amount when compared with the aforementioned studies, the absence of differences for NEAT throughout the intervention was expectable. Nevertheless, our results go along with other studies with larger WL (∼10%) (Leibel et al., Citation1995; Levine et al., Citation2005), with no reduction in NEAT after a WL intervention. Such findings may be justified by the preservation of FFM, and consequently skeletal muscle mass, which are known to have a role in mediating the alterations in EE under the occurrence of WL (Leibel et al., Citation1995).
Another non-biological factor that may explain the discrepancy among investigations concerns to the methodologies used to measure EE, in particular NEAT. While some studies measured EE components with respiratory chambers, others used METs algorithms calculated from accelerometers used under free-living conditions (Silva et al., Citation2018). Despite respiratory chambers being the current gold-standard for assessing human EE, its use to measure NEAT may compromise the time expended in voluntary PA, underestimating PAEE and, consequently, TDEE (Rosenbaum et al., Citation1996). Consequently, a growing number of investigations has been examining accelerometer-derived EE, as it provides more practical and realistic estimation of total PAEE based on free-living conditions. On the other hand, some challenges may arise when estimating NEAT during an intervention, considering that changes in this component may either reflect changes in the muscular efficiency (Rosenbaum et al., Citation2003) and/or changes in NEPA (time spend and/or intensity) (Levine et al., Citation2001; Redman et al., Citation2009). Therefore, further studies are needed to better understand how accelerometers EE prediction is influenced by a caloric restriction intervention.
Despite this interesting discussion, the limitations of this study should be addressed. First, the percentage of dropouts should be considered, as ∼30% of participants were lost to follow up that may influence the findings of the study. Second, even though no compensations in NEPA and predictive equation derived NEAT were found, we cannot state that decreases in NEPA or a lower-than-expected decrease in NEAT would still not occur if participants lost larger amounts of weight. Third, as an alternative to respiratory chambers, known as the gold- standard for assessing human PAEE, we used specific METs algorithms calculated from the ActiGraph GT3X+ accelerometer (ActiGraph, Pensacola, FL). Despite PAEE being indirectly estimated through accelerometry, when compared with reference methods, this technique accurately predicts the PAEE over a wide range of PA (Kumahara et al., Citation2004) and provides a more representative measure of free-living PAEE. Fourth, since EE components were assessed through accelerometry, where participants were asked to remove the device only when sleeping and water-based activities (e.g. bathing and swimming), there may have led to an underestimation of PAEE and TDEE. Even though we were not able to objectively measure EE during these activities, an additional logbook was given to the participants to record the type and duration of activities performed without the accelerometer.
Lastly, since this investigation was based on the Self-Determination Theory, where participants were taught about the importance to increase PA and decrease the time in sedentary behaviours following the principles of autonomy, competence, and relatedness (Deci & Ryan, Citation2000), there may have existed different individual responses in terms of the intensity, duration and practice location of PA. However, the research team ensured that all participants had access to the same information throughout the whole intervention.
Therefore, despite no differences at the group level were found for NEAT after a moderate WL, the large variability should be taken into account when studying the potential energy conservation in this component. Therefore, health-related professionals should consider the potential reduction of energy expenditure during free-living PA beyond that expected when implementing lifestyle-induced weight loss interventions.
Author contributions
The Champ4Life project led by Primary Investigator A.M.S. obtained funding for the research. C.L.N and A.M.S. conceptualised and designed the study. C.L.N, G.B.R and F.J acquired the data. C.L.N. performed the data analysis and interpretation. C.L.N and G.B.R. wrote the first draft of the manuscript. All authors revised the manuscript critically and contributed to the final approval of the version to be submitted.
Supplemental Material
Download MS Word (17.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ainsworth, B. E., Haskell, W. L., Herrmann, S. D., Meckes, N., Bassett, D. R., Tudor-Locke, C., Greer, J. L., Vezina, J., Whitt-Glover, M. C., & Leon, A. S. (2011). 2011 compendium of physical activities: A second update of codes and MET values. Medicine & Science in Sports & Exercise, 43(8), 1575–1581. https://doi.org/10.1249/MSS.0b013e31821ece12
- Aronne, L. J., Hall, K. D., Jakicic, J. M., Leibel, R. L., Lowe, M. R., Rosenbaum, M., & Klein, S. (2021). Describing the weight-reduced state: Physiology, behavior, and interventions. Obesity, 29(S1), S9–S24. https://doi.org/10.1002/oby.23086
- Atkinson, G., & Batterham, A. M. (2015). True and false interindividual differences in the physiological response to an intervention. Experimental Physiology, 100(6), 577–588. https://doi.org/10.1113/EP085070
- Bonafiglia, J. T., Islam, H., Preobrazenski, N., Ma, A., Deschenes, M., Erlich, A. T., Quadrilatero, J., Hood, D. A., & Gurd, B. J. (2021). Examining interindividual differences in select muscle and whole-body adaptations to continuous endurance training. Experimental Physiology, 106(11), 2168–2176. https://doi.org/10.1113/EP089421
- Deci, E. L., & Ryan, R. M. (2000). The “what” and “why” of goal pursuits: Human needs and the self-determination of behavior. Psychological Inquiry, 11(4), 227–268. https://doi.org/10.1207/S15327965PLI1104_01
- de Groot, L. C., van Es, A. J., van Raaij, J. M., Vogt, J. E., & Hautvast, J. G. (1990). Energy metabolism of overweight women 1 mo and 1 y after an 8-wk slimming period. The American Journal of Clinical Nutrition, 51(4), 578–583. https://doi.org/10.1093/ajcn/51.4.578
- Dent, R., McPherson, R., & Harper, M.-E. (2020). Factors affecting weight loss variability in obesity. Metabolism, 113, 154388. https://doi.org/10.1016/j.metabol.2020.154388
- Dhurandhar, E. J., Kaiser, K. A., Dawson, J. A., Alcorn, A. S., Keating, K. D., & Allison, D. B. (2015). Predicting adult weight change in the real world: A systematic review and meta-analysis accounting for compensatory changes in energy intake or expenditure. International Journal of Obesity, 39(8), 1181–1187. https://doi.org/10.1038/ijo.2014.184
- Fedewa, M. V., Hathaway, E. D., Williams, T. D., & Schmidt, M. D. (2017). Effect of exercise training on non-exercise physical activity: A systematic review and meta-analysis of randomized controlled trials. Sports Medicine, 47(6), 1171–1182. https://doi.org/10.1007/s40279-016-0649-z
- Griffin, J. R., Maxwell, T. M., & Griffin, L. (2016). The prevalence and consequences of obesity in athletes. Current Orthopaedic Practice, 27(2), 129–134. https://doi.org/10.1097/BCO.0000000000000346
- Herrmann, S. D., Willis, E. A., Honas, J. J., Lee, J., Washburn, R. A., & Donnelly, J. E. (2015). Energy intake, nonexercise physical activity, and weight loss in responders and nonresponders: The midwest exercise trial 2. Obesity (Silver Spring), 23(8), 1539–1549. https://doi.org/10.1002/oby.21073
- Heymsfield, S. B., Harp, J. B., Reitman, M. L., Beetsch, J. W., Schoeller, D. A., Erondu, N., & Pietrobelli, A. (2007). Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. The American Journal of Clinical Nutrition, 85(2), 346–354. https://doi.org/10.1093/ajcn/85.2.346
- Hopkins W. G. (2015). Individual responses made easy. Journal of Applied Physiology, 118(12), 1444–1446. https://doi.org/10.1152/japplphysiol.00098.2015
- King, N. A., Caudwell, P., Hopkins, M., Byrne, N. M., Colley, R., Hills, A. P., Stubbs, J. R., & Blundell, J. E. (2007). Metabolic and behavioral compensatory responses to exercise interventions: Barriers to weight loss. Obesity (Silver Spring), 15(6), 1373–1383. https://doi.org/10.1038/oby.2007.164
- Kumahara, H., Schutz, Y., Ayabe, M., Yoshioka, M., Yoshitake, Y., Shindo, M., Ishii, K., & Tanaka H. (2004). The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: A validation study against whole-body indirect calorimetry. The British Journal of Nutrition, 91(2), 235–243. https://doi.org/10.1079/BJN20031033
- Leibel, R. L., Rosenbaum, M., & Hirsch, J. (1995). Changes in energy expenditure resulting from altered body weight. New England Journal of Medicine, 332(10), 621–628. https://doi.org/10.1056/NEJM199503093321001
- Levine, J., Melanson, E. L., Westerterp, K. R., & Hill, J. O. (2001). Measurement of the components of nonexercise activity thermogenesis. American Journal of Physiology-Endocrinology and Metabolism, 281(4), E670–E6E5. https://doi.org/10.1152/ajpendo.2001.281.4.E670
- Levine, J. A., Eberhardt, N. L., & Jensen, M. D. (1999). Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science, 283(5399), 212–214. https://doi.org/10.1126/science.283.5399.212
- Levine, J. A., Lanningham-Foster, L. M., McCrady, S. K., Krizan, A. C., Olson, L. R., Kane, P. H., Jensen, M. D., & Clark, M. M. (2005). Interindividual variation in posture allocation: Possible role in human obesity. Science, 307(5709), 584–586. https://doi.org/10.1126/science.1106561
- Martin, C. K., Das, S. K., Lindblad, L., Racette, S. B., McCrory, M. A., Weiss, E. P., DeLany, J. P., & Kraus, W. E. (2011). Effect of calorie restriction on the free-living physical activity levels of nonobese humans: Results of three randomized trials. Journal of Applied Physiology, 110(4), 956–963. https://doi.org/10.1152/japplphysiol.00846.2009
- Nunes, C. L., Casanova, N., Francisco, R., Bosy-Westphal, A., Hopkins, M., Sardinha, L. B., & Silva, A. M. (2021). Does adaptive thermogenesis occur after weight loss in adults? A systematic review. British Journal of Nutrition, 1–43. https://doi.org/10.1017/S0007114521001094.
- Nunes, C. L., Jesus, F., Francisco, R., Matias, C. N., Heo, M., Heymsfield, S. B., Bosy-Westphal, A., Sardinha, L. B., Martins, P., Minderico, C. S., & Silva, A. M. (2022). Adaptive thermogenesis after moderate weight loss: Magnitude and methodological issues. European Journal of Nutrition, 61(3), 1405–1416. https://doi.org/10.1007/s00394-021-02742-6
- Ostendorf, D. M., Caldwell, A. E., Creasy, S. A., Pan, Z., Lyden, K., Bergouignan, A., MacLean, P. S., Wyatt, H. R., Hill, J. O., Melanson, E. L., & Catenacci, V. A. (2019). Physical activity energy expenditure and total daily energy expenditure in successful weight loss maintainers. Obesity (Silver Spring), 27(3), 496–504. https://doi.org/10.1002/oby.22373
- Park, Y. W., Heymsfield, S. B., & Gallagher, D. (2002). Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity, 26(7), 978–983. https://doi.org/10.1038/sj.ijo.0801982.
- Racette, S. B., Schoeller, D. A., Kushner, R. F., Neil, K. M., & Herling-Iaffaldano, K. (1995). Effects of aerobic exercise and dietary carbohydrate on energy expenditure and body composition during weight reduction in obese women. The American Journal of Clinical Nutrition, 61(3), 486–494. https://doi.org/10.1093/ajcn/61.3.486
- Redman, L. M., Heilbronn, L. K., Martin, C. K., de Jonge, L., Williamson, D. A., Delany, J. P., & Ravussin, E. (2009). Metabolic and behavioral compensations in response to caloric restriction: Implications for the maintenance of weight loss. PloS one, 4(2), e4377. https://doi.org/10.1371/journal.pone.0004377
- Riou, M.-E., Jomphe-Tremblay, S., Lamothe, G., Finlayson, G. S., Blundell, J. E., Décarie-Spain, L., Gagnon, J.-C., & Doucet, É. (2019). Energy compensation following a supervised exercise intervention in women living with overweight/obesity is accompanied by an early and sustained decrease in non-structured physical activity. Frontiers in Physiology, 10, 1048. https://doi.org/10.3389/fphys.2019.01048
- Rosenbaum, M., Ravussin, E., Matthews, D. E., Gilker, C., Ferraro, R., Heymsfield, S. B., Hirsch, J., & Leibel, R. L. (1996, March). A comparative study of different means of assessing long-term energy expenditure in humans. American Journal of Physiology, 270(3 Pt 2), R496–R504. https://doi.org/10.1152/ajpregu.1996.270.3.R496.
- Rosenbaum, M., Vandenborne, K., Goldsmith, R., Simoneau, J.-A., Heymsfield, S., Joanisse, D. R., Hirsch, J., Murphy, E., Matthews, D., Segal, K. R., & Leibel, R. L. (2003). Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 285(1), R183–RR92. https://doi.org/10.1152/ajpregu.00474.2002
- Sasaki, J. E., John, D., & Freedson, P. S. (2011). Validation and comparison of ActiGraph activity monitors. Journal of Science and Medicine in Sport, 14(5), 411–416. https://doi.org/10.1016/j.jsams.2011.04.003
- Silva, A. M., Júdice, P. B., Carraça, E. V., King, N., Teixeira, P. J., & Sardinha, L. B. (2018). What is the effect of diet and/or exercise interventions on behavioural compensation in non-exercise physical activity and related energy expenditure of free-living adults? A systematic review. British Journal of Nutrition, 119(12), 1327–1345. https://doi.org/10.1017/S000711451800096X
- Silva, A. M., Nunes, C. L., Jesus, F., Francisco, R., Matias, C. N., Cardoso, M., Santos, I., Carraça, E. V., Silva, M. N., Sardinha, L. B., Martins, P., & Minderico, C. S. (2021). Effectiveness of a lifestyle weight-loss intervention targeting inactive former elite athletes: The Champ4Life randomised controlled trial. British Journal of Sports Medicine, 12(2). https://doi.org/10.3390/nu12020286.
- Silva, A. M., Nunes, C. L., Matias, C. N., Jesus, F., Francisco, R., Cardoso, M., Santos, I., Carraça, E. V., Silva, M. N., Sardinha, L. B., Martins, P., & Minderico, C. S. (2020). Champ4life study protocol: A one-year randomized controlled trial of a lifestyle intervention for inactive former elite athletes with overweight/obesity. Nutrients, 12(2), 286. https://doi.org/10.3390/nu12020286
- Stubbs, R. J., Hughes, D. A., Johnstone, A. M., Horgan, G. W., King, N., & Blundell, J. E. (2004). A decrease in physical activity affects appetite, energy, and nutrient balance in lean men feeding ad libitum. The American Journal of Clinical Nutrition, 79(1), 62–69. https://doi.org/10.1093/ajcn/79.1.62
- Swift, D. L., McGee, J. E., Earnest, C. P., Carlisle, E., Nygard, M., & Johannsen, N. M. (2018). The effects of exercise and physical activity on weight loss and maintenance. Progress in Cardiovascular Diseases, 61(2), 206–213. https://doi.org/10.1016/j.pcad.2018.07.014
- Swinton, P. A., Hemingway, B. S., Saunders, B., Gualano, B., & Dolan, E. (2018). A statistical framework to interpret individual response to intervention: Paving the way for personalized nutrition and exercise prescription. Frontiers in Nutrition, 5. https://doi.org/10.3389/fnut.2018.00041
- von Loeffelholz, C., & Birkenfeld, A. (2000). The role of non-exercise activity thermogenesis in human obesity. MDText.com, Inc. Retrieved April 9, 2018, from https://www.ncbi.nlm.nih.gov/books/NBK279077/
- Weir, C. B., & Jan, A. (2022). BMI classification percentile and cut off points. Statpearls. StatPearls Publishing. Retrieved April 20, 2019.
- Weststrate J. A. (1993). Resting metabolic rate and diet-induced thermogenesis: A methodological reappraisal. The American Journal of Clinical Nutrition, 58(5), 592–601. https://doi.org/10.1093/ajcn/58.5.592
- World Medical Association. (2008). Declaration of Helsinki – ethical principles for medical research involving human subjects. WMJ, 54(4), 122–125. Retrieved 15 September, 2022, https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/doh-oct2008/.